Abstract
The medaka, Oryzias latipes, is a well-recognized fish model for biomedical research. An understanding of gamete characteristics is necessary for experimental manipulations such as artificial fertilization and sperm cryopreservation. The goal of this study was to investigate sperm characteristics of motility initiation, duration, and retention in medaka. First, motility was initiated by osmolality values ranging from 25 to 686 mOsm/kg, which included deionized water and hypotonic, isotonic, and hypertonic Hanks’ balanced salt solution. The percentage of motile sperm was >80% when osmolality was <315 mOsm/kg and decreased as osmolality increased. This is different from most fish with external fertilization in which sperm motility can be initiated by hypotonic (for freshwater fish) or hypertonic (for marine fish) solutions or by altering the concentration of specific ions such as potassium (e.g., in salmonids). Second, upon activation, the sperm remained continuously motile, with reserve capacity, for as long as 1 wk during storage at 4 °C. This was also different from other externally fertilizing fish, in which motility is typically maintained for seconds to several minutes. Third, after changing the osmolality to 46 to 68 mOsm/kg by adding deionized water, the motility of sperm held at 274 to 500 mOsm/kg was higher than the original motility (P ≤ 0.035) after 24, 48, and 72 h of storage at 4 °C. Fourth, the addition of glucose had no effect on maintaining sperm motility during refrigerated storage. To our knowledge, this combination of sperm motility characteristics is reported for the first time in fish and may be unique to medaka or may represent an undescribed modality of sperm behavior within euryhaline fish.
Keywords: Fish, Medaka, Motility initiation, Oryzias latipes, Sperm, Swimming duration
1. Introduction
The medaka, Oryzias latipes, has external fertilization and is generally considered to be a euryhaline species, although it cannot withstand direct transfer from freshwater into seawater [1]. It is a well-recognized fish model for biomedical research because of features such as small body size, daily spawning activity, transparency of embryos, short generation time, small genome size, and established transgenic techniques [2–4]. With extensive use of this fish in various research fields, techniques for gamete handling have become necessary for experimental manipulations such as intracytoplasmic sperm injection, in vitro fertilization, and gamete cryopreservation. Currently, most research on gamete characteristics in medaka has focused on eggs, and little information is available for sperm, especially sperm motility.
Generally, sperm motility initiation in fish can be grouped roughly into the following modes: (1) For most fish with external fertilization, sperm motility can be initiated by either hypotonic (for freshwater fish) or hypertonic (for marine fish) osmolalities [5,6]. Once activated, the sperm generally have a short period of motility (30 s to 5 min), and usually the swimming duration of sperm from marine fish is longer than that of sperm from freshwater fish [7]. (2) Sperm motility can be initiated by alteration of the concentration of certain ions, such as in salmonid fishes, where motility can be initiated by reducing the concentration of potassium ions [8,9]. Or, as another example, in tilapia, Oreochromis mossambicus, intracellular calcium is required for activation of sperm motility and can prolong the motility period [10]. (3) For live-bearing (internal fertilization) fish, such as those of the freshwater genus Xiphophorus [11] and the marine ocean pout Macrozoarces americanus [12,13], sperm motility can be initiated by isotonic osmolalities but not by hypotonic or hypertonic osmolalities. Once initiated, the sperm of these species can remain continuously motile for as long as 1 wk [14]. To date, the motility initiation of sperm in fish was generally considered to be an osmotic-shock–dependent or ionic-signal–dependent event [15].
In this study, we investigated the characteristics of sperm motility initiation, duration, and retention during refrigerated storage in medaka. At present, only two meeting abstracts [16,17] and one research report [1] have mentioned the motility initiation of medaka sperm, but no detailed study was reported. Our objectives were to evaluate (1) sperm motility initiation after suspension in deionized water and different osmolalities of Hanks’ balanced salt solution (HBSS); (2) duration of continuous motility at various osmolalities of HBSS; (3) retention of motility capacity of sperm held at different osmolalities during refrigerated storage at 4 °C for as long as 168 h; and (4) the effect of glucose in the storage buffer on maintaining sperm motility.
