Abstract
The phosphatidylinositol 3-kinase/Akt (PI3K) pathway regulates hypoxia-inducible factor (HIF) activity. Higher expression of HIF-1α and carbonic anhydrase IX (CAIX), a hypoxia-inducible gene, in HT10806TG fibrosarcoma cells (mutant N-ras allele), compared to derivative MCH603 cells (deleted mutant N-ras allele), correlated with increased PI3K activity. Constitutive activation of the PI3K pathway in MCH603/PI3Kact cells increased HIF-1α but, surprisingly, decreased CAIX levels. The cell-type specific inhibitory effect on CAIX was confirmed at the transcriptional level whereas epigenetic modifications of CA9 were ruled out. In summary, our data do not substantiate the generalization that PI3K upregulation leads to increased HIF activity.
Keywords: PI3K/Akt, hypoxia, CAIX, VEGF, HIF
Introduction
The hypoxia-inducible factor (HIF) is a key transcriptional regulator in response to lowered oxygen concentration (hypoxia). HIF is a heterodimer consisting of a constitutive β(HIF-1β) and one of the regulated α(HIF-1α, HIF-2α, and HIF-3α) subunits (reviewed in [1]). HIF is regulated by two types of oxygen sensor control. The first control regulates stability of HIF-α via hydroxylation at the prolines 462 and 564 (in HIF-1α) within the oxygen-dependent degradation domain (ODD) [2]. Under normoxic conditions, hydroxylation of the prolines by a family of prolyl-4-hydroxylases that require O2, Fe(II), and 2-oxoglutarate for activity, allows specific recognition of HIF-α by the von Hippel Lindau protein (VHL) [3,4]. VHL and other protein components, that form the E3 ubiquitin-ligase complex, then mediate ubiquitinylation that leads to degradation of the HIF-α protein by the 26S proteasome. In addition to the ODD, HIF-1α also contains two transcriptional activation domains (AD), the N-terminal NAD and the C-terminal CAD. The second oxygen sensor control regulates transcriptional activity of the CAD via the oxygen-dependent asparaginyl hydroxylase factor inhibiting HIF-1 (FIH-1), which hydroxylates asparagine N803 (in HIF-1α) within the CAD, thus preventing interaction with the transcriptional coactivators p300/CBP [5].
Under hypoxia, prolyl and asparaginyl hydroxylases are inhibited and the stabilized HIF-α subunit translocates to the nucleus, heterodimerizes with HIF1-β, and recruits cofactors [6-8]. The HIF complex then binds to hypoxia-responsive elements (HRE) on target genes and activates their transcriptions. Besides proline hydroxylation, other regulatory pathways, including the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, have been implicated in the control of HIF-α protein expression [9,10]. The PI3K/Akt pathway is commonly activated in human cancers via the loss of the tumor suppressor phosphatase and tensin homologue deleted on chromosome 10 (PTEN) or deregulation of growth factor signaling [11]. A number of studies have suggested that activation of the PI3K pathway exerts increased HIF-1α translation through mammalian target of rapamycin (mTOR) [7,12-14]. There are other reports, however, showing that Akt can increase HIF-1α translation through an mTOR-independent pathway [9,15]. Although most reports have shown a direct correlation between increased PI3K/Akt signaling and HIF-1α activity, there are other studies which indicate that this pathway is not required for hypoxic stabilization of HIF-1α and its effect is more likely to be cell-type specific [16,17]. This controversy has led us to investigate the effects of the PI3K/Akt pathway on HIF function in a model of human fibrosarcoma cell lines with various levels of PI3K/Akt activity [18,19], and other cancer cell lines. To achieve this, we examined the levels of HIF-1α and HIF-2α protein expression, and the levels of expression of their target genes, namely carbonic anhydrase IX (CAIX) and vascular endothelial growth factor (VEGF), in these cell lines under various experimental conditions.
Materials and Methods
Cell lines and culture
HT10806TG and MCH603 cells are variants of HT1080 human fibrosarcoma cells which contain mutant and wild-type N-ras alleles, respectively [20]. MCH603/PI3Kact are MCH603 cells stably transfected with the PI3Kact pCMV(hyg)P110CAAX5’myc plasmid which gave rise to cells with constitutively active PI3-kinase [19]. Breast adenocarcinoma MCF-7 and osteosarcoma Saos-2 cell lines were also used. Cells were grown in Dulbecco’s modified Eagle’s medium (BioWhittaker) supplemented with 10% fetal calf serum (Life Technologies), 1 × 102 U/ml penicillin (Sigma), and 1 × 102 μg/ml streptomycin (ICN). The effects of cell density and hypoxia on CAIX, VEGF, and HIF-α expression were tested with cells that had been 3-times subcultured at 10,000/cm2 [21]. For sparse and dense conditions, the cells were plated at 20,000 and 200,000 cells/cm2, respectively. Once the cells attached, the plates were either exposed to normoxia or a 0.1% O2 environment in a ProOx in vitro chamber (BioSpherix), controlled by ProOx model 110 (BioSpherix), for 48 h.
