Abstract
Immunization with vaccinia virus causes long-term immunity. Efforts have been made to characterize the T cells responsible for this protection. Recently, T cell subsets were described that not only co-express multiple cytokines, but also show increased per cell cytokine productivity. These highly productive cells are often considered to be the most protective. We used ELISPOT assays to measure per cell IFN-γ productivity of vaccinia specific T cells in childhood immunized adults immediately before and at different time points after vaccinia re-vaccination. Apart from an increase in frequency, we found a marked increase of IFN-γ productivity following vaccinia re-vaccination. However, these changes were short-lived as both parameters quickly returned to baseline values within 22 days after re-vaccination. Therefore, increased per cell IFN-γ productivity seems to be a sign of recent in vivo T cell activation rather than a stable marker of a distinct T cell subset responsible for long-term immune protection.
Keywords: Vaccinia, T cell memory, Interferon gamma, Cytokine productivity, ELISPOT
1. Introduction
The T cell system responds with clonal expansion to antigen stimulation, leading to an increase of antigen-specific T cells in lymphoid tissues and in the peripheral blood [1,2]. Therefore, frequency measurements of specific T cells have established themselves as a method of quantifying the magnitude of T cell immunity, particularly when assessing the success of immunizations. Initially, limiting dilution assays were used for this purpose, but these have been increasingly replaced by techniques that provide more accurate frequency information with less experimental effort. Among these newer generation techniques, ELISPOT assays and intracytoplasmic staining (ICS) have gained wide use [3]. Supernatant-based cytokine measurements, such as ELISA, cytokine bead arrays, or measurements of cytokine mRNA, can offer semi-quantitative information on the magnitude of the T cell response, but do not provide direct frequency information.
In addition to the frequency of antigen-specific T cells, per cell cytokine productivity has recently drawn attention as a potential marker of protective immunity. The notion has evolved that those T cells that are most effective in mediating immunity in infections such as influenza, vaccinia, CMV or EBV are those T cells that co-express multiple cytokines [4-7]. Furthermore, these multiple-cytokine producing T cells have also been shown to produce a larger amount of cytokine per cell [5-7]. This observation has led to the hypothesis that in addition to memory T cells, that may or may not be able to contribute directly to host defense, there is a distinct subset of highly productive multiple-cytokine effector cells that actually convey immune protection [8-11]. These findings, however, result from studies performed with ICS, for which cells need to be treated with secretion inhibitors in order to prevent the release of cytokine. Unfortunately, this pharmacological manipulation of cells alters the very end point to be measured: the actual release of cytokine. In order to avoid this alteration in our study, we chose to assess per cell cytokine productivity using ELISPOT assays that directly measure cytokine secretion by pharmacologically untreated cells.
For this purpose, we focused on the IFN-γ productivity of vaccinia-specific T cells. Childhood immunization with vaccinia virus is thought to result in long-term protection [12-16]. Therefore, in addition to antibodies, vaccinia-specific T cells in immunized individuals can also be considered a prototype of protective T cells [17-20]. In this study, adults vaccinated in childhood were re-vaccinated and the frequencies of vaccinia-specific T cells as well as their per cell IFN-γ secretion were measured in ELISPOT assays. We observed that per cell IFN-γ productivity strongly increased fourteen (14) days after re-vaccination. However, this increase was only transient in nature, and returned to baseline levels within twenty-two (22) days post re-vaccination. Therefore, we conclude that increased per cell IFN-γ productivity of T cells is not a steady characteristic of a certain protective T cell subset, but rather indicates a temporary T cell activation state. In classic terms, these findings are compatible with memory cells secreting low amounts of cytokine, and effector memory cells secreting a larger amount of cytokine per cell. Therefore, cytokine productivity may be used as a helpful indicator of recent in vivo T cell reactivation. It does not appear suitable, however, for assessing long-term protection.
2. Materials and methods
2.1. Subjects and sample collection
Four healthy adult members of our lab (2 males, 2 females, ages 33-53) who had been vaccinated in childhood with a single IM dose of vaccinia virus vaccine (Dryvax™) volunteered to be re-vaccinated with a single IM dose of Dryvax™. Peripheral blood was drawn immediately prior to re-vaccination (day 0) as well as on days 14 and 22 after re-vaccination. For all participants, PBMC were isolated from 40 to 100 ml of heparinized blood by standard Ficoll density-gradient centrifugation (Isoprep, Robbins Scientific, Sunnyvale, CA) and cryo-preserved. PBMC samples from different time points were tested side by side in ELISPOT assays as described below.