2. Materials and methods
2.1. Fish
The medaka used in this study were 6 to 8 mo old with standard lengths of 2.7 ± 0.1 cm (mean ± SD) and body weights of 0.375 ± 0.090 g. They were cultured in freshwater aquaria at the University of Georgia and obtained by overnight shipping to Baton Rouge, Louisiana. Upon arrival, the fish were maintained in freshwater aquaria (1 fish/L) with water flow recirculated through an upwelling bead filter at 26 °C and were fed twice daily with commercial flakes (Aquatic Eco-system, Apopka, FL, USA) and live Artemia larvae grown from cysts (INVE group; Grantsville, UT, USA). The photoperiod was set at 14 h light:10 h dark. Guidelines from the Institutional Animal Care and Use Committee of the Louisiana State University Agricultural Center were followed for animal care in this study.
2.2. Sperm collection
Sperm was collected by crushing of dissected testis. Male fish were anesthetized on ice for 1 min and blotted with a paper towel to dry the body. The testes (2.4 ± 0.8 mg) were removed and separated from surrounding lipid tissues while viewing with a dissecting microscope (×10 magnification), and transferred to 1.5-mL centrifuge tubes for weighing. Hanks’ balanced salt solution (HBSS; 0.137 M NaCl, 5.4 mM KCl, 1.3 mM CaCl2, 1.0 mM MgSO4, 0.25 mM Na2HPO4, 0.44 mM KH2PO4, 4.2 mM NaHCO3, and 5.55 mM glucose, pH = 7.2) was used as the extender (storage buffer) in this study. Various osmolalities of HBSS (from 92 to 900 mOsm/kg) were prepared by adjusting the volume of deionized water and were verified by use of a vapor pressure osmometer (Model 5520; Wescor Inc., Logan, UT, USA).
2.3. Motility estimation
The motility of sperm was estimated visually by observing at ×200 magnification using dark-field microscopy (Optiphot 2; Nikon Inc., Garden City, NY, USA) with an accuracy of ±5%. The motility was expressed as the percentage of sperm that moved actively in a forward direction; sperm that vibrated in place were not counted as motile. For each sample, the sperm motility was estimated at least two times with three fields observed each time.
2.4. Sperm motility initiation in HBSS with different osmolalities
Because of the tiny volume (microliters) of sperm available from these small-bodied fish, testes from nine males were used, with three individuals pooled as each replicate. After collection, testes in each replicate were gently dissociated with scissors and forceps, and 0.2 μL dry sperm (undiluted) was placed on a slide and observed by use of a microscope. For activation, sperm were mixed on the slide with 20 μL HBSS at each osmolality from 25 to 900 mOsm/kg at intervals of ~50 mOsm/kg. The osmolality of 25 mOsm/kg was deionized water only, and the osmolalities from 92 to 900 mOsm/kg were diluted or condensed HBSS buffer, prepared by adjusting the volume of deionized water and verified by use of a vapor pressure osmometer.
2.5. Duration of sperm continuous motility
To evaluate continuous motility duration, after being suspended in HBSS with osmolalities ranging from 25 to 900 mOsm/kg, sperm suspensions were stored refrigerated (4° C), and motility was observed at 4, 24, 48, 72, and 168 h without dilution.
2.6. The retention of motility capability during refrigerated storage
To test the motility capability after refrigerated storage, 1 μL from each sperm sample extended at the 15 osmolalities was taken and placed on an individual slide, 20 μL of deionized water was added, and motility was observed immediately. After observation of motility, 10 μL of the mixture was removed from the slide for measurement of the final osmolality, which was 25 to 67 mOsm/kg.
2.7. Effect of exogenous carbohydrate on maintaining sperm motility
To test the effect of exogenous carbohydrate, testes from six males were used, with sperm from two individuals pooled as each replicate. Sperm suspensions were prepared by crushing the testes in HBSS prepared without glucose (265 mOsm/kg) at a volume of 10 times the testis weight. Immediately, aliquots of this sperm suspension were diluted with HBSS (with supplemented glucose) at a ratio of 1:1 to yield final concentrations of glucose at 5.55, 11.10, 27.75, and 55.50 mM and final osmolalities of 270, 272, 290, and 316 mOsm/kg. Motility of sperm was estimated at 0, 4, 24, 48, 72, and 168 h after refrigerated storage (4° C).
