Abstract
Neuropathic pain affects millions of people globally and could be a disease on its own right. Current treatments focus on blocking neurotransmission and have resulted in limited success. Recent progress points to an important role of neuroinflammation in the pathogenesis of neuropathic pain. Matrix metalloproteases (MMPs) comprise a large family of zinc endopeptidases that have been implicated in the generation of neuroinflammation via cleavage of extracellular matrix proteins and activation of proinflammatory cytokines and chemokines. However, little is known about the role of MMPs in chronic pain regulation. Our recent study has shown that neuropathic pain development in the early and late phase requires MMP-9 and MMP-2, respectively. Inhibition of MMP-9 or MMP-2 may provide a new strategy for the prevention and treatment of neuropathic pain.
Introduction
Chronic neuropathic pain is caused by lesions in the peripheral nervous system (PNS, e.g., peripheral nerves) or the central nervous system (CNS, e.g., spinal cord and thalamus). Neuropathic pain comes in many forms and can be induced by trauma (e.g., spinal cord injury, stroke), disease conditions (e.g., diabetic neuropathy, HIV-associated neuropathy), and major surgeries (e.g., thoracotomy, amputation) [1-4] (Table 1). Chemotherapy also produces neuropathic pain. For example, vincristine and paclitaxel, two of the most effective drugs to fight against cancer, produce painful peripheral neuropathy [5]. Neuropathic pain can manifest as spontaneous pain, which is described as shooting, lancinating or burning pain. Neuropathic pain is also characterized by evoked pain, such as hyperalgesia (increased responsiveness to noxious stimuli) to mechanical and cold stimuli. In particular, mechanical allodynia or tactile allodynia, i.e. painful responses to normally innocuous tactile stimuli, is probably the most distinct symptom of neuropathic pain. Thus, patients feel pain when they walk and wear clothes. Currently available drugs for neuropathic pain include antidepressants, anticonvulsants, sodium channel blockers, NMDA receptor antagonists, and opioids (Table 1). Unfortunately, these drugs were designed to target neuronal targets and focus on blocking neurotransmission. They can treat pain symptoms but not the underlying pathology of neuropathic pain. They only provide a transient relief of neuropathic pain in only a fraction of patients and produce severe CNS side effects [1-4] (Table 1). Our incomplete understanding of mechanisms underlying the induction and maintenance of neuropathic pain has hindered more effective treatment of this pain.
Table 1.
Characteristics of neuropathic pain
| Indications | |
| Trauma | |
| Nerve injury, spinal cord injury, stroke | |
| Diseases | |
| Diabetic neuropathy, viral infection (AIDS), trigeminal neuralgia, multiple sclerosis |
|
| Major surgeries | |
| Amputation, thoracotomy | |
| Chemotherapy | |
| Paclitaxel, Vincristine | |
| Symptoms | |
| Spontaneous pain | |
| Shooting pain, burning pain | |
| Evoked pain | |
| Hyperalgesia, allodynia | |
| Treatment | |
| Anticonvulsants, antidepressants opioids, sodium channel blockers |
|
| NMDA receptor antagonists | |
| Mechanisms | |
| Peripheral sensitization Central sensitization |
|
| Immune/glial regulation |
Studies on neuropathic pain mechanisms have greatly benefited from the development of animal models in which the sciatic nerve and its branches, or the spinal nerves are intentionally damaged [6-9]. Early studies focused on neuronal mechanisms of neuropathic pain and established that neuropathic pain is an expression of neural plasticity both in the PNS (peripheral sensitization) and CNS (central sensitization) [10-12]. After nerve injury, proinflammatory cytokines such as IL-1β, IL-6, and TNF-α are induced in the nerve and dorsal root ganglion (DRG) and play an essential role in the generation of hyperexcitability in DRG neuronal cell bodies and axons leading to peripheral sensitization [13-15]. In particular, TNF-α and IL-1β have been shown to increase the sensitivity and excitability of sensory neurons by activating the TTX-resistant sodium channels Nav1.8 [16, 17]. Central sensitization in spinal cord dorsal horn neurons, which can be induced both by an increase in excitatory synaptic transmission mediated via the glutamate NMDA and AMPA receptors and a decrease or loss of inhibitory synaptic transmission (disinhibition) mediated via GABA and glycine receptors [1, 18], is particularly important for the persistence of neuropathic pain and spread of pain beyond initial injury site [12]. Proinflammatory cytokines also contribute importantly to the generation of central sensitization [19]. For example, TNF-α can enhance excitatory synaptic transmission in superficial dorsal horn neurons whereas IL-6 can suppress inhibitory synaptic transmission in these neurons. Remarkably, IL-1β. is very dynamic: it not only increases excitation but also decrease inhibition. TNF-α, IL-1β, and IL-6 can further produce long-term effects on synaptic plasticity by inducing gene transcription [19].