Western blot analysis
Western blot analysis of HIF-1α, HIF-2α, CAIX, and ß-actin expression was performed as described previously [21]. Akt, pAkt, FoxO1 and pFoxO1 antibodies were from Cell Signaling Technology.
VEGF assay
VEGF levels in cell culture media were assayed with a VEGF sandwich enzyme-linked immunosorbent assay kit (Chemicon), according to the manufacturer’s protocol and expressed as pg protein/mg of total protein.
Reverse transcriptase PCR (RT-PCR)
MCH603 and MCH603/PI3Kact cells were seeded as described above, followed by exposure to normoxia or 0.5% O2 for 48 h, when total RNA was isolated with an RNeasy mini kit (QIAGEN). cDNA was synthesized with Oligo dT primer and the ImProm-II Reverse Transcription System (Promega). cDNA fragments were amplified with the following primer pairs: CA9 (GenBank accession no. NM_001216), CTGTCACTGCTGCTTCTGAT (residues 121 to 140), sense, and TCCTCTCCAGGTAGATCCTC (residues 321 to 301), antisense; for VEGF (GenBank accession no. M32977), GCCTTGCTGCTCTACCTC (residues 93 to 110), sense, and GGCACACAGGATGGCTTG (residues 292 to 275), antisense; and for ß-actin (GenBank accession no. NM_001101), ACAACGGCTCCGGCATGTGCAA (residues 105 to 126), sense, and CGGTTGGCCTTGGGGTTCAG (residues 420 to 402), antisense. PCRs were performed with a GeneAmp PCR system 9700 (PE Applied Biosystems) for 30 cycles (with the exception of β-actin [25 cycles]) at 95°C for 40 s, annealing at 56°C for 40 s and extension at 72°C for 1 min with a final extension of 7 min at 72°C. Products were analyzed on a 1.5% agarose gel.
Transient transfection assay
Cells were cotransfected with the [-173;+31] (respective to the transcription start site) CA9 promoter fragment in firefly luciferase expressing pGL2-basic (Promega) and the pRL-CMV expressing Renilla luciferase (internal control for transfection efficiency) as described previously [22]. After exposure to the transfection mixture for 16 h, the cells were trypsinized, plated at required concentrations, and allowed to adhere for 5 h. The cells were then exposed to normoxia or 0.1% O2 for 48 h prior to harvesting. Reporter assays were performed as described previously [21] and measured in the Sirius luminometer (Berthold Detection Systems). Promoter activities were expressed as the average ratios of firefly to Renilla luciferase activities (±SD) from at least three independent experiments.
Infection with Adenovirus-PI3K
Cells were infected with PI3Kact in Adeno-X virus [23] at various multiplicities of infection and incubated for 48 h under indicated experimental conditions. Control cells were exposed to empty Adeno-X virus (Clontech).
Results and Discussion
The role of the PI3K/Akt pathway on the regulation of HIF activity is controversial. Due to this fact, we set out to investigate the effects of PI3K/Akt activation in several cancer cell lines. We examined the effect of modulating the activity of the PI3K pathway on HIF function by observing levels of HIF-1α and HIF-2α, and expression levels of their target genes, namely CAIX and VEGF. Correlation between HIF-1α and CAIX expression under conditions of hypoxia and high cell density has been well established [21,22,24]. In the present study, we used the HT10806TG fibrosarcoma cells, which harbor the mutant form of N-ras, and their derivative MCH603 cells which carry the wild-type N-ras, and MCH603/PI3Kact cells with a constitutively activated PI3K/Akt pathway [18,19].
At first, we assessed activation of the PI3K pathway in these cell lines by testing the steady-state levels of pAkt, the downstream target of PI3K [25,26], under conditions of various oxygen concentrations and/or cell density. Previously, we have shown that HT10806TG cells have constitutively active PI3K/Akt pathway due to the mutant form of N-ras [19]. As expected, the level of pAkt in this cell line was high under all the conditions tested, with maximal activity observed in the dense samples (Fig. 1A). This observation agreed with our previous report which showed that PI3K/Akt activity was two to three times higher in dense compared to sparse cultures of HeLa cells [21]. In the present study, we found that under both normoxic and hypoxic conditions, the level of pAkt was equally high in dense culture (Fig. 1A). In MCH603 cells, which carry the wild type N-ras alleles, pAkt levels were the lowest in sparse cells under normoxic conditions (Fig. 1A). The level then increased slightly either under hypoxic or dense conditions alone. When these two stimuli were combined, as in the dense hypoxic culture, pAkt levels increased dramatically. MCH603/PI3Kact1 cells, with the constitutively active PI3K/Akt pathway, displayed a five-fold higher level of pAkt than MCH603 in sparse normoxic cells. This level was comparable to that seen in the N-ras mutant HT10806TG cells. This observation also corroborated our earlier finding that the level of pAkt in MCH603/PI3Kact1 was significantly higher compared to the parental MCH603 cells when cells were cultured under subconfluent conditions in ambient oxygen concentration [19]. These results showed that activation of the PI3-kinase pathway by either ectopic expression of the PI3Kact cDNA or by the upstream mutant N-ras resulted in similarly increased levels of Akt phosphorylation.
