Abstract
Background
The A1 allele of the TaqIA polymorphism (rs1800497) in the dopamine D2 receptor gene (DRD2) has been associated with substance use. It is unclear whether this allele is a marker for an underlying propensity for specifically developing a substance use disorder, or more generally to developing an externalizing psychiatric disorder highly correlated with substance use. It is also possible that DRD2 is related to a behavioral phenotype common to externalizing disorders and substance use.
Method
Data was obtained from 104 psychiatrically hospitalized adolescents in a larger assessment study. Adolescents were genotyped for the DRD2 TaqIA site, grouped as carriers of the A1 allele (A1+) or homozygous for the A2 allelle (A1−). Associations of the presence of the A1 allele with externalizing disorders, the intermediate phenotype of impulsivity, and measures of alcohol and drug use were examined.
Results
A diagnosis of conduct disorder and impulsive behavior were both associated with severity of problem drinking and/or drug use. Further, interaction effects were found between the DRD2 TaqIA polymorphism and conduct disorder (trend level) as well as A1+ status and impulsivity, such that adolescents who were carriers of the A1 allele, and had conduct disorder or impulsive behavior, reported higher levels of problematic alcohol use than those who were non-carriers (A2/A2 or A1−). The same interaction effect between this polymorphism and impulsivity was found for severity of problem drug use. In contrast, no interaction effects were found between the DRD2 allele status and ADHD on severity of problem drinking or drug use.
Discussion
These results suggest that the well documented relationship between conduct disorder, the behavioral phenotype of impulsivity, and problematic alcohol/drug use among adolescents may be moderated by A1 carrier status of the DRD2 gene.
Keywords: DRD2, adolescents, alcohol, drugs, ADHD, conduct disorder, impulsivity
Introduction
A number of large epidemiologic studies, e.g., the National Epidemiologic Survey on Alcohol and Related Conditions (Stinson et al., 2005), have demonstrated associations between substance use disorders (SUD) and externalizing psychiatric disorders such as Conduct Disorder (CD) and Attention Deficit Hyperactivity Disorder (ADHD). These associations, in turn, have led researchers to present evidence from both adolescent and adult twin studies that these disorders are related to an underlying shared genetic liability (Kendler, Prescott, Myers & Neale, 2003; Knopik et al, this issue; Kreuger et al., 2002; Young, Stallings, Corley, Krauter, & Hewitt, 2000).
Genes in several biological systems (e.g., dopamine, serotonin, GABA, glutamate) have been investigated in relation to both substance use disorders and externalizing psychiatric disorders, albeit with mixed results. Dopaminergic function in particular seems to underlie all drugs of abuse (Kalivas & Volkow, 2005), and variation in dopaminergic genes may impact dependence rates across multiple substances, including alcohol and marijuana use. A number of studies have found a relationship between the DRD4 gene and substance use. For example, Laucht, Becker, Blomeyer, and Schmidt (2007) found that male adolescents with the 7-repeat allele of DRD4 drank more alcohol per occasion and had higher rates of lifetime heavy drinking than males without this allele. Novelty seeking was found to moderate this relationship for males but not females. Several studies have also found a specific relationship between a DRD2 polymorphism and substance abuse. For example, one study found that the presence of the A1 allele of the TaqIA polymorphism close to the dopamine D2 receptor in the brains of adult alcoholics correctly classified 77% of alcoholics, relative to non-alcoholics (Blum et al., 1990). The authors concluded that this gene infers a genetic susceptibility to alcoholism, in this case a severe form of alcoholism, as most of the patients in this sample died due to the effects of alcohol.
Since the original paper by Blum et al. (1990), there have been a number of other studies implicating the role of this polymorphism (rs1800497) in alcohol abuse and dependence (see Noble, 2000). The findings, however, are not consistent. Variation in the findings across studies may reflect the fact that alcohol use disorders take many forms and have differing etiologies. The DRD2 gene may only be related to specific subtypes of alcoholism or specific characteristics of alcohol use disorders (Lu, Lee, Ko & Lin, 2001). Alternatively, the influence of genetic factors may differ depending on the stage of substance use, e.g., initiation to regular use/abuse to dependence (Kreek, Nielsen, Butelman & LaForge, 2005). For example, Poelen et al (2008) found that genetic factors were more important in explaining initiation of alcohol use in 12 to 15 year olds than environmental factors, while common environmental factors explained most of the variance in drinking frequency. Additional evidence has been found to suggest that historical DRD2 associations may have in fact been driven by variation in nearby genes. For example, Yang et al. (2008) report associations with alcohol and drug dependence and the “NTAD cluster” that includes NCAM1, TTC12, ANKK1 and DRD2. These results follow several other findings that suggest that the inconsistent literature on DRD2 TaqIA may in part be driven by variation in other genes (Dick et al., 2007, Gelernter et al., 2006, Yang et al., 2007).
Most studies in the literature use heterogeneous samples of individuals with SUD and do not examine stage of substance use. Limiting an investigation to adolescents helps to control some of this heterogeneity. Although adolescents may have a history of alcohol use, this history will be shorter than the histories of the participants in the studies described above. Positive associations of the DRD2 A1 allele and alcohol and drug use behaviors in an adolescent sample would suggest that either relatively shorter consumption histories still manifest the expected genetic differences or that the DRD2 polymorphism is related to some underlying vulnerability. Alternatively, the notion that the influence of genes may vary at different stages of development (Rende & Plomin, 1995) may be applicable here with gene penetrance being prominent even in adolescence.
There have been a number of studies of clinical and nonclinical populations that have found a significant percentage of adults with SUD also have a diagnosis of ADHD. Data from the National Comorbidity study indicated that adults with ADHD had significantly higher rates of SUDs than adults without ADHD (Kessler et al., 2006). About one-quarter of psychologically hospitalized adolescents also report a SUD (DeMilio, 1989). Similar to that found with substance use, recent studies also suggest a relationship between the dopamine transporter gene and ADHD (Faraone et al., 2005; Todd et al., 2005). For example, an association between ADHD and the A1 allele of the DRD2 gene has been found in a study of a Finnish birth cohort population sample (Nyman et al., 2007).
