Abstract
Introduction
Extracellular matrix changes occur in many heart valve pathologies. For example, myxomatous mitral valves are reported to contain excess proteoglycans (PGs) and hyaluronan (HA). However, it is unknown which specific PGs are altered in myxomatous valves. Because PGs perform varied functions in connective tissues, this study was designed to identify and localize three matrix-associated PGs as well as HA and the HA receptor for endocytosis (HARE) within myxomatous and normal mitral valves.
Methods
Human mitral posterior leaflets (control n=6−9, myxomatous n=14−21, mean age 61 for all groups) were histochemically stained for PG core proteins, HA, and HARE. Stain intensity was semi-quantitatively graded to determine differences in marker abundance betweennormal and myxomatous valves. The PGs were localized to different regions of the leaflet by correspondence to parallel Movat stained sections
Results
The PGs decorin, biglycan and versican were more abundant in myxomatous valves than in normal controls (p<0.03). There was a gender effect on PG presence but no age related trends were observed. HA and HARE were distributed throughout all valves. There was no significant difference in HA between groups, but HARE expression was reduced in myxomatous valves compared to normal controls (p<0.002).
Conclusion
Excess decorin, biglycan and versican may be associated with the remodeling of other matrix components in myxomatous mitral valves. Decreased expression of HARE in myxomatous valves suggests that HA metabolism could be altered in myxomatous mitral valve disease. These finding contribute towards elucidating the pathogenesis of myxomatous mitral valve disease and developing potential new therapies.
Keywords: Myxomatous mitral valve, Proteoglycan, Hyaluronan, Remodeling, Extracellular matrix
1. Introduction
Myxomatous mitral valve disease is a common valve abnormality with an incidence of 2.4−5% [1]. Myxomatous degeneration is the most common cause of mitral regurgitation in older patients [2], but the cause and progression of myxomatous disease remains unclear. Myxomatous mitral valves are characterized by floppy leaflets and elongated or ruptured chordae tendineae, which have profoundly weakened material behavior compared to normal valve tissues [3]. Previous histological studies of myxomatous valves have revealed collagen disruption [4], elastic fiber fragmentation [5], and the accumulation of matrix metalloproteinases and glycosaminoglycans (GAGs) [6]. Currently, the only treatment for this disease is surgical repair; no medical therapies are available to treat the matrix changes associated with myxomatous degeneration. Although there are a number of different theories regarding the pathogenesis of myxomatous mitral valve disease, including the diminished healing response of aging valve tissue to mechanical stress [2], genetic abonormalities [7], collagen and matrix dissolution [6] or abnormal accumulation of proteoglycans (PGs) [8], there is no consensus as to the mechanism of myxomatous degeneration. It is, however, evident by histological examination that GAGs and PGs, as a general matrix class, are abnormally distributed within myxomatous mitral valves, but exactly which PGs are overabundant within myxomatous valves has not been previously investigated.
Given the diverse nature of different PGs and their associated GAG chains, and their functions in mediating extracellular matrix organization [9], it is compelling to ask which specific PG or PGs could be involved in the remodeling and resultant dysfunction of myxomatous mitral valves. PGs consist of at least one GAG chain attached to a core protein. All GAG types exist as components of a PG, except for hyaluronan (HA), which is unsulfated and frequently present as a free molecule, although HA may be non-covalently bound to the core protein of some of the larger hyalectin PGs through a link protein, also known as HA binding protein (HABP). The diverse group of PGs, as well as HA, performs many functions related to the growth, development and pathology of tissues [10], and likely performs many such roles in heart valves as well. It has been shown that three particular PGs (decorin, biglycan, and versican) are present in the mitral valve and that their relative abundance varies according to the type of loading experienced by specific valve regions [11]. In the regions that experienced tension, the small leucine-rich PGs decorin and biglycan were more abundant; these PGs mediate collagen fibrillogenesis [12] and sequester transforming growth factor-beta [13]. The large hyalectin PG versican was also found in the mitral valve; versican can aggregate with HA [14] to provide hydrated compressive resistance to tissues. Versican often co-localizes with elastic fibers and numerous cell associated molecules to regulate cell adhesion, proliferation and migration [14]. We have previously reported that there are elevated concentrations of selected GAG classes within myxomatous mitral valve chordae and posterior leaflets as compared to normal control tissues [15], but have not investigated specific classes of PGs within these valves. Therefore, the primary purpose of this study was to assess the location and abundance of specific PGs and the GAG HA within myxomatous and normal mitral valves to improve our understanding of this common valve disease and contribute towards developing novel treatments for this disease.