Recent studies have also pointed to non-neuronal mechanisms such as immune and glial regulation for neuropathic pain [20-22]. Nerve injury induces a profound activation of glial cells including microglia and astrocytes in the spinal cord, and both microglia and astrocytes contribute to neuropathic pain sensitization via glial-neural interaction [23, 24]. Inhibitors of microglia and astrocytes are able to attenuate neuropathic pain [25, 26]. Thus, neuroinflammation mediated by the activation of glial cells in the CNS is also critical to the development of neuropathic pain [27]. In the spinal cord proinflammatory cytokines are predominantly produced by glial cells and contribute to neuropathic pain development [15, 28-30]. The production of proinflammatory cytokines is controlled by the mitogen-activated protein kinases (MAPKs) including extracellular signal-regulated kinase (ERK), p38, and c-Jun N-terminal kinase (JNK). These MAPKs are differentially activated in spinal microglia and astrocytes after peripheral nerve injury and important for neuropathic pain development [26, 31, 32]. Interestingly, activation of MAPKs in different types of glial cells after nerve injury may define different phases of neuropathic pain. For example, activation (phosphorylation) of p38 (or ERK) in spinal microglia is important for early-phase development of neuropathic pain [31, 32], whereas activation of ERK (or JNK) in astrocytes is essential for late-phase development of neuropathic pain [26, 32]. The opinion we will be presenting in this review is MMPs represent novel mechanisms and therapeutic targets for neuropathic pain.
MMPs and neuroinflammation
Matrix metalloproteases (MMPs) consist of a large family of endopeptidases that require Zn2+ for their enzyme activity. MMPs play a critical role in inflammation through the cleavage of the extracellular matrix proteins, cytokines, and chemokines [33-36]. MMP inhibitors have been developed to target different kinds of inflammation-related diseases such as arthritis, atherosclerosis, periodontitis, and cancer [37]. Although there are more than 20 members in the MMP family, the gelatinases MMP-9 and MMP-2 are two of the best-studied family members. After secretion MMP-9 and MMP-2 are found in the extracellular matrix, cerebrospinal fluid, and serum. MMP-9 and MMP-2 are activated by other proteases. For instance, MMP-9 is activated by plasminogen/plasmin and MMP-3, and MMP-2 is activated by MMP-14 (Table 2). The activity of the MMP-2 and MMP-9 can be easily detected using gelatin-substrate zymography assay. While MMP-2 is constitutively expressed in many tissues, MMP-9 is highly inducible. The activity of MMPs is regulated by endogenous inhibitors, TIMPs (tissue inhibitors of metalloproteases). For example, the activity of MMP-9 and MMP-2 is inhibited by TIMP-1 and TIMP-2, respectively [38].
Table 2.