Epidemiologic studies have also documented a relationship between DRD2 and other externalizing disorders and behaviors (Kandel et al., 1997). In one such study, the DRD2 A1 allele was related to pathological gambling (Ratsma, Vanderstelt, Schoffelmeer, Westerveld & Gunning, 2001). Comings et al. (2000) examined the relationship of the dopamine, serotonin, and nonadrenaline genes to ADHD and CD and found that the DRD2 gene was partially related to CD. However, like the studies reviewed above with alcohol and other drugs, not all studies support this relationship between ADHD or externalizing disorders in general, and the DRD2 A1 status.
The heterogeneity of symptom presentation in individuals with the same diagnosis may also contribute to the variability in genetic findings. Consequently, in addition to examining psychiatric diagnoses, examination of intermediate phenotypes is critical to furthering our understanding of what behaviors underlie the relationship between diagnoses and genes. For example, the diagnostic criteria for alcohol dependence do not distinguish between persons who drink alcohol problematically for different reasons, e.g., as a means to self-medicate versus impulsively drinking to excess with peers. Variations in intermediate phenotypes may also contribute to differences across individuals in initiation and transitions across stages of substance use.
Impulsivity is a potential intermediate phenotype that is associated with alcohol and drug use in adolescents and adults (Colder & Chassin, 1997; Labouvie & McGee, 1986; Noble, 1998). Blum et al. (2000) postulated that the DRD2 gene may mediate the preference for immediate rewards in impulsive persons. Several studies have examined the role of impulsivity and the DRD2 gene in substance use with conflicting findings. On the one hand, Eisenberg et al. (2007) examined the DRD2 TaqI A locus classified as having one copy of the A1 allele (A1+) or not (A1−) in 195 college students. Each participant completed a delayed discounting task, a behavioral test of impulsivity. A main effect of A1+ status on delayed discounting was found, suggesting that those individuals with the A1+ allele exhibited greater impulsivity than those with the A1− allele. On the other hand, Limosin et al. (2003) administered the Barrett Impulsiveness Scale to 92 alcohol-dependent inpatients and found that the DRD2 TaqIA polymorphism was associated with lower impulsivity in this sample. Limosin et al. (2003) suggest that alcohol may be taken to stimulate dopamine activity in order to increase reward-related impulsivity.
Impulsivity is a cardinal symptom of both a CD and ADHD diagnosis (Sagvolden, Johansen, Aase & Russell, 2005). Twin studies have also found that impulsivity has a significant heritable component (e.g., Seroczymski, Bergeman, & Coccaro, 1999). In addition, genetic studies have also linked impulsivity evident in ADHD to the dopamine system (Li, Sham, Owen, & He, 2006). Given that the symptom presentation in ADHD is variable, i.e. not all patients show the same degree of attentional problems or impulsivity, it is possible that impulsivity may be a specific link between SUD and ADHD. Similarly, diagnoses of CD may differ in the extent to which social norms do or do not affect problem behaviors. Thus, impulsivity may be a key factor in the subset of adolescents with CD who progress to SUDs (Nigg et al., 2006).
In this study we examine a subset of adolescents from a larger assessment study that characterizes alcohol and drug use in a sample of youth at high risk for the development of alcohol and drug dependence, adolescents with psychiatric disorders. We were interested in examining three major questions. First, is the DRD2 TaqIA polymorphism associated with more severe problem drinking and drug use in a sample of high risk adolescents? Second, does the DRD2 TaqIA polymorphism affect the well documented relationship between externalizing disorders, i.e. CD and ADHD, and alcohol or drug related problems? And third, does the DRD2 TaqIA polymorphism affect the nature of the relationship between the endophenotype of impulsivity, which is related to both CD and ADHD, and severity of problem drinking and drug use. We hypothesized that carriers of the A1+ allele of the DRD2 TaqIA polymorphism would report more severe problem drinking and drug use. Further, we hypothesized that those adolescents who met diagnostic criteria for ADHD, conduct disorder, or exhibited significant impulsivity, and were carriers of the A1+ allele of the DRD2 TaqIA polymorphism, would report more severe alcohol and drug use problems than those who exhibited these externalizing diagnoses/behavior but were non-carriers of the A1+ allele of the DRD2 TaqIA polymorphism.
METHOD
Participants
One hundred and forty-nine adolescents hospitalized on an acute adolescent psychiatric inpatient unit and their parents/guardians were asked to participate on a voluntary basis. The large majority of adolescents were hospitalized due to suicidal thoughts or behavior. Adolescents were recruited from a child psychiatric hospital located in the Northeast which accepts both uninsured and privately insured youth. Of those approached for participation, 118 (79%) were successfully recruited. However, 4 participants subsequently withdrew from the study and 10 provided inadequate DNA samples, leaving a final sample of 104 participants. Adolescents ranged in age from 13 to 18 years, with a mean age of 14.9 years (SD = 1.3). Participants were 71% female and were 84.6% White, 1.9% African American, 1.9% Asian, 3.8% Native American and 7.7% other ethnicity and approximately 8% of the sample was of Hispanic/Latino ethnicity.
Procedure
Adolescents admitted to an adolescent psychiatric inpatient unit over the course of a two year period as part of a larger assessment study were eligible for participation. Adolescents met inclusion criteria for participation if they: 1) were English speaking; 2) adolescent assent and parental consent were provided; and 3) had a Verbal IQ estimate ≥ 70 as assessed via the Kaufman Brief Intelligence Test (Kaufman & Kaufman, 1990). Exclusion criteria included current psychosis or full placement in state custody due to child abuse or neglect, as documented in the inpatient admission materials.
Adolescents and their parents/guardians were approached for recruitment by a trained bachelor level research assistant after family meetings or during family visits on the adolescent inpatient unit. If parental consent and adolescent assent were provided, the family was enrolled in this assessment study. Adolescents and their parent/guardian completed the assessments while the adolescent was hospitalized on the inpatient unit. The research assistant administered the assessment battery with the exception of the diagnostic interview which was administered by masters and doctoral level clinicians who completed training in this interview provided by CES. Parent and adolescent assessments were conducted separately. The parent version of the diagnostic interview and assessments was administered in a two hour session. The child version of the diagnostic interview and intelligence test was administered in a separate two hour session.
All adolescents received four movie tickets and their parent/guardian received a $50 money order for their participation. A feedback form summarizing responses to clinical measures (with the exception of substance related information) was placed in each adolescent’s inpatient file upon completion of the full assessment battery so that it could be reviewed by the adolescent’s inpatient treatment team to aid in treatment and discharge planning. Substance related information was not shared because teens self-reported substance use has been shown to be accurate and reliable under conditions of confidentiality (Needle, McCubbin, Lorence, & Hochhaser, 1983). This study was approved by the affiliated University and Hospital Institutional Review Boards.