The secondary purpose of this study was an initial step towards investigating the mechanisms of turnover of PGs and GAGs, including HA, within heart valves. Understanding how the synthesis and degradation of PGs and GAGs are regulated within these tissues is important not only for myxomatous mitral valve disease but also for calcific aortic valve disease [16] and the design of bioprosthetic valves. In addition, understanding this regulation may lead to the development of novel medical therapies for valve disease or offer opportunities for earlier intervention. Because gaining this knowledge will be a profound undertaking, in this particular study we only investigated the potential for the turnover of HA in mitral valves via the HA receptor for endocytosis or HARE [17]. HARE, a.k.a. stabilin-2, is a cell receptor that enables the scavenging and clearance of HA and other GAGs from the blood [18]. Most abundant in lymph nodes, spleen, and liver, HARE has also been found in the heart valves of mice [19], but is absent from any other cardiovascular tissues. Based on the previously reported excess of HA in myxomatous tissues it was speculated that the abundance of HARE in human myxomatous heart valves, might be different from that of control valves. Therefore, in this study the abundance of specific PGs, HA, and HARE within normal and myxomatous mitral valves was measured using histochemical techniques.
2. Materials and Methods
2.1 Tissue Procurement
Normal control mitral valve posterior leaflets were obtained after autopsy. Control subjects demonstrating any cardiovascular disease were excluded from this study. Myxomatous mitral valve posterior leaflets were surgically resected from patients undergoing a surgical repair procedure to correct mitral regurgitation resulting from primary myxomatous degeneration (all had regurgitation grades of 3+/4+). The demographic makeup of all groups is shown in Table 1. Tissues for this study were provided by the Cleveland Clinic Foundation (Cleveland, OH), St. Luke's Episcopal Hospital (Houston, TX), Ben Taub Hospital (Houston, TX), and the Cooperative Human Tissue Network. The research use of these tissues was approved by the Institutional Review Boards at all institutions. Radially oriented segments of the leaflets were cut from the annulus to free edge and fixed immediately in 10% formalin to minimize autolysis. After overnight fixation, the tissue sections were paraffin embedded, cut in 5 μm full thickness sections, and mounted on glass slides according to standard procedures.
Table 1.
Subject demographics.
| Group | Subjects* |
Gender (M/F) |
Age (years) |
|---|---|---|---|
| PGs | |||
| Normal | 9 | 7/2 | 61 ± 15 |
| Myxomatous | 17 | 9/8 | 61 ± 10 |
| HA | |||
| Normal | 8 | 6/2 | 61 ± 15 |
| Myxomatous | 14 | 5/9 | 61 ± 11 |
| HARE | |||
| Normal | 6 | 5/1 | 60 ± 12 |
| Myxomatous | 21 | 9/12 | 61 ± 12 |
Data represented as mean ± standard deviation.
Due to the small size of some tissues, it was not possible to obtain histological sections for all stains from all subjects’ tissue blocks.
2.2 Histochemistry
Immunohistochemistry (IHC) was performed to localize the PGs decorin, biglycan and versican within the valve tissues using antibodies against their respective core proteins. Briefly, tissue slides were processed through a series of graded alcohols and hydrated to water. All slides were pretreated with chondroitinase ABC (200 mU/ml, 37°C, 1 hour) to remove the GAG chains from the PG core proteins. Sections were blocked with 10% goat-serum (Sigma, St. Louis, MO), then incubated with primary antibodies against PGs overnight at 4°C. The rabbit anti-human decorin (LF-136, dilution 1:800) and biglycan antibodies (LF-51, dilution 1:2000) were generously provided by Larry Fisher, Ph.D., NIH [20]; the murine antibody against mammalian versican (clone 2-B-1, dilution 1:5000) was purchased from Associates of Cape Cod (East Falmouth, MA). After the slides had been rinsed in phosphate buffered saline, biotinylated secondary antibodies (goat anti-mouse or anti-rabbit IgG; Jackson Immunoresearch, Inc., West Grove, PA) were applied for 1 h at room temperature. Postive staining was demonstrated by a chromogen reaction using Vectastain Elite ABC and diaminobenzidine kits (Vector Laboratories, Burlingame, CA). All samples were counterstained with hematoxylin.
