Abstract
Background
Reducingexposure to household dust inhalant allergens has been proposed as one strategy to reduce asthma.
Objective
To examine the dose response relationships and health impact of five common household dust allergens on disease severity, quantified using both symptom frequency and medication use, in atopic and non-atopic asthmatic children.
Methods
Asthmatic children (N=300) aged 4–12 years were followed for one year. Household dust samples from two indoor locations were analyzed for allergens including dust mite (Der p 1, Der f 1), cat (Fel d 1), dog (Can f 1), cockroach (Bla g 1). Daily symptoms and medication use were collected in monthly telephone interviews. Annual disease severity was examined in models including allergens, specific IgE sensitivity and adjusted for age, gender, atopy, ethnicity, and mother’s education.
Results
Der p 1 house dust mite allergen concentration of 2.0 + μg/g from the main room and the child’s bed was related to increased asthma severity independent of allergic status (respectively, OR 2.93, 95% CI 1.37, 6.30 for 2.0 –10.0 μg/g and OR 2.55 95% CI 1.13, 5.73 for ≥ 10.0 μg/g). Higher pet allergen levels were associated with greater asthma severity, but only for those sensitized (cat OR 2.41 95% CI 1.19, 4.89; dog OR 2.06 95% CI 1.01, 4.22).
Conclusion
Higher levels of Der p 1 and pet allergens were associated with asthma severity, but Der p 1 remained an independent risk factor after accounting for pet allergens and regardless of Der p 1 specific IgE status.
Keywords: pediatric asthma, household dust allergens, Der p1, dust mite, pet allergens
Introduction
House dust commonly includes allergens from dust mites (Der p 1, Der f 1), cockroach (e.g., Bla g 1) and pets (e.g., dog [Can f 1], cat [Fel d 1]). In a multinational epidemiological study of asthmatics and non-asthmatics, house dust mite was the antigen most consistently associated with the presence of asthma or increased bronchial reactivity (Janson et al. 2001). The amounts of antigen in homes are regionally variable, and depend on climate, location (i.e., urban, suburban, rural) and pet ownership. For example, while Dermatophagoides pteronyssinus (D pteronyssinus) predominates in house dust samples collected in New Zealand (Erwin et al. 2005) and Australia (Dharmage et al. 1999;Marks et al. 1995), Dermatophagoides farinae (D farinae) is most common in German homes (Gehring et al. 2001) and Blattella germanica (B germanica) is a major constituent in inner city house dust in urban US (Gruchalla et al. 2005;Morgan et al. 2004). High levels of pet allergen depend primarily on current pets in residence, although significant amounts may be found in homes for months or years following a pet’s removal (Gehring et al. 2004), and may also be detected in homes without pets where allergens may enter the home on residents’ clothing (Bollinger et al. 1996).
Although dust mite and other home antigen levels may vary seasonally in some locations, there is evidence to suggest that the between-home variability due to housing type, pet ownership, and other household factors is far greater than within-home seasonal variability (Chew et al. 1999;Heinrich et al. 2003;van Strien et al. 2004;Zock et al. 2006). Repeated allergen measurements in a household, in the absence of specific intervention attempts, have been shown to be highly correlated, and levels measured at one point in time are generally representative of levels that can be found for at least a year (Chew et al. 1999;Heinrich et al. 2003). Thus, though levels may vary, asthmatic children living in homes where antigens have been detected at one time are likely to be exposed to similar levels of antigens for up to a year. It is possible that for asthmatics, chronic exposure to household allergens maintains a certain level of background respiratory inflammation (Simpson et al. 1999).
Exposure to common household allergens takes place concurrently, however, few studies have examined multiple allergen co-exposures together in multivariate models. Studies that have included more than one allergen in statistical models are Gehring et al., (Gehring et al. 2001), who reported some models that included levels of Der p 1, Der f 1 and/or Fel d 1, and Blanc et al. (Blanc et al. 2005), who reported multivariable models that included Can f 1 , Fel d 1 and the weight of dust sampled.
We prospectively followed a cohort of asthmatic children ages 4–12 for one year and examined associations between level of household allergens measured at the time of study enrollment, allergen-specific IgE, and asthma severity. Outcome measures of asthma severity included symptom frequency, type and amount of medication use as well as a severity score quantified according to modified Global Initiatives for Asthma (GINA) guidelines (United States Department of Health and Human Services February 2002) which considers both symptoms and medication use. We examined the impact of concurrent exposures to five common household allergens on asthma severity in multivariate models that included all measured household allergens.
