Abstract
Purpose
To evaluate the relationship between accommodation, visual acuity, and emmetropization in human infancy.
Methods
Defocus at distance and near (57cm) was assessed using Mohindra and dynamic retinoscopy, respectively, in 262 normal birthweight infants at 3, 9, and 18 months of age. Preferential looking provided acuity data at the same ages. The spherical equivalent refractive error was measured by cycloplegic retinoscopy (cyclopentolate 1%).
Results
Univariate linear regression analyses showed no associations between the change in refractive error and defocus at distance or near. Change in refractive error was linearly related to the accommodative response at distance (R2 = 0.17, p<0.0001) and near (R2 = 0.13, p<0.0001). The ten subjects with the poorest emmetropization relative to the change predicted by the linear effects of their refractive error had higher average levels of hyperopic defocus at distance and near (p-values <0.043). Logistic regression showed a decrease in the odds of reaching +2.00D or less hyperopia by 18 months with increasing levels of hyperopia at 3 months, or if Mohindra retinoscopy was myopic combined with acuity better than the median level of 1.25 logMAR (area under the receiver operating characteristic curve = 0.78 (95% CI = 0.68, 0.88)).
Conclusions
The level of cycloplegic refractive error was the best single factor for predicting emmetropization by 18 months of age, with smaller contributions from visual acuity and Mohindra retinoscopy. The lack of correlation between defocus and change in refractive error does not support a simple model of emmetropization in response to the level of hyperopic defocus. Infants were capable of maintaining accurate average levels of accommodation across a range of moderate hyperopic refractive errors at 3 months of age. The association between the change in refractive error and accommodative response suggests that the amount of accommodation is a plausible visual signal for emmetropization.
Keywords: emmetropization, refractive error, accommodation, infant vision, visual development
Refractive error represents a mismatch between the eye's focal and axial lengths. At birth the average cycloplegic refractive error for human infants typically ranges from +1.00 D to +2.50 D with standard deviations on the order of ±1.50 D to ±2.50 D.1-6 Myopic refractive errors are less common in newborns with a wide range of estimates in prevalence, from 0% to 25%.2, 3 Infant eyes undergo a process of emmetropization whereby the average amount and the variance in the distribution of early refractive errors are both reduced. Emmetropization is bi-directional; reduction in early refractive error occurs for both myopic and hyperopic infants.7, 8 The majority of refractive change and reduction in variance during emmetropization is completed within the first year of life.9-11 Within the first year, the bulk of emmetropization appears to take place in normal infants between 3 and 9 months of age, as there is little change in refractive error between birth and 3 months of age.9-11
The mechanism responsible for the coordination between the optical and structural development of the emmetropizing eye appears to be visually-based. The eye appears able to detect and respond to its own refractive error, as the amount of refractive change and axial growth during emmetropization occur in proportion to the initial ametropia.7, 12 Considerable evidence from animal experiments suggests that the emmetropization process is guided by feedback from visual input. The eyes of young animals of numerous species compensate for the refractive errors induced by spectacle lenses by increasing or decreasing the rate of axial growth.13-19 Eye growth displays sensitivity to the sign and magnitude of the defocusing lens. Myopic blur from positive lenses slows the rate of axial growth and, particularly in birds, stimulates the expansion of the choroid, resulting in a compensatory hyperopia.20 Hyperopic blur from minus lenses increases the rate of axial growth and results in myopia. Particular emphasis is given to hyperopic defocus as neonates are more commonly hyperopic and, in monkeys, the effective range of simulated refractive errors that can be compensated for extends further into hyperopia than it does into myopia.16 The level of exposure to hyperopic defocus therefore is a candidate for the visual signal by which the eye could detect the magnitude of its own refractive error and make compensatory proportional increases in axial length to reduce hyperopia.
Important recent findings from animal studies highlight the non-linear temporal integration of defocus signals. Several studies show that brief interruptions of exposure to hyperopic defocus negate the expected excessive axial elongation.21, 22 In addition, hyperopic defocus in the chick is not equal to myopic defocus in its ability to modulate ocular growth as a function of time of exposure. Alternating blur experiments show that as little as minutes a day of exposure to myopic defocus negates the effect of hours of exposure to hyperopic defocus.23 Mammals also display this non-linear integration, but images closer to being in focus rather than myopically defocused may act as the more consistent “stop” signal counteracting the “go” signal from hyperopic defocus.18 These experiments suggest that the more constant the defocus signal, the more effective it will be in stimulating axial growth to reduce initially hyperopic refractive errors.
Exposure to hyperopic defocus in hyperopic human infants will be a function of the underlying refractive error and accommodation. The infant steady state accommodative error is variable; accommodative microfluctuations have been found to be larger across viewing distances in infants (rms less than 0.50 D) than in adults (rms = 0.08 D to 0.28 D).24 Dynamic accommodative capabilities appear to be present by 8 weeks of age.25 Demand-dependent changes in accommodative velocity occur, albeit with variability, showing latencies that are within 0.4 seconds of adult values.25 Infant accommodation displays immaturities in terms of variability in the accommodative response slope and the steady-state focus error. Both of these immaturities improve up to the age of 3 to 4 months, either reaching an adult level of performance,26 or still showing occasional immaturity at 3 months.27, 28 Braddick et al. (1979) observed consistent focus behavior emerging between 3 and 6 months of age in response to a low accommodative demand of 1.3 D (75 cm).29 Infants presented with greater accommodative demands generally showed improvement in the accommodative response slope with age, but not to adult levels even by the age of 5 months.30, 31 Howland et al. (1987) observed values for the accommodative response slope in infants of about 0.6 with no change between 2 and 7 months.32 Accommodative accuracy may be limited in part by sensory immaturities in acuity that increase the depth of field of the infant eye.33 For example, Sokol et al. (1983) found an age-dependent absence of accommodative responses to finer check targets due to poor visual resolution.31 Larger and more spatially complex targets may override these sensory immaturities, however. In a sample of infants ranging in age from 3 to 12 months, average accommodative responses to targets with a broad spatial frequency amplitude spectrum were similar to those found in school-aged children, were unrelated to age, and were independent of the variation in normal levels of acuity.34 A somewhat accurate yet variable accommodative response, therefore, appears to be in place at the time when the emmetropization process begins.
The efficacy of constant defocus signals and the variability in infant accommodation suggest that the visual basis for the emmetropization process might be chronic exposure to hyperopic defocus from errors of accommodation at distance and near. Infants exposed to higher levels of hyperopic defocus would undergo greater amounts of refractive change during emmetropization. To our knowledge, this hypothesis has not been evaluated in human infants in a longitudinal study. The Berkeley Infant Biometry Study (BIBS) monitored refractive error and ocular component development in a cohort of human infants followed longitudinally. The purpose of the current analysis is to examine the relationships in BIBS infants between infant accommodation, more specifically the defocus from accommodative errors at distance and near, and changes in refractive error during the emmetropization period. It should be noted that this emmetropization refers to spherical equivalent. A previous analysis of this dataset has shown that emmetropization of astigmatism took place over a longer time course, involved corneal and lenticular rather than axial length changes, and was unrelated to the emmetropization of spherical equivalent.10
METHODS
Subjects for BIBS were recruited from several sources, including advertisements in diaper service newsletters, word-of-mouth, and letters sent to parents of newborns identified from birth records in Contra Costa County, CA. Parents provided written informed consent according to the tenets of the Declaration of Helsinki after all procedures were explained. The BIBS protocol was reviewed and approved by the Institutional Review Boards for The Ohio State University, the University of California, Berkeley, and the State of California. Inclusion criteria were both genders, any refractive error, birthweight over 2500 grams, and confirmation that the baby was under the general care of a pediatrician. An existing strabismus was allowable, although no baby entered the study with strabismus. Infants were excluded if they had a previous history of difficulty with pupil dilation, a history of cardiac, liver, asthma, or other respiratory disease, or a history of ocular pathology or active ocular inflammation. A total of 302 infants were recruited to be seen at study entry at age 3 ± 1 month with follow-up examinations at 9 ± 1 month, and 18 ± 2 months. Follow-up continued up to the age of 8 years; data beyond 18 months were not analyzed for this report. Of the 302 infants initially enrolled, 40 were not included in the first visit: 37 were tested outside the first visit window, two were later found by survey to be below the entry criterion for birthweight, and one inadvertently received tropicamide 1% at the first visit. The number of subjects by visit is shown in Table 1. The primary reasons for variation in numbers of subjects at 9 and 18 months were losses to follow-up, visits outside of window, or inability to cooperate with the measurements.