Measures
Schedule for Affective Disorders and Schizophrenia for School- Age Children - Present and Lifetime Version (K-SADS- PL; Kaufman et al., 1997) is a widely used semi-structured diagnostic interview that provides a reliable and valid measurement of DSM-IV psychopathology in children and adolescents. Interrater agreement for scoring screens and diagnoses is high (range: 93% to 100%). Test-retest reliability and kappa coefficients are in the excellent range for diagnoses of major depressive, bipolar, generalized anxiety, conduct, and oppositional defiant disorders (.77 to 1.00) and in the good range for other diagnoses (.63 to .67) (Kaufman et al., 1997). Only the current mood, anxiety, disruptive behavior, alcohol/substance use, and eating disorder sections were administered to both the adolescent and parent. All interviews were audiotaped and 10% were randomly selected and rated for reliability. Only data for the ADHD and conduct disorder diagnoses is presented here. Kappa coefficients reflected strong agreement for both disorders (Kappa = 1.0).
All cases were staffed during weekly clinical consensus team meetings. This clinical consensus team comprised doctoral level child psychologists in addition to the interviewers. During this meeting, all K-SADS-PL symptoms and assessment data were reviewed. A best-estimate clinical consensus procedure was used to resolve discrepancies between adolescent and parent report as well as confirm diagnoses. A best-estimate clinical consensus procedure is commonly used to reconcile discrepancies (Cantwell et al., 1997, Klein et al., 1994; 2001) and yields good to excellent reliability (Klein et al., 1994; 2001).
K-SADS –PL Impulsivity Rating
As we were interested in examining impulsivity in particular, we also examined responses regarding impulsivity from the K-SADS-PL ADHD screener which asked participants to respond to the following question: Since you were little, have you gotten into trouble or gotten hurt because you rushed into things without thinking (e.g., run into street without looking). Can you give me some examples?” Parents were asked the same question with regard to their child’s behavior, with wording adjusted appropriately. Those participants whose responses met the threshold for this item “often impulsive, problem has moderate to severe effect on functioning” were coded as impulsive based on the best estimate procedure described above to confirm K-SADS-PL diagnoses and symptoms. This item was also examined in relation to other psychological measures to establish convergent and divergent validity. Specifically, this item was found to be significantly correlated with hyperactive and combined inattentive/hyperactive subtypes of ADHD (r =.65, p< .00) but not the inattentive subtype of ADHD (r = .06, p > .05). Further, it was significantly correlated with an impulsivity summary score (r = .27, p < .01) created by summing likert-type items that tap impulsivity on the parent rated Behavioral Assessment Scale for Children (BASC; Reynolds, & Kamphaus, 1992). The BASC impulsivity summary score included items such as “acts without thinking’, “cannot wait to take turn”, “interrupts parents when talking on the phone”, and “interrupts when others are speaking”.
Kaufman Brief Intelligence Test (K-BIT; Kaufman & Kaufman, 1990) provides a brief estimate of intelligence for individuals 4–90 years of age. It contains 2 subtests, Vocabulary and Matrices, which provide estimated Verbal IQ and Performance IQ, respectively. Split half reliability and test-retest reliability estimates for the subtests are acceptable and the IQ scores have demonstrated adequate convergent validity with other measures of verbal intelligence and achievement (Spreen & Strauss, 1998). Only the Vocabulary subtests were administered in the present study as a screening tool.
Substance Abuse Subtle Screening Inventory Adolescent Version 2 (SASSI A-2; Miller & Lazowski, 2001) contains 72 true-false items and 28 multiple choice questions that assess frequency of substance misuse, problems associated with substance misuse, attitudes toward substance misuse, and related contextual factors. This instrument contains 9 subscales: face valid alcohol scale, face valid other drug scale, family-friends risk scale, attitudes scale, symptoms scale, obvious attributes scale, supplemental addiction measure, subtle attributes scale, and defensiveness scale. This instrument was developed and cross validated with 1,244 adolescents and has excellent psychometric properties (Miller & Lazowski, 2001). In the present study, the total scores from the face valid alcohol and other drug scales were examined. These scales ask participants to indicate how often they have experienced symptoms and problems associated with alcohol and/or drug misuse over the course of the last 6 months using a 4-point likert scale. Sample items include, “How often have you taken a drink or drinks to help you talk about your feelings and ideas? How often have you gotten into trouble in school, at home, on the job, or with the police because of your drug use?”.
Candidate genotyping
After obtaining secondary consent for DNA collection for genetic testing, DNA collection was performed at the final visit in the parent study. Genomic DNA was collected and isolated from buccal swabs using published procedures (Freeman et al., 1997; Lench, Stanier & Williamson, 1988). The DRD2 TaqIA polymorphism was assayed using a Taqman genotyping assay (Applied Biosystems). Participants were grouped by whether they possessed at least one copy of the A1 allele (A1+) or not (A1−). All genotyping was performed by technicians blinded to participant characteristics. Quality control procedures for genotyping included separate genotype calls by two independent lab technicians, and rerunning ten percent (randomly determined) of samples to check for reliability. Successful calls were made for all samples and there was full agreement in genotyping calls made by both raters.
Results
Participant Characteristics
Approximately 32% of participants met criteria for conduct disorder and 39% for Attention-Deficit/Hyperactivity Disorder (35% inattentive subtype, 5% hyperactive subtype, 60% combined inattentive/hyperactive subtype). Approximately 32% of participants endorsed the impulsivity item from the ADHD screen. Further, 38.5% of the sample was found to be carriers of the A1 allele of the DRD2 TaqIA polymorphism (A1+) and 61.5% were non-carriers (A1−). The mean SASSI face valid alcohol total raw score (SASSI-ALC) was 3.4 (SD = 6.2), and the mean SASSI face valid other drugs total raw score (SASSI-OD) was 7.4 (SD = 11.3). Genotype frequencies did not vary from Hardy Weinberg equilibrium.