Similar and distinct features of MMP-9 and MMP-2 in health and disease
| MMP-9 | MMP-2 | |
|---|---|---|
| Alternative names | Gelatinase-B | Gelatinase-A |
| Activators [36] | Plasminogen/plasmin, MMP-3 | MMP-14 (MT1-MMP) |
| Substrates [34-37] | Extracellular matrix proteins (gelatin, Type IV/V collagens) Cytokines (IL-1β, TNF-α) Chemokines (SDF-1/CXCL12, CCL7) |
|
| CCL11, CCL17, CXCL8 TGF-β, VEGF |
CX3CL1 | |
| Inhibitors [37,38] | Hydroxamates | |
| TIMP-1, minocycline | TIMP-2 | |
| Physiological roles [35,47] | Synaptic plasticity | |
| Development, regeneration | ||
| Pathological roles [33-37] | Brain-blood barrier degradation, stroke, epilepsy, arthritis Spinal cord injury, demyelination, cancer and metastasis |
|
| After nerve injury [15] | ||
| Induction pattern | Rapid but transient | Delayed but persistent |
| Tissue expression | DRG (neurons) | DRG (satellite cells) Spinal cord (astrocytes) |
| Role in neuropathic pain | Early phase development | Late-phase development |
MMPs especially MMP-9 and MMP-2 have also play important roles in neuroinflammation and are involved in a wide range of CNS diseases including Alzheimer’s, amyotrophic lateral sclerosis, multiple sclerosis, brain and spinal cord trauma, epilepsy, and stroke. By degrading extracellular matrix in the CNS, MMPs damage the blood-brain barrier, resulting in edema and vascular leakage. By interrupting cell-cell and cell-matrix homeostasis, MMPs can also trigger anoikis-like pathways of brain cell death. Consistently, MMP-9 and MMP-2 are upregulated in the CNS in these disease conditions [33, 34, 39, 40]. Besides targeting matrix, MMPs can process a variety of growth factors and other extracellular cytokines and signals. In this regard, MMPs may contribute to neurovascular remodeling that accompanies chronic CNS injury [40]. It is important to point out that apart from pathological roles, MMP-9 and MMP-2 also have physiological roles in regulating development and regeneration (Table 2). Depending on whether functional or dysfunctional remodeling occurs, the result might be recovery or the induction of aberrant neuronal circuits. Finally, it is worthwhile to mention that the tetracycline minocycline has been proposed to treat different types of neurological diseases and happens to be an inhibitor of MMP-9 [41, 42].
MMPs and neuropathic pain
Accumulating evidence suggests that proteases play an important role in the development of neuropathic pain. For example, the lysosomal cysteine protease cathepsin S contributes to neuropathic pain facilitation via cleavage of the chemokine CX3CL1 [43]. Neuropathic pain is impaired in mice lacking membrane-type 5 MMP (MMP-24) [44]. Further, MMP-9 is upregulated in the sciatic nerve after nerve crush, leading to nerve dymyelination by degradation of myelin basic protein [45]. We investigated the distinct roles of MMP-9 and MMP-2 in neuropathic pain in the spinal nerve ligation (SNL) model [15]. First, we examined whether and how MMP-9 regulates neuropathic pain. Spinal nerve ligation (SNL) at the L5 level produces rapid (<1 day) and persistent (> 21 d) neuropathic pain symptoms (e.g., mechanical allodynia) in rats and mice [8,15]. Gelatin zymography was used to check MMP-9 activity and expression in the L5 DRGs collected from naïve animals and injured animals (6 h to 21 d after SNL) and demonstrates that SNL induces a rapid (< 1 d) but transient (< 3 d) upregulation of MMP-9 in the DRG. Further, MMP-9 expression increases in DRG neurons on SNL day one [15]. To define the specific role of MMP-9 in neuropathic pain development, we employed four different approaches to inhibit MMP-9: (1) small synthetic inhibitor of MMP-9, (2) endogenous peptide inhibitor of MMP-9 (TIMP-1), (3) small interfering RNA against MMP-9, and (4) MMP-9 knockout mice. To target MMP-9 in the DRG and spinal cord and avoid systemic effects of drugs, all the drugs were delivered to spinal fluid via intrathecal administration. Results from these four different approaches are highly consistent, showing that MMP-9 inhibition or deletion can attenuate neuropathic pain in the early phase. Remarkably, the endogenous inhibitor TIMP-1 is extremely potent in blocking neuropathic pain in the early phase (e.g., day 1) but has no effect in the late phase (e.g., day 10). Conversely, intrathecal injection of exogenous MMP-9 is sufficient to induce neuropathic symptoms such as mechanical allodynia [15].