Table 1.
Number (%) of subjects by gender and age group.
| 3 months | 9 months | 18 months | |
|---|---|---|---|
| Male | 127 (48.5) | 114 (46.9) | 120 (46.7) |
| Female |
135 (51.5) |
129 (53.1) |
137 (53.3) |
| Total | 262 | 243 | 257 |
Acuity was measured binocularly without any refractive correction using Teller acuity cards. The subject was observed through the center peephole of the acuity cards for evidence of preferential looking. Successively smaller patterns were presented until the subject either no longer displayed preferential looking or lost interest in the task. Subjects at 3 months of age were presented with the 0.32 cycles/cm card initially, while 9 and 18 month-old subjects began with the 1.3 cycles/cm testing card. Acuity was recorded as cycles per degree for the last card where preferential looking was observed. Visual acuity scores were transformed to logMAR values prior to all analyses.
Refractive error was measured by two observers performing retinoscopy on each eye 25 minutes after instillation of one drop of proparacaine 0.5%, followed by two drops of cyclopentolate 1.0% in each eye with five minutes between cyclopentolate drops. The second examiner's measurement was made without knowledge of the first examiner's findings. Refractive error is reported as the average of the two examiners’ results for the cycloplegic (wet) spherical equivalent refractive error of the right eye (WSEQ). The 95% limits of agreement between the two examiners have been reported to be −0.71 to +0.98 D for spherical equivalent.10 Cycloplegic refractive errors over +4.00 D were referred for clinical care from community eyecare providers. No subject had a refractive correction prescribed during this portion of BIBS.
Mohindra retinoscopy (MRET) was performed as an estimate of non-cycloplegic distance defocus.35-39 Dynamic retinoscopy (DRET) was used to estimate near defocus error. Each technique was done once per visit by one examiner only. DRET has been used in several studies to characterize infant accommodation.26, 27, 30 For each technique, the examiner introduced hand-held trial lenses over the infant's right eye to neutralize the movement of the retinoscopic reflex when the baby's attention was directed at the target for DRET and towards the retinoscope for MRET. No adjustment was made to DRET values but +0.75 D was subtracted as a tonus allowance for MRET.39 Room lights were off for MRET. The traditional technique for Mohindra retinoscopy is for the contralateral eye to be covered,35 but this was abandoned early in our protocol due to infants’ poor tolerance for occlusion. Both eyes were open in each technique. The horizontal meridian was neutralized first followed by the vertical meridian. A positive sign for DRET indicates accommodative lag and a minus sign indicates accommodative lead. The DRET target, a black-on-white cartoon mouse face, was on a card viewed binocularly at the plane of the retinoscope at 57 cm (1.75 D). The examiner's face was also visible behind the card. Measures were made with the room lights on with a target luminance of 16.5 cd/m2. The spatial frequency content of the target was analyzed using the Fourier transform feature in Mathematica (v. 4.0, Wolfram Research, Champaign, IL). Spatial frequencies ranged from 0.2 to 7.0 cpd with a falloff of about 1.8 log units of relative amplitude per log unit increase in spatial frequency. For infants in this age range, these frequencies were within the acuity limit of roughly 5 cpd to 12 cpd,40 and within the range for peaks in the contrast sensitivity functions of 0.375 cpd to 3.0 cpd.41 Targets with large checks (0.5 deg or 1 deg),28, 31 stripes with spatial frequencies of 0.75 cpd to 1.5 cpd,30 or random check sizes with a wide range of spatial frequencies27 have been used as effective stimuli for accommodation in previous infant studies. Results from dynamic retinoscopy are in general agreement with those from photorefraction29, 32 and from autorefraction in a study of adults.42 The method used for DRET was recently compared to PowerRefractor measurements of accommodative errors in infants. Mean lag estimates from the two methods were not significantly different from each other (0.50 D for the PowerRefractor and 0.48 D for DRET), but the 95% limits of agreement were wide at ±1.78 D.34
Data were transmitted to the Optometry Coordinating Center at The Ohio State University for dual data entry. PC SAS v. 8.0 was used for verification of ranges and missing information as well as for data analysis. Mixed linear modeling was used for assessing change in refractive error and accommodation measures with error rates in post-hoc comparisons between age groups controlled at the 0.05 level (Tukey adjustment). Regression analyses were used to assess the linear relationship between defocus, refractive error, and acuity variables. Logistic regression was used to assess defocus, refractive error, and acuity as risk factors for reaching an emmetropization outcome of +2.00 D or less hyperopia by 18 months of age. The correlated variances for refractive error and dynamic retinoscopy at each age were compared using the method described by Cox and Hinkley.43 Orthogonal regression was performed using SAS JMP (v. 4, SAS Institute, Cary NC).
RESULTS
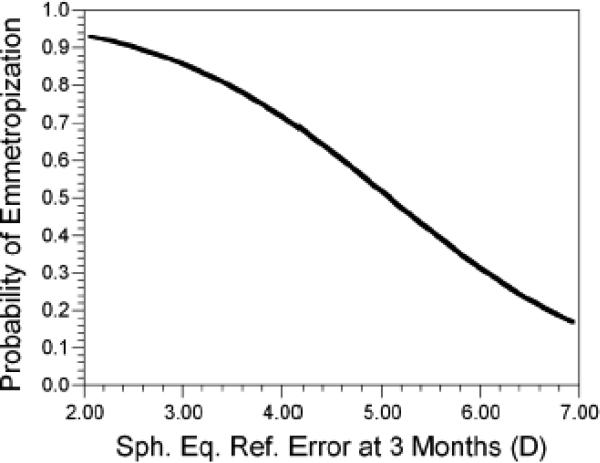
Infants were moderately hyperopic in their cycloplegic spherical equivalent refractive error at 3 months of age (WSEQ in Table 2). As reported previously for this sample,10 there was significant emmetropization that took place between 3 and 18 months of age with the majority of the change taking place by 9 months of age. The average level of hyperopia decreased by 1.03 D between 3 and 18 months of age, comprised of a substantial change of 0.81 D between 3 and 9 months and a smaller change of 0.23 D between 9 and 18 months (both p<0.0001). There was also a significant reduction in the variability in refractive error between 3 and 9 (p<0.0001) and between 9 and 18 months of age (p<0.001). As expected, the level of WSEQ at 3 months was a significant factor in emmetropization. More hyperopic infants were less likely to reach an emmetropization outcome of ≤+2.00 D by 18 months of age (OR = 0.42, 95% CI = 0.25, 0.71). The probability of reaching this emmetropization outcome as a function of WSEQ at 3 months of age is shown in Figure 1. Probabilities for emmetropization were high at 0.80 for hyperopia at 3 months of +3.50 D, moderate at 0.50 for +5.00 D, and fell to 0.30 for +6.00 D.
Table 2.
Mean ± SD spherical equivalent retinoscopy results by age. Number of subjects for each measurement and age group is in parentheses.
| Age in months | Cycloplegic retinoscopy | Dynamic retinoscopy | Mohindra retinoscopy |
|---|---|---|---|
| 3 | +2.13 ± 1.31 (262) |
+0.57 ± 1.25 (240) |
+0.08 ± 1.62 (260) |
| 9 | +1.32 ± 1.07 (243) |
+1.04 ± 0.89 (242) |
+0.78 ± 0.93 (243) |
| 18 | +1.10 ± 0.90 (257) |
+1.02 ± 0.72 (256) |
+0.71 ± 0.86 (255) |
Figure 1.