Data Analyses
Preliminary analyses conducted to examine the distributional properties of variables revealed that the SASSI-ALC and SASSI-OD were significantly skewed. A square root transformation was conducted on these measures that improved normality to an acceptable level. T-tests, correlations, and one-way ANOVAs were conducted to further examine the data. Preliminary analyses did not reveal significant differences across age, gender, race, or ethnicity on the alcohol or substance use scales. Table 1 presents the correlations among predictor and criterion variables.
Table 1.
Means, Standard Deviations, and Correlations for DRD2 Status, Externalizing Disorders/Symptoms, and Problematic Alcohol and Other Drug Use
| (1) | (2) | (3) | (4) | (5) | (6) | |
|---|---|---|---|---|---|---|
| (1) DRD2 status | --- | .06 | −.14 | −.16 | .09 | .09 |
| (2) Conduct Disorder | --- | .18 | .20* | .52** | .57** | |
| (3) ADHD | --- | .61** | .18 | .23* | ||
| (4) Impulsivity | --- | .21* | .29** | |||
| (5) SASSI-ALC | --- | .81** | ||||
| (6) SASSI-OD | --- | |||||
|
| ||||||
| M | 0.38 | 0.32 | 0.38 | 0.32 | 1.14 | 1.75 |
| (SD) | (0.49) | (0.46) | (0.48) | (0.47) | (1.46) | (2.10) |
Note. SASSI-ALC = Subtle Substance Abuse Screening Inventory Face Valid Alcohol Scale; SASSI-OD = Subtle Substance Abuse Screening Inventory Face Valid Other Drug Scale.
p < .05.
p < .01.
A series of hierarchical linear regression analyses were performed to examine whether DRD2 status (A1+ or A1−) predicted problematic alcohol and other drug use as well as whether DRD2 status moderated the association between externalizing disorders/symptoms (conduct disorder, ADHD, impulsivity) and problematic alcohol and other drug use. Interaction terms were computed by obtaining the cross product of diagnostic and psychosocial variables. The criterion for entry into and for being retained in the regression equations were p = .05 and p = .10, respectively. To minimize the type I error rate due to multiple comparisons, the alpha level for statistical significance was set at p ≤ .01.
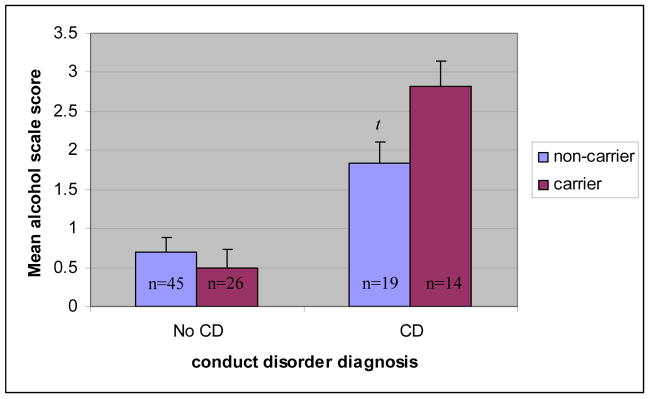
The first set of hierarchical regression analyses tested the association between DRD2 status, conduct disorder, and problematic alcohol and other drug use. Results are presented in Table 2. The main effect of DRD2 status was not associated with problematic alcohol or other drug use on the SASSI-ALC (t = .73, p > .05), or the SASSI-OD (t = .66, p > .05). However, the main effect of conduct disorder was associated with problematic alcohol and other drug use on the SASSI-ALC (t = 6.09, p < .01) and the SASSI-OD (t = 6.90, p < .01). Moreover, the interaction of DRD2 status X conduct disorder was associated with problematic alcohol use on the SASSI-ALC at a trend level (t = 2.27, p < .05) but not problematic drug use on the SASSI-OD (t = .1.13, p > .05). The latter results suggest that while conduct disorder was significantly associated with more severe problem drinking behavior in the sample as a whole, it also interacted at a trend level with DRD2 status indicating a potentially stronger association between conduct disorder and problematic alcohol use among youth with A1+ versus A1− carrier status (See Figure 1 for mean differences by group). Specifically, in our sample, genetic effects accounted for 8% and 1% of the variance in problematic alcohol use among adolescents with and without conduct disorder, respectively
Table 2.
Hierarchical Regression Analysis for DRD2 Carrier Status, Conduct Disorder, and Problematic Alcohol and Other Drug Use
| Variable | B | SEB | β | R2 | ΔR2 |
|---|---|---|---|---|---|
| SASSI-ALC | |||||
| Step 1 | .28** | ||||
| DRD2 | 0.19 | .25 | .06 | ||
| Conduct Disorder | 1.62 | .27 | .52** | ||
| Step 2 | .31* | .04 | |||
| DRD2 × Conduct Disorder | 1.20 | .53 | .28* | ||
|
| |||||
| SASSI-OD | |||||
| Step 1 | .33** | ||||
| DRD2 | 0.23 | .35 | .05 | ||
| Conduct Disorder | 2.54 | .37 | .57** | ||
| Step 2 | .33 | .01 | |||
| DRD2 × Conduct Disorder | 0.84 | .75 | .14 | ||
Note. B = unstandardized beta at entry. SE B = standard error at entry. β = standardized beta at entry. SASSI-ALC = Subtle Substance Abuse Screening Inventory Face Valid Alcohol Scale; SASSI-OD = Subtle Substance Abuse Screening Inventory Face Valid Other Drug Scale.
p < .05.
p < .01.
Figure 1.
Interaction between DRD2 carrier status and conduct disorder diagnosis on the square root transformed SASSI Face Valid Alcohol Scale (SASSI-ALC).
Note. t = trend (p< .05). Error bars represent standard error of measurement.
The second set of hierarchical regression analyses tested the association between DRD2 status, ADHD, and problematic alcohol and other drug use. Results are presented in Table 3. The main effect of DRD2 status was not associated with problematic alcohol or other drug use on the SASSI-ALC (t = 1.19, p > .05) or the SASSI-OD (t = 1.23, p > .05). The main effect of ADHD was not associated with problematic alcohol use on the SASSI-ALC (t = .1.96, p > .05) but it was associated with problematic drug use on the SASSI-OD at a trend level (t = 2.49, p < .05). The interaction of DRD2 status X ADHD was not associated with problematic alcohol or drug use on the SASSI-ALC (t = .0.52, p > .05) or the SASSI-OD (t = .0.64, p > .05).
Table 3.