2.8. Data analysis
Data were analyzed using SYSTAT 12. The effects of treatments were tested by use of ANOVA. Percentage data were arcsine-transformed before analysis, and post hoc Tukey’s test was used to locate differences. The significance level was set at P < 0.050.
3. Results
3.1. Sperm motility initiation with osmolalities
Dry sperm were immotile. After suspending in HBSS, the initiation of sperm motility generally decreased as the osmotic pressure of the HBSS increased (Fig. 1). When osmolality ranged from 25 to 227 mOsm/kg, motility of activated sperm was >90%, and the swimming motion was progressive. As osmolality increased from 315 to 373 mOsm/kg, motility decreased from 85% to 67% (P = 0.023) and to 27% when osmolality increased to 454 mOsm/kg (P < 0.001). When the osmolality increased to above 500 mOsm/kg, the motility decreased to <7%, and at ≥ 750 mOsm/kg or above, sperm motility was completely inhibited.
Fig. 1.
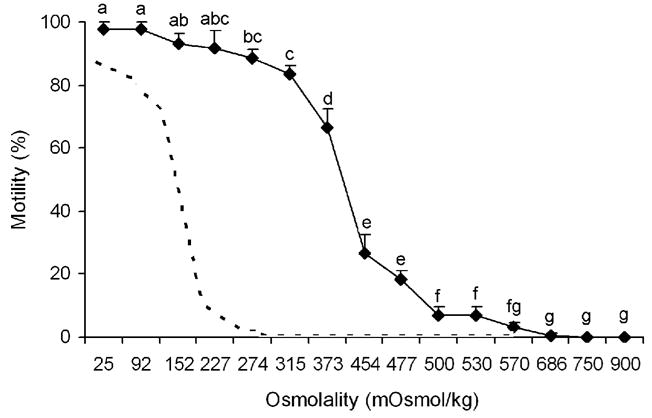
Motility of sperm (mean ± SD, n = 3) from medaka (Oryzias latipes) activated at various osmolalities of HBSS and deionized water (25 mOsm/kg). Note: The dotted line is the sperm activation curve of zebrafish [18], provided for comparison with the activation profile of a typical externally fertilizing freshwater fish. a–gMeans without a common superscript differed (P < 0.05).
3.2. Duration of sperm continuous motility
After 4 h of storage, the motility of sperm in all of the osmolalities decreased (Fig. 2). The motility of sperm in 25 mOsm/kg (deionized water) declined to zero, and in osmolalities ≥454 mOsm/kg, motility decreased to less than 8%. With additional storage for 24, 48, and 72 h, the motility of sperm continued to decrease (Fig. 2). The sperm motility at osmolalities between 92 and 373 mOsm/kg declined to ~20% at 72 h. After 168 h of storage, the motility of sperm in all of the osmolalities dropped to 0 to 2% (Fig. 2). Therefore, after activation, medaka sperm remained motile (albeit with decreasing intensity) for as long as 1 wk.
Fig. 2.
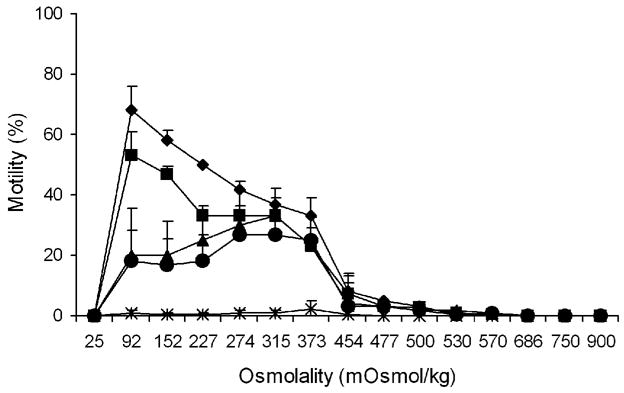
Continuous motility duration of sperm (mean ± SD, n = 3) evaluated at 4 h (diamonds), 24 h (squares), 48 h (triangles), 72 h (circles), and 168 h (stars) after activation in medaka (Oryzias latipes) at various osmolalities of HBSS.