The probability of reaching +2.00D by 18 months of age as a function of the level of cycloplegic refractive error at 3 months of age.
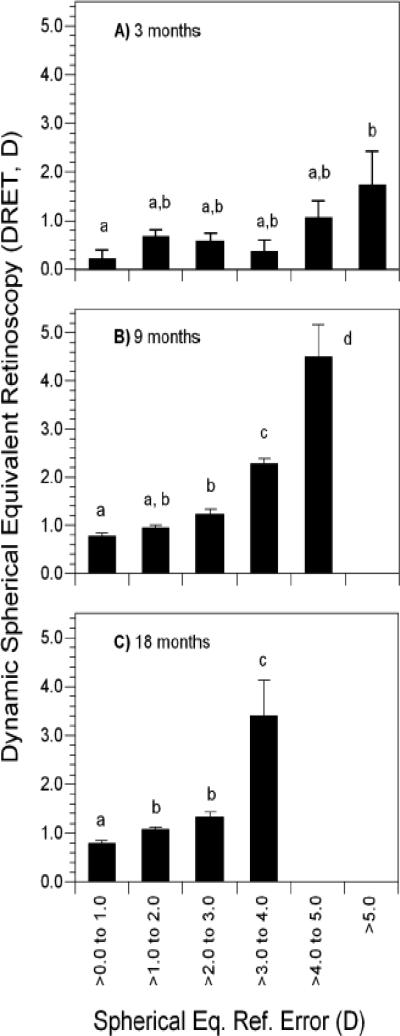
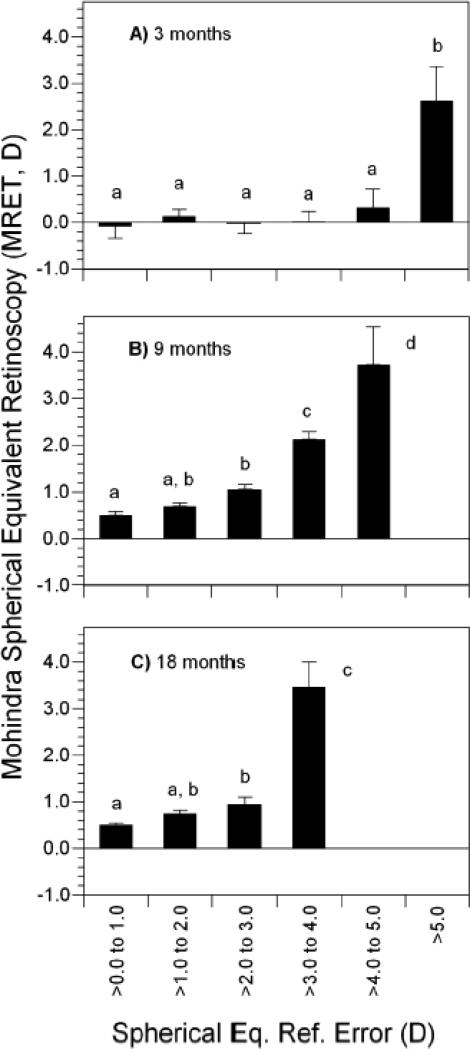
The average DRET result at 3 months of age was roughly +0.50 D and the average MRET was essentially plano (Table 2). Each showed a significant increase toward an average level of mild hyperopic defocus (0.47 D increase for DRET and 0.70 D increase for MRET, each p<0.0001) with a concurrent reduction in variability between 3 and 9 months of age (each p<0.0001). Results at 18 months were not significantly different from those at 9 months for the average level of DRET (p = 0.93) and MRET (p = 0.43) or the standard deviation for MRET (p = 0.34). The standard deviation for DRET decreased by a small amount between 9 and 18 months (p = 0.002). Both DRET and MRET showed significant positive correlations with WSEQ across age groups; more hyperopic defocus at distance and near was associated with more hyperopic refractive errors (Table 3). Because emmetropization is proportional to WSEQ,7, 12 and because DRET and MRET are related to WSEQ, each was analyzed as a potential visual signal for emmetropization. Average values of each as a function of WSEQ (with WSEQ categorized in 1-D intervals) are shown in Figures 2 and 3. In general, DRET and MRET were the most hyperopic at the highest levels of WSEQ but less hyperopic and more uniform over a range of moderate levels of hyperopia. The amount of moderate hyperopia over which defocus was similar varied with age, generally extending over a wider interval at younger ages and a narrower one at older ages.
Table 3.
Pearson correlations with WSEQ cycloplegic refractive error by age (p values in parentheses).
| Age (months) | Dynamic retinoscopy | Mohindra retinoscopy |
|---|---|---|
| 3 | 0.21 (0.0013) | 0.22 (0.0003) |
| 9 | 0.57 (<0.0001) | 0.57 (<0.0001) |
| 18 | 0.55 (<0.0001) | 0.45 (<0.0001) |
Figure 2.
Dynamic retinoscopy results in each of three age groups as a function of cycloplegic refractive error in each of three age groups: A) 3 months, B) 9 months, and C) 18 months. Bars with shared letters above are not significantly different. No shared letters indicates a significant difference. Error bars represent the SEM.
Figure 3.
Mohindra retinoscopy results in each of three age groups as a function of cycloplegic refractive error in each of three age groups: A) 3 months, B) 9 months, and C) 18 months. Bars with shared letters above are not significantly different. No shared letters indicates a significant difference. Error bars represent the SEM.
At 3 months, DRET varied significantly as a function of WSEQ category (Figure 2, F5, 232 = 2.64, p=0.024). DRET was uniform across WSEQ values between plano and +5.00 D. The significance of the ANOVA appeared to be driven by the comparison between the least hyperopic category of plano to +1.00 D and most hyperopic category of hyperopia greater than +5.00 D (p = 0.052; Figure 2). At 9 and at 18 months, DRET did not vary by more than 0.50 D over a narrower interval of WSEQ values from plano to +3.00 D. DRET was statistically and clinically meaningfully higher, reaching 3.0 D to 4.0 D, on average, for WSEQ values greater than +3.00 D (F4, 233 = 43.2, p<0.0001 at 9 months and F3, 250 = 33.2, p<0.0001 at 18 months).
At 3 months, MRET did not vary significantly as a function of WSEQ values between plano and +5.00 D (Figure 3). Significantly higher values of MRET were found in subjects with the most hyperopic amounts of WSEQ, >+5.00 D, compared to subjects in each of the five less hyperopic WSEQ categories (F5, 252 = 3.35, p=0.006). As with DRET, average values of MRET at 9 and 18 months only varied by about 0.50 D for WSEQ values between plano to +3.0 D. Significantly larger values of MRET occurred in infants with WSEQ more hyperopic than +3.00 D (F4, 234 = 29.3, p<0.0001 at 9 months and F3, 249 = 25.5, p<0.0001 at 18 months).