A set of mitral valves was also stained for HARE and HA (Table 1). The immunohistochemical staining for HARE was performed as described above except that the pretreatment was performed using 0.1 N HCl for 15 minutes at 37°C and the mAb159 antibodies for human HARE were developed in Dr. Weigel's laboratory [17]. The presence of HA was demonstrated by histochemical binding to an HA binding protein (HABP) [21], which contains the “link” domain protein that normally binds HA to aggrecan or versican in the formation of a PG aggregate. This HA staining procedure required blocking with 2% fetal bovine serum, treating with biotinylated HABP (exactly as described by Lara et al. [21]), then performing chromogenic detection as described above. To minimize variability, all normal and myxomatous tissues were stained together in one batch.
Negative controls for all markers were performed in the absence of the primary antibody. After staining, samples were blinded and graded on a scale of 0−4 to evaluate overall intensity. Grade “0” was chosen for no staining, “1” for weak stain in <50% of tissue, “2” for weak stain in >50% of tissue or strong stain in <10% of tissue, “3” for strong stain in >10% but <50% of tissue and “4” for strong stain in >50% of tissue [6]. Grading was also performed on the negative controls to assess the amount of nonspecific staining. Between 1 to 3 sections of a given valve sample were stained for a given marker and the staining intensity of all tissue sections was graded twice. Replicates were averaged and the staining and grading variability was calculated to be less than 15%.
2.3 Localization
Parallel sections of the posterior leaflets were also stained with Movat pentachrome to co-localize the different PGs, which stained blue/green, with collagen and elastin, which were stained yellow and black, respectively. Mapping the ECM composition of these sections also enabled the comparison of PG localization between the deep spongiosa layer and the more superficial atrialis and fibrosa layers of the mitral valve.
2.4 Data Analysis
A one-way ANOVA (SigmaStat, San Jose, CA) was used to compare immunohistochemical data between the normal and myxomatous valve groups. A two-way ANOVA with post hoc Tukey testing was performed to examine the effect of gender by choosing factor 1 as valve condition (normal vs. myxomatous) and factor 2 as gender (male vs. female). A Spearman rank order correlation was performed to determine if there were any age related trends in the data. In all cases, the level of significance was chosen as 0.05.
3. Results
3.1 IHC for decorin, biglycan and versican
Myxomatous valve sections showed overall higher PG staining intensity, with significantly greater abundance of decorin (p<0.001), biglycan (p<0.003) and versican (p<0.03) as compared to normal valves (Figs. 1 and 2). The staining intensity of versican in the myxomatous mitral valves was almost a full grade stronger than in the normal valves and the staining intensities of decorin and biglycan were more than a full grade stronger in the myxomatous valves. The intensity of non-specific staining in the samples stained for the various PGs, which was evaluated by staining the negative controls with the secondary antibody only, was graded as 0.16 ± 0.28 (mean ± standard deviation, n=24) for normal valves and 0.07 ± 0.24 (n=22) for myxomatous valves.
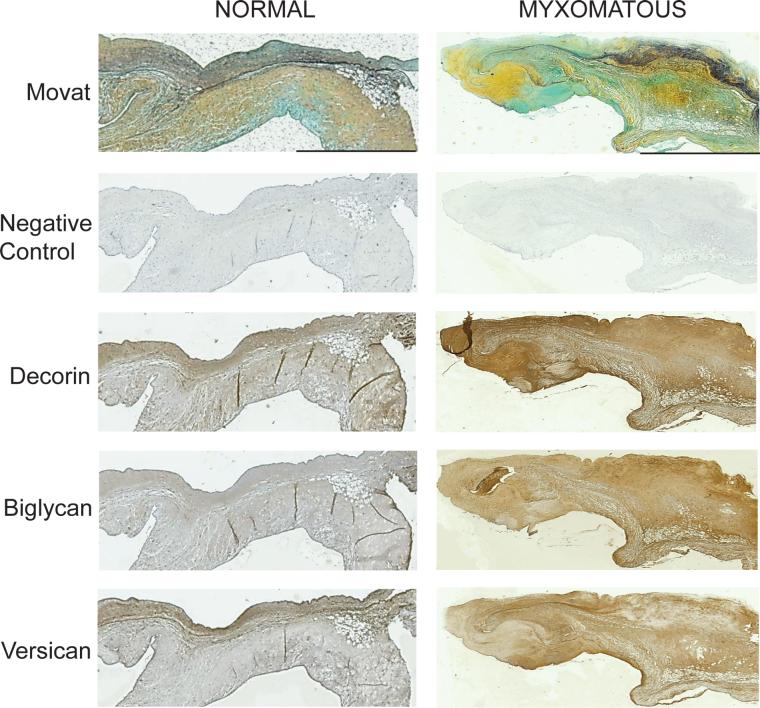
Figure 1.