MMP-9 appears to induce neuropathic pain symptom via IL-1β, because IL-1β neutralizing antibody can partially block MMP-9-induced allodynia. Interestingly, MMP-9 and IL-1β are co-expressed in the same DRG neurons [15]. IL-1β is synthesized as a precursor (31 kDa) and subsequently cleaved into a partially processed form (28 kDa) and completely processed or mature form (17 kDa). The precursor form of IL-1β shows no biological activity. Caspase-1 is well known as an IL-1β converting enzyme, but this enzyme is not regulated after nerve injury in the DRG and spinal cord. Other enzymes such as MMP-9 and MMP-2 were also implicated in IL-1β cleavage [46]. SNL induces a marked IL-1β activation (cleavage) in the DRG in the early phase (day 1). But this activation is abrogated in mice lacking Mmp9, suggesting that MMP-9 is needed for IL-1β activation after nerve injury. Injection of MMP-9 is also sufficient to induce IL-1β activation in the DRG. We hypothesize that nerve injury-induced spontaneous discharge in sensory neurons releases MMP-9 and pro-IL-1β to the extracellular matrix, where MMP-9 cleaves pro-IL-1β to produce active IL-1β, which then acts on adjacent nociceptive neurons to produce hyperexcitability. MMP-9 in DRG soma is transported to central terminals in the dorsal horn to activate microglia. At spinal level, MMP-9 is sufficient to activate microglia even in a low dose that does not produce demyelination and apoptosis [15]. As summarized in Fig. 1, MMP-9 induces early phase neuropathic pain by activating IL-1β and microglia in the early phase. In addition, a recent study shows that MMP-9 is required for the development of aberrant neuronal connections in the hippocampus, which underlies epileptogenesis [47]. MMP-9 may also induce aberrant neuronal connections in the spinal cord that is important for the development of neuropathic pain [1, 44].
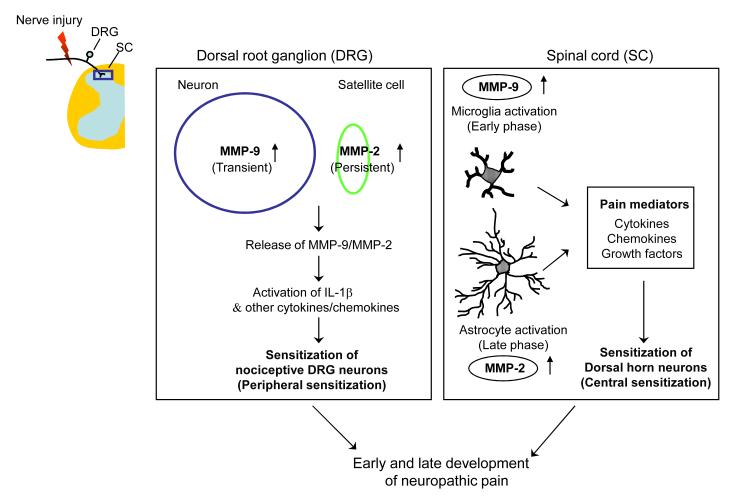
Figure 1.
Schematic showing MMP-9 and MMP-2 upregulation in the DRG (left box) and spinal cord (right box) after peripheral nerve injury and their contribution to early- and late-phase development of neuropathic pain. Transient upregulation of MMP-9 in DRG neurons and persistent MMP-2 upregulation in DRG satellite cells result in IL-1β activation and peripheral sensitization in the early- and late-phase, respectively. After nerve injury, activation of spinal microglia in the early phase by MMP-9 and activation of spinal astrocytes in the late-phase by MMP-2 also contribute to early- and late-phase development of central sensitization and neuropathic pain sensitization, respectively.
Second, we examined whether and how MMP-2 regulates neuropathic pain. Nerve injury also induces an upregulation of MMP-2, but the induction pattern of MMP-2 is quite different from that of MMP-9, because (a) SNL produces a delayed (>7 d) and persistent (> 21 d) upregulation of MMP-2 in the DRG, (b) MMP-2 is induced in small satellite cells surrounding DRG neurons, and (c) MMP-2 upregulation is persistently induced in spinal cord astrocytes [15]. Importantly, neuropathic pain in the late phase is blocked by small synthetic inhibitor of MMP-2, endogenous inhibitor (TIMP-2), and MMP-2 siRNA. On the other hand, injection of exogenous MMP-2 is able to induce neuropathic pain symptom. While MMP-9 induces IL-1β cleavage and activation in the early phase, MMP-2 appears to do this in the late phase. MMP-2 is also important for the activation of spinal cord astrocytes, since MMP-2 inhibition can suppress pERK induction in spinal cord astrocytes in the late phase [15]. Taken together, MMP-2 contributes to late phase neuropathic pain development by activating IL-1β and astrocytes in the late phase (Fig. 1).