The potential role for DRET as a visual signal for emmetropization was evaluated by regression, with DRET treated either as a value at a particular age (3, 9, or 18 months) or as the change in DRET between 3 and 9, 9 and 18, or 3 and 18 months. WSEQ as an outcome was evaluated either as the value at 18 months or as the change in WSEQ between 3 and 9 or 3 and 18 months. DRET at each of the three ages was positively associated with WSEQ at 18 months; i.e., a more hyperopic DRET at 3, 9, and 18 months was found in infants who were more hyperopic in WSEQ at 18 months (R2 values between 0.027 and 0.030, p-values between <0.0001 and 0.017). Change in DRET in two of the three intervals was associated with WSEQ at 18 months; greater decreases in DRET between 3 and 9 and between 3 and 18 months were seen in the least hyperopic infants at 18 months (R2 = 0.025 and 0.029, p-values = 0.031 and 0.014, respectively). Change in DRET between 9 and 18 months was not associated with WSEQ at 18 months. DRET at any of the three ages was not associated with change in WSEQ between 3 and 9 or between 3 and 18 months (R2 values between 0.003 and 0.006, p-values between 0.14 and 0.45), nor was change in DRET (R2 values between 0.002 and 0.013, p-values between 0.11 and 0.52). These findings suggest that a more hyperopic DRET was associated with an infant's current hyperopic refractive error but was not associated with an infant's future loss of hyperopic refractive error. MRET was analyzed in a similar manner. Like DRET, MRET at each of the three ages was positively associated with WSEQ at 18 months; e.g., a more hyperopic MRET at 3, 9, and 18 months was found in infants who were more hyperopic in WSEQ at 18 months (R2 between 0.040 and 0.25, p-values between <0.0001 and 0.003). Unlike DRET, change in MRET in any of the three intervals was not associated with WSEQ at 18 months (R2 values <0.002 and p-values <0.80). MRET at any of the three ages was not associated with change in WSEQ between 3 and 9 or between 3 and 18 months (R2 values between 0.014 and 0.000, p-values between 0.082 and 0.93), nor was change in MRET (R2 values between 0.016 and 0.007, p-values between 0.062 and 0.25). These findings suggest that, as with DRET, a more hyperopic MRET was associated with an infant's current hyperopic refractive error but was not associated with an infant's future loss of hyperopic refractive error.
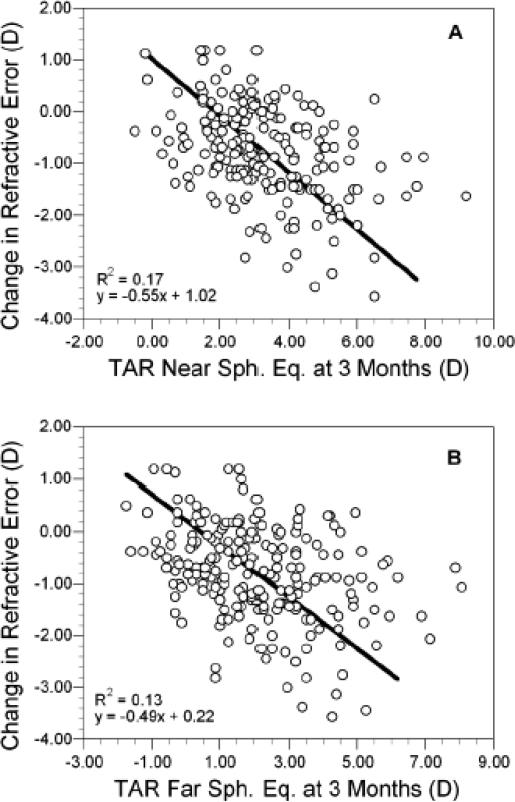
The similarity of MRET and DRET across a range of moderate levels of hyperopia, as depicted in Figures 2 and 3, suggests that the infants were able to accommodate to the combined dioptric demand of their refractive error and the targets. WSEQ and defocus data were combined into an estimate of the total accommodative response (TAR) at near and at distance as shown below, then evaluated for their relationship with change in refractive error using regression.
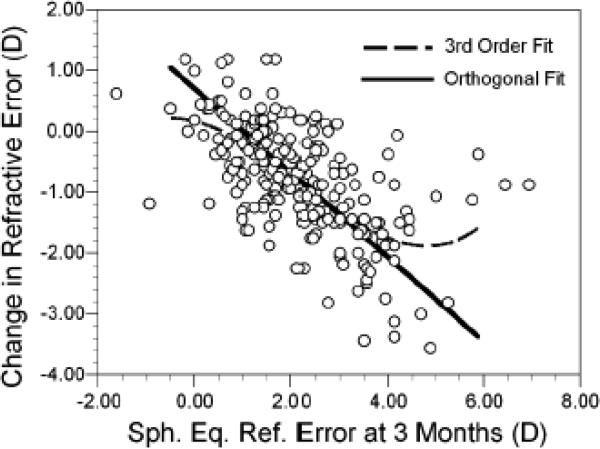
The 1.75 D in TAR (near) is the vergence of the near target while TAR (far) assumes that MRET is a non-cycloplegic distance refractive error measure. Both TAR (near) and TAR (far) at 3 months of age were linearly related to the change in WSEQ between 3 and 9 months of age (Figure 4). The R2 value for TAR (near) was 0.17 (p<0.0001) and for TAR (far) was 0.13 (p<0.0001). Attempts at fitting higher order polynomials did not significantly improve the R2 value. Because neither variable in Figure 4 was fixed, slopes were calculated using orthogonal regression. Each diopter increase in TAR (near) was associated with a loss of −0.55 D in WSEQ between 3 and 9 months (95% CI for slope = −0.76 to −0.40). Likewise, each diopter increase in TAR (far) was associated with a loss of −0.49 D in WSEQ between 3 and 9 months (95% CI for slope = −0.71 to −0.34). TAR at 3 months was also related to the change in WSEQ between 3 and 18 months, both at near (R2 = 0.26) and at far (R2 = 0.22, p<0.0001 for each).
Figure 4.
The change in refractive error between 3 and 9 months of age plotted as a function of the total accommodative response (TAR) at near (A) and far (B) at 3 months of age.
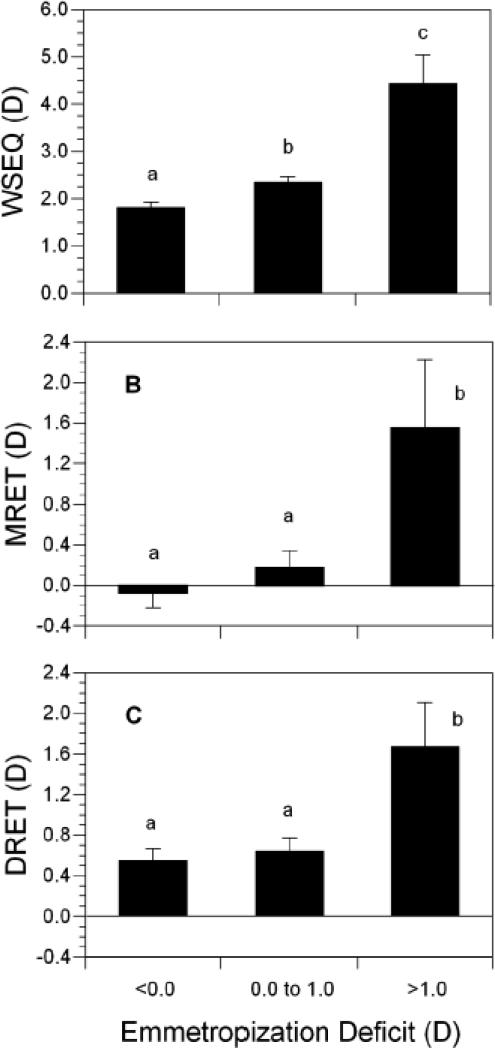
The relationship between the amount of the accommodative response and the degree of change in refractive error was evaluated further using linear and orthogonal regression. As previously reported,12 there was a significant third-order nonlinear relationship between the initial WSEQ at 3 months and the change in WSEQ between 3 and 9 months (R2 = 0.41; Figure 5). While the majority of infants fall into a linear zone of refractive error change for initial levels of hyperopia between +1.00 D and +4.00 D, there is a non-linear portion to this curve at higher levels of hyperopia. This nonlinearity can be appreciated by comparison of the third-order regression line to the line obtained using orthogonal regression. Residuals from the orthogonal regression were then obtained. These values might be thought of as an “emmetropization deficit;” a positive sign indicates that the degree of emmetropization was less than that predicted from the linear relationship between WSEQ at 3 months and refractive error change. These residual values were analyzed in relation to the amount of defocus as measured by MRET and DRET. These was a significant positive correlation between these residuals and MRET (r = 0.22, p = 0.001) and DRET (r = 0.15, p = 0.036) at 3 months, indicating that more hyperopic levels of defocus at distance and at near were associated with poorer emmetropization than that predicted by the underlying level of WSEQ. These residual values were also analyzed as categorical variables in a one-way ANOVA: 155 infants had residuals less than 0.00 D, 95 had residuals between 0.00 and 1.00 D, and 10 had residuals greater than 1.00 D. The ten subjects with the poorest emmetropization relative to their underlying WSEQ at 3 months had substantially more hyperopia (roughly 2.0 D more) and significantly more hyperopic levels of MRET and DRET at 3 months (Figure 6, p-values less than 0.043 with Bonferroni correction). Acuity did not differ across categories of emmetropization deficit (p-values <0.67). These results indicate that the majority of variance in emmetropization between 3 and 9 months can be explained through its linear relationship with WSEQ. The nonlinear response to WSEQ, i.e., the poorer emmetropization in the most hyperopic subjects, was related to higher levels of hyperopic defocus at distance and at near from a deficient accommodative response.