Fig. 1.
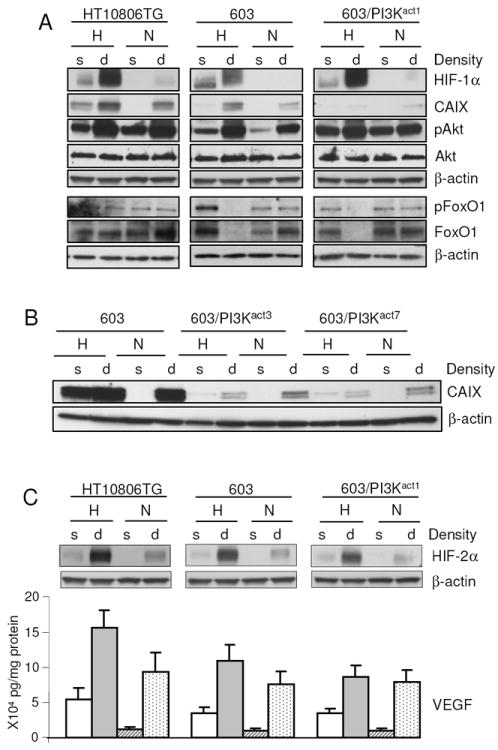
Effects of density and oxygen concentrations on pAkt, HIF-1α, CAIX, HIF-2α and VEGF expression levels in HT10806TG, MCH603 cells and MCH603/PI3Kact clones. (A) Cells were seeded at the stated densities and incubated at normoxic (N) or hypoxic (0.1% O2, H) conditions. After 48 h, cells were harvested and the total cell lysates were probed by Western blotting with specific antibodies as indicated (B) Effects of cell density and oxygen concentrations on CAIX expression in the additional clones of MCH603/PI3Kact. (C) Samples from the HT10806TG, MCH603 cells and MCH603/PI3Kact1 were also probed for HIF-2α Blots were later reprobed with β-actin Ab. For comparison purposes, similar β-actin bands were shown in (A) and (C). Culture media of these cells were used for the VEGF assay. The data represent the mean (±SD) of two experiments performed in triplicate. s; sparse, d; dense.
We had earlier shown that the elevated level of PI3K activity in the MCH603/PI3Kact1 cells resulted in cross-talk activation of the MAPK and JNK pathways [19]. We further analyzed this effect of higher levels of PI3K activity on phosphorylation of the transcriptional regulator, FoxO1. This is a recognized target of Akt phosphorylation but, in addition, it has been shown that the FoxO1 protein is degraded by the proteasome in cells with elevated levels of PI3K or pAkt activity [27]. This degradation of total FoxO1 protein was seen in both MCH603 and MCH603/PI3Kact1 and, to a lesser degree, HT1080 under conditions of high density and hypoxia that produced the highest levels of pAkt (Fig. 1A).