Methods
Subjects were drawn from a study population of 466 children aged 4–12 when enrolled between 2000 and 2003; 454 (93.1%) completed one-year follow-up (Gent et al. 2003). Eligibility criteria included physician-diagnosed asthma, age less than 12 years and asthma symptoms or medication use in the previous 12 months. Subjects in the present analysis (N=300) include those who had allergy test results and lived for at least 7 months in the home where dust samples were collected. The Yale Human Investigation Committee approved the study, and mothers of subjects gave informed consent.
During the enrollment visit, a research assistant administered a questionnaire to the child’s mother to collect demographic information, medical history, and home environment information. Mothers recorded daily symptoms (wheeze, persistent cough, shortness of breath, chest tightness, night symptoms) and medication use (rescue medication including bronchodilators, and controller medication including steroids, cromolyn sodium, and leukotriene inhibitors) on study calendars, and reported this information during monthly telephone interviews.
Dust samples
Dust samples, taken from the floor in the main living area and from the child’s bed during the enrollment visit using a protocol described previously (Belanger et al. 2003;Gehring et al. 2004;Leaderer et al. 2002), were assayed by ELISA (enzyme-linked immunosorbent assay) for allergens (dust mite (Der p 1, Der f 1), cat (Fel d 1), dog (Can f 1), and cockroach (Bla g 1)) (Gehring et al. 2004;Leaderer et al. 2002).
Specific IgE
Although not required, 336 participants (72%) provided blood samples for a 10-item allergen-specific IgE panel: Der p1, Der f 1, Fel d 1, Can f 1, Bla g 1, Alternaria, mouse, grass, ragweed, egg. Serum was analyzed using the UniCAP® system. IgE 0.35 kU/L or greater was considered positive. General atopy was defined as a positive response to one or more allergens.
Asthma severity
Three measures of asthma severity were calculated. A 5-category severity score based on both symptom frequency (including night symptoms) and medication use (Global Initiatives for Asthma [GINA] guidelines (United States Department of Health and Human Services February 2002)) was assigned for each month of follow-up, then an annual arithmetic mean was calculated. Scores ranged from 0 (no symptoms or medication) to 4 (severe persistent asthma) (see Table 1). We defined, a priori, two additional measures of annual asthma severity: 30 or more days of wheeze, and controller medication use for 9 or more months.
Table 1.
Personal characteristics and asthma severity for children participating in a year-long prospective study (N = 300). CT, western MA, 2000 – 2004.
| Asthma severity during 12 month study period | |||||||
|---|---|---|---|---|---|---|---|
| GINA score* (mean monthly) | Wheeze (total days) | Controller medication use (total months) | |||||
| Variables | N (%) | Moderate/Severe (%) | p-value† | ≥30 days (%) | p-value† | ≥9 months (%) | p-value† |
| All subjects | 300 | 98 (32.7) | 53 (17.7) | 125 (41.7) | |||
| Gender | |||||||
| Male | 191 (63.7) | 33.0 | 0.88 | 17.3 | 0.81 | 42.9 | 0.55 |
| Female | 109 (36.3) | 32.1 | 18.4 | 39.4 | |||
| Age (yrs) | |||||||
| 4 to < 8 | 124 (41.3) | 30.6 | 0.53 | 13.7 | 0.13 | 37.1 | 0.18 |
| 8 to 12 | 176 (58.7) | 34.1 | 20.4 | 44.9 | |||
| Ethnicity | |||||||
| White(190)/Asian(3)/Other(6) | 199 (66.3) | 36.2 | 0.17 | 17.6 | 0.99 | 44.2 | 0.41 |
| Black | 46 (15.3) | 28.3 | 17.4 | 39.1 | |||
| Hispanic | 55 (18.3) | 23.6 | 18.2 | 34.6 | |||
| Maternal education (yrs) | |||||||
| < 12 | 23 (7.7) | 39.1 | 0.49 | 39.1 | 0.02 | 52.2 | 0.36 |
| 12 – 15 | 162 (54.2) | 29.6 | 14.8 | 38.3 | |||
| ≥16 | 114 (33.1) | 35.1 | 17.5 | 43.9 | |||
| Exposed to smoking | |||||||
| No | 274 (92.6) | 32.8 | 0.59 | 17.5 | 0.64 | 42.3 | 0.33 |
| Yes | 22 (7.4) | 27.3 | 13.6 | 31.8 | |||
| General atopy‡ | |||||||
| No | 111 (37.0) | 21.6 | 0.002 | 9.9 | 0.007 | 26.1 | 0.0001 |
| Yes | 189 (63.0) | 39.2 | 22.2 | 50.8 | |||
| Asthma severity (GINA score*) | |||||||
| 0 No symptoms/medication | 9 (3.0) | 0 | 0.0001 | 0 | 0.0001 | ||
| 1 Intermittent | 135 (45.0) | 3.7 | 1.5 | ||||
| 2 Mild persistent | 58 (19.3) | 24.1 | 48.3 | ||||