Figure 5.
Change in refractive error between 3 and 9 months plotted as a function of initial refractive error (WSEQ) at 3 months. The dotted line represents the best fit third-order polynomial. The solid line is the best linear fit using orthogonal regression.
Figure 6.
Average values of WSEQ, MRET, and DRET at 3 months as a function of the “Emmetropization Deficit” (categorized residuals from the orthogonal regression in Figure 5). Bars with shared letters above are not significantly different. No shared letters indicates a significant difference. Error bars are the SEM.
Visual acuity results are presented in Table 4. Visual acuity improved with age as expected, but averages tended to be in the lower end of the normal range at each age.44, 45 Visual acuity was analyzed for its relationship with DRET, MRET, and WSEQ. As might be expected, there was a significant relationship at 3 months of age between poorer acuity (higher logMAR) and more hyperopic values of distance defocus (MRET), but only at 3 months of age (Table 5). Some infants did not respond to the preferential looking task. These missing acuity data were also analyzed in order to determine if the lack of response might be the result of higher levels of defocus. At 3 months missing acuity was more likely with higher hyperopic values of DRET and MRET, but not WSEQ (p = 0.03, p<0.0001, and p = 0.16, respectively). At the beginning of the emmetropization period at 3 months of age, therefore, greater hyperopic defocus was related both to poorer visual acuity and a reduced ability to perform the visual acuity task. At 9 and 18 months of age, missing acuity was not related to DRET or MRET (p = 0.74 and p = 0.16, respectively).
Table 4.
Mean (± SD) visual acuity by age in logMAR and cycles per degree (cpd, transformed from the logMAR mean).
| Age (months) | 3 | 9 | 18 |
|---|---|---|---|
| Average acuity (logMAR) | 1.31 | 0.97 | 0.85 |
| (SD in octaves) | (0.76) | (0.62) | (0.70) |
| Average acuity (cpd) | 1.47 | 3.21 | 4.24 |
Table 5.
Pearson correlations between acuity in logMAR and retinoscopic findings at each age (p-values in parentheses, significant p-value in bold).
| 3 months | 9 months | 18 months | |
|---|---|---|---|
| Dynamic retinoscopy | 0.13 (0.065) |
0.11 (0.14) |
0.08 (0.29) |
| Mohindra retinoscopy |
0.20 (0.005) |
0.05 (0.55) |
0.08 (0.28) |
| Cycloplegic retinoscopy | 0.12 (0.095) |
0.01 (0.89) |
0.02 (0.83) |
Visual acuity was analyzed further for its relationship to emmetropization by regression, with acuity treated either as a value at a particular age (3, 9, or 18 months) or as the change in acuity between 3 and 9, 9 and 18, or 3 and 18 months. WSEQ as an outcome was evaluated as the value at 18 months or as the change in WSEQ between 3 and 9 or 3 and 18 months. Worse visual acuity at 3 months was related to more hyperopic WSEQ at 18 months (r = 0.18, p = 0.021). Acuity at 9 and 18 months was not related to WSEQ at 18 months (p-values = 0.20 and 0.83, respectively). Change in acuity over any interval was not related to WSEQ at 18 months (p-values range from 0.25 to 0.53). Acuity at 3 and 9 months was not related to change in WSEQ between 3 and 9 months (p-values = 0.86 and 0.61, respectively), nor was acuity at 3, 9, or 18 months related to change in WSEQ between 3 and 18 months (p values between 0.22 and 0.99). Change in acuity over any interval was not related to change in WSEQ between either 3 and 9 or 3 and 18 months (p-values range from 0.45 to 0.97).
Visual acuity was analyzed for any potential modification of the effect of WSEQ on emmetropization. The finding above that poorer acuity at 3 months was related to a more hyperopic refractive error at 18 months might be due to the relationship between defocus and acuity at 3 months (Table 5), a relationship between accommodative response and acuity, or might indicate that poor acuity modifies the effect of refractive error in emmetropization. Infants with poorer early levels of acuity might be at risk for not emmetropizing by being less sensitive to any visual signals. On the other hand, poor acuity might increase the odds of emmetropization because the reduction in acuity indicates the child is experiencing more hyperopic defocus. The influence of visual acuity on emmetropization was analyzed further through logistic regression. Visual acuity was dichotomized at 3 months into two groups based on a median level of 1.25 logMAR (1.7 cpd) and logistic regression performed using children at 3 months of age with WSEQ >+2.00 D. Variables in the model were acuity group, WSEQ, MRET, and DRET all at 3 months of age. A more hyperopic WSEQ was associated with a reduced likelihood of reaching an emmetropization outcome of ≤+2.00 D by 18 months of age (OR = 0.42, 95% CI = 0.25, 0.71). Acuity group was not associated with the emmetropization outcome (p = 0.60). No significant interaction between WSEQ and acuity group was found with respect to the likelihood of emmetropization (p-value for interaction = 0.70). There was also no difference in acuity at 18 months between infants who reached the emmetropization outcome of ≤+2.00 D by 18 months (0.81 logMAR) and those who did not (0.85 logMAR, p = 0.49). There was also no significant interaction between DRET and acuity (p-value for interaction = 0.77) or a significant main effect for DRET in emmetropization (p = 0.17). DRET was excluded from the final logistic model.
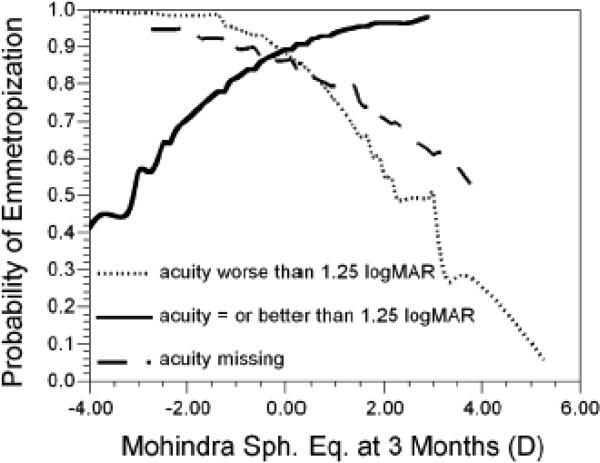
Acuity did significantly modify the effect of MRET on emmetropization in this logistic model (p-value for interaction = 0.006; Figure 7). If acuity was worse than the median level of 1.25 logMAR at 3 months, the odds of emmetropization were lower for more hyperopic values of MRET (OR = 0.40, 95% CI = 0.18, 0.88). If acuity was at or better than the median level of 1.25 logMAR at 3 months, the odds of emmetropization were higher with more hyperopic values of MRET (OR = 1.86, 95% CI = 1.004, 3.44). This difference between the two acuity groups in the effect of MRET on the odds of emmetropization did not appear to be the result of differences in the underlying level of hyperopia. In infants with acuity at or better than the median level of 1.25 logMAR at 3 months, the average level of hyperopia at 3 months was +3.08 D if MRET was myopic and +3.24 D if MRET was plano or hyperopic. Infants with unmeasurable, missing acuity data at 3 months appeared to follow the pattern for infants with poorer than median acuity (Figure 7).