Previous studies have shown that HIF is stabilized by upregulated PI3K/Akt [13, 28-30]. Thus, if upregulation of the PI3K/Akt pathway leads to stabilization of HIF, we would be able to observe an increase in CAIX levels in the HT10806TG and MCH603/PI3Kact1 but not MCH603 cells. To test this possibility, we investigated the levels of HIF-1α and its target gene CA9 in these cell lines. As expected, HIF-1α was found to be strongly induced by hypoxia in all three cell lines with maximal induction observed under dense hypoxic conditions. In normoxic dense cultures, only minimal stabilization of HIF-1α was noted. This modest HIF-1α accumulation under dense condition was due to the existence of pericellular hypoxia even when cells were cultured under normoxic condition [31]. As previously reported [32], CAIX was expressed under this condition (Fig. 1A). Comparatively, more CAIX was expressed in HT10806TG than in MCH603 cells. Similar to HIF-1α, the highest level of CAIX expression was found in dense hypoxic samples in both cell lines. This result is in agreement with our previous study which showed that the induction of CAIX by density and hypoxia is additive, although the exact pathways have not been elucidated [21]. Intriguingly, in MCH603/PI3Kact1 cells the increased pAkt level did not stimulate CAIX expression in any of the conditions tested (Fig. 1A). The expression, however, remained hypoxia- or density-induced, albeit at a reduced level compared to MCH603. This observation is intriguing since the majority of previous reports suggested a direct correlation between PI3K and HIF-α activities [13,28,29] and hence, CAIX protein expression. Despite this general view of the direct correlation, there are studies that showed no correlation between PI3K activity and HIF protein levels, leading to the suggestion that the relationship is purely cell-type specific [16,17]. Arsham and colleagues showed that constitutively activated Akt was able to increase the level of HIF-1α protein in glioblastoma but not hepatoma cells [17]. In their study, they proposed that Akt activity itself was insufficient for the induction of HIF target gene transcription. Similar observations were noted in the present study in the sparse normoxic samples of both HT10806TG and MCH603/PI3Kact1 cells. In these samples, there was no CAIX expression despite the high levels of pAkt. Under hypoxia and density, HIF-1α accumulation was similarly observed in the two cells lines but different levels of CAIX were expressed. In MCH603/PI3Kact1 cells, CAIX expression was markedly reduced even though HIF-1α levels were as high as in HT10806TG cells. Since upregulation of PI3K/Akt activity in MCH603/PI3Kact1 cells failed to stimulate CAIX expression, its increase in the parental HT10806TG cells was likely to be due to activation of other pathways downstream of the constitutively active N-ras and not the PI3K/Akt pathway per se. The observation of reduced expression of HIF target proteins due to increased PI3K/Akt signaling has not been reported previously. To confirm that the effect of reduced CAIX expression was not due to clonal variation of MCH603/PI3Kact cells, we repeated the experiment using additional clones, designated MCH603/PI3Kact3 and MCH603/PI3Kact7. Similar to MCH603/PI3Kact1, these additional clones also showed reduction in the levels of CAIX protein in all of the conditions tested compared to the parental MCH603 cells (Fig. 1B). Our study is thus the first evidence suggesting that not only there is no direct correlation between PI3K upregulation and increased HIF activity, but we also show that in certain cells the upregulation may counterintuitively lead to reduced HIF activity, as evidenced by the decreased expression of a HIF target gene.
To see whether the same applies for HIF-2α and VEGF, the similar samples were probed for HIF-2α while the secreted VEGF protein levels were measured in the culture media. Under conditions of combined hypoxia and high cell density, we observed that the HIF-2α level in the parental HT10806TG cell line was highest, followed by MCH603 cells (Fig. 1C). The constitutively active PI3K in MCH603/PI3Kact1 failed to increase HIF-2α levels, instead the expression was slightly lowered. In contrast to CAIX, no marked downregulation of VEGF expression was observed in the MCH603/PI3Kact1 compared to the MCH603. Similar to HIF-2α, only a slight decrease was observed. This result suggested that the constitutively active PI3K in the MCH603/PI3Kact1 did not affect the overall levels or activity of HIF-2. This observation is supported by previous reports showing preferential regulation of HIF-1α by the PI3K/Akt pathway, without apparent affect on HIF-2α[33] and HIF-1β[7].
Next, we performed semi-quantitative RT-PCR analysis to see the effects of PI3K on transcription of CA9 and VEGF in MCH603 and all three clones of MCH603/PI3Kact. Dense hypoxic cells (Fig. 2A) and dense normoxic cells (Fig. 2B) showed reduced levels of CA9 transcript in all of the MCH603/PI3Kact cell clones compared to the parental MCH603 cells. Reduced transcript levels correlated well with reduced CAIX protein expression levels in these cells. This result suggests that downregulation of CA9 occurred at the transcription level. For VEGF, similar to secreted VEGF levels, a slight decrease in the transcript was observed in dense hypoxic MCH603/PI3Kact cell clones compared to MCH603, but no marked differences were noted in dense normoxic cells. Our results thus far strongly suggested the potential deregulation of HIF-1α activity in MCH603/PI3Kact cells, particularly its effects on CA9 transcription.
Fig. 2.
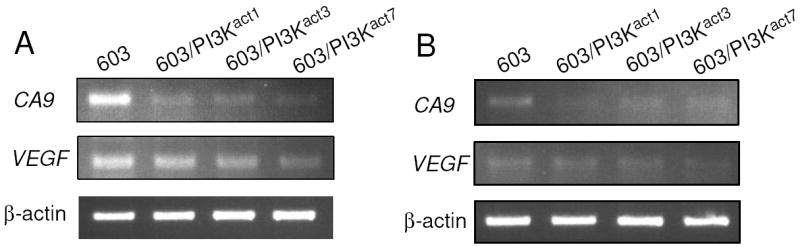
Semi-quantitative RT-PCR analyses of the CA9 and VEGF transcript levels in MCH603 and MCH603/PI3Kact clones under dense condition. (A) hypoxia, (B) normoxia. Total RNA was reverse-transcribed, amplified with gene-specific primers, and analyzed on agarose gels.