| 3 Moderate persistent | 61 (20.3) | 26.2 | 95.1 | ||||
| 4 Severe persistent | 37 (12.3) | 48.6 | 100 | ||||
| Wheeze (days per year) | |||||||
| None | 70 (23.3) | 12.9 | 0.0001 | 15.7 | 0.0001 | ||
| 1 – 7 | 71 (23.7) | 18.3 | 32.4 | ||||
| 8 – 14 | 50 (16.7) | 32.0 | 40.0 | ||||
| 15 – 21 | 29 (9.7) | 55.2 | 69.0 | ||||
| 22 – 29 | 27 (9.0) | 37.0 | 37.0 | ||||
| 30 or more | 53 (17.7) | 64.2 | 77.4 | ||||
| Controller medication (mos per year) | |||||||
| None | 35 (11.7) | 0 | 0.0001 | 0 | 0.0001 | ||
| 1 – 2 | 48 (16.0) | 2.1 | 2.1 | ||||
| 3 – 5 | 49 (16.3) | 0.0 | 6.1 | ||||
| 6 – 8 | 43 (14.3) | 4.6 | 18.6 | ||||
| 9 or more | 125 (41.7) | 76.0 | 32.8 | ||||
A 5-point asthma severity score (from 0 [no symptoms or medication use] to 4 [severe persistent]), based on the Global Initiative for Asthma (GINA) guidelines (United States Department of Health and Human Services February 2002) was calculated for each month of the year-long prospective study. The mean severity was the arithmetic mean of the 12 monthly severity scores.
p-values from χ2 tests. Significant p-values (< 0.05) shown in bold.
Atopy defined as a positive response to one or more of the panel of 10-allergens tested: dust mite (Der f 1, Der p 1), cat (Fel d 1), cockroach (Bla g 1), dog (Can f 1), Alternaria, mouse, grass, ragweed, egg.
Data analysis
Unadjusted associations between asthma severity and other variables were examined with χ2 analyses and Cochran-Armitage test for linear trend (for allergen level categorized a priori (Ingram et al. 1995;Leaderer et al. 2002;Platts-Mills et al. 1997)). SAS version 9 (Cary, NC) was used for all analyses. Spearman correlations between allergens were calculated (10×10 matrix produced 100 comparisons, Bonferroni-corrected α = 0.0005). Logistic regression models simultaneously included dust mite (Der p 1 or Der f 1), Fel d 1, Can f 1 and Bla g 1. Final models included dust mite and cockroach allergens as categorical variables with four and two concentration levels, respectively; pet allergens as binary variables according to specific sensitization and exposure (sensitized AND exposure above detection vs. non-sensitized OR exposure below detection). Models were adjusted for age, gender, general atopy, ethnicity and mother’s education.
Results
Mean (SD) age at enrollment was 8.6 (2.0) years. Boys outnumbered girls in the study by nearly 2 to 1. During the year of follow-up, equal percentages of each gender experienced moderate to severe asthma (one-third), wheezed for 30 or more days (nearly one-fifth), and used asthma controller medication for 9 or more months (over one-third) (Table 1). There were no significant differences in age, ethnic distribution or exposure to smoking within the three measures of asthma severity (Table 1). Maternal education was modestly associated with amount of child’s wheezing (p < 0.02): however this could be a chance finding due to the many associations examined. Importantly, atopic children were approximately twice as likely to experience each outcome and the associations for all three outcomes were very strong (Table 1).
The distribution of allergen levels found in the main living areas and the beds is shown in Table 2. Three-quarters of all samples had measurable levels of pet allergen: Fel d 1 allergen concentrations of 8 μg/g or more were found in 23% of both main room and bed samples; Can f 1 allergen concentrations of ≥ 10 μg/g were found in 32% of the main room and 31% of the bed samples. Der f 1 allergen was the more common of the two dust mite allergens and was found in 59% of the main room and 71% of the bed samples with 18% and 16%, respectively, at levels of 10 μg/g or more. Der p 1 allergen was found in 41% of both main room and bed samples with 12% of the main room and 9% of the bed samples at levels of 10 μg/g or more. Cockroach allergen (Bla g 1) was found in the fewest homes: only 14% of the main room samples (2% were at levels ≥ 10 Ug/g) and 11% of the bed samples (1% at levels ≥ 10 Ug/g). For each allergen, levels measured in the main room were significantly correlated with levels measured in the child’s bed with Spearman r-values below 0.7 for Der p 1 (r=0.6) and Der f 1 (r= 0.4); and above 0.7 for Can f 1, Fel d 1 and Bla g 1.