Normal and myxomatous valve sections immunohistochemically stained for the core proteins of decorin, biglycan and versican. The scale bar for each valve represents 1 mm. Images of the same valve stained with Movat pentachrome (collagen — yellow, elastin — black, proteoglycans — blue/green) are provided to demonstrate the leaflet's layered microstructure.
Figure 2.
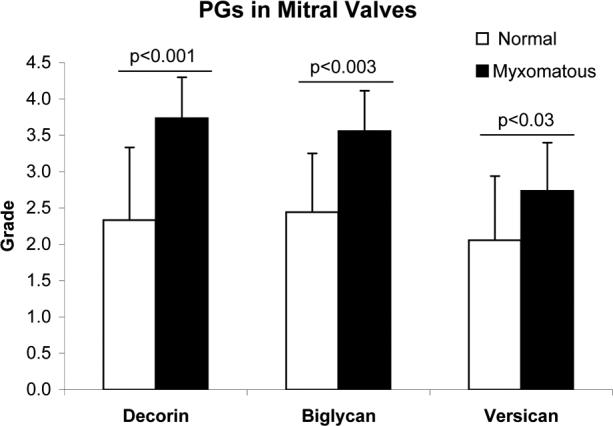
Abundances of decorin, biglycan and versican in normal and myxomatous mitral valves. Grading was performed on immunohistochemically stained valve sections using a scale of 0 to 4. Data represented as mean ± standard deviation.
3.2 IHC for HA and HARE
There was no significant difference found in HABP staining for HA between normal and myxomatous valves (Figs. 3 and 4). However, there was almost one full grade less HARE expression in myxomatous valves than in normal valves (p<0.002). In the negative controls, the intensity of non-specific staining was 0.22 ± 0.42 (n=24) in the samples stained for HA and 0.13 ± 0.35 (n=20) in the samples stained for HARE.
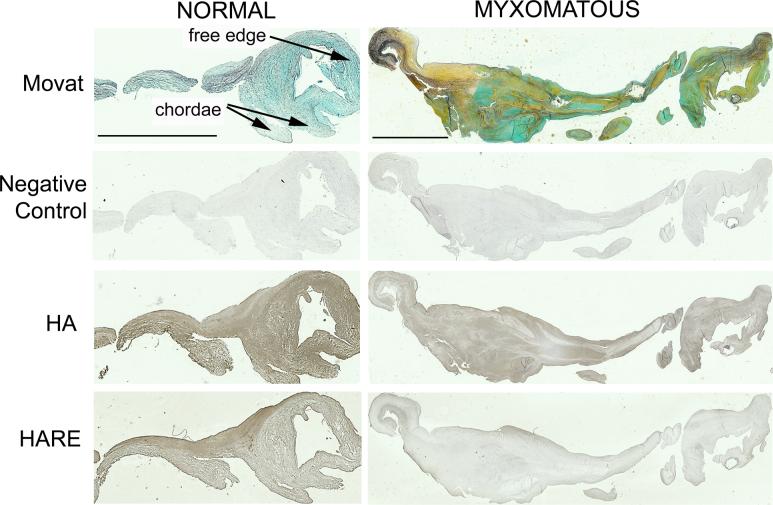
Figure 3.
Normal and myxomatous valve sections histochemically stained for HA and HARE. The scale bar for each valve represents 500 μm. Images of the same valve stained with Movat pentachrome (collagen — yellow, elastin — black, proteoglycans — blue/green) are provided to demonstrate the leaflet's layered microstructure.
Figure 4.