To ensure that this effect on CA9 transcription was not due to modifications of its endogenous promoter, we introduced the CA9 minimal promoter region [22] into MCH603 and MCH603/PI3Kact1 cells. The transfectants were then cultured under either sparse or dense hypoxic conditions. The CA9 promoter activity was reduced in MCH603/PI3Kact1 compared to the MCH603 cells (Fig. 3A). Although the reduction was not as strong as in the endogenous CAIX protein levels, these results confirmed that activity of the exogenously introduced CA9 promoter is also affected in MCH603/PI3Kact cells. The reduced level was not due to methylation of the promoter since addition of 5-azacytidine, an epigenetic modifier often used to reactivate methylation-dependent transcriptionally silent genes [34], failed to recover CAIX expression (data not shown). To investigate whether similar effects can be observed via transiently activated PI3K, we co-transfected the parental MCH603 cells with the PI3Kact plasmid construct [21] and the CA9 promoter reporter plasmids. Results showed that the CA9 promoter activity was also reduced significantly in the transiently co-transfected PI3Kact cells, compared to cells co-transfected with the empty plasmid vector (Fig 3B). These results suggest that the effects of lowered CA9 expression observed in MCH603/PI3Kact were specifically due to the increased PI3K activity in the cells.
Fig. 3.
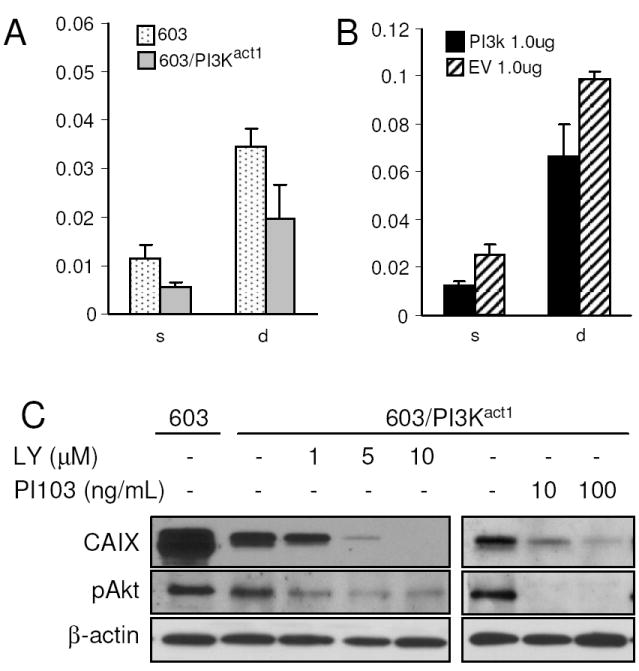
Effects of density and oxygen concentrations on exogenous CA9 promoter, and effects of PI3K inhibitors in MCH603 and MCH603/PI3Kact1 cell lines. (A) Cells were transfected with plasmids carrying the CA9 promoter and the internal control. (B) PI3Kact expression plasmid and an empty vector (EV) control were co-transfected with the CA9 promoter construct into MCH603 cells and the reporter levels were measured. Promoter activities were expressed as the average ratios of firefly to Renilla luciferase activities (±SD) from at least three independent experiments. (C) Effects of PI3K inhibitors on pAkt and CAIX expression in MCH603/PI3Kact1 cells.
Given that increased PI3K activity in MCH603/PI3Kact cells results in the inhibition of HIF-1 activity, even when large amounts of stable HIF-1α are present in hypoxic conditions, we investigated whether inhibition of the PI3K activity in these cells would restore HIF-1 activation of CAIX expression. We used two inhibitors, LY294002 and the more specific PI103 [35]. Both inhibitors decreased pAkt expression and concomitantly decreased CAIX expression (Fig. 3C). This is a very interesting result when coupled with the overexpression data seen in Fig. 1A. It shows, that whereas high levels of PI3K activity lead to inhibition of HIF-1 activity, there is a threshold level of PI3K activity that is needed for CAIX expression that may be independent of HIF-1α levels. This latter observation has been reported previously [21]. This notion of threshold effects has been noted with other oncoproteins, for example c-Myc [36].
To investigate the effects of PI3K/Akt activation on HIF activity in other cell lines, we used the MCF-7 breast cancer and Saos-2 osteosarcoma cell lines. We achieved this by infecting the cells with adenoviral vector expressing PI3Kact [23]. Infection using 20 and 100 multiplicities of infection (MOI) caused increased PI3K/Akt activity under both normoxic and hypoxic conditions (Fig. 4). Activation of PI3K/Akt in MCF-7 (Fig. 4A) and Saos-2 cells (Fig. 4B) did not downregulate hypoxia-induced CAIX expression, instead it increased the level. This increase corroborated previous studies of increased HIF-1 protein levels correlating with upregulated PI3K/Akt activity [13,28]. However, when MCH603 cells were infected with the virus, reduced hypoxic CAIX expression was observed (Fig. 4C). Altogether, results from this study indicate that the PI3K/Akt pathway can affect CAIX expression at the transcriptional level perhaps through reduced HIF-1 activity. Although only one out of the three types of tumor cell lines tested in this study displayed such characteristics, this is the first evidence showing negative effects of the constitutively active PI3K/Akt pathway on HIF activity, despite increased levels of HIF-1α protein.