Figure 7.
The probability of reaching +2.00D in refractive error by 18 months of age as a function of the level of visual acuity group and Mohindra retinoscopy results at 3 months of age.
The final logistic model for achieving the emmetropization outcome of ≤+2.00 D WSEQ at 18 months of age was a function of acuity group, WSEQ, and MRET at 3 months: If acuity was unmeasurable at 3 months:
If acuity was worse than 1.25 logMAR at 3 months:
If acuity was equal to or better than 1.25 logMAR at 3 months:
The probability of achieving the emmetropization outcome of ≤+2.00 D WSEQ at 18 months of age can be found by computing the logit within an acuity group and then applying the formula:
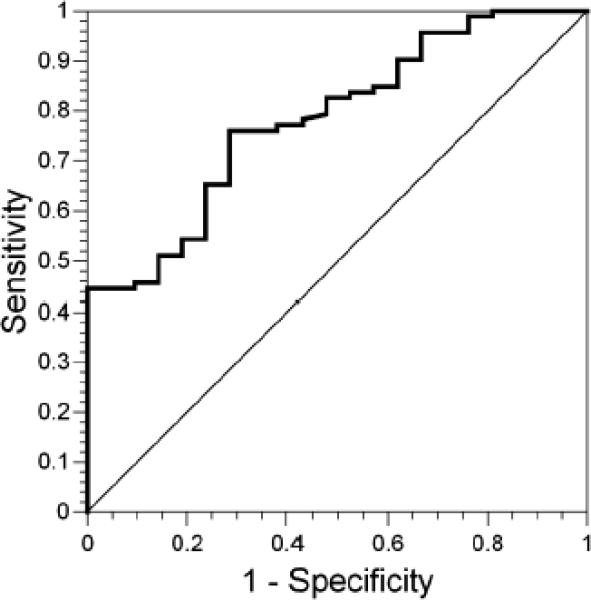
The ROC curve associated with this logistic model is shown in Figure 8. The ROC curve is a function of sensitivity versus (1 – specificity) across the range of all possible values of WSEQ, MRET, and acuity group. On the y-axis is sensitivity, or the true-positive fraction. On the x-axis is (1 – specificity), the false-positive fraction. The area under the curve was 0.78 (95% CI = 0.68, 0.88). The area under the curve is the probability that for two randomly selected subjects with different emmetropization outcomes, the subject who emmetropized had the higher logit. The null hypothesis that these variables have no predictive value is represented by the 1:1 line and a probability of 0.50. As shown in Figure 8, the ROC does not fall on the 1:1 line, consistent with the statistical significance of the 95% CI for the area under the curve not including 0.50.
Figure 8.
The receiver operating characteristic curve associated with the logistic model incorporating cycloplegic refractive error, visual acuity group, and Mohindra retinoscopy results, all at 3 months of age.
DISCUSSION
As previously reported from BIBS data, the majority of emmetropization occurred in this sample by 9 months of age.12 Average values for DRET and MRET findings were generally low levels of hyperopic defocus of 1.00 D or less, consistent with normal values reported for infants in these age ranges.26, 27, 30, 34 Decreases in variability for DRET and MRET with age might indicate the effects of emmetropization decreasing variability in hyperopic distance refractive error, but the impact of WSEQ on DRET and MRET seemed limited except at the highest levels of WSEQ. Improvement in control of accommodation, therefore, might be a better explanation for the reduced variability in DRET and MRET with age. DRET and MRET values were uniform across a range of moderate refractive errors at each age. Correlations between WSEQ, DRET, and MRET shown in Table 3 seemed to be driven by the extremes of refractive error: about +5.00 D at 3 months and +3.00 D at 9 and 18 months (Figures 2 and 3). Of clinical note, these may represent age-dependent limits for normal levels of hyperopia, above which higher amounts might challenge accommodation and convergence. This “flat” shape to defocus as a function of underlying refractive error at 3 months is noteworthy as this is also the range of refractive errors where emmetropization was occurring most effectively, between +1.00 D and +4.00 D.12 Accurate accommodation for infants with initial refractive errors in this range would effectively damp any dose-dependent defocus signals for emmetropization. Although each was a potential defocus signal, neither DRET nor MRET was correlated with the amount of refractive change during emmetropization. Infants’ capacity for accurate accommodative responses at 3 months of age and the reduction in defocus that follows are not consistent with a simple defocus model for emmetropization where the level of defocus controls the amount of refractive change.
Other defocus-driven emmetropization models might be considered. A consistent level of hyperopic defocus above a certain threshold might trigger rapid growth and a reduction in hyperopic distance refractive error. Emmetropization could be achieved if eyes lost hyperopia initially during this phase of faster growth, then switched to slower growth once emmetropia was achieved and defocus fell below the threshold value. Constant, rapid growth rates for various periods of time rather than various growth rates over one period of time could be the basis for emmetropization. The duration of rapid growth would be proportional to the initial level of refractive error and limited by the arrival of a “stop” signal. Minimal distance defocus is one candidate “stop” signal.18 One argument against this model is that MRET was closest to plano, the putative “stop” signal, at the beginning of the emmetropization phase at 3 months of age. The similarity between DRET and MRET at 9 and 18 months is also not consistent with DRET being a “go” and MRET being a “stop” signal. This model also predicts that there would be a correlation between change in MRET and either WSEQ at 18 months or the change in WSEQ, yet neither correlation was significant.
Another model for emmetropization might be that the rate of axial growth is controlled by the accommodative effort expended. The level of hyperopic refractive error could be a dose-dependent signal for emmetropization through the stimulus to accommodation rather than through defocus, or lack of accommodation. Results from this study are consistent with this model. Similar levels of defocus were found across the range of moderate refractive errors where emmetropization took place, suggesting that an accurate accommodative response occurred when emmetropization was effective. This finding addresses the issue of the operating range for emmetropization. The linear operating range for emmetropization in response to WSEQ extends from about +1.00 D to +4.00 D (Figure 5). The results in Figure 3 suggest that this operating range for emmetropization only extends across levels of WSEQ over which infants could accommodate with accuracy. Another piece of evidence is that while the response to WSEQ was non-linear, the results in Figure 4 show that the relationship between accommodative effort and emmetropization was linear. Finally, this non-linear deficient emmetropization at higher average levels of hyperopic refractive error was related to poorer accommodation and higher levels of defocus (Figure 6). This finding is opposite to predictions that emmetropization should be promoted rather than deficient when there are higher levels of hyperopic defocus.
Accommodative effort may be a visual signal for emmetropization but these data are not conclusive evidence. The sample does not have a large number of high hyperopes. It is also uncertain whether the association between poor accommodation and poor emmetropization is cause or effect. A common factor could inhibit both emmetropization and accommodation. Animal studies might shed light on the issue of accommodative effort vs. error by pursuing the interesting observation in monkeys that “...eyes that failed to compensate for large negative lenses...appeared to exhibit no effort to accommodate for the imposed error.”16 Another argument against this model is the lack of any proposed mechanism connecting accommodative effort and ocular growth rates in infants. Perhaps accommodation might influence refraction through changes in ocular shape46 or by influencing ciliary muscle.47 An accommodative effort model would also predict varying growth rates over time as a function of the changing amount of underlying refractive error. Examining whether the rate of ocular growth is relatively constant until emmetropia is reached or varies in relation to either defocus or accommodative effort might help to distinguish between these models. The current data with measures at only 3 and 9 months, however, lack the temporal resolution needed to yield precise growth rates.