2.2. ELISPOT assays and digital image analysis
ImmunoSpot® plates (Cellular Technology Limited, Cleveland, OH) were coated with IFN-γ capture antibody mAb M700A (Endogen, Woburn, MA) in PBS (3 μg/ml) and placed at 4 °C overnight. The plates were then blocked with PBS containing 1 % BSA (Sigma, St. Louis, MO) for 1 h and washed three times with PBS. PBMC were plated in RPMI-1640 (BioWhittaker, Walkersville, MD) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine and 10 % pooled heat-inactivated human AB serum. For all experiments 2×105 PBMC were plated per well in the presence of live vaccinia Lister (Applied Biosystems, Foster City, CA; used at 1 MOI) or individual vaccinia peptides (PANATecs GmbH, Tübingen, Germany; used at 5 μg/ml). Refer to Table 1 for peptide numbers assigned, amino acid sequences, and HLA restriction [21-25]. CEF peptide pool (Cellular Technology Limited, Cleveland, OH) contained MHC-I peptides derived from Cytomegalovirus, Epstein-Barr virus, and Influenza virus and was used at a final concentration of 2 μg/ml of each peptide. Phytohemagglutinin (PHA) was obtained from Sigma and used as a positive control in all assays (10 μg/ml). Negative control wells contained PBMC with medium only. After 24 h of incubation at 37 °C and 7 % CO2, the plates were washed with PBS and PBS/TWEEN, and IFN-γ biotinylated detection mAb M701 (Endogen, 2 μg/ml) was added. The antibody was diluted in PBS containing 1 % BSA and 0.025 % TWEEN 20 (Fisher Scientific International Inc., Hampton, NH). The plates were incubated at 4 °C overnight. The plates were then washed three times with PBS/TWEEN and subsequently streptavidin-HRP conjugate (DAKO, Carpinteria, CA) was added at 1/2000 dilution, incubated for 2 h at room temperature, and removed by washing twice with PBS and PBS/TWEEN. The spots were visualized by adding HRP substrate 3-amino-9-ethylcarbozole (Pierce, Rockford, IL). The reaction was stopped by rinsing the plate with distilled water when distinct spots were visible macroscopically. Plates were dried overnight and images of the ELISPOT wells were captured with an ImmunoSpot® Series 5 Analyzer (Cellular Technology Limited, Cleveland, OH). Image analysis of ELISPOT results was performed with the ImmunoSpot® 5.0 Analysis Software (Cellular Technology Limited, Cleveland, OH), allowing precise spot size measurements down to spot sizes of 0.001 mm2. Spot size distribution curves as well as mean spot sizes were also calculated using the ImmunoSpot® 5.0 Analysis Software (Cellular Technology Limited, Cleveland, OH).
Table 1.
Vaccinia peptides a
| Peptide ID | Sequence | HLA restriction |
|---|---|---|
| 1 | VSVNNVCHMY | A*2601 |
| 2 | SQSDTVFDY | A*0101 |
| 3 | QSDTVFDYY | A*0101 |
| 4 | VTDTNKFDNY | A*0101 |
| 5 | CMLTEFLHY | A*2601 |
| 6 | FTIDFKLKY | A*2601 |
| 7 | GTHVLLPFY | A*0101 |
| 8 | DMCDIYLLY | A*0101, *2601 |
| 9 | FGDSKEPVPY | A*0101, *2601 |
| 10 | FLSMLNLTKY | A*0101, *2601 |
| 11 | QSITRSLIY | A*2601 |
| 12 | AMLNGIYV | A*0201 |
| 13 | VLPFDIKKL | A*0201 |
| 14 | NLWNGIVPM | A*0201 |
| 15 | ILDDNLYKV | A*0201, *0202, *0203, *0206 |
| 16 | YVNAILYQI | A*0201 |
| 17 | SLSAYIIRV | A*0201 |
| 18 | KVDDTFYYV | A*0201 |
| 19 | NLFDIPLLTV | A*0201 |
| 20 | FLTSVINRV | A*0201 |
| 21 | KIDYYIPYV | A*0201 |
| 22 | FLNISWFYI | A*0201 |
| 23 | GLNDYLHSV | A*0201 |
| 24 | SMHFYGWSL | A*0201 |
| 25 | YLYTEYFLFI | A*0201 |
| 26 | MMLVPLITV | A*0201 |
| 27 | YIYGIPLSL | A*0201 |
| 28 | AVYGNIKHK | A*0301 |
| 29 | KVLHVTDNK | A*0301 |
| 30 | ATSLDVINY | A*1101 |
| 31 | KLKIISNDYK | A*0301 |
| 32 | KVMFVIRFK | A*0301, *1101 |
| 33 | IVFNLPVSK | A*0301 |
| 34 | NQVKFYFNK | A*0301 |
| 35 | KTKNFTIDFK | A*0301 |
| 36 | YLLVKWYRK | A*3303 |
| 37 | VTSSGAIYK | A*1101 |
| 38 | GTIAGGVCYY | A*1101 |
| 39 | AVFKDSFLRK | A*1101 |
| 40 | VWINNSWKF | A*2402 |
| 41 | RYRFAFLYLI | A*2402 |
| 42 | RYYDGNIYE | A*2407 |
| 43 | KPKPAVRFAI | B*0702 |
| 44 | APNPNRFVI | B*0702 |
| 45 | RPMSLRSTII | B*0702 |
| 46 | MPAYIRNTL | B*0702 |
| 47 | GESKSYCEL | B*4001 |
| 48 | DELVDPINY | B*3701 |
| 49 | TEYDDHINL | B*4001 |
| 50 | HPRHYATVM | B*0702 |
2.3. Intracytoplasmic staining and flow cytometry
Intracellular cytokine staining was performed as previously described [26]. PBMC (4×106/ml) were cultured overnight with or without live vaccinia virus (1 MOI) in complete RPMI medium. The next day, duel-color flow cytometry was performed to establish the frequency of IFN-γ producing cells within the CD3 cell population. For staining of IFN-γ, FITC-labeled IFN-γ Ab (Becton Dickinson, San Jose, CA) was used; for CD3, peridinin chlorophyl protein-labeled anti-human CD3 Abs (Becton Dickinson, San Jose, CA). The isotype-matched control monoclonal antibodies were also from Becton Dickinson. The samples were analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA). As a protein transport inhibitor, we used Monensin or brefeldin A (PharMingen, City, State).