3.3. The retention of motility capability during refrigerated storage
After 4 h of storage at 4° C, motility of sperm extended in all osmolalities was activated by the addition of deionized water, except for the samples held at 25 and 900 mOsm/kg HBSS (Fig. 3), and the motility of sperm extended above 274 mOsm/kg was higher than the initial motility at the samples (P ≤ 0.039). After 24 h of storage, only samples held between 274 and 530 mOsm/kg were able to respond to the addition of deionized water with an increase in motility compared with the initial motilities (P ≤ 0.05; Fig. 3). The same situation occurred after 48 h of storage (P ≤ 0.04; Fig. 3). After 72 h of storage, the motility (after addition of deionized water) of samples held at osmolalities between 152 and 500 mOsm/kg was higher than the initial motilities (P ≤ 0.035; Fig. 3). After 168 h of storage, motility was less than 10% in all samples before and after addition of deionized water (Fig. 3).
Fig. 3.
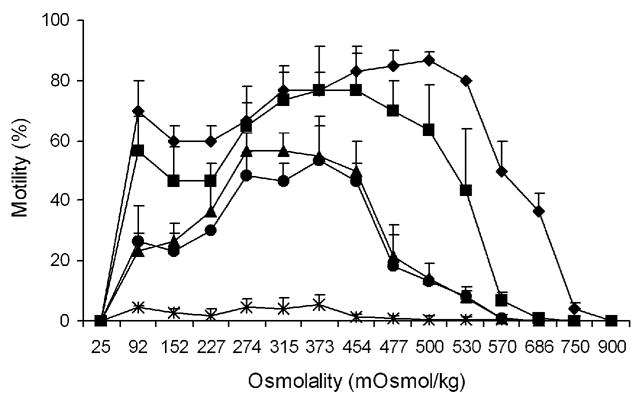
Retention of motility capacity of medaka (Oryzias latipes) sperm (mean ± SD, n = 3) after refrigerated storage for 4 h (diamonds), 24 h (squares), 48 h (triangles), 72 h (circles), and 168 h (stars) at various osmolalities of HBSS. Motility was activated by reducing the storage osmolality to 46 to 68 mOsm/kg by addition of deionized water (1:20).
3.4. Effect of exogenous carbohydrate on sperm motility
The osmolalities of the HBSS with glucose were 265 mOsm/kg (0 mM glucose), 270 mOsm/kg (5.55 mM), 272 mOsm/kg (11.10 mM), 290 mOsm/kg (27.75 mM), and 316 mOsm/kg (55.50 mM); all of these were in the range suitable to initiate sperm motility. The motility of sperm held in HBSS with various glucose concentrations decreased from >90% to 14% to 17% after 168 h of storage at 4 °C (Fig. 4). There were no differences in motility of sperm held in HBSS with the five concentrations of glucose immediately after suspension (P ≥ 0.950) and after storage for 4 (P ≥ 0.927), 24 (P ≥ 0.999), 48 (P ≥ 0.988), 72 (P ≥ 0.997), and 168 h (P ≥ 0.999). Therefore, exogenous glucose did not affect the duration of motility of medaka sperm.
Fig. 4.
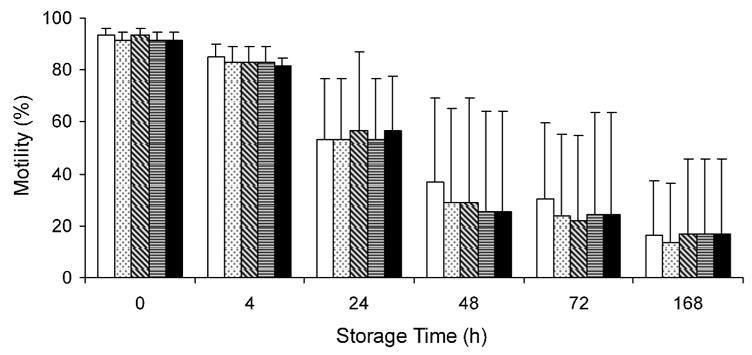
Motility of sperm (mean ± SD, n = 3) held in HBSS with glucose concentrations of 0 (clear), 5.55 mM (dotted), 11.10 mM (slashed), 27.75 mM (horizontal lined), and 55.50 mM (black) after storage at 4 °C for 0, 4, 24, 48, 72, and 168 h. No significant differences were detected in sperm motility among the glucose concentrations at each storage time.