Conclusions and future directions
Millions of people suffer from neuropathic pain, which is a disease on its own right. Current treatments are limited, because they focus on blocking neurotransmission but ignore the pathology underlying this pain and different phases dividing this pain. Therefore, these treatments only transiently attenuate the pain symptoms but do not modify the progression of the disease. MMPs contribute importantly to the generation of neuroinflammation, which is believed to underlie the progression of the disease. Our finding indicates a critical role of MMP-9 in the development of early phase neuropathic pain after nerve injury. Thus, MMP-9 inhibition may be used to prevent neuropathic pain induced after major surgeries (e.g., amputation, thoracotomy). In addition to nerve injury-induced peripheral neuropathic pain, spinal cord injury and brain trauma will also cause central neuropathic pain. In future studies, we will examine whether MMP-9 is also required for the development of central neuropathic pain in animal models. As a MMP-9 inhibitor, minocycline may be used to prevent or delay neuropathic pain. Our finding also reveals an essential role of MMP-2 for the development of late phase neuropathic pain. Therefore, MMP-2 inhibition may be used to treat established neuropathic pain after diabetes, viral infection, and chemotherapy. Although all these conditions produce nerve injury, animals studies are still needed to test whether MMP-2 inhibition can reverse neuropathic pain in animal models of these conditions.
It is worthy to notice that MMPs belong to a very large family and different MMPs will play different roles in neuropathic pain. Thus, a broad inhibitor of MMPs such as GM6001 may not be very effective and will produce severe side effects, since MMPs also have normal physiological functions. Specific targeting of MMP-9 or/and MMP-2 with small molecule inhibitors as well as monoclonal antibodies, siRNAs, and peptide inhibitors may reduce side effects of broad MMP inhibitors. A dual inhibitor of MMP-9/2 may be used to treat neuropathic pain at different phases. However, caution should be taken when long-term treatment of MMP-2/9 inhibitor is required, because minocycline, when given in a late stage of disease progression, made some patients worse in an ALS clinical trial [48].
We should emphasize that MMP-9 or MMP-2 inhibitor should have different profiles and mechanisms for neuropathic pain treatment than IL-1β inhibitor, although some effects of MMP-9/2 on neuropathic pain are mediated by IL-1β. In addition to IL-1β, MMPs can cleave other substrates such as chemokines (Table 2) that are also important for neuropathic pain sensitization.
The importance of MMPs for neuropathic pain has been further emphasized in a recent microarray study. A Comparison of gene expression profiles in neuropathic and inflammatory pain reveals that the endogenous inhibitor of MMP-9, TIMP-1 is one of the four genes highly induced in the DRG and spinal cord in neuropathic but inflammatory pain condition [49]. A balance between MMPs and TIMPs (e.g., MMP-9/TIMP-1) is extremely important to limit the detrimental effects of MMP over-production. Given the high potency of TIMP-1 and TIMP-2 in suppressing neuropathic pain at different phases, TIMPs and their derivates may also have therapeutic potentials for treating neuropathic pain, apart from small molecule inhibitors and humanized monoclonal antibodies against MMP-9/2.
Acknowledgements
This work was supported by NIH grants R01-NS54932 and R01-DE17794 to R.R.J and R01-NS48422, R01-NS56458, R37-NS37074 and P01-NS55104 to E.H.L and X.W.
Footnotes
Conflict of interests: All the authors have no conflict of interest.