3. Results
3.1. Vaccinia virus-induced IFN-γ secreting T cells
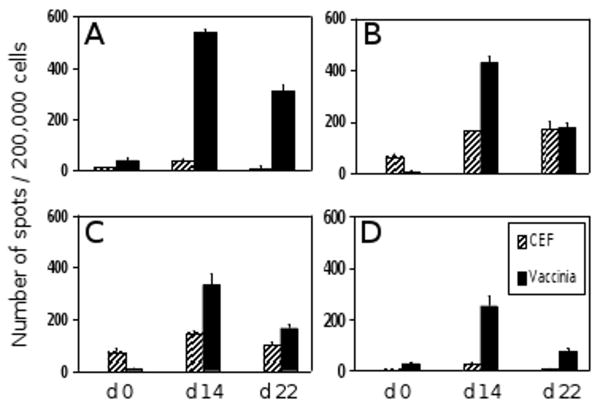
Four healthy adult donors were selected for this study. All four had been vaccinated in childhood with vaccinia live virus vaccine. When tested before re-vaccination (day 0), all four donors showed a weak recall response to vaccinia virus, ranging from 5 to 40 IFN-γ producing cells per 200,000 PBMC (Fig. 1). These donors also responded to CEF peptide pool. When the donors were tested on day 14 after re-vaccination, the numbers of vaccinia-induced IFN-γ spots increased significantly, ranging between 250 and 540 spots per 200,000 PBMC. This corresponds to a frequency increase between 10-fold and 50-fold compared to day 0. The numbers of CEF-induced IFN-γ spots also increased slightly in all four donors. However, this increase was less than 2.3-fold in each case. When tested on day 22, the numbers of vaccinia specific cells had already dropped to 30-58 % of their day 14 frequency. The frequency of CEF reactive cells remained unchanged or dropped to day 0 values. These data are consistent with a vaccinia-induced expansion of specific T cells, clearly detectable on day 14 post re-vaccination, and an immediate subsequent contraction phase as indicated by markedly reduced frequencies by day 22. The moderate increase of CEF-reactive cells most likely results from bystander proliferation [27].
Fig. 1.
Frequencies of vaccinia specific T cells before and after vaccinia re-vaccination. The numbers of IFN-γ spots per 200,000 PBMC, measured by ELISPOT immediately prior to re-vaccination (day 0) as well as 14 and 22 days after re-vaccination, are shown for each of the four donors. Panels A – D correspond to donors 1 – 4. IFN-γ responses to CEF peptide pool served as control.
ICS was performed in parallel to ELISPOT assays in order to determine the cell type that produces IFN-γ after antigen stimulation (Fig. 2). The vaccinia-stimulated cells were counterstained with anti-CD3. As can be seen in Fig. 2A, in un-stimulated cells, 13 per 100,000 gated events were measured within the IFN-γ+/CD3+ quadrant. In the vaccinia-stimulated population (Fig. 2B), 71 out of 100,000 gated events occurred in the IFN-γ+/CD3+ quadrant and 12 in the IFN-γ+/CD3- quadrant. These data show that the majority of IFN-γ producing cells were CD3+ T cells and that the frequency of such cells was 0.0580 % within the PBMC tested. In the ELISPOT assay performed in parallel, the medium control was 0 spots for the un-stimulated cells (Fig. 2C), compared to 115 spots per 200,000 cells after vaccinia stimulation (Fig. 2D). The resulting frequency of vaccinia-induced antigen specific T cells detected by ELISPOT was 0.0575 %; essentially identical to ICS. Therefore, using the IFN-γ ELISPOT assay, T cells can be detected at single cell resolution. The vaccinia-induced cytokine production was strictly antigen specific, since none of the 12 vaccinia-naïve donors had in vitro vaccinia induced cytokine spots over medium background (data not shown).
Fig. 2.