Fig. 4.
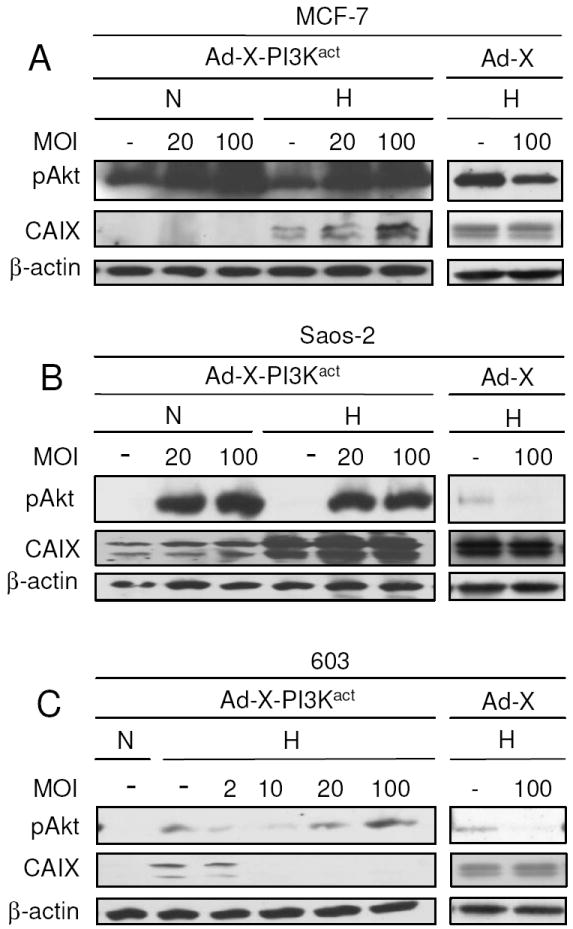
Effects of PI3Kact/Adeno-X virus infection on CAIX expression in MCF-7, Saos-2, and MCH603 cells. MCF-7 breast cancer (A), Saos-2 osteosarcoma (B) and MCH603 (C) cell lines were infected with the virus at the indicated multiplicity of infection (MOI) and incubated for 48 h under the indicated experimental conditions. Protein samples were harvested and probed with pAkt, CAIX and β-actin antibodies. Empty adenovirus vector (Ad-X) was used as control.
The different effects of PI3K/Akt upregulation on HIF-1, reflected by CAIX expression levels in the different cell lines, confirmed that the effects are purely cell-type specific. Currently, the mechanism for this counterintuitive decrease in the level of CA9 transcription, and corresponding low levels of CAIX protein expression in the presence of significantly increased PI3K/Akt signaling in MCH603/PI3Kact cells, is unknown. Although it is possible that the effect is due to specific features of MCH603 cells, results from this study suggest that PI3K/Akt is involved in this phenomenon. Therefore, additional mechanistic studies on its regulation of HIF will potentially unravel specific components that can be exploited for cancer drug discovery. In this regard it should be noted that proteasome inhibitors, e.g. bortezomib, have been shown to stabilize HIF-1α protein although the HIF transcription factor remains inactive [22,37]. Bortezomib, of course, stabilizes many proteins in addition to HIF-1α One candidate may be a putative co-repressor protein [22]. The elevated PI3K activity may also affect this putative co-repressor interaction with HIF-1. Whatever the mechanism this more physiological inhibitory effect on HIF-1 activity suggests a potential therapeutic target.