Table 2.
Distribution of allergens measured in dust samples from main living areas and beds in homes of asthmatic children.* CT, western MA, 2000–2004.
| Allergens | Main living area N (%) | Bed N (%) | Main room and bed Correlation† |
|---|---|---|---|
| Der p (μg/g) | 0.60 | ||
| < 0.10 | 177 (59.0) | 172 (58.3) | |
| 0.10 to < 2.0 | 46 (15.3) | 51 (17.3) | |
| 2.0 to < 10.0 | 42 (14.0) | 46 (15.6) | |
| ≥ 10.0 | 35 (11.7) | 26 (8.8) | |
| Der f (μg/g) | 0.44 | ||
| < 0.10 | 123 (41.0) | 85 (28.8) | |
| 0.10 to < 2.0 | 69 (23.0) | 82 (27.8) | |
| 2.0 to < 10.0 | 55 (18.3) | 80 (27.1) | |
| ≥ 10.0 | 53 (17.7) | 48 (16.3) | |
| Fel d (μg/g) | 0.71 | ||
| < 0.12 | 82 (27.4) | 79 (26.8) | |
| 0.12 to < 2.0 | 109 (36.4) | 116 (39.3) | |
| 2.0 to < 8.0 | 38 (12.7) | 33 (11.2) | |
| ≥ 8.0 | 70 (23.4) | 67 (22.7) | |
| Can f (μg/g) | 0.79 | ||
| < 0.12 | 64 (21.4) | 61 (20.7) | |
| 0.12 to < 2.0 | 88 (29.4) | 87 (29.5) | |
| 2.0 to < 10.0 | 52 (17.4) | 56 (19.0) | |
| ≥ 10.0 | 95 (31.8) | 91 (30.8) | |
| Bla g I (Ug/g) | |||
| < 0.60 | 259 (86.3) | 261 (88.8) | 0.76 |
| 0.60 to < 2.0 | 29 (9.7) | 22 (7.5) | |
| 2.0 to < 10.0 | 6 (2.0) | 8 (2.7) | |
| ≥ 10.0 | 6 (2.0) | 3 (1.0) |
N=300 children lived for 7 or more months in the home where the dust was sampled.
Spearman correlations, p-values < 0.0001.
Correlations among different allergens were moderately positive (r=0.3 to 0.4) for dust mite allergens Der p 1 and Der f 1, and among either house dust mite or either pet allergen. Correlations between cat and dog allergens ranged from 0.2 to 0.4 within and between measurement locations. Bla g 1 allergen was significantly negatively correlated with Can f 1 (in the main room, r = −0.2) and Fel d 1 (in the bed, r = −0.2). Bla g 1 was not significantly correlated to either dust mite allergen.
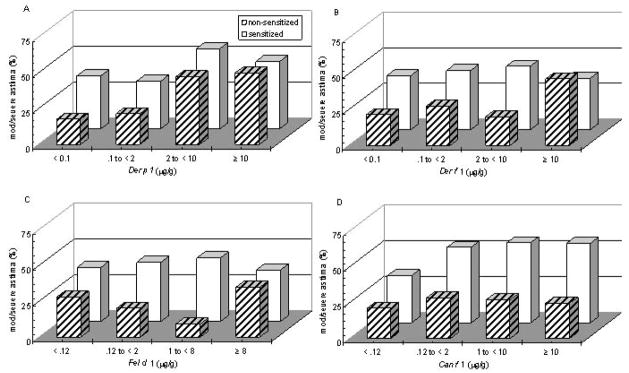
Unadjusted associations between asthma severity measured with the GINA score and dust mite and pet allergens measured in the main rooms in homes of children with (≥ 0.35 kU/L) and without (<0.35 kU/L) serum-specific IgE sensitivities are displayed in Figure 1. For children with positive IgE responses to each specific allergen, there appears to be a relationship between that allergen level and asthma severity, but it does not appear to be strongly linear for any allergen. Levels above 2 μg/g for Der p 1 (Fig. 1A), and any detectable amount of either pet allergen (Fig. 1C, 1D) are associated with more severe asthma. For subjects without a positive IgE response to a specific allergen, a linear trend between level of allergen and asthma severity is particularly striking for Der p 1 (p=0.0003) (Fig 1A). Among non-sensitized subjects, there is an association, but not linear, between moderate to severe asthma and the highest allergen levels (≥ 10 μg/g or ≥ 8 μg/g, respectively) for Der f 1 (p=.03) (Fig. 1B) and Fel d 1 (Fig. 1C). For non-sensitized children there appears to be no association between level of Can f 1 (Fig. 1D) or Bla g 1 and asthma severity.