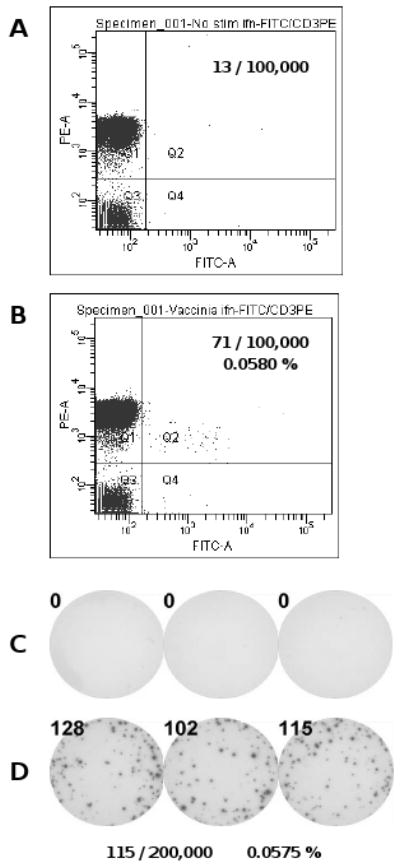
Comparison of IFN-γ ELISPOT and ICS. Frequencies of vaccinia specific T cells were measured for one donor on day 0 using IFN-γ ELISPOT and ICS in parallel. Measurements obtained with ICS are shown without (A) and with (B) in vitro vaccinia stimulation. The number of events collected in quadrant 2 (Q2), representing CD3+/IFN-γ+ cells, are given along with the resulting frequency of vaccinia specific T cells in percent. ELISPOT was performed with the same PBMC samples. Well images are shown without (C) and with (D) in vitro challenge with live vaccinia virus. Spot numbers are given for each well along with the resulting mean spot number and the frequency of vaccinia specific T cells in percent.
3.2. Measurement of vaccinia virus-induced per cell IFN-γ productivity
The striking observation made in this study was the fact that along with the frequency increase described above, the size of IFN-γ spots induced by vaccinia also changed fundamentally between day 0 and day 14. We used digital image analysis to measure the exact spot size distribution for each donor and time point. The size distribution curve for day 0 samples of the four donors are shown in Fig. 3A with an adjoining representative well. Day 14 results are shown in Fig. 3B along with a corresponding well. Already by day 21, the spot size distribution had returned to values similar to those on day 0 (Fig. 3C). Jointly, these data clearly show that on day 14, after re-vaccination, the mean spot size was significantly increased relative to day 0, and that this increase was transient, returning to baseline value by day 22 (Fig. 3F). Therefore, increased per cell IFN-γ productivity seems to be a transient feature of recently activated antigen-specific T cells.
Fig. 3.
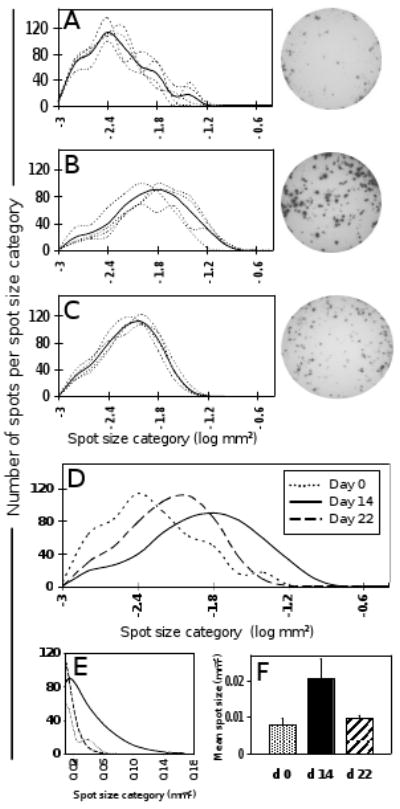
IFN-γ spot size distribution using live vaccinia virus as recall antigen. Panel A shows the individual IFN-γ spot size distribution curves for donors 1 – 4 (dotted lines), obtained with live virus in vitro recall of day 0 PBMC. The solid line indicates the mean spot size distribution of all four donors. For comparability, all curves are adjusted to cover a total of 500 spots. The same information is given for days 14 (B) and 22 (C). Representative well images are depicted for each time point. Panel D directly compares the mean spot size distribution curves from panels A – C, corresponding to the three time points (dotted line, day 0; solid line, day 14; dashed line, day 22). Panel E illustrates the day 14 spot size shift by showing the upper range of the three curves from panel D on a linear scale. Panel F shows the mean spot sizes (mean of all four donors) for the three time points.