Although poorer acuity was related to more hyperopic WSEQ and MRET at 3 months, acuity outcomes were similar in this sample whether infants reached a WSEQ of +2.00 D by 18 months or not. Measurement of acuity at 3 months provided some useful prognostic information in the logistic models of emmetropization, along with WSEQ and MRET data. Regardless of acuity, the best prognostic sign for emmetropization to +2.00 D or less by 18 months, obviously, was not being highly hyperopic initially. Emmetropization was also more likely if acuity was at or above the median at 3 months and MRET values were hyperopic. Poor prognostic signs at 3 months of age for eventual emmetropization to +2.00 D or less by 18 months included having a high degree of hyperopia in addition to poor or unmeasurable acuity combined with hyperopic MRET values. Emmetropization was also less likely if acuity was at or above the median at 3 months with myopic MRET values. DRET was not useful as a prognostic factor. The results in Figure 7 when acuity is below the median or unmeasurable seem to parallel those in Figure 1 for WSEQ alone, suggesting that they are driven by the underlying amount of WSEQ and its association with MRET. Even though the eye grows faster at higher levels of WSEQ,12 high levels of hyperopia reduce the likelihood for successful emmetropization when they exceed its operating range.
The results when acuity was above the median are more difficult to explain. Why would a myopic MRET in this group reduce the likelihood of emmetropization? One possibility is that acuity was good because the eye was myopically defocused during both MRET and acuity measurement. This suggests poor control and an inability to relax accommodation in this group. Good acuity plus accommodative control, as indicated by a normal amount of lag at distance and near, may be a positive factor in emmetropization. An alternate explanation for the low likelihood of emmetropization might be that myopic defocus during MRET is a potent “stop” signal that interferes with emmetropization,18 but only in the group with good acuity and sensitivity to visual signals.
MRET was performed to provide measures comparable to distance non-cycloplegic refractive error. The correlation between these two measures in adults has been reported to be 0.99.36 More studies have compared Mohindra retinoscopy and cycloplegic measures. The agreement has been reported to be good to excellent with correlations of 0.80 to 0.93.35, 37-39 Even when rated as not producing good agreement, near retinoscopy had a correlation of at least 0.56 with cycloplegic retinoscopy.48 The current study results, except for the lower correlation at 3 months, are more consistent with this modest correlation. The reported correlations between Mohindra retinoscopy and tonic accommodation further confuse the issue of what exactly is being measured.49, 50 The retinoscope light has been described as a degraded stimulus to accommodation, insufficiently degraded to fully open the accommodative loop,42 but not as compelling a stimulus as the target and retinoscopist during the closed-loop DRET. MRET may therefore represent some intermediate value between full cycloplegic distance refractive error and tonic accommodation that might approximate the intended estimate of distance defocus. Whatever MRET is actually measuring, the technique as used in this study resulted in information that had value in predicting the probability of emmetropization. Relative accommodation caused by the introduction of plus lenses is an additional issue for both MRET and DRET. This explanation has been offered in a recent report showing that MEM overestimates lag by 0.32 D to 0.41 D compared to Nott retinoscoopy.51 Although the current study examiners were trained initially to record the midpoint between the high and low neutral points, no such range was ever observed. Therefore relative accommodation did not appear to be a contaminating factor in estimating distance or near defocus in BIBS.
In summary, retinoscopic measurement of accommodation in infants during emmetropization showed that infants were capable of accurate average levels of accommodation across a range of moderate initial refractive errors. The existence of similar amounts of defocus across moderate levels of initial hyperopia does not support a simple model of emmetropization in response to the level of hyperopic defocus. Study results suggest that accommodative effort may be a visual signal for emmetropization. Collection of data at more frequent intervals to investigate the rates of growth and change in refractive error in more detail may help to distinguish between models of visually guided ocular growth. While the precise visual controllers of ocular growth and change in refractive error may not have been identified here, clinicians may find that cycloplegic refractive error, visual acuity, and Mohindra retinoscopy at 3 months of age are useful predictive factors for estimating the probability of a hyperopic infant reaching emmetropia by 18 months of age.
ACKNOWLEDGMENTS
We wish to acknowledge the contributions of Stephanie J. Kirschbaum, OD, J. Daniel Twelker OD, PhD, and Robert I. Sholtz, MS to earlier phases of this project. We are also grateful to Angela M. Brown, PhD for the Fourier analysis of the accommodative target.
Supported by grants R01-EY11801 (DM) and R24-EY014792 (KZ) from the National Institutes of Health, Bethesda, Maryland
REFERENCES
- 1.Santonastaso A. La rifrazione oculare nei primi anni di vita. Ann Ottalmol Clin Oculist. 1930;58:852–84. [Google Scholar]
- 2.Cook RC, Glasscock RE. Refractive and ocular findings in the newborn. Am J Ophthalmol. 1951;34:1407–13. doi: 10.1016/0002-9394(51)90481-3. [DOI] [PubMed] [Google Scholar]
- 3.Luyckx J. [Measurement of the optic components of the eye of the newborn by ultrasonic echography]. Arch Ophtalmol Rev Gen Ophtalmol. 1966;26:159–70. [PubMed] [Google Scholar]
- 4.Goldschmidt E. Refraction in the newborn. Acta Ophthalmol (Copenh) 1969;47:570–8. doi: 10.1111/j.1755-3768.1969.tb08143.x. [DOI] [PubMed] [Google Scholar]
- 5.Zonis S, Miller B. Refractions in the Israeli newborn. J Pediatr Ophthalmol. 1974;11:77–81. [Google Scholar]
- 6.Wood IC, Hodi S, Morgan L. Longitudinal change of refractive error in infants during the first year of life. Eye. 1995;9:551–7. doi: 10.1038/eye.1995.138. [DOI] [PubMed] [Google Scholar]
- 7.Saunders KJ, Woodhouse JM, Westall CA. Emmetropisation in human infancy: rate of change is related to initial refractive error. Vision Res. 1995;35:1325–8. doi: 10.1016/0042-6989(94)00222-8. [DOI] [PubMed] [Google Scholar]
- 8.Ehrlich DL, Atkinson J, Braddick O, Bobier W, Durden K. Reduction of infant myopia: a longitudinal cycloplegic study. Vision Res. 1995;35:1313–24. doi: 10.1016/0042-6989(94)00228-e. [DOI] [PubMed] [Google Scholar]
- 9.Mayer DL, Hansen RM, Moore BD, Kim S, Fulton AB. Cycloplegic refractions in healthy children aged 1 through 48 months. Arch Ophthalmol. 2001;119:1625–8. doi: 10.1001/archopht.119.11.1625. [DOI] [PubMed] [Google Scholar]
- 10.Mutti DO, Mitchell GL, Jones LA, Friedman NE, Frane SL, Lin WK, Moeschberger ML, Zadnik K. Refractive astigmatism and the toricity of ocular components in human infants. Optom Vis Sci. 2004;81:753–61. doi: 10.1097/00006324-200410000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Mutti DO. To emmetropize or not to emmetropize? The question for hyperopic development. Optom Vis Sci. 2007;84:97–102. doi: 10.1097/OPX.0b013e318031b079. [DOI] [PubMed] [Google Scholar]