Hierarchical Regression Analysis for DRD2 Carrier Status, ADHD, and Problematic Alcohol and Other Drug Use
| Variable | B | SEB | β | R2 | ΔR2 |
|---|---|---|---|---|---|
| SASSI-ALC | |||||
| Step 1 | .05 | ||||
| DRD2 | 0.35 | .29 | .12 | ||
| ADHD | 0.58 | .29 | .19 | ||
| Step 2 | .05 | .00 | |||
| DRD2 × ADHD | 0.32 | .62 | .07 | ||
|
| |||||
| SASSI-OD | |||||
| Step 1 | .07* | ||||
| DRD2 | 0.51 | .42 | .12 | ||
| ADHD | 1.04 | .42 | .24* | ||
| Step 2 | .07 | .00 | |||
| DRD2 × ADHD | .57 | .88 | .09 | ||
Note. B = unstandardized beta at entry. SE B = standard error at entry. β = standardized beta at entry. SASSI-ALC = Subtle Substance Abuse Screening Inventory Face Valid Alcohol Scale; SASSI-OD = Subtle Substance Abuse Screening Inventory Face Valid Other Drug Scale.
p < .05.
p < .01.
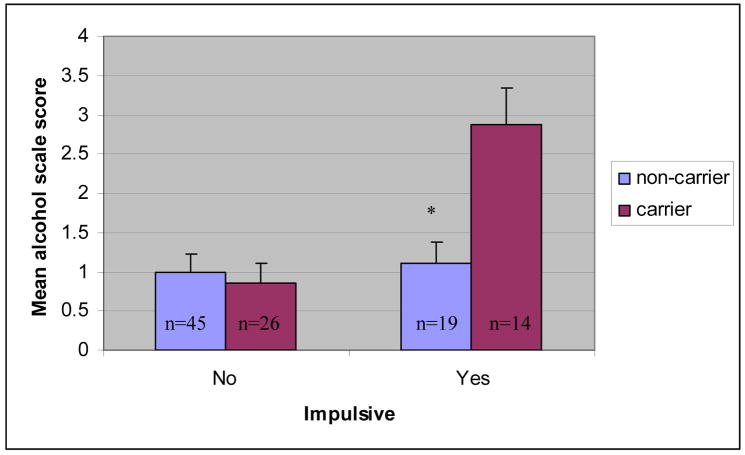
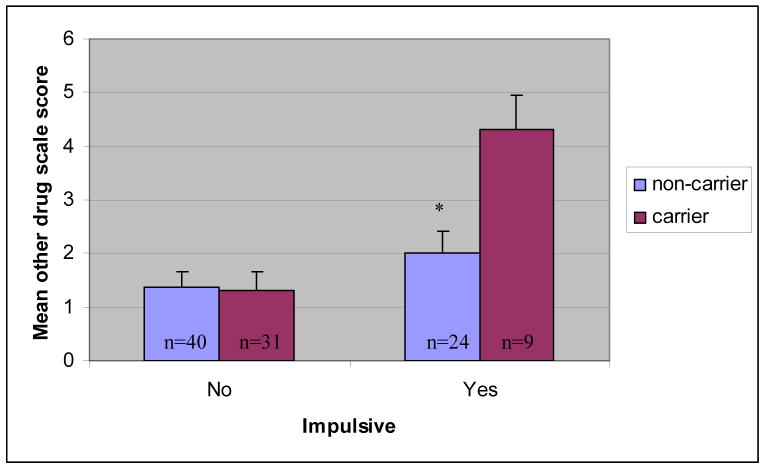
The third set of hierarchical regression analyses tested the association between DRD2 status, impulsivity, and problematic alcohol and other drug use. Results are presented in Table 4. The main effect of DRD2 status was not associated with problematic alcohol or other drug use on the SASSI-ALC (t = 1.29, p > .05) or the SASSI-OD (t = 1.41, p > .05). The main effect of impulsivity was associated with problematic alcohol use at a trend level on the SASSI-ALC (t = 2.33, p < .05). It was also associated with problematic drug use on the SASSI-OD (t = 3.29, p < .01). Further, the interaction of DRD2 status X impulsivity was associated with problematic alcohol and other drug use on the SASSI-ALC (t = 3.06, p < .01) and the SASSI-OD (t = .2.62, p = .01). These latter results suggest that while impulsivity was associated with more severe problem drinking (at a trend level) and drug use, respectively, in the sample as a whole, it also interacted significantly with DRD2 status indicating a significantly stronger association between impulsivity and problematic alcohol and drug use among youth with A1+ versus A1− carrier status (See Figures 2–3 for mean differences by group). Specifically, genotype accounted for 23% of the variance in SASSI-ALC and 18% of the variance in the SASSI-OD score in impulsive adolescents. Genetic effects for these two outcomes (in terms of total variance accounted for) were negligible in non-impulsive adolescents.
Table 4.
Hierarchical Regression Analysis for DRD2 Carrier Status, Impulsivity, and Problematic Alcohol and Other Drug Use
| Variable | B | SEB | β | R2 | ΔR2 |
|---|---|---|---|---|---|
| SASSI-ALC | |||||
| Step 1 | .06* | ||||
| DRD2 | 0.38 | .29 | .13 | ||
| Impulsivity | 0.71 | .31 | .23* | ||
| Step 2 | .14** | .08 | |||
| DRD2 × Impulsivity | 1.93 | .63 | .37** | ||
|
| |||||
| SASSI-OD | |||||
| Step 1 | .10** | ||||
| DRD2 | 0.58 | .41 | .13 | ||
| Impulsivity | 1.41 | .43 | .31** | ||
| Step 2 | .16* | .06 | |||
| DRD2 × Impulsivity | 2.35 | .90 | .32* | ||
Note. B = unstandardized beta at entry. SE B = standard error at entry. β = standardized beta at entry. SASSI-ALC = Subtle Substance Abuse Screening Inventory Face Valid Alcohol Scale; SASSI-OD = Subtle Substance Abuse Screening Inventory Face Valid Other Drug Scale.
p < .05.
p < .01.
Figure 2.
Interaction between DRD2 carrier status and impulsivity on the square root transformed SASSI Face Valid Alcohol Scale (SASSI-ALC).
Note. * p < .01. Error bars represent standard error of measurement.
Figure 3.
Interaction between DRD2 carrier status and impulsivity on the square root transformed SASSI Face Valid Other Drug Scale (SASSI-OD).