3.3. Vaccinia peptide-induced IFN-γ recall responses
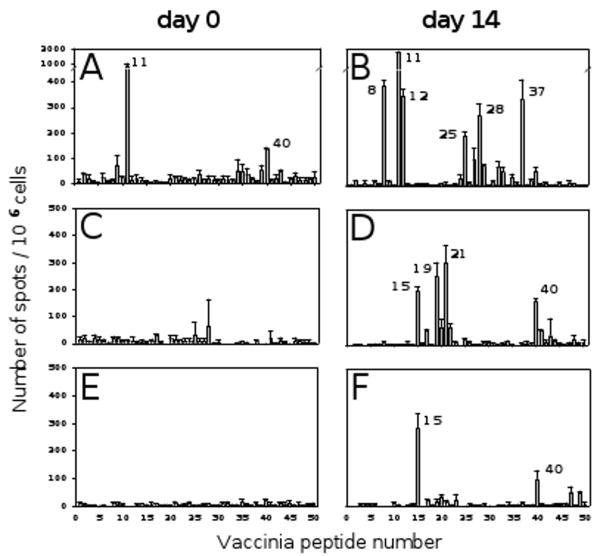
The live vaccinia virus we used in the experiments above is a highly complex antigen system. We therefore extended our studies to the use of “clean” and simple antigenic subunits: vaccinia peptides. For this purpose, we chose 50 vaccinia peptides that had been described in the literature as T cell determinants and had them commercially synthesized [21-25]. The number assigned to each peptide along with its amino acid sequence and HLA restriction is listed in Table 1. The individual peptides were tested on three of the donors on day 0 and day 14 (Fig. 4). On day 0, the peptides that did induce a recall response over medium control elicited only low frequency IFN-γ secreting cells, with the exception of peptide number 11 in donor 1 (Fig. 4A). However, on day 14 after re-vaccination, several of the peptides triggered strong recall responses in all three donors (Fig. 4B, 4D, and 4F). Although it is not the scope of this publication to match the peptide-induced responses with each donor's HLA type based on each peptide's HLA restriction, this information can be extracted from the data provided in Fig. 4 and Tables 1 and 2. Those 12 peptides that induced substantial responses on day 14 were subsequently used for spot size analysis.
Fig. 4.
IFN-γ recall response to individual vaccinia peptides. The 50 individual vaccinia peptides listed in Table 1 were used as recall antigens to detect vaccinia peptide specific T cell responses in donors 1 – 3 before (day 0) and after re-vaccination (day 14). Donor 1: panels A and B. Donor 2: panels C and D. Donor 3: panels E and F.
Table 2.
HLA genotypesa
| HLA Class I | Donor 1 | Donor 2 | Donor 3 |
|---|---|---|---|
| A1 | A3-0301 | A2-0201 | A2-0201 |
| A2 | A11-1101 | A24-2402 | A24-2402 |
| B1 | B35-3511 | B44-4403 | B15-1535 |
| B2 | B55-5513 | B51-5101 | B40-4001 |
| C1 | Cw9-0303 | Cw1-0102 | Cw7-0702 |
| C2 | Cw4-0401 | Cw4-0401 | Cw15-1502 |
The table lists the HLA genotypes of donors 1 – 3.
The vaccinia peptide-induced responses were once again antigen specific since they did not induce significant IFN-γ responses over medium background when tested individually in 10 naïve subjects (data not shown).
3.4. Measurement of vaccinia peptide-induced per cell IFN-γ productivity
We systematically compared IFN-γ spot sizes induced by vaccinia peptides on days 0, 14, and 22 (Fig. 5). The sizes of these spots were not only compared between time points but also relative to those induced by CEF peptide pool. As can be seen in Fig. 5A, vaccinia peptide-induced spots on day 0 (decades after childhood immunization) were tiny (mean spot size 0.0036 mm2) relative to CEF peptide-induced spots (mean spot size 0.0335 mm2). On day 14 after re-vaccination, the vaccinia peptide-induced spots showed a dramatic increase in size for all three donors and all of the selected peptides (Fig. 5B). The size distribution of spots induced by the peptides showed considerable variability between individual peptides. However, each peptide curve was shifted significantly to the right compared to day 0. By day 22, the peptide-induced spots had already decreased to sizes comparable to those spots induced by CEF peptide pool (Fig. 5C). Representative well images are shown for each time point next to the corresponding spot size distribution curve.
Fig. 5.
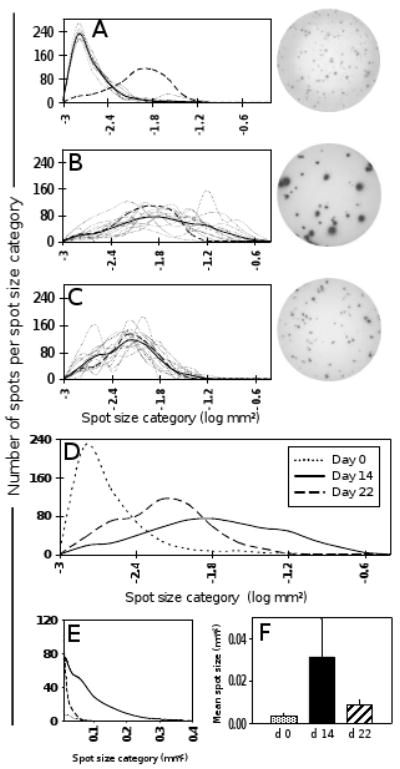
IFN-γ spot size distribution using individual vaccinia peptides as recall antigens. Peptide antigens that elicited a marked frequency increase in Fig. 4 were chosen for detailed spot size analysis (n = 12). Panel A shows the individual IFN-γ spot size distribution curves elicited by each of the chosen peptides (dotted lines) when used as recall antigen on day 0 PBMC. The solid line indicates the mean spot size distribution calculated from all 12 peptides (combined data from donors 1 – 3). The dashed line shows the spot size distribution obtained with CEF peptide pool as control. For comparability, all curves are adjusted to cover a total of 500 spots. The same information is given for days 14 (B) and 22 (C). Representative well images are depicted for each time point. Panel D directly compares the mean spot size distribution curves from panels A – C, corresponding to the three time points (dotted line, day 0; solid line, day 14; dashed line, day 22). Panel E illustrates the day 14 spot size shift by showing the upper range of the three curves from panel D on a linear scale. Panel F shows the mean spot sizes (mean of all 12 peptides) for the three time points.