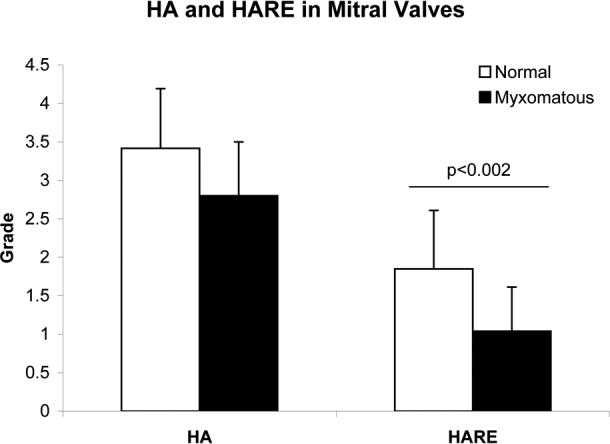
Abundances of HA and HARE in normal and myxomatous mitral valves. Grading was performed on histochemically stained valve sections using a scale of 0 to 4. Data represented as mean ± standard deviation.
3.3 Co-localization of specific PGs and other extracellular matrix components
Comparison of Movat Pentachrome stained valve tissues with the histochemically stained tissues revealed many interesting patterns regarding the location of PGs. The Movat stain demonstrated the three layers of the normal mitral valve: the collagen-rich fibrosa (lower outer layer), the PG-rich spongiosa (deep inner layer), and the atrialis (upper outer layer), which contains several thin layers of elastic fibers (Fig. 1). It was difficult to distinguish the layered structure in Movat stains of myxomatous valves as these valves were deformed, irregularly shaped and had disorganized ECM. In both normal and myxomatous valve sections, staining for decorin was co-localized with collagen (Fig. 1). However, biglycan was found in locations that also stained strongly for both collagen and elastin. Interestingly, versican staining corresponded to leaflet regions that were rich in PG and elastic fibers for both normal and myxomatous valves.
HA staining was found to be greater in the presence of elastin but lower in the presence of collagen (Fig. 3). Similarly, HARE binding was strong in elastin rich regions (Fig. 3).
3.4 Effect of gender and age on abundance of PGs
Of all the PGs, only the abundance of versican had any relation to gender. This association with gender was found in both normal and myxomatous valves. Interestingly, versican staining was stronger for females (compared to males) in normal controls (male 1.7±0.5 vs. female 3.2±1.1; p<0.01) but showed a trend of being lower in myxomatous valves (male 3.0±0.7 vs. female 2.4±0.5; p=0.07). Also, when data describing the abundance of each PG were grouped together, myxomatous valves from males showed a trend of greater PG abundance than found in valves from females (3.5±0.6 vs. 3.1±0.8, p=0.06). There was no significant correlation between age and the abundance of specific PGs. However, HARE expression in normal valves significantly increased with age (R2 = 0.62; Spearman correlation, p<0.001).
4. Discussion
This study demonstrated differences in the abundance and localization of specific PGs within normal and myxomatous mitral valves. Decorin, biglycan and versican, three PGs previously found in normal mitral valves [11], were present in significantly greater abundance in myxomatous valves. We did not find significant differences in the presence of HA within normal and myxomatous valve sections, although the abundance of HARE was stronger in normal valves. This is the first study to demonstrate the presence of HARE in human heart valves.
The stronger immunohistochemical staining for all three PGs within myxomatous valves was unexpected, given our previous analysis of GAGs using fluorophore—assisted carbohydrate electrophoresis (FACE) [15]. FACE analysis showed that myxomatous valves are particularly rich in 6-sulfated GAGs, which are strongly associated with the PG versican [22], but not decorin or biglycan. We speculate that these differences between the FACE and immunohistochemical results arose because FACE measures GAGs, whereas these valve samples were stained for the core proteins of the different PGs. The amount, type, or sulfation pattern of the GAGs associated with the different PGs may be responsible for these differences. More specifically, decorin and biglycan have 1 and 2 GAG chains respectively, so their GAG content may represent only a small percentage of total leaflet GAGs measured by FACE, even if their core proteins are shown to be abundant within the tissues. Versican, in contrast, contains 15−20 GAG chains and hence the numerous GAGs from versican may be disproportionally abundant in a FACE analysis. The relative amount of GAG per PG may also explain the location of these PGs within the different layers of the valve. Versican immunostaining was strong in areas of the leaflet that also stained strongly with alcian blue from the Movat pentachrome stain, indicating an abundance of GAGs, but decorin and biglycan were not strongly stained in the blue regions. There are also significant differences in the preparation of the tissue between these two methods. In the future, it will be important to confirm these patterns of PG abundance using biochemical methods such as western blotting.