Note. * p < .01. Error bars represent standard error of measurement.
Discussion
The first aim of this study was to investigate whether the DRD2 TaqI polymorphism was associated with problem drinking and drug use in a group of adolescents with psychiatric disorders. There was no direct relationship found between the DRD2 TaqIA polymorphism and problem drinking or drug use behavior. These results suggest that the DRD2 TaqIA polymorphism may not be directly associated with problem drinking or drug use among adolescents.
With regard to externalizing diagnoses, a direct relationship was found between the diagnosis of CD and alcohol and drug use. This finding is consistent with the literature which indicates a strong relationship between CD and substance use (Boyle & Oxford, 1991; Bukstein, Brent & Kaminer, 1989). In contrast, while a trend was found for the relationship between ADHD and problematic drug use, no direct association was found with problematic alcohol use. Epidemiologic studies have also reported a stronger relationship between ADHD and drug dependence than alcohol use or dependence (Kessler et al., 2006). A direct relationship was also found between the endophenotype of impulsivity and problem drinking (at a trend level) and drug use. These findings are again consistent with the literature (Nigg et al., 2006)
Most research to date has examined the main effects of the DRD2 gene and externalizing behavior on alcohol and drug use. Building upon this research, the present study also sought to examine whether A1 carrier status of the DRD2 gene and externalizing behaviors interact to better predict problem drinking and drug use. Results were mixed. A trend toward an interaction effect was found among adolescents diagnosed with CD. Specifically, there was a trend for adolescents diagnosed with CD who possessed the A1 allele to report higher levels of problematic drinking than adolescents with CD who were non-carriers. This relationship was not found on the drug use measure. However, the most consistent and striking findings were among impulsive youth. Similar to that found with conduct disordered youth, impulsive youth who were A1 carriers reported more severe problem drinking and drug use than impulsive youth who were non-carriers. Therefore, it appears that while a direct association was found between conduct disorder and problematic drinking, as well as impulsivity and problem drinking and drug use, the relationship is stronger among those who are A1 carriers of the DRD2 TaqIA polymorphism.
In contrast to results found in relation to CD and impulsivity, A1 carrier status of the DRD2 TaqIA polymorphism did not interact with ADHD to predict substance use. In exploratory analyses, we also examined whether these results varied for youth diagnosed with predominantly hyperactive-impulsive type or those with the combined type of ADHD, but results remained consistent. In contrast, as noted above, impulsivity, a behavioral phenotype common to ADHD, was directly associated with problem drinking and drug use, and interacted with the A1 carrier status to predict problem drinking and drug use. There results suggest that perhaps it is impulsivity, not the diagnosis of ADHD per se, which is related to substance use among adolescents with ADHD who use alcohol and drugs.
Relatedly, it has been suggested that CD might eventually result in alcohol and other drug abuse/dependence via impulsivity (Wiers, Sergeant, & Gunning, 1994). It has also been suggested that the TaqI allele of the DRD2 TaqIA polymorphism may be associated with a reward deficiency syndrome which helps explain its relation to substance use (Blair et al., 1999). Sagvolden et al. (2005) hypothesized that a less than optimally functioning dopamine system results in alterations in behavioral reinforcement and extinction which, in turn, leads to learning deficits and subsequent impulsive behaviors. The present results, as well as prior research which reported a relationship between DRD2 and impulsivity (e.g., Frank et al., 2004), provide partial support for the possibility that the association of the DRD2 TaqIA polymorphism with alcohol use and other externalizing disorders may be due to an underlying propensity toward impulsivity. However, this study included a sample of convenience and is by no means an authoritative examination of this possibility.
As is evident, examinations of the relation between the DRD2 gene and externalizing disorders/behavior leads to a more fine grained understanding of how these constructs may be related to problem drinking and drug use. This preliminary study, with limited power to detect genetic main effects, provides additional evidence of a relationship between the DRD2 TaqIA polymorphism and conduct disorder, as well as impulsivity, and suggests the need for further characterization of the role of the DRD2 TaqIA polymorphism in larger adolescent samples. Our power calculations indicate that, in order to find small to medium genetic main effects accounting for 1%, 2%, 3%, 4%, or 5% of the variance, we would need a sample size of 350, 418, 560, 850, and 1700 adolescents, respectively. Thus, while power to detect small genetic effects is low in this sample, we can use these preliminary results as a guide for designing future research studies with larger sample sizes.
This study also has several limitations that should be noted including the limited power discussed above to detect effects due to small cell sizes, a reliance on self report, and only a single interview item used to assess impulsivity. However, with regard to this third limitation, the impulsivity rating was based on consensus of parent and adolescent report, staffed during a clinical consensus meeting, and was significantly correlated with ADHD diagnosis as well as impulsivity items included on a parent rated behavior checklist. The sample itself also needs to be considered when evaluating the results given that it is drawn from a psychiatric inpatient unit as opposed to the community or a substance use treatment program. Nonetheless, the likelihood of placement on a psychiatric inpatient unit increases with the severity of substance abuse problems, as research suggests that suicidality increases with more severe substance abuse (Esposito-Smythers & Spirito, 2004). Further, psychiatric inpatient units regularly admit youth from substance abuse treatment facilities who report suicidality in the context of their stay. They also regularly make referrals for youth to outpatient and residential substance abuse treatment programs upon discharge. Another limitation is the possibility that some of the adolescents who did not report problematic alcohol or drug use in the present study may develop these problems in the future. The standard disclaimers for genetic association studies regarding population stratification and linkage disequilibrium and other unmeasured third variables also apply (e.g., Hutchison et al., 2004). Moreover, this study was limited to the examination of the historically studied DRD2 TaqIA polymorphism despite recent reports suggesting that these findings may be ultimately related to variation in the NCAM1-TTC12-ANKK1 gene cluster (e.g., Dick et al., 2007, Gelernter et al., 2006, Huang et al., 2008).