4. Discussion
In this study, sperm motility in medaka was initiated across a broad range of osmolalities varying from deionized water (25 mOsm/kg) and HBSS with hypotonic, isotonic, and hypertonic osmolalities ranging from 92 to 686 mOsm/kg. In previous publications, medaka sperm were found to be functional in freshwater but not in seawater (equal to an osmolality of ~900 mOsm/kg) [1], and it was mentioned that motility of medaka sperm could be activated by a wide range of osmolality [16,17], but the specific osmolalities for sperm activation were not described. This motility activation mode defined in the current study in medaka sperm is different from the typical mode in freshwater species, in which motility is activated by hypotonic solutions (<300 mOsm/kg) [5], such as in zebrafish Danio rerio (shown as the dotted line in Fig. 1 for comparison) [18], although the medaka used in this experiment originated from and were maintained in freshwater. Also, this mode is different from that described in most externally fertilizing marine fish [6,9] and in fish with internal fertilization [11,14]. Thus, the broad osmotic range of motility activation in medaka is distinctive from the modes described previously in fish. Generally, motility activation relates to the natural conditions where spermatozoa function during spawning. Medaka live in freshwater but can acclimate to brackish water or even seawater (by gradually increasing the salinity) and are considered to be euryhaline fish [1]. Therefore, the motility activation mode reported here is likely linked to the adaptability of medaka to salinity changes [1] and most importantly could be representative for other euryhaline fish.
In tilapia, Oreochromis mossambicus, another euryhaline fish, sperm had a similar but attenuated pattern of motility activation. The particular fish studied originated from brackish water and were acclimated to freshwater [10] or seawater [19]. For the freshwater-acclimated tilapia, sperm motility could be activated by osmolalities ranging from 0 to 400 mOsm/kg, with or without electrolytes, and Ca2+ was found to be required for motility activation [10]. For the seawater-acclimated tilapia, sperm motility could be activated by osmolalities from 0 to 500 mOsm/kg of NaCl with 10 mM HEPES, and the addition of Ca2+ caused increased motility in the presence of high osmolalities (1000 to 1400 mOsm/kg [19]). In Fundulus grandis, another euryhaline fish that lives in brackish water, sperm motility was activated at osmolalities between that of freshwater and seawater, excluding freshwater and full-strength seawater (our unpublished data). Therefore, we inferred that motility activation in euryhaline fish can involve complicated mechanisms and may have different modes, depending on the ability to acclimate to different or variable salinities. More investigation is necessary to define the sperm activation mechanisms in euryhaline species.
Ions, such as potassium and calcium, have been shown to control the initiation of sperm motility [9,20]. For example, in chum salmon, Oncorhynchus keta, low concentrations of potassium ion (<10 mM) can override osmotic pressure to trigger motility [8]; potassium ion at a lower concentration (0.01 mM) controlled sperm activation in paddlefish Polyodon spathula and shovelnose sturgeon Scaphirhynchus platyorynchus [21]; and in tilapia, calcium ion enhanced sperm motility [10,19]. In medaka, extracellular Ca2+ has been mentioned in one abstract to be required for motility activation [17] but without detailed description. However, in this study, deionized freshwater at 25 mOsm/kg initiated sperm motility. Therefore, motility activation in medaka sperm may depend on osmolality rather than certain ions. More investigation is needed for estimating the function of the Ca2+ in motility activation of medaka sperm. Special attention should be given to osmotic and ionic conditions of the water from which experimental animals are obtained. Different results may occur and confound comparisons across studies if test animals are held in divergent conditions before analysis.
The long motility duration after activation was another distinguishing characteristic in medaka sperm. Typically, once activated, sperm will remain motile for a short time (seconds to several minutes) for most freshwater and marine fishes with external fertilization [7,9]. In contrast, sperm motility of fishes with internal fertilization, such as freshwater species of the genus Xiphophorus [11,14] and marine species Macrozoarces americanus [12,13], can remain continuously motile for 3 to 7 d. For medaka, the swimming duration after activation was for as long as 7 d at all osmolalities tested, except for 25 mOsm/kg (deionized water). To our knowledge, this phenomenon of protracted motility has not been reported in other externally fertilizing fish.