Although there has been very little investigation of the upregulation of specific types of PGs in myxomatous valves, our results are consistent with a previous report showing increased decorin gene expression in myxomatous leaflets [23]. In this study, decorin was strongly co-localized with collagen fibers in both normal and myxomatous valves; localization of decorin with collagen fibers has also been shown by others [12, 24]. This co-localization is understandable given that decorin is known to regulate collagen fibrillogenesis [12]. Biglycan, which is a small leucine-rich PG closely related to decorin, was found in both collagen and elastin rich regions of the leaflets. Biglycan has been shown to bind to tropoelastin and may influence elastic fiber formation [25]. Versican, which was strongly co-localized with elastic fibers, has been shown to bind to fibulin and fibrillin, microfibrillar proteins that are components of elastic fibers [14]. Interestingly, abnormal fibrillin distribution has been proposed as a factor in the development of myxomatous degeneration [4]. Collagen and elastin fragmentation has been shown before during myxomatous remodeling [4, 5] and the link between PGs and these ECM fibrous proteins may explain the compensatory mechanism during myxomatous valve remodeling. The fact that each of these three proteoglycans — each of which is differentially regulated and likely to perform a distinct function within the ECM — was more abundant demonstrates the complexity of matrix changes in myxomatous valve remodeling. These numerous matrix changes suggest that there may not be a single primary cause of myxomatous degeneration.
We did not find significant differences in HA staining between normal and myxomatous leaflets, which is consistent with our previous biochemical findings using FACE [15]. Although our previous FACE analyses demonstrated elevated amounts of HA in myxomatous chordae, that study also showed that the myxomatous posterior leaflets did not contain more HA than normal posterior leaflets.
This is the first study to demonstrate the presence of HARE in human heart valves. Previously, HARE has been found in the heart valves of mice [19]. HARE is a cell receptor that enables the clearance of HA and thought to be associated with HA turnover [18]. In addition, it was recently demonstrated that the binding of HA to HARE causes phosphorylation of extracellular signal-regulated kinases 1 and 2 (ERK1/2) in a dose- and time-dependent manner [26]. The finding of less HARE expression in myxomatous mitral valves suggests that diseased valves may have alterations in HA metabolism or intracellular signaling. In general, the demonstration of HARE in these tissues opens new avenues for research into the turnover and function of HA in valve remodeling and pathology. HA also interacts with other receptors such as CD44, which have been previously demonstrated in heart valves [27], and the receptor for HA-mediated motility (RHAMM) during cell migration and ECM remodeling [28]. HA turnover is also regulated by enzymes such as the HA synthases and hyaluronidases [29]. Any one or more of these receptors and enzymes may regulate the abundance and activity of HA during myxomatous degeneration, and should be examined in greater detail in future studies.
Other limitations of this study included our difficulty in identifying the distinct characteristic leaflet layers within the myxomatous valve sections, which restricted us to PG localization based on collagen or elastin as opposed to layers. In addition, some of the valve sections showed clear “onlays” or superficial plaques [30] that were not part of any valve layer, but stained for PGs. These onlays were found in sections from 3 normal valves and 4 myxomatous valves. Compared to the interior portions of the valve sections, onlays in normal valves tended to have greater expression of decorin and versican, whereas in myxomatous valve sections the onlays only showed greater expression of biglycan. It has been previously reported that myxomatous valve onlays contain similar amounts of GAGs and collagen as found in the rest of the valve [30].
In conclusion, the PGs decorin, biglycan and versican were significantly more abundant in myxomatous mitral valves compared to normal valves, a difference which may either contribute to or be a consequence of the valve remodeling. Although HA expression was unaltered between two leaflet groups, HARE expression was lower in myxomatous valves, suggesting that HA homeostasis may be altered in myxomatous mitral valve disease. In the future, it will be important to investigate the regulation of matrix synthesis and degradation within these diseased valves in order to identify the regulatory molecules or cell population(s) responsible for valve remodeling. Such information will be essential for the development of medical therapies to target the responsible agents and would provide a platform to treat myxomatous degeneration without requiring valve surgery.