- 12.Mutti DO, Mitchell GL, Jones LA, Friedman NE, Frane SL, Lin WK, Moeschberger ML, Zadnik K. Axial growth and changes in lenticular and corneal power during emmetropization in infants. Invest Ophthalmol Vis Sci. 2005;46:3074–80. doi: 10.1167/iovs.04-1040. [DOI] [PubMed] [Google Scholar]
- 13.Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988;28:639–57. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- 14.Irving EL, Sivak JG, Callender MG. Refractive plasticity of the developing chick eye. Ophthalmic Physiol Opt. 1992;12:448–56. [PubMed] [Google Scholar]
- 15.Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995;35:1175–94. doi: 10.1016/0042-6989(94)00233-c. [DOI] [PubMed] [Google Scholar]
- 16.Smith EL, 3rd, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999;39:1415–35. doi: 10.1016/s0042-6989(98)00229-6. [DOI] [PubMed] [Google Scholar]
- 17.Graham B, Judge SJ. The effects of spectacle wear in infancy on eye growth and refractive error in the marmoset (Callithrix jacchus). Vision Res. 1999;39:189–206. doi: 10.1016/s0042-6989(98)00189-8. [DOI] [PubMed] [Google Scholar]
- 18.Norton TT, Siegwart JT, Jr., Amedo AO. Effectiveness of hyperopic defocus, minimal defocus, or myopic defocus in competition with a myopiagenic stimulus in tree shrew eyes. Invest Ophthalmol Vis Sci. 2006;47:4687–99. doi: 10.1167/iovs.05-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen W, Sivak JG. Eyes of a lower vertebrate are susceptible to the visual environment. Invest Ophthalmol Vis Sci. 2007;48:4829–37. doi: 10.1167/iovs.06-1273. [DOI] [PubMed] [Google Scholar]
- 20.Wallman J, Wildsoet C, Xu A, Gottlieb MD, Nickla DL, Marran L, Krebs W, Christensen AM. Moving the retina: choroidal modulation of refractive state. Vision Res. 1995;35:37–50. doi: 10.1016/0042-6989(94)e0049-q. [DOI] [PubMed] [Google Scholar]
- 21.Napper GA, Brennan NA, Barrington M, Squires MA, Vessey GA, Vingrys AJ. The duration of normal visual exposure necessary to prevent form deprivation myopia in chicks. Vision Res. 1995;35:1337–44. doi: 10.1016/0042-6989(94)00226-c. [DOI] [PubMed] [Google Scholar]
- 22.Smith EL, 3rd, Hung LF, Kee CS, Qiao Y. Effects of brief periods of unrestricted vision on the development of form-deprivation myopia in monkeys. Invest Ophthalmol Vis Sci. 2002;43:291–9. [PubMed] [Google Scholar]
- 23.Zhu X, Winawer JA, Wallman J. Potency of myopic defocus in spectacle lens compensation. Invest Ophthalmol Vis Sci. 2003;44:2818–27. doi: 10.1167/iovs.02-0606. [DOI] [PubMed] [Google Scholar]
- 24.Candy TR, Bharadwaj SR. The stability of steady state accommodation in human infants. J Vis. 2007;7:4 1–16. doi: 10.1167/7.11.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tondel GM, Candy TR. Human infants’ accommodation responses to dynamic stimuli. Invest Ophthalmol Vis Sci. 2007;48:949–56. doi: 10.1167/iovs.06-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haynes H, White BL, Held R. Visual accommodation in human infants. Science. 1965;148:528–30. doi: 10.1126/science.148.3669.528. [DOI] [PubMed] [Google Scholar]
- 27.Banks MS. The development of visual accommodation during early infancy. Child Dev. 1980;51:646–66. [PubMed] [Google Scholar]
- 28.Currie DC, Manny RE. The development of accommodation. Vision Res. 1997;37:1525–33. doi: 10.1016/s0042-6989(97)85022-5. [DOI] [PubMed] [Google Scholar]
- 29.Braddick O, Atkinson J, French J, Howland HC. A photorefractive study of infant accommodation. Vision Res. 1979;19:1319–30. doi: 10.1016/0042-6989(79)90204-9. [DOI] [PubMed] [Google Scholar]
- 30.Brookman KE. Ocular accommodation in human infants. Am J Optom Physiol Opt. 1983;60:91–9. doi: 10.1097/00006324-198302000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Sokol S, Moskowitz A, Paul A. Evoked potential estimates of visual accommodation in infants. Vision Res. 1983;23:851–60. doi: 10.1016/0042-6989(83)90052-4. [DOI] [PubMed] [Google Scholar]
- 32.Howland HC, Dobson V, Sayles N. Accommodation in infants as measured by photorefraction. Vision Res. 1987;27:2141–52. doi: 10.1016/0042-6989(87)90128-3. [DOI] [PubMed] [Google Scholar]
- 33.Green DG, Powers MK, Banks MS. Depth of focus, eye size and visual acuity. Vision Res. 1980;20:827–35. doi: 10.1016/0042-6989(80)90063-2. [DOI] [PubMed] [Google Scholar]
- 34.Gabriel GM, Mutti DO. Comparison of dynamic retinoscopy and binocular photoretinoscopy for measurement of accommodative response in infants. Optom Vis Sci. 2005;82 E-Abstract 050033. [Google Scholar]
- 35.Mohindra I. A non-cycloplegic refraction technique for infants and young children. J Am Optom Assoc. 1977;48:518–23. [PubMed] [Google Scholar]
- 36.Mohindra I. Comparison of “near retinoscopy” and subjective refraction in adults. Am J Optom Physiol Opt. 1977;54:319–22. [PubMed] [Google Scholar]
- 37.Mohindra I, Molinari JF. Near retinoscopy and cycloplegic retinoscopy in early primary grade schoolchildren. Am J Optom Physiol Opt. 1979;56:34–8. doi: 10.1097/00006324-197901000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Borghi RA, Rouse MW. Comparison of refraction obtained by “near retinoscopy” and retinoscopy under cycloplegia. Am J Optom Physiol Opt. 1985;62:169–72. doi: 10.1097/00006324-198503000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Saunders KJ, Westall CA. Comparison between near retinoscopy and cycloplegic retinoscopy in the refraction of infants and children. Optom Vis Sci. 1992;69:615–22. doi: 10.1097/00006324-199208000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Birch EE, Swanson WH. Probability summation of acuity in the human infant. Vision Res. 1992;32:1999–2003. doi: 10.1016/0042-6989(92)90059-r. [DOI] [PubMed] [Google Scholar]
- 41.Gwiazda J, Bauer J, Thorn F, Held R. Development of spatial contrast sensitivity from infancy to adulthood: psychophysical data. Optom Vis Sci. 1997;74:785–9. doi: 10.1097/00006324-199710000-00017. [DOI] [PubMed] [Google Scholar]
- 42.Rosenfield M, Portello JK, Blustein GH, Jang C. Comparison of clinical techniques to assess the near accommodative response. Optom Vis Sci. 1996;73:382–8. doi: 10.1097/00006324-199606000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Cox DR, Hinkley DV. Theoretical Statistics. Chapman and Hall; London: 1974. [Google Scholar]
- 44.Mayer DL, Beiser AS, Warner AF, Pratt EM, Raye KN, Lang JM. Monocular acuity norms for the Teller Acuity Cards between ages one month and four years. Invest Ophthalmol Vis Sci. 1995;36:671–85. [PubMed] [Google Scholar]
- 45.Salomao SR, Ventura DF. Large sample population age norms for visual acuities obtained with Vistech-Teller Acuity Cards. Invest Ophthalmol Vis Sci. 1995;36:657–70. [PubMed] [Google Scholar]
- 46.Walker TW, Mutti DO. The effect of accommodation on ocular shape. Optom Vis Sci. 2002;79:424–30. doi: 10.1097/00006324-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Bailey MD, Sinnott LT, Mutti DO. Ciliary body thickness and refractive error in children. Invest Ophthalmol Vis Sci. 2008;49:4353–60. doi: 10.1167/iovs.08-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maino JH, Cibis GW, Cress P, Spellman CR, Shores RE. Noncycloplegic vs cycloplegic retinoscopy in pre-school children. Ann Ophthalmol. 1984;16:880–2. [PubMed] [Google Scholar]
- 49.Owens DA, Mohindra I, Held R. The effectiveness of a retinoscope beam as an accommodative stimulus. Invest Ophthalmol Vis Sci. 1980;19:942–9. [PubMed] [Google Scholar]
- 50.Bullimore MA, Gilmartin B, Hogan RE. Objective and subjective measurement of tonic accommodation. Ophthalmic Physiol Opt. 1986;6:57–62. [PubMed] [Google Scholar]
- 51.del Pilar Cacho M, Garcia-Munoz A, Garcia-Bernabeu JR, Lopez A. Comparison between MEM and Nott dynamic retinoscopy. Optom Vis Sci. 1999;76:650–5. doi: 10.1097/00006324-199909000-00023. [DOI] [PubMed] [Google Scholar]