Figure 1.
Der p 1 (A), Der f 1 (B), Fel d 1 (C) and Can f 1 (D) allergen levels measured in the main living area in homes of children sensitized (specific IgE ≥ .35 kU/L, solid white squares) and non-sensitized (specific IgE ≥ .35 kU/L, striped squares) and their association with moderate to severe GINA asthma severity scores (United States Department of Health and Human Services February 2002). The increase in percentage of children with severe asthma with increases in allergen level was significant for Der p 1 (A) regardless of sensitization (p < 0.0001, Cochran-Armitage test for linear trend), but was most striking for those non-sensitized (p < 0.0003).
Preliminary regression models simultaneously included house dust allergen levels as main effects and as interactions with specific allergic status. The main effect of house dust mite allergen (Der p 1) was significant in the main room model for GINA score (p=0.01) and medication use (p=0.04), indicating significant associations between measured level of Der p 1 and asthma severity. Neither of the house dust mite and dust mite specific allergy interaction terms nor the measures of association with asthma severity for either pet allergen were significant. Interaction terms were significant for dog allergen and marginally significant for cat allergen. None of the main effects or interaction terms was significant for cockroach allergen.
Results of the final models for the main living area and bed including Der p 1 as the dust mite allergen variable are shown in Table 3. All models included all allergens and were adjusted for age, gender, general atopy (which, by definition, includes specific IgE responses to any one or more of all allergens tested), ethnicity and mother’s education. Children exposed in the main room to levels of Der p 1 allergen above 2.0 μg/g were two to three times more likely to have moderate to severe GINA severity scores and increased likelihood of 9 or more months of medication use compared to children living in homes with undetectable levels, regardless of Der p 1 specific sensitization. Of the two pet allergens, the most consistent results were observed for Can f 1: children were two to nearly three times as likely to suffer from severe asthma, no matter how severity was measured, if they were living in homes with detectable levels of dog allergen and they tested positive for a specific allergy to Can f 1. Regression results for the main room levels of dog allergen with Der f 1 allergen included in the models in place of Der p 1 (data not shown) were similar to those shown in Table 3 (for GINA score OR 2.21, 95% CI 1.09, 4.47; for wheeze OR 2.65, 95% CI 1.15, 6.09; and for medication use OR 2.52, 95% CI 1.24, 5.08). Significant associations were also observed between cat allergen (Fel d 1) in the main room and GINA score with Der p 1 allergen in the model (Table 3) or with Der f 1 allergen in the model (OR 2.18, 95% CI 1.09, 4.35). There were no significant associations between levels of Der f 1 or Bla g 1 allergen measured in the main room and measures of asthma severity.
Table 3.
Adjusted odds ratios (OR) from logistic regression models relating asthma severity to dust allergens in homes of asthmatic children*. CT, western MA, 2000–2004.
| Asthma severity during 12 month study period |
|||
|---|---|---|---|
| Allergens | Moderate/Severe GINA score OR (95% CI) | Wheeze ≥ 30 days OR (95% CI) | Controller meds ≥ 9 mos OR (95% CI) |
| Main living area | |||
| Der p 1 (μg/g) | |||
| <0.10 | 1.0 | 1.0 | 1.0 |
| 0.10 to < 2.0 | 0.93 (0.41, 2.10) | 1.05 (0.38, 2.84) | 0.61 (0.27, 1.35) |
| 2.0 to < 10.0 | 2.93 (1.37, 6.30) | 1.55 (0.62, 3.85) | 2.52 (1.17, 5.42) |
| ≥ 10.0 | 2.55 (1.13, 5.73) | 2.01 (0.78, 5.19) | 2.17 (0.97, 4.86) |
| Can f 1 Exposure/Sensitization | |||
| Below detection OR allergy absent | 1.0 | 1.0 | 1.0 |
| Above detection AND allergy present | 2.06 (1.01, 4.22) | 2.68 (1.17, 6.12) | 2.43 (1.18, 5.00) |
| Fel d 1 Exposure/Sensitization | |||
| Below detection OR allergy absent | 1.0 | 1.0 | 1.0 |
| Above detection AND allergy present | 2.41 (1.19, 4.89) | 0.99 (0.44, 2.25) | 1.76 (0.87, 3.55) |
| Bla g 1(Ug/g) | |||
| < 0.60 | 1.0 | 1.0 | 1.0 |
| ≥ 0.60 | 1.53 (0.70, 3.34) | 1.17 (0.47, 2.92) | 1.65 (0.77, 3.55) |
| Bed | |||
| Der p 1 (μg/g) | |||
| < 0.10 | 1.0 | 1.0 | 1.0 |