In this study, medaka sperm showed a successive phase of motility increase that could be induced by reducing the osmolality to 48 to 67 mOsm/kg with the addition of deionized water. This was especially true for the motility of sperm held at between 274 and 500 mOsm/kg, which was significantly increased after storage at 4° C for 24, 48, and 72 h. A similar phenomenon was observed in turbot Psetta maxima (a marine fish), in which motility was initiated by exposure to seawater, arrested by transfer to seminal fluid, and then reactivated by addition of seawater [22]. This phenomenon was also observed for Xiphophorus helleri, in which sperm immobilized for storage by suspension in higher osmolality (500 mOsm/kg) and activated by exposure to isotonic solutions had the same fertility as continuously motile (isotonic) suspension activated sperm after cryopreservation [11,23]. This characteristic of medaka sperm could assist the choice of appropriate extender osmolalities to maintain motility potential during storage, which is necessary in manipulations such as artificial fertilization and sperm cryopreservation. For most fish with external fertilization, the extender used for sperm handling and storage during sperm cryopreservation is usually a salt buffer with an appropriate osmolality to immobilize sperm, which are activated by increasing (for marine species) or decreasing (for freshwater species) the osmolality [6,7,9,18,24]. In contrast, due to the long duration of continuous motility in medaka sperm, the extender osmolality for storage of sperm could lie between 274 and 500 mOsm/kg in which motility can be maintained at 47% to 53% after 72 h.
In oviparous fish, previous studies have shown that sperm obtain energy for movement through the oxidation of endogenous substrates, not exogenous sources such as carbohydrate, and mitochondria are the source of the endogenous energy for the sperm motility of most fish species [25]. However, in viviparous fish, sperm have been reported to be capable of using external energy sources, such as the metabolism of carbohydrate [26,27]. In Xiphophorus helleri, the addition of glucose yielded higher and longer motility for fresh and cryopreserved sperm, but there was a plateau, and this effect was not seen when the concentration of glucose was higher than 11.10 mM [28]. For medaka sperm in this study, the presence or absence of glucose did not have a significant effect on motility, which suggests that medaka sperm does not make use of extracellular glucose as an energy source for motility. The mechanism responsible for enabling the long swimming duration in medaka sperm requires further investigation.
In summary, the distinguishing characteristics of medaka sperm identified in this study were the following: (1) motility that could be initiated by a wide range of osmolalities (from 25 to 686 mOsm/kg) including, hypertonic, isotonic, and hypertonic pressures; (2) upon activation, sperm could remain continuously motile for as long as 7 d; (3) motility capacity could be enhanced by reduced osmotic pressure when held in HBSS at osmolalities between 274 and 500 mOsm/kg; and (4) glucose was not necessary as an exogenous energy source for maintaining the long duration of sperm motility.
This combination of sperm characteristics was distinctive. Medaka are widely distributed in nature throughout freshwater systems in China, Japan, and Korea [29,30] and are found in brackish and coastal waters as well [31]. Unlike the strictly freshwater zebrafish, medaka has the ability to be acclimated from freshwater (25 mOsm/kg) to brackish water (between 25 mOsm/kg and 900 mOsm/kg) and even seawater (~900 mOsm/kg) [1]. These habitat features suggest an explanation for the distinctive sperm characteristics described in this study. Medaka may be adapted to live and spawn in an osmotic range of natural habitats that span from hypotonic to hypertonic. They could be unique in this regard, or potentially are representative of euryhaline species. These species, especially small-bodied species, have not received much study of sperm characteristics, and further research is warranted based on these and other observations in medaka [1,16,17]. Furthermore, these sperm characteristics found in medaka seemed to be more related to those of internal fertilizing fish than to those of externally fertilizing species [32] and provide a basis for hypotheses that address whether estuarine or euryhaline species have traits that could have served as preadaptations or initiating steps to the processes that led to the evolution of internal fertilization in freshwater fish.