Consistent with the observations made using live virus for in vitro recall, these data show that also with individual vaccinia peptides as recall antigens, IFN-γ spot size significantly increased on day 14 after re-vaccination. Again, this increase was clearly transient, as by day 22, spot sizes had returned to baseline values (Fig. 5F).
The data obtained with “clean” vaccinia peptide antigens therefore confirm the results obtained with live virus: The increased per cell IFN-γ productivity after re-vaccination seems to be merely a transient feature of recently activated antigen-specific T cells and not a stable marker of long-term protection.
4. Discussion
ELISPOT assays are widely used to measure antigen-specific T cell immunity ex vivo. Such studies typically focus on establishing frequencies of antigen-specific cells within the PBMC population. As shown in Fig. 2 of this publication, the frequencies measured by ELISPOT and by ICS match up rather closely, suggesting that both assays accurately measure the antigen specific recall responses to Vaccinia if the frequencies are high enough. While ICS has a detection limit around 1 in 10,000 cells, ELISPOT assays have substantially lower detection limits [28;29]. This is well-illustrated in Fig. 2, where the test sample showed a frequency of vaccinia-reactive cells at a frequency of approximately 0.06 % which was easily detectable by ELISPOT but reached the detection limit of ICS. Therefore, ELISPOT's sensitivity and ease of use gives it a unique position for frequency measurements of antigen-specific T cells.
To our knowledge, there have been no published studies that took advantage of the additional strength of ELISPOT assays, namely to monitor cytokine productivity per cell. Typically, only spot counts are used, neglecting useful spot size information. Among all techniques that permit to measure antigen-induced cytokine production, ELISPOT is the only one that detects the actual release of cytokine by each individual cell. As the cell secretes, the cytokine is immediately captured around the cell during the entire culture period. Size and density of this “footprint” are a direct reflection of the amount of cytokine secreted per cell over the measuring period. It is important to note that only that fraction of cytokine is being captured on the membrane that diffuses towards the membrane while the rest diffuses into the supernatant. In an approximation, one can therefore compare the spot left on the membrane with a cross section through a cytokine sphere. As shown by Guerkov et al., the total amount of cytokine released can be calculated based upon the diameter of the spot [30]. Therefore, using ELISPOT assays in our study enabled us to precisely detect the increase of per cell IFN-γ secretion based upon the changes in spot size we measured.
In earlier studies, we showed that even for a T cell clone, per cell IFN-γ productivity follows a close to perfect log normal curve [31]. This indicates that even within a clonal population of T cells, individual cells substantially differ in the amount of cytokine they secrete. Some cells are high producers, some are low producers, and the majority lie in between. Therefore, for all the data we analyzed in this study, we used digital image analysis software (ImmunoSpot®) with built-in algorithms to calculate spot size distribution curves. We then compared these curves in order to assess changes in per cell productivity. As seen in Fig. 3 and 5, significant shifts of size distribution were noted due to their magnitudes expressed on a logarithmic scale. These data clearly show that the per cell cytokine productivity of vaccinia-specific T cells strongly increases by day 14 after immunization relative to day 0 values, and subsequently declines by day 22. To our knowledge, this is the first report describing the short-lived nature of this increase in T cell cytokine productivity following antigen activation in vivo.
The amount of cytokine produced by T cells is a function of the signal strength. When studying a T cell clone, we found that the stronger the T cell stimulation was (increasing concentrations of in vitro recall antigen), the more cytokine the T cells produced, indicated by a marked right-shift of the log normally distributed spot size curves [31]. Notably, in this study, identical antigen and peptide concentrations were used for all time points. In another publication we described that the type of antigen presenting cell (APC) that activates a T cell substantially influences the T cell's cytokine productivity [32]. T cells stimulated on dendritic cells (DC) not only engaged more rapidly in cytokine production, but also produced more cytokine per cell compared to T cells activated by B cells. Macrophages led to an intermediate level of T cell activation. Based on this information, the increased per cell IFN-γ productivity seen on day 14 in the present study could have also been caused by changes in the APC population: An increase of PBMC and DC caused by inflammation and leukocytosis could have led to enhanced T cell activation due to a higher density of professional APC. In that case, the increased per cell IFN-γ productivity would not be an inherent property of the T cells themselves, but rather induced by APC fluctuations. If APC changes had indeed contributed to the increased per cell productivity in our study, a right shift in spot size distribution should have also been seen in the CEF peptide-induced spots. As illustrated in Fig. 5A vs. 5B, the CEF peptide-induced curves perfectly overlap for days 0 and 14. Only the vaccinia-induced spots underwent the size increase. Therefore, we conclude that the increased per cell productivity is in fact a property of the activated T cells themselves.