| 0.10 to < 2.0 | 0.99 (0.47, 2.08) | 1.70 (0.68, 4.22) | 1.35 (0.66, 2.73) |
| 2.0 to < 10.0 | 2.73 (1.32, 5.64) | 1.60 (0.64, 4.00) | 2.16 (1.04, 4.48) |
| ≥ 10.0 | 1.19 (0.46, 3.08) | 3.58 (1.28, 9.97) | 1.41 (0.57, 3.46) |
| Can f 1 Exposure/Sensitization | |||
| Below detection OR allergy absent | 1.0 | 1.0 | 1.0 |
| Above detection AND allergy present | 1.84 (0.92, 3.68) | 1.56 (0.69, 3.53) | 2.00 (1.00, 3.98) |
| Fel d 1 Exposure/Sensitization | |||
| Below detection OR allergy absent | 1.0 | 1.0 | 1.0 |
| Above detection AND allergy present | 1.39 (0.71, 2.75) | 1.62 (0.72, 3.66) | 1.29 (0.66, 2.52) |
| Bla g 1 (Ug/g) | |||
| < 0.60 | 1.0 | 1.0 | 1.0 |
| ≥ 0.60 | 0.87 (0.36, 2.11) | 0.47 (0.14, 1.52) | 0.76 (0.33, 1.79) |
Models included all allergens shown here and were adjusted for age, gender, atopy, ethnicity and mother’s education. Separate analyses were performed for each sampled area (main room or bed) and each of the three severity measures. Significant results (p < 0.05) shown in bold.
Results for models using allergen samples from the child’s bed were similar: elevated levels of Der p 1 allergen were associated with all three asthma severity measures. Children exposed and allergic to Can f 1 were twice as likely to have used controller medication for 9 or more months (Table 3). No significant associations were observed between Fel d 1, Der f 1 or Bla g 1 allergen levels measured in the child’s bed and measures of asthma severity.
Discussion
In our study of five common indoor allergens, children were over twice as likely to have moderate to severe asthma in homes with Der p 1 allergen levels of 2 μg/g or more compared to children in homes with undetectable levels in logistic regression models that also included allergen levels for cat (Fel d 1), dog (Can f 1) and cockroach (Bla g 1) (Table 3). This association did not depend on specific IgE response to Der p 1, which indicates a possible non- allergic component of its mechanism of action. Only children exposed to cat or dog allergen who also had specific IgE responses to cat or dog were at increased risk of severe asthma. No significant associations were found between severe asthma and levels of cockroach allergen (Bla g 1) or house dust mite allergen Der f 1 measured in the main living area or child’s bed although Bla g 1 exposures were relatively uncommon in this cohort.
Study strengths include our extensive assessment of symptoms and medication use, the large number of children for whom specific allergy test results were available, and consideration of other major allergen exposures in the same model. A recent population-based study of multiple allergens in US housing found that most of the 831 units surveyed had detectable levels of at least six of seven measured allergens, and concluded that studies that focus on a single exposure are likely confounded by the presence of other allergens (Salo et al. 2007).
Of many indoor dust allergens with potentially potent health effects, we limited the focus of our study to six of the most common in urban and suburban households in the Northeastern US, namely, dust mites (Der p 1 and Der f 1), cat, dog and cockroach, for which assays are readily available. A potential limitation of this study is our one-time measurement of dust levels in homes. However, the seasonal variability within homes in our region has been shown to be far out-weighed by the variability between homes due to housing type and other household characteristics (Chew et al. 1999;Leaderer et al. 2002;van Strien et al. 2004). Although repeated measurements might provide more accurate estimates of annual exposures, levels measured at one time are likely to be representative of exposure magnitude over the course of a year. It is possible that the very low number of homes with detectable cockroach allergen and relatively low number with high levels of dust mites limited our ability to detect interactions between exposure and sensitization status. It is also possible that some families may have been engaged in intervention strategies including attempted amelioration of allergen levels and pet management during the symptom follow-up year after the enrollment visit when dust samples were taken. Nonetheless, if this had occurred, it was not sufficient to prevent the associations observed in the study.