Acknowledgments
We thank R. Winn and M. Norris from the Aquatic Biotechnology and Environmental Laboratory of the University of Georgia for providing medaka and valuable discussion. We thank N. Novelo and E. Hu for help with the experiments. This work was supported in part by funding from the NIH National Center for Research Resources, the U.S. Department of Agriculture, the ACRES–LSU Collaborative Research Program, and the National and Louisiana Sea Grant College Programs. The manuscript of this article was approved for publication by the director of the Louisiana Agricultural Experiment Station as No. 2008-244-1711.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Inoue K, Takei Y. Diverse adaptability in Oryzias species to high environmental salinity. Zool Sci. 2002;19:727–734. doi: 10.2108/zsj.19.727. [DOI] [PubMed] [Google Scholar]
- 2.Naruse K, Sakaizumi M, Shima A. Medaka as a model organism for research in experimental biology. Fish Biol J Medaka. 1994;6:47–52. [Google Scholar]
- 3.Ishikawa Y. Medaka fish as a model system for vertebrate developmental genetics. Bioessays. 2000;22:487–495. doi: 10.1002/(SICI)1521-1878(200005)22:5<487::AID-BIES11>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Wittbrodt J, Shima A, Schartl M. Medaka - A model organism from the Far East. Nat Rev Genet. 2002;3:53–64. doi: 10.1038/nrg704. [DOI] [PubMed] [Google Scholar]
- 5.Morisawa M, Suzuki K. Osmolality and potassium ion - their roles in initiation of sperm motility in teleosts. Science. 1980;210:1145–1147. doi: 10.1126/science.7444445. [DOI] [PubMed] [Google Scholar]
- 6.Cosson J, Groison AL, Suquet M, Fauvel C, Dreanno C, Billard R. Marine fish spermatozoa: racing ephemeral swimmers. Reproduction. 2008;136:277–294. doi: 10.1530/REP-07-0522. [DOI] [PubMed] [Google Scholar]
- 7.Cosson J, Groison AL, Suquet M, Fauvel C, Dreanno C, Billard R. Studying sperm motility in marine fish: an overview on the state of the art. J Appl Ichthyol. 2008;24:460–486. [Google Scholar]
- 8.Morisawa M, Suzuki K, Morisawa S. Effects of potassium and osmolality on spermatozoan motility of salmonid fishes. J Exp Biol. 1983;107:105–113. doi: 10.1242/jeb.107.1.105. [DOI] [PubMed] [Google Scholar]
- 9.Cosson J. The ionic and osmotic factors controlling motility of fish spermatozoa. Aquacult Int. 2004;12:69–85. [Google Scholar]
- 10.Morita M, Takemura A, Okuno M. Requirement of Ca2+ on activation of sperm motility in euryhaline tilapia Oreochromis mossambicus. J Exp Biol. 2003;206:913–921. doi: 10.1242/jeb.00153. [DOI] [PubMed] [Google Scholar]
- 11.Yang H, Hazelwood L, Walter RB, Tiersch TR. Effect of osmotic immobilization on refrigerated storage and cryopreservation of sperm from a viviparous fish, the green swordtail Xiphophorus helleri. Cryobiology. 2006;52:209–218. doi: 10.1016/j.cryobiol.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Crim LW. Seasonal changes in the biochemistry of seminal plasma and sperm motility in the ocean pout, Macrozoarces americanus. Fish Physiol Biochem. 1997;16:77–83. [Google Scholar]
- 13.Yao Z, Crim LW, Richardson GF, Emerson CJ. Motility, fertility and ultrastructural changes of ocean pout Macrozoarces americanus L. sperm after cryopreservation Aquaculture. 2000;181:361–375. [Google Scholar]
- 14.Huang C, Dong Q, Walter RB, Tiersch TR. Initial studies on sperm cryopreservation of a live-bearing fish, the green swordtail Xiphophorus helleri. Theriogenology. 2004;62:179–194. doi: 10.1016/j.theriogenology.2003.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morisawa M. Adaptation and strategy for fertilization in the sperm of teleost fish. J Appl Ichthyol. 2008;24:362–370. [Google Scholar]
- 16.Kawaguchi K, Takeda Y, Namiki H, Okuno M. Activation mechanism for sperm motility in medaka Oryzias latipes. Zool Sci. 2004;21:1282. [abstract] [Google Scholar]