Acknowledgement
This research was funded by American Heart Association Scientist Development Grant 0235216N (KJGA) and NIH grants HL081558 (KJGA) and GM69961 (PHW).
Funding This research was funded by American Heart Association Scientist Development Grant 0235216N (KJGA) and NIH grants HL081558 (KJGA) and GM69961 (PHW).
References
- 1.Freed LA, Acierno JS, Jr., Dai D, Leyne M, Marshall JE, Nesta F, Levine RA, Slaugenhaupt SA. A locus for autosomal dominant mitral valve prolapse on chromosome 11p15.4. Am J Hum Genet. 2003;72:1551–9. doi: 10.1086/375452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins P, Cotton RE, Duff RS. Symptomatic mitral myxomatous transformation in the elderly. Thorax. 1976;31:765–70. doi: 10.1136/thx.31.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barber JE, Kasper FK, Ratliff NB, Cosgrove DM, Griffin BP, Vesely I. Mechanical properties of myxomatous mitral valves. J Thorac Cardiovasc Surg. 2001;122:955–62. doi: 10.1067/mtc.2001.117621. [DOI] [PubMed] [Google Scholar]
- 4.Nasuti JF, Zhang PJ, Feldman MD, Pasha T, Khurana JS, Gorman JH, 3rd, Gorman RC, Narula J, Narula N. Fibrillin and other matrix proteins in mitral valve prolapse syndrome. Ann Thorac Surg. 2004;77:532–6. doi: 10.1016/S0003-4975(03)01584-4. [DOI] [PubMed] [Google Scholar]
- 5.Akhtar S, Meek KM, James V. Ultrastructure abnormalities in proteoglycans, collagen fibrils, and elastic fibers in normal and myxomatous mitral valve chordae tendineae. Cardiovasc Pathol. 1999;8:191–201. doi: 10.1016/s1054-8807(99)00004-6. [DOI] [PubMed] [Google Scholar]
- 6.Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104:2525–32. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- 7.Trochu JN, Kyndt F, Schott JJ, Gueffet JP, Probst V, Benichou B, Le Marec H. Clinical characteristics of a familial inherited myxomatous valvular dystrophy mapped to xq28. J Am Coll Cardiol. 2000;35:1890–7. doi: 10.1016/s0735-1097(00)00617-3. [DOI] [PubMed] [Google Scholar]
- 8.Olsen EG, Al-Rufaie HK. The floppy mitral valve. Study on pathogenesis. Br Heart J. 1980;44:674–83. doi: 10.1136/hrt.44.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinsella MG, Bressler SL, Wight TN. The regulated synthesis of versican, decorin, and biglycan: Extracellular matrix proteoglycans that influence cellular phenotype. Crit Rev Eukaryot Gene Expr. 2004;14:203–34. doi: 10.1615/critreveukaryotgeneexpr.v14.i3.40. [DOI] [PubMed] [Google Scholar]
- 10.Wight TN, Heinegard D, Hascall VC. Proteoglycans: structure and function. In: Hay ED, editor. Cell Biology of Extracellular Matrix. 2nd ed. Plenum Press; New York: 1991. pp. 45–78. [Google Scholar]
- 11.Grande-Allen KJ, Calabro A, Gupta V, Wight TN, Hascall VC, Vesely I. Glycosaminoglycans and proteoglycans in normal mitral valve leaflets and chordae: Association with regions of tensile and compressive loading. Glycobiology. 2004;14:621–33. doi: 10.1093/glycob/cwh076. [DOI] [PubMed] [Google Scholar]
- 12.Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J. 2002;19:249–55. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- 13.Kresse H, Schonherr E. Proteoglycans of the extracellular matrix and growth control. J Cell Physiol. 2001;189:266–74. doi: 10.1002/jcp.10030. [DOI] [PubMed] [Google Scholar]
- 14.Wight TN. Versican: A versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol. 2002;14:617–23. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- 15.Grande-Allen KJ, Griffin BP, Ratliff NB, Cosgrove DM, Vesely I. Glycosaminoglycan profiles of myxomatous mitral leaflets and chordae parallel the severity of mechanical alterations. J Am Coll Cardiol. 2003;42:271–7. doi: 10.1016/s0735-1097(03)00626-0. [DOI] [PubMed] [Google Scholar]