Another potential limitation is the reliance on mothers’ reports of study subjects’ daily symptoms and medication use. However, each mother was provided with a calendar and instructed to record her child’s asthma symptoms and medications each day. Recall error was further minimized with frequent (monthly) phone interviews to collect symptom and medication use data from the preceding month.
Consideration of household allergens in multivariate models
Two recent studies have examined simultaneous risk of allergen exposure in adults (Blanc et al. 2005;Gehring et al. 2001) but unlike our analysis, neither accounted for potential interaction of antigen and specific allergy. Blanc et al. (Blanc et al. 2005) used multivariate models to predict asthma severity (N=199), and included many potential risk factors: amount of dust collected, dog or cat ownership, specific allergy to dog or cat, and dog and cat allergen concentration, as well as variables for endotoxin level, general atopy, and housing characteristics. Dog ownership was related to more severe asthma, while level of dog antigen was related to less severe asthma, but there were no terms in the model to account for potential interactions between antigen level and specific allergy. Gehring et al., (Gehring et al. 2001) examined the association of respiratory symptoms to household Der p 1, Der f 1, and Fel d 1 allergen levels. The authors found that subjects (N=405) exposed to high concentrations (over 10 μg/g for either dust mite allergen, over 8 μg/g for cat allergen) of at least two of the three allergens studied, had up to a seven-fold increase in the risk of respiratory symptoms compared to nonexposed subjects (Gehring et al. 2001).
Atopy, household allergen exposure and asthma severity
We found that children with atopy were significantly more likely to have more severe asthma compared to children without atopy (Table 1 and Fig. 1), which is consistent with many other studies of asthma in children or adults (Al-Mousawi et al. 2004;Anonymous 1992;Erwin et al. 2005;Noertjojo et al. 1999;Sporik et al. 1999). Our finding that Der p 1 allergen at levels above 2 μg/g is associated with asthma severity regardless of sensitization status is consistent with Zock et al. (Zock et al. 1994), who found a positive association between asthma severity, measured as symptom frequency, and Der p 1 levels measured in bedroom floor dust: for all subjects (N=228 asthmatic children), regardless of dust mite allergic status, an increase in Der p 1 from 2 to 10 μg/g was associated with a 25% increase in shortness of breath (p < 0.05) (Zock et al. 1994). In our models, Der p 1 remained an independent risk factor for asthma severity even after accounting for pet allergen exposure and sensitization. These epidemiological findings continue to suggest that exposure to dust mite antigen, in particular, is an important environmental factor in the severity of asthma. These findings are also consistent with a recent investigation of cytokine production from human eosinophils where house dust mite extract (specifically, Der p 1) induced interleukin-9 expression from eosinophils from sensitized as well as non-sensitized donors (Fujisawa et al. 2008). Their conclusion was that accumulated eosinophils in the airways in asthma may directly react with house dust mite allergen and produce interleukin-9 to further promote Th2-type immune responses (Fujisawa et al. 2008).
Nitschke et al. (Nitschke et al. 2006) found that levels of Der p 1 of 10 μg/g or greater measured in the beds of mite-sensitized subjects (N=88 of 174 asthmatic children studied) were associated with wheeze at night, cough during the day, and daytime asthma attacks. The authors did not report results from subjects who were not mite sensitive, but mean concentration in their beds was significantly lower than in beds of mite sensitized subjects. Mite levels observed in this Australian study were much higher than in our study: 99% of the homes had measurable levels of Der p 1, and one-fourth were exposed to levels of 10 μg/g or greater compared to 41% detectable and 12% above 10 μg/g in our study. We did not find a significant interaction between mite level and allergic status, i.e., our subjects were affected by Der p 1 concentration regardless of sensitization.
The most striking results from Gruchalla et al. (Gruchalla et al. 2005), who followed 234 inner city children with asthma in the US for two years, were for exposure to cockroach allergen (Bla g 1): 39.5% of the subjects were exposed, and subjects who were both exposed to high levels (over 2 U/g) and sensitized had the most symptoms, missed the most school, and had a significantly higher rate of unscheduled health care visits compared to all other subjects. Our subjects came from a variety of neighborhood environments including but not limited to inner city families. The number of our subjects exposed to levels of allergen Bla g 1 above 2 U/g was very small (4%), which may have contributed to the fact that we did not observe an association with asthma severity.