Future research with adolescents might employ behavioral tasks of impulsivity, such as the delayed discounting task used by Eisenberg et al. (2007), to better characterize this endophenotype. More complete characterization of the DRD2 gene and its neighbors was limited by available funding. In light of recent evidence that the DRD2 TaqIA may be in linkage disequilibrium with other polymorphisms in DRD2 and neighboring genes, this is an important next step. Future studies might also examine the role of other related endophenotypes, such as novelty seeking and the dopamine genes at the various stages of substance use uptake (Berman, Ozkaragoz, Young & Noble, 2002). And finally, phenotypes defined by behavior, such as psychiatric diagnoses including SUD, CD and ADHD, are complex and affected by multiple genes as well as gene-by-gene interactions. This study did not examine the potential role of gene by gene interactions (e.g., DRD2 x DRD4) on substance use. Such interactions have been found to be related to conduct problems in a large epidemiologic sample (Beaver et al, 2007) and could be another fruitful area for further study.
Acknowledgments
Research supported by a grant from the National Institute of Mental Health (NIMH) 5R01MH065885-03 (CES) and a Research Career Development Award from the Medical Research Service of the Department of Veterans Affairs (JEM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beaver KM, Wright JP, DeLisi M, et al. A gene × gene interaction between DRD2 and DRD4 is associated with conduct disorder and antisocial behavior in males. Behav Brain Funct. 2007;3:30. doi: 10.1186/1744-9081-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman S, Ozkaragoz T, Young RMcD, Noble E. D2 dopamine receptor gene polymorphism discriminates two kinds of novelty seeking. Pers Indiv Dif. 2002;33:867–882. [Google Scholar]
- Blum K, Braverman ER, Holder JM, et al. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs. 2000;32:1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- Blum K, Noble EP, Sheridan PJ, et al. Allelic association of human dopamine D2 receptor gene in alcoholism. JAMA. 1990 Apr;263(15):2055–2060. [PubMed] [Google Scholar]
- Boyle MH, Offord DR. Psychiatric disorder and substance use in adolescence. Can J Psychiatry. 1991;36:699–705. [PubMed] [Google Scholar]
- Bukstein OG, Brent DA, Kaminer Y. Comorbidity of substance abuse and other psychiatric disorders in adolescents. Am J Psychiatry. 1989;146:1131–1141. doi: 10.1176/ajp.146.9.1131. [DOI] [PubMed] [Google Scholar]
- Cantwell DP, Lewinsohn PM, Rohde P, Seeley JR. Correspondence between adolescent report and parent report of psychiatric diagnostic data. J Am Acad Child Adolesc Psych. 1997;36:610–619. doi: 10.1097/00004583-199705000-00011. [DOI] [PubMed] [Google Scholar]
- Colder CR, Chassin L. Affectivity and impulsivity: temperament risk for adolescent alcohol involvement. Psychol Addict Behav. 1997;11(2):83–97. [Google Scholar]
- Comings DE, Gade-Andavolu R, Gonzalez N, et al. Comparison of the role of dopamine, serotonin, and noradrenaline genes in ADHD, ODD and conduct disorder: multivariate regression analysis of 20 genes. Clin Genet. 2000;57 (3):178–196. doi: 10.1034/j.1399-0004.2000.570304.x. [DOI] [PubMed] [Google Scholar]
- DeMilio L. Psychiatric syndromes in adolescent substance abusers. Am J Psychiatry. 1989;146:1212–1214. doi: 10.1176/ajp.146.9.1212. [DOI] [PubMed] [Google Scholar]
- Dick DM. Identification of genes influencing a spectrum of externalizing psychopathology. JAMA. 1990 Apr;263(15):2055–2060. [Google Scholar]
- Dick DM, Wang JC, Plunkett J, et al. Family-based association analyses of alcohol dependence phenotypes across DRD2 and neighboring gene ANKK1. Alcohol Clin Exp Res. 2007;31:1645–53. doi: 10.1111/j.1530-0277.2007.00470.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg DTA, MacKillop J, Modi M, et al. Examining impulsivity as an endophenotype using a behavioral approach: a DRD2 Taql A and DRD4 48-bp VNTR association study. Behav Brain Funct. 2007;3 doi: 10.1186/1744-9081-3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito-Smythers C, Spirito A. Adolescent suicidal behavior and substance use: A review with implications for treatment research. Alcohol Clin Exp Res. 2004;28 (supplement):77S–88S. doi: 10.1097/01.alc.0000127417.99752.87. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Frank Y, Pergolizzi RG, Perilla MJ. Dopamine D4 receptor gene and attention deficit hyperactivity disorder. Pediatr Neurol. 2004;31(5):345–8. doi: 10.1016/j.pediatrneurol.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Freeman B, Powell J, Ball D, Hill L, Craig I, Plomin R. DNA by mail: an inexpensive and noninvasive method for collecting DNA samples from widely dispersed populations. Behav Genet. 1997;27:251–257. doi: 10.1023/a:1025614231190. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Yu Y, Weiss R, et al. Haplotype spanning TTC12 and ANKK1, flanked by the DRD2 and NCAM1 loci, is strongly associated to nicotine dependence in two distinct American populations. Hum Mol Genet. 2006;15(24):3498–507. doi: 10.1093/hmg/ddl426. [DOI] [PubMed] [Google Scholar]
- Heath AC, Todorov AA, Nelson EC, Madden PAF, Bucholz KK, Martin NG. Gene-environment interaction effects on behavioral variation and risk of complex disorders: the example of alcoholism and other psychiatric disorders. Twin Research. 2002;5:30–43. doi: 10.1375/1369052022875. [DOI] [PubMed] [Google Scholar]
- Huang W, Payne TJ, Ma JZ, Beuten J, Dupont RT, Inohara N, Li MD. Significant Association of ANKK1 and Detection of a Functional Polymorphism with Nicotine Dependence in an African-American Sample. Neuropsychopharmacology. 2008;34:319–330. doi: 10.1038/npp.2008.37. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, McGeary J, Smolen A, Bryan A, Swift RM. The DRD2 VNTR polymorphism moderates craving after alcohol consumption. Health Psychol. 2002;21:139–146. [PubMed] [Google Scholar]