Increased per cell productivity has previously been detected by ICS and linked to a multi-cytokine producing T cell phenotype [5-7]. A series of recent publications tried to make the point that multi-cytokine producing T cells constitute the T cell subset critical for host defense [4-11]. Some of these publications also showed that these multi-cytokine producing T cells are characterized by an increased per cell cytokine productivity. What has remained unclear, however, is whether this multi-cytokine per increased-productivity phenotype is a stable marker of a cell lineage that carries immunological memory and protection, or whether it merely reflects a transient T cell activation state. Our data do not attempt to resolve this issue. However, some conclusions can clearly be drawn. Studying the four individuals that we examined in detail - along with more than 50 additional donors immunized in childhood, but not re-vaccinated (data not shown) - we could generate substantial information on long-term memory cells. All of the spot size distributions we measured in these individuals decades after immunization were similar to those shown in Fig. 3A, where none of the spots exceeded a size threshold of 0.08 mm2 (corresponding to 10-1.1 on the logarithmic scale). Therefore, high productivity cells (causing spots larger than this) that were commonly seen on day 14 and occasionally still on day 22, occur (if at all) in undetectably low frequencies in the long-lived memory T cell pool known to provide long-term protection. These high productivity cells were only detectable transiently after re-vaccination and then disappeared. We therefore conclude that the high producing cell type is not a protective memory T cell lineage of its own but rather represents a transiently increased activation state of long-lived memory T cells. Even if high productivity cells constituted a distinct T cell subset or blast, they would be too short-lived to convey the long-term protection seen after vaccinia immunization. The simplest explanation of our data is that long-term resting memory cells show low per cell productivity, but when stimulated with antigen, transform into effector memory cells with high cytokine productivity. This high productivity, however, is quickly lost as the effector memory cells either die or revert to a resting memory phenotype [33].
By no means do our data attempt an extensive analysis of cytokine productivity or memory T cell biology in general. The data emerged from a limited re-vaccination project and, in view of these striking results, are communicated with two intents: First, to introduce cytokine productivity measurements as a promising new application for T cell diagnostics and to show how easily it can be added to the standard frequency measurements; second, to draw attention to the fact that increased per cell productivity may signify recently in vivo activated T cells rather than a distinct subset of long-term memory T cells. Therefore, rather than using productivity measurements to assess long-term immune protection, one should add it to frequency measurements in order to distinguish between long-term memory and recently activated T cells, and thus be able to detect actively ongoing immune processes, be it in infection, allergy or autoimmune disease.
Acknowledgments
The authors wish to thank Ms. Andrea Csipak for her most valuable assistance with image analysis and statistical calculations.
This work was supported by NIAID contract N01-AI-400948.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doherty PC. The numbers game for virus-specific CD8+ T cells. Science. 1998;280:227. doi: 10.1126/science.280.5361.227. [DOI] [PubMed] [Google Scholar]
- 3.Perfetto SP, Chattopadhyay PK, Roederer M. Seventeen-colour flow cytometry: unravelling the immune system. Nat Rev Immunol. 2004;4:648–655. doi: 10.1038/nri1416. [DOI] [PubMed] [Google Scholar]
- 4.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 6.Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol. 2007;81:8468–8476. doi: 10.1128/JVI.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, Asher TE, Douek DC, Harari A, Pantaleo G, Bailer R, Graham BS, Roederer M, Koup RA. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med. 2007;204:1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casazza JP, Betts MR, Price DA, Precopio ML, Ruff LE, Brenchley JM, Hill BJ, Roederer M, Douek DC, Koup RA. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med. 2006;203:2865–2877. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harari A, Dutoit V, Cellerai C, Bart PA, Du Pasquier RA, Pantaleo G. Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol Rev. 2006;211:236–254. doi: 10.1111/j.0105-2896.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- 10.Makedonas G, Betts MR. Polyfunctional analysis of human t cell responses: importance in vaccine immunogenicity and natural infection, Springer Semin. Immunopathol. 2006;28:209–219. doi: 10.1007/s00281-006-0025-4. [DOI] [PubMed] [Google Scholar]
- 11.Pantaleo G, Harari A. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nat Rev Immunol. 2006;6:417–423. doi: 10.1038/nri1840. [DOI] [PubMed] [Google Scholar]