References
- 1.Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 2.Dworkin RH, et al. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60:1524–1534. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- 3.Ji RR, Strichartz G. Cell signaling and the genesis of neuropathic pain. Sci STKE. 2004:reE14. doi: 10.1126/stke.2522004re14. 2004. [DOI] [PubMed] [Google Scholar]
- 4.Kehlet H, et al. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 5.Polomano RC, Bennett GJ. Chemotherapy-evoked painful peripheral neuropathy. Pain Med. 2001;2:8–14. doi: 10.1046/j.1526-4637.2001.002001008.x. [DOI] [PubMed] [Google Scholar]
- 6.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 7.Seltzer Z, et al. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- 8.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 9.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 10.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 11.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 12.Ji RR, et al. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Schafers M, et al. Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-alpha after spinal nerve ligation. J Neurosci. 2003;23:3028–3038. doi: 10.1523/JNEUROSCI.23-07-03028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawasaki Y, et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14:331–336. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin X, Gereau R.W.t. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci. 2006;26:246–255. doi: 10.1523/JNEUROSCI.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Binshtok AM, et al. Nociceptors are interleukin-1beta sensors. J Neurosci. 2008;28:14062–14073. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coull JA, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 19.Kawasaki Y, et al. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watkins LR, et al. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 21.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 22.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuda M, et al. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Ji RR, et al. Possible role of spinal astrocytes in maintaining chronic pain sensitization: review of current evidence with focus on bFGF/JNK pathway. Neuron Glia Biol. 2006;2:259–269. doi: 10.1017/S1740925X07000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raghavendra V, et al. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 26.Zhuang ZY, et al. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci. 2006;26:3551–3560. doi: 10.1523/JNEUROSCI.5290-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- 28.Milligan ED, et al. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sweitzer S, et al. Intrathecal interleukin-1 receptor antagonist in combination with soluble tumor necrosis factor receptor exhibits an anti-allodynic action in a rat model of neuropathic pain. Neuroscience. 2001;103:529–539. doi: 10.1016/s0306-4522(00)00574-1. [DOI] [PubMed] [Google Scholar]
- 30.Guo W, et al. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin SX, et al. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhuang ZY, et al. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39:279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- 34.Parks WC, et al. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 35.Yong VW. Metalloproteinases: mediators of pathology and regeneration in the CNS. Nat Rev Neurosci. 2005;6:931–944. doi: 10.1038/nrn1807. [DOI] [PubMed] [Google Scholar]
- 36.Manicone AM, McGuire JK. Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol. 2008;19:34–41. doi: 10.1016/j.semcdb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu J, et al. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007;6:480–498. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]
- 38.Murphy G, Willenbrock F. Tissue inhibitors of matrix metalloendopeptidases. Methods Enzymol. 1995;248:496–510. doi: 10.1016/0076-6879(95)48032-3. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, et al. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci. 2000;20:7037–7042. doi: 10.1523/JNEUROSCI.20-18-07037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao BQ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 41.Yong VW, et al. The promise of minocycline in neurology. Lancet Neurol. 2004;3:744–751. doi: 10.1016/S1474-4422(04)00937-8. [DOI] [PubMed] [Google Scholar]
- 42.Murata Y, et al. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke. 2008;39:3372–3377. doi: 10.1161/STROKEAHA.108.514026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark AK, et al. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc Natl Acad Sci U S A. 2007;104:10655–10660. doi: 10.1073/pnas.0610811104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Komori K, et al. Absence of mechanical allodynia and Abeta-fiber sprouting after sciatic nerve injury in mice lacking membrane-type 5 matrix metalloproteinase. FEBS Lett. 2004;557:125–128. doi: 10.1016/s0014-5793(03)01458-3. [DOI] [PubMed] [Google Scholar]
- 45.Chattopadhyay S, et al. Cytokine regulation of MMP-9 in peripheral glia: implications for pathological processes and pain in injured nerve. Brain Behav Immun. 2007;21:561–568. doi: 10.1016/j.bbi.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schonbeck U, et al. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol. 1998;161:3340–3346. [PubMed] [Google Scholar]
- 47.Wilczynski G, et al. Important role of matrix metalloproteinase 9 in epileptogenesis. J Cell Biol. 2008;180:1021–35. doi: 10.1083/jcb.200708213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordon PH, et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007;6:1045–1053. doi: 10.1016/S1474-4422(07)70270-3. [DOI] [PubMed] [Google Scholar]
- 49.Parkitna J. Rodriguez, et al. Comparison of gene expression profiles in neuropathic and inflammatory pain. J Physiol Pharmacol. 2006;57:401–414. [PubMed] [Google Scholar]