- 17.Kawaguchi K, Okuno M. Analysis of medaka sperm motility under the hyperosmolality and motility inhibition by CO2. Zool Sci. 2005;22:1445. [abstract] [Google Scholar]
- 18.Yang H, Carmichael C, Varga ZM, Tiersch TR. Development of a simplified and standardized protocol with potential for high-throughput for sperm cryopreservation in zebrafish Danio rerio. Theriogenology. 2007;68:128–136. doi: 10.1016/j.theriogenology.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morita M, Takemura A, Okuno M. Acclimation of sperm motility apparatus in seawater-acclimated euryhaline tilapia Oreochromis mossambicus. J Exp Biol. 2004;207:337–345. doi: 10.1242/jeb.00748. [DOI] [PubMed] [Google Scholar]
- 20.Alavi SMH, Cosson J. Sperm motility in fishes. (II) Effects of ions and osmolality: a review. Cell Biol Int. 2006;30:1–14. doi: 10.1016/j.cellbi.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Cosson J, Linhart O, Mims SD, Shelton WL, Rodina M. Analysis of motility parameters from paddlefish Polyodon spathula and shovelnose sturgeon Scaphirhynchus platorynchus spermatozoa. J Fish Biol. 2000;56:1348–1367. [Google Scholar]
- 22.Dreanno C, Cosson J, Suquet M, Seguin F, Dorange G, Billard R. Nucleotide content, oxidative phosphorylation, morphology, and fertilizing capacity of turbot Psetta maxima spermatozoa during the motility period. Mol Reprod Dev. 1999;53:230–243. doi: 10.1002/(SICI)1098-2795(199906)53:2<230::AID-MRD12>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 23.Yang H, Hazlewood L, Heater SJ, Guerrero PA, Walter RB, Tiersch TR. Production of F1 interspecies hybrid offspring with cryopreserved sperm from a live-bearing fish, the swordtail Xiphophorus helleri. Biol Reprod. 2007;71:401–406. doi: 10.1095/biolreprod.106.056549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suquet M, Dreanno C, Fauvel C, Cosson J, Billard R. Cryopreservation of sperm in marine fish. Aquac Res. 2000;31:231–243. [Google Scholar]
- 25.Gwo JC. Cryopreservation of sperm of some marine fishes. In: Tiersch TR, Mazik PM, editors. Cryopreservation in Aquatic Species. World Aquaculture Society; 2000. pp. 138–160. [Google Scholar]
- 26.Stoss J. Fish gamete preservation and spermatozoan physiology. In: Hoar WS, Randall DJ, Donaldson EM, editors. Fish Physiology, Part B, Behavior and Fertility Control. Academic Press; 1983. pp. 305–350. [Google Scholar]
- 27.Billard R, Cosson MP. The energetics of fish motility. In: Gagnon C, editor. Control of Sperm Motility: Biological and Clinical Aspects. CRC Press; 1990. pp. 153–173. [Google Scholar]
- 28.Dong Q, Huang C, Tiersch TR. Post-thaw amendment of cryopreserved sperm for use in artificial insemination of a viviparous fish, the green swordtail Xiphophorus helleri. Aquaculture. 2006;259:403–414. doi: 10.1016/j.aquaculture.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naruse K, Shima A, Matsuda M, Sakaizumi M, Iwamatsu T, Soeroto B, Uwa H. Distribution and phylogeny of rice fish and their relatives belonging to the suborder Adrianichthyoidei in Sulawesi, Indonesia. Fish Biol J Medaka. 1993;5:11–15. [Google Scholar]
- 30.Naruse K. Classification and phylogeny of fishes of the genus Oryzias and its relatives. Fish Biol J Medaka. 1996;8:1–9. [Google Scholar]
- 31.Miyamoto T, Machida T, Kawashima S. Influence of environmental salinity on the development of chloride cells of freshwater and brackish water medaka Oryzias latipes. Zool Sci. 1986;3:859–865. [Google Scholar]
- 32.Yang H, Tiersch TR. Current status of sperm cryopreservation in biomedical research fish models: zebrafish, medaka, and Xiphophorus. Comp Biochem Physiol C. 2008 doi: 10.1016/j.cbpc.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