- 12.Combadiere B, Boissonnas A, Carcelain G, Lefranc E, Samri A, Bricaire F, Debre P, Autran B. Distinct time effects of vaccination on long-term proliferative and IFN-gamma-producing T cell memory to smallpox in humans. J Exp Med. 2004;199:1585–1593. doi: 10.1084/jem.20032083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 14.Demkowicz WE, Littaua RA, Wang J, Ennis FA. Human cytotoxic T-cell memory: long-lived responses to vaccinia virus. J Virol. 1996;70:2627–2631. doi: 10.1128/jvi.70.4.2627-2631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frey SE, Newman FK, Yan L, Lottenbach KR, Belshe RB. Response to smallpox vaccine in persons immunized in the distant past. JAMA. 2003;289:3295–3299. doi: 10.1001/jama.289.24.3295. [DOI] [PubMed] [Google Scholar]
- 16.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 17.Chaudhri G, Panchanathan V, Bluethmann H, Karupiah G. Obligatory requirement for antibody in recovery from a primary poxvirus infection. J Virol. 2006;80:6339–6344. doi: 10.1128/JVI.00116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang M, Sigal LJ. Antibodies and CD8+ T cells are complementary and essential for natural resistance to a highly lethal cytopathic virus. J Immunol. 2005;175:6829–6836. doi: 10.4049/jimmunol.175.10.6829. [DOI] [PubMed] [Google Scholar]
- 19.Karupiah G, Buller RM, Van RN, Duarte CJ, Chen J. Different roles for CD4+ and CD8+ T lymphocytes and macrophage subsets in the control of a generalized virus infection. J Virol. 1996;70:8301–8309. doi: 10.1128/jvi.70.12.8301-8309.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol. 2004;172:6265–6271. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]
- 21.Dong Y, Denny TN. HLA-A2-restricted human CD8(+) cytotoxic T lymphocyte responses to a novel epitope in vaccinia virus that is conserved among orthopox viruses. J Infect Dis. 2006;194:168–175. doi: 10.1086/505224. [DOI] [PubMed] [Google Scholar]
- 22.Drexler I, Staib C, Kastenmuller W, Stevanovic S, Schmidt B, Lemonnier F, Rammensee HG, Busch DH, Bernhard H, Erfle V, Sutter G. Identification of vaccinia virus epitope-specific HLA-A*0201-restricted T cells and comparative analysis of smallpox vaccines. Proc Natl Acad Sci U S A. 2003;100:217–222. doi: 10.1073/pnas.262668999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oseroff C, Kos F, Bui HH, Peters B, Pasquetto V, Glenn J, Palmore T, Sidney J, Tscharke DC, Bennink JR, Southwood S, Grey HM, Yewdell JW, Sette A. HLA class I-restricted responses to vaccinia recognize a broad array of proteins mainly involved in virulence and viral gene regulation. Proc Natl Acad Sci U S A. 2005;102:13980–13985. doi: 10.1073/pnas.0506768102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snyder JT, Belyakov IM, Dzutsev A, Lemonnier F, Berzofsky JA. Protection against lethal vaccinia virus challenge in HLA-A2 transgenic mice by immunization with a single CD8+ T-cell peptide epitope of vaccinia and variola viruses. J Virol. 2004;78:7052–7060. doi: 10.1128/JVI.78.13.7052-7060.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terajima M, Cruz J, Raines G, Kilpatrick ED, Kennedy JS, Rothman AL, Ennis FA. Quantitation of CD8+ T cell responses to newly identified HLA-A*0201-restricted T cell epitopes conserved among vaccinia and variola (smallpox) viruses. J Exp Med. 2003;197:927–932. doi: 10.1084/jem.20022222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Openshaw P, Murphy EE, Hosken NA, Maino V, Davis K, Murphy K, O'Garra A. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J Exp Med. 1995;182:1357–1367. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 28.Helms T, Boehm BO, Asaad RJ, Trezza RP, Lehmann PV, Tary-Lehmann M. Direct visualization of cytokine-producing recall antigen-specific CD4 memory T cells in healthy individuals and HIV patients. J Immunol. 2000;164:3723–3732. doi: 10.4049/jimmunol.164.7.3723. [DOI] [PubMed] [Google Scholar]
- 29.Hofstetter HH, Targoni OS, Karulin AY, Forsthuber TG, Tary-Lehmann M, Lehmann PV. Does the frequency and avidity spectrum of the neuroantigen-specific T cells in the blood mirror the autoimmune process in the central nervous system of mice undergoing experimental allergic encephalomyelitis? J Immunol. 2005;174:4598–4605. doi: 10.4049/jimmunol.174.8.4598. [DOI] [PubMed] [Google Scholar]
- 30.Guerkov RE, Targoni OS, Kreher CR, Boehm BO, Herrera MT, Tary-Lehmann M, Lehmann PV, Schwander SK. Detection of low-frequency antigen-specific IL-10-producing CD4(+) T cells via ELISPOT in PBMC: cognate vs. nonspecific production of the cytokine. J Immunol Methods. 2003;279:111–121. doi: 10.1016/s0022-1759(03)00240-0. [DOI] [PubMed] [Google Scholar]
- 31.Hesse MD, Karulin AY, Boehm BO, Lehmann PV, Tary-Lehmann M. A T cell clone's avidity is a function of its activation state. J Immunol. 2001;167:1353–1361. doi: 10.4049/jimmunol.167.3.1353. [DOI] [PubMed] [Google Scholar]
- 32.Ott PA, Tary-Lehmann M, Lehmann PV. The secretory IFN-gamma response of single CD4 memory cells after activation on different antigen presenting cell types. Clin Immunol. 2007;124:267–276. doi: 10.1016/j.clim.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]