- 16.Grande-Allen KJ, Osman N, Ballinger ML, Dadlani H, Marasco S, Little PJ. Glycosaminoglycan synthesis and structure as targets for the prevention of calcific aortic valve disease. Cardiovasc Res. 2007;76:19–28. doi: 10.1016/j.cardiores.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Zhou B, Weigel JA, Fauss L, Weigel PH. Identification of the hyaluronan receptor for endocytosis (HARE). J Biol Chem. 2000;275:37733–41. doi: 10.1074/jbc.M003030200. [DOI] [PubMed] [Google Scholar]
- 18.Zhou B, McGary CT, Weigel JA, Saxena A, Weigel PH. Purification and molecular identification of the human hyaluronan receptor for endocytosis. Glycobiology. 2003;13:339–49. doi: 10.1093/glycob/cwg029. [DOI] [PubMed] [Google Scholar]
- 19.Falkowski M, Schledzewski K, Hansen B, Goerdt S. Expression of stabilin-2, a novel fasciclin-like hyaluronan receptor protein, in murine sinusoidal endothelia, avascular tissues, and at solid/liquid interfaces. Histochem Cell Biol. 2003;120:361–9. doi: 10.1007/s00418-003-0585-5. [DOI] [PubMed] [Google Scholar]
- 20.Fisher LW, Stubbs JT, 3rd, Young MF. Antisera and cdna probes to human and certain animal model bone matrix noncollagenous proteins. Acta Orthop Scand Suppl. 1995;266:61–5. [PubMed] [Google Scholar]
- 21.Lara SL, Evanko SP, Wight TN. Morphological evaluation of proteoglycans in cells and tissues. Methods Mol Biol. 2001;171:271–90. doi: 10.1385/1-59259-209-0:271. [DOI] [PubMed] [Google Scholar]
- 22.Schonherr E, Jarvelainen HT, Sandell LJ, Wight TN. Effects of platelet-derived growth factor and transforming growth factor-beta 1 on the synthesis of a large versican-like chondroitin sulfate proteoglycan by arterial smooth muscle cells. J Biol Chem. 1991;266:17640–7. [PubMed] [Google Scholar]
- 23.Radermecker MA, Limet R, Lapiere CM, Nusgens B. Increased mRNA expression of decorin in the prolapsing posterior leaflet of the mitral valve. Interact Cardiovasc Thorac Surg. 2003;2:389–94. doi: 10.1016/S1569-9293(03)00107-5. [DOI] [PubMed] [Google Scholar]
- 24.Scott JE. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988;252:313–23. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reinboth B, Hanssen E, Cleary EG, Gibson MA. Molecular interactions of biglycan and decorin with elastic fiber components: Biglycan forms a ternary complex with tropoelastin and microfibril-associated glycoprotein 1. J Biol Chem. 2002;277:3950–7. doi: 10.1074/jbc.M109540200. [DOI] [PubMed] [Google Scholar]
- 26.Kyosseva SV, Harris EN, Weigel PH. The hyaluronan receptor for endocytosis (HARE) mediates hyaluronan-dependentsignal transduction via extracellular signal-regulated kinases (ERK). J Biol Chem. 2008;283:15047–55. doi: 10.1074/jbc.M709921200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hellstrom M, Johansson B, Engstrom-Laurent A. Hyaluronan and its receptor CD44 in the heart of newborn and adult rats. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:587–92. doi: 10.1002/ar.a.20332. [DOI] [PubMed] [Google Scholar]
- 28.Savani RC, Cao G, Pooler PM, Zaman A, Zhou Z, DeLisser HM. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J Biol Chem. 2001;276:36770–8. doi: 10.1074/jbc.M102273200. [DOI] [PubMed] [Google Scholar]
- 29.Allison DD, Grande-Allen KJ. Review. Hyaluronan: A powerful tissue engineering tool. Tissue Eng. 2006;12:2131–40. doi: 10.1089/ten.2006.12.2131. [DOI] [PubMed] [Google Scholar]
- 30.McDonald PC, Wilson JE, McNeill S, Gao M, Spinelli JJ, Rosenberg F, Wiebe H, McManus BM. The challenge of defining normality for human mitral and aortic valves: Geometrical and compositional analysis. Cardiovasc Pathol. 2002;11:193–209. doi: 10.1016/s1054-8807(01)00102-8. [DOI] [PubMed] [Google Scholar]