Acknowledgments
This study was supported by the Avon Foundation-American Association for Cancer Research International Scholar Award in Breast Cancer Research (NS and EJS), NIH grant CA-104214 (EJS) and Malaysian Ministry of Science, Technology and Innovation grants 02-01-04-SF0788 and 04-11-08-62FR (NS and EJS). We thank Dr. Jerry Olefsky for the gift of PI3Kact/Adeno-X.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith TG, Robbins PA, Ratcliffe PJ. The human side of hypoxia-inducible factor. Br J Haematol. 2008;141:325–34. doi: 10.1111/j.1365-2141.2008.07029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan DA, Sutphin PD, Yen SE, Giaccia AJ. Coordinate regulation of the oxygen-dependent degradation domains of hypoxia-inducible factor 1 alpha. Mol Cell Biol. 2005;25:6415–26. doi: 10.1128/MCB.25.15.6415-6426.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG. HIF-alpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–8. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 4.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–724. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 5.Arany Z, Huang LE, Eckner R, Bhattacharya S, Jiang C, Goldberg MA, Bunn HF, Livingston DM. An essential role for p300/CBP in the cellular response to hypoxia. Proc Natl Acad Sci U S A. 1996;93:12969–73. doi: 10.1073/pnas.93.23.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 7.Jiang BH, Jiang G, Zheng JZ, Lu Z, Hunter T, Vogt PK. Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor. Cell Growth Differ. 2001;12:363–9. [PubMed] [Google Scholar]
- 8.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 9.Pore N, Jiang Z, Shu HK, Bernhard E, Kao GD, Maity A. Akt1 activation can augment hypoxia-inducible factor-1alpha expression by increasing protein translation through a mammalian target of rapamycin-independent pathway. Mol Cancer Res. 2006;4:471–9. doi: 10.1158/1541-7786.MCR-05-0234. [DOI] [PubMed] [Google Scholar]
- 10.Jiang BH, Liu LZ. AKT signaling in regulating angiogenesis. Curr Cancer Drug Targets. 2008;8:19–26. doi: 10.2174/156800908783497122. [DOI] [PubMed] [Google Scholar]
- 11.Chalhoub N, Baker SJ. PTEN and the PI3-Kinase Pathway in Cancer. Annu Rev Pathol. 2008 doi: 10.1146/annurev.pathol.4.110807.092311. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang BH, Zheng JZ, Aoki M, Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci U S A. 2000;97:1749–53. doi: 10.1073/pnas.040560897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zundel W, Schindler C, Haas-Kogan D, Koong A, Kaper F, Chen E, Gottschalk AR, Ryan HE, Johnson RS, Jefferson AB, Stokoe D, Giaccia AJ. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 2000;14:391–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of hypoxia-inducible factor 1 expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–14. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brugarolas JB, Vazquez F, Reddy A, Sellers WR, Kaelin WG. TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell. 2003;4:147–58. doi: 10.1016/s1535-6108(03)00187-9. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez-Tejado M, Alfranca A, Aragones J, Vara A, Landazuri MO, del Peso L. Lack of evidence for the involvement of the phosphoinositide 3-kinase/Akt pathway in the activation of hypoxia-inducible factors by low oxygen tension. J Biol Chem. 2002;277:13508–17. doi: 10.1074/jbc.M200017200. [DOI] [PubMed] [Google Scholar]
- 17.Arsham AM, Plas DR, Thompson CB, Simon MC. PI3K/Akt signaling is neither required for hypoxic stabilization of HIF-1 nor sufficient for HIF-1-dependent target gene transcription. J Biol Chem. 2002;277:15162–70. doi: 10.1074/jbc.M111162200. [DOI] [PubMed] [Google Scholar]
- 18.Gupta S, Plattner R, Der CJ, Stanbridge EJ. Dissection of Ras-dependent signaling pathways controlling aggressive tumor growth of human fibrosarcoma cells: evidence for a potential novel pathway. Mol Cell Biol. 2000;20:9294–306. doi: 10.1128/mcb.20.24.9294-9306.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta S, Stuffrein S, Plattner R, Tencati M, Gray C, Whang YE, Stanbridge EJ. Role of phosphoinositide 3-kinase in the aggressive tumor growth of HT1080 human fibrosarcoma cells. Mol Cell Biol. 2001;21:5846–56. doi: 10.1128/MCB.21.17.5846-5856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plattner R, Anderson MJ, Sato KY, Fasching CL, Der CJ, Stanbridge EJ. Loss of oncogenic ras expression does not correlate with loss of tumorigenicity in human cells. Proc Natl Acad Sci U S A. 1996;93:6665–70. doi: 10.1073/pnas.93.13.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaluz S, Kaluzová M, Chrastina A, Olive PL, Pastoreková S, Pastorek J, Lerman MI, Stanbridge EJ. Lowered oxygen tension induces expression of the hypoxia marker MN/carbonic anhydrase IX in the absence of hypoxia-inducible factor 1 alpha stabilization: a role for phosphatidylinositol 3’-kinase. Cancer Res. 2002;62:4469–77. [PubMed] [Google Scholar]