- Hutchison KE, Stallings M, McGeary J, Bryan A. Population stratification in the candidate gene study: fatal threat or red herring? Psychol Bull. 2004 Jan;130(1):66–79. doi: 10.1037/0033-2909.130.1.66. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psych. 2005 Aug;162(8):1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kandel D, Johnson J, Bird H, et al. Psychiatric disorders associated with substance use among children and adolescents: Findings from the Methods for the Epidemiology of Child and Adolescent Mental Disorders (MECA) study. J Abnorm Child Psychol. 1997;25:121–132. doi: 10.1023/a:1025779412167. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school-age children - present version and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psych. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman brief intelligence test manual. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DN, Ouimette PC, Kelly HS, Ferro T, Riso LP. Test-retest reliability team consensus best-estimate diagnoses of Axis I and II disorders in a family study. Am J Psych. 1994;151:1043–1047. doi: 10.1176/ajp.151.7.1043. [DOI] [PubMed] [Google Scholar]
- Klein DN, Lewinsohn PM, Seeley JR, Rohde P. A family study of major depressive disorder in a community sample of adolescents. Arch General Psych. 2001;58:13–20. doi: 10.1001/archpsyc.58.1.13. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Bucholz KK, Madden PAF, Waldron M. Genetic and environmental influences on externalizing behavior and alcohol problems in adolescence: A female twin study. Pharmacology, Biochemistry, and Behavior. 2009 doi: 10.1016/j.pbb.2009.03.011. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen CA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8(11):1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abn Psych. 2002;111:411–424. [PubMed] [Google Scholar]
- Labouvie EW, McGee CR. Relation of personality to alcohol and drug-use in adolescence. J Consult Clin Psychol. 1986;54(3):289–293. doi: 10.1037//0022-006x.54.3.289. [DOI] [PubMed] [Google Scholar]
- Laucht M, Becker K, Blomeyer D, Schmidt M. Novelty seeking involved in mediating the association between the dopamine D4 Receptor Gene Exon III polymorphism and heavy drinking in male adolescents: Results from a high-risk community sample. Biol Psychiatry. 2007;61(1):87–92. doi: 10.1016/j.biopsych.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Lench N, Stanier P, Williamson R. Simple non-invasive method to obtain DNA for gene analysis. Lancet. 1988;18:1356–1358. doi: 10.1016/s0140-6736(88)92178-2. [DOI] [PubMed] [Google Scholar]
- Li D, Sham PC, Owen MJ, He L. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD) Hum Mol Genet. 2006;15(14):2276–2284. doi: 10.1093/hmg/ddl152. [DOI] [PubMed] [Google Scholar]
- Limosin F, Loze JY, Dubertret C, Gouya L, Ades J, Rouillon F, Gorwood P. Impulsiveness as the intermediate link between the dopamine receptor D2 gene and alcohol dependence. Psych Genetics. 2003;13:127–129. doi: 10.1097/01.ypg.0000066963.66429.00. [DOI] [PubMed] [Google Scholar]
- Miller FG, Lazowski LE. The adolescent SASSI-A2 manual: identifying substance use disorders. Springville, IN: The SASSI Institute; 2001. [Google Scholar]
- Needle R, McCubbin H, Lorence J, Hochhauser M. Reliability and validity of adolescent self-reported drug use in a family based study: a methodological report. Int J Addictions. 1983;18:901–912. doi: 10.3109/10826088309033058. [DOI] [PubMed] [Google Scholar]
- Nigg J, Wong M, Martel M, et al. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(4):468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Noble EP. The D-2 dopamine receptor gene: a review of association studies in alcoholism and phenotypes. Alcohol. 1998;16(1):33–45. doi: 10.1016/s0741-8329(97)00175-4. [DOI] [PubMed] [Google Scholar]
- Nyman ES, Ogdie MN, Loukola A, et al. ADHD candidate gene study in a population-based birth cohort: association with DBH and DRD2. J Am Acad Child Adolesc Psychiatry. 2007;46:1614–1621. doi: 10.1097/chi.0b013e3181579682. [DOI] [PubMed] [Google Scholar]
- Poelen EPP, Derks EM, Engels R, et al. The Relative contribution of genes and environment to alcohol use in early adolescents: Are similar factors related to initiation of alcohol use and frequency of drinking? Alcohol Clin Exp Res. 2008;32:975–982. doi: 10.1111/j.1530-0277.2008.00657.x. [DOI] [PubMed] [Google Scholar]
- Ratsma JE, van der Stelt O, Schoffelmeer ANM, Westerveld A, Gunning WB. P3 event-related potential, dopamine D2 receptor A1 allele, and sensation-seeking in adult children of alcoholics. Alcohol Clin Exp Res. 2001;25(7):960–967. [PubMed] [Google Scholar]
- Rende R, Plomin R. Nature, nurture, and the development of psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology. Vol. 1 theory and methods. New York: Wiley; pp. 291–314. [Google Scholar]
- Reynolds WM, Kamphaus RW. Behavioral Assessment Scale for Children. American Guidance Service, Circle Pines; MN: 1992. [Google Scholar]
- Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperacive/impulsive and combined subtypes. Behav Brain Sci. 2005;28(3):397–468. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- Seroczynski AD, Bergeman CS, Coccaro EF. Etiology of the impulsivity aggression relationship: genes or environment? Psychiatry Res. 1999;86(1):41–57. doi: 10.1016/s0165-1781(99)00013-x. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford University Press; NY: 1998. [Google Scholar]
- Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, Saha T. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiological Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2005;80:105–116. doi: 10.1016/j.drugalcdep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Todd RD, Huang H, Smalley SL, et al. Collaborative analysis of DRD4 and DAT genotypes in population-defined ADHD subtypes. J Child Psychol Psychiatry. 2005;46:1067–1073. doi: 10.1111/j.1469-7610.2005.01517.x. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Sergeant JA, Gunning WB. Psychological mechanisms of enhanced risk of addiction in children of alcoholics: A dual pathway? Acta Paediatrica. 1994;404(Supp):9–13. doi: 10.1111/j.1651-2227.1994.tb13377.x. [DOI] [PubMed] [Google Scholar]
- Yang BZ, Kranzler HR, Zhao H, Gruen JR, Luo X, Gelernter J. Association of haplotypic variants in DRD2, ANKK1, TTC12 and NCAM1 to alcohol dependence in independent case control and family samples. Hum Mol Genet. 2007;16:2844–53. doi: 10.1093/hmg/ddm240. [DOI] [PubMed] [Google Scholar]
- Yang BZ, Kranzler HR, Zhao H, Gruen JR, Luo X, Gelernter J. Haplotypic Variants in DRD2, ANKK1, TTC12, and NCAM1 are Associated With Comorbid Alcohol and Drug Dependence. Alcohol Clin Exp Res. 2008;32(12):2117–2127. doi: 10.1111/j.1530-0277.2008.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. Am J Med Gen. 2000;86:684–695. [PubMed] [Google Scholar]