- 22.Kaluz S, Kaluzová M, Stanbridge EJ. Proteasomal inhibition attenuates transcriptional activity of hypoxia-inducible factor 1 (HIF-1) via specific effect on the HIF-1alpha C-terminal activation domain. Mol Cell Biol. 2006;26:5895–907. doi: 10.1128/MCB.00552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma PM, Egawa K, Huang Y, Martin JL, Huvar I, Boss GR, Olefsky JM. Inhibition of phosphatidylinositol 3-kinase activity by adenovirus-mediated gene transfer and its effect on insulin action. J Biol Chem. 1998;273:18528–37. doi: 10.1074/jbc.273.29.18528. [DOI] [PubMed] [Google Scholar]
- 24.Kaluz S, Kaluzová M, Stanbridge EJ. The role of extracellular signal-regulated protein kinase in transcriptional regulation of the hypoxia marker carbonic anhydrase IX. J Cell Biochem. 2006;97:207–16. doi: 10.1002/jcb.20633. [DOI] [PubMed] [Google Scholar]
- 25.Duronio V, Scheid MP, Ettinger S. Downstream signalling events regulated by phosphatidylinositol 3-kinase activity. Cell Signal. 1998;10:233–239. doi: 10.1016/s0898-6568(97)00129-0. [DOI] [PubMed] [Google Scholar]
- 26.Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 27.Aoki M, Jiang H, Vogt PK. Proteasomal degradation of the FoxO1 transcriptional regulator in cells transformed by the PI3k and Akt oncoproteins. Proc Natl Acad Sci U S A. 2004;101:13613–7. doi: 10.1073/pnas.0405454101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin HO, An S, Lee HC, Woo SH, Seo SK, Choe TB, Yoo DH, Lee SB, Um HD, Lee SJ, Park MJ, Kim JI, Hong SI, Rhee CH, Park IC. Hypoxic condition- and high cell density-induced expression of Redd1 is regulated by activation of hypoxia-inducible factor-1alpha and Sp1 through the phosphatidylinositol 3-kinase/Akt signaling pathway. Cell Signal. 2007;19:1393–403. doi: 10.1016/j.cellsig.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Lee BL, Kim WH, Jung J, Cho SJ, Park JW, Kim J, Chung HY, Chang MS, Nam SY. A hypoxia-independent up-regulation of hypoxia-inducible factor-1 by AKT contributes to angiogenesis in human gastric cancer. Carcinogenesis. 2008;29:44–51. doi: 10.1093/carcin/bgm232. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka H, Yamamoto M, Hashimoto N, Miyakoshi M, Tamakawa S, Yoshie M, Tokusashi Y, Yokoyama K, Yaginuma Y, Ogawa K. Hypoxia-independent overexpression of hypoxia-inducible factor 1alpha as an early change in mouse hepatocarcinogenesis. Cancer Res. 2006;66:11263–70. doi: 10.1158/0008-5472.CAN-06-1699. [DOI] [PubMed] [Google Scholar]
- 31.Sheta EA, Trout H, Gildea JJ, Harding MA, Theodorescu D. Cell density mediated pericellular hypoxia leads to induction of HIF-1alpha via nitric oxide and Ras/MAP kinase mediated signaling pathways. Oncogene. 2001;20:7624–34. doi: 10.1038/sj.onc.1204972. [DOI] [PubMed] [Google Scholar]
- 32.Kaluzová M, Kaluz S, Stanbridge EJ. High cell density induces expression from the carbonic anhydrase 9 promoter. Biotechniques. 2004;36:228–30. doi: 10.2144/04362BM05. [DOI] [PubMed] [Google Scholar]
- 33.Blancher C, Moore JW, Robertson N, Harris AL. Effects of ras and von Hippel-Lindau (VHL) gene mutations on hypoxia-inducible factor (HIF)-1alpha, HIF-2alpha, and vascular endothelial growth factor expression and their regulation by the phosphatidylinositol 3’-kinase/Akt signaling pathway. Cancer Res. 2001;61:7349–55. [PubMed] [Google Scholar]
- 34.El-Osta A. DNMT cooperativity- the developing links between methylation, chromatin structure and cancer. Bioessays. 2003;25:1071–1084. doi: 10.1002/bies.10345. [DOI] [PubMed] [Google Scholar]
- 35.Raynaud FI, Eccles S, Clarke PA, Hayes A, Nutley B, Alix S, Henley A, Di-Stefano F, Ahmad Z, Guillard S, Bjerke LM, Kelland L, Valenti M, Patterson L, Gowan S, de Haven Brandon A, Hayakawa M, Kaizawa H, Koizumi T, Ohishi T, Patel S, Saghir N, Parker P, Waterfield M, Workman P. Pharmacologic characterization of a potent inhibitor of class I phosphatidylinositide 3-kinases. Cancer Res. 2007;67:5840–50. doi: 10.1158/0008-5472.CAN-06-4615. [DOI] [PubMed] [Google Scholar]
- 36.Murphy DJ, Junttila MR, Pouyet L, Karnezis A, Shchors K, Bui DA, Brown-Swigart L, Johnson L, Evan GI. Distinct thresholds govern Myc’s biological output in vivo. Cancer Cell. 2008;14:447–57. doi: 10.1016/j.ccr.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. J Biol Chem. 1997;272:22642–7. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]