Our findings are consistent with some studies enrolling asthmatic adults (Bjornsson et al. 1995;Lewis et al. 2002;Tunnicliffe et al. 1999). Bjornsson et al. (Bjornsson et al. 1995) found that asthma-related symptoms in the previous year (from 88 adults) were significantly associated with the presence of house dust mite allergen (species not specified). Tunnicliffe et al. (Tunnicliffe et al. 1999) enrolled 54 asthmatic adults and found that compared to mild asthmatics, severe asthmatics had significantly more allergies to house dust mite (Der p 1), cat (Fel d 1), and/or dog (Can f 1). They also found that with the exception of Fel d 1, severe asthmatics were exposed to significantly higher levels of the allergens to which they were sensitized (Tunnicliffe et al. 1999). Lewis et al. (Lewis et al. 2002) found that women (N=140 followed for one year) both sensitized and exposed to cat allergen (Fel d 1 at levels 8 μg/g or greater) were more than twice as likely to use steroid medication and nearly seven times more likely to wheeze without a cold compared to asthmatic women who were either not sensitized or in low exposure homes (Fel d 1 less than 8 μg/g), or both. We also found significant associations with cat allergen among those sensitized, but at lower levels (any detectable allergen).
Further health implications of chronic exposure to high levels of household allergens: Risk of reduced lung function, respiratory viral infections and hospitalization
For asthmatic children and adults, chronic exposure to household allergens is associated not only with risk of respiratory symptoms and increased medication use, but is also associated with increased risk of impaired lung function (Langley et al. 2005), increased risk of respiratory viral infections and hospitalization for asthma (Bjornsson et al. 1995;Lewis et al. 2002;Tunnicliffe et al. 1999). Langley et al. (Langley et al. 2005) found significantly reduced lung function and more severe bronchial reactivity among adults (N=248) exposed to high household levels of dust mite allergen or dog allergen compared to those exposed to low levels (Langley et al. 2005). The authors did not observe increased bronchial reactivity among subjects exposed to high levels of cat allergen. As in our study, these results suggest that chronic exposure to high levels of house dust mite can have an adverse effect on respiratory health of asthmatics independent of specific allergic status, but that high levels of cat allergen will not affect asthmatics who are not sensitized to this allergen. Unlike our study, Langley et al. (Langley et al. 2005) also found that exposure to high levels of dog allergen in non-sensitized asthmatics is associated with respiratory effects. Interestingly, a recent community-based study of adults found no association between bronchial reactivity and exposure to high levels of cat allergen among subjects with specific IgE sensitivity to Fel d 1, but did find a significant increase in bronchial reactivity with increased exposure to cat allergen among subjects with positive IgE responses to any of four allergens tested, including cat (Chinn et al. 2007).
Asthma severity and reduction of household dust allergens
In some studies where reduction in household allergen levels has been measured, aggressive intervention efforts have been associated with modest, yet significant, improvements in respiratory health status (Carswell et al. 1996;Kercsmar et al. 2006;Lee 2003;Morgan et al. 2004), but not always (Erwin et al. 2005;Gotzsche et al. 2004). Such disparity in intervention results suggests that greater reductions in mite allergen exposures might be necessary in order to measure health effects, or that interactions with other home environmental exposures may interfere with any health improvements due to mite reduction. Given our finding that asthmatic children chronically exposed to levels of Der p 1 allergenof 2.0 μg/g or more have more severe disease and that Der p 1 allergen remains an independent risk factor for disease severity, regardless of atopic status and even after accounting for pet allergen exposures, our results suggest that disease severity could be reduced in all asthmatic children by reduction of dust mite levels.
Acknowledgments
Funding source: Funded by NIH: ES011013, ES05410, ES01247
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Human Investigation Committee Approval: The Human Investigation Committee of Yale University School of Medicine reviewed and approved this research (HIC Protocol number 0004011756).
References
- Al-Mousawi MSH, Lovel H, Behbehani N, Arifhodzic N, Woodcock A, Custovic A. Asthma and sensitization in a community with low indoor allergen levels and low pet- keeping frequency. J Allergy Clin Immunol. 2004;114:1389–1394. doi: 10.1016/j.jaci.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Anonymous. Dust mite allergens and asthma: Report of a second international workshop. J Allergy Clin Immunol. 1992;89(5):1046–1060. doi: 10.1016/0091-6749(92)90228-t. [DOI] [PubMed] [Google Scholar]
- Belanger K, Beckett W, Triche E, Bracken MB, Holford T, Ren P, et al. Symptoms of wheeze and persistent cough in the first year of life: Associations with indoor allergens, air contaminants and maternal history of asthma. Am J Epidemiol. 2003;158:195–202. doi: 10.1093/aje/kwg148. [DOI] [PubMed] [Google Scholar]
- Bjornsson E, Norback D, Janson C, Widstrom J, Palmgren U, Strom G, et al. Asthmatic symptoms and indoor levels of micro-organisms and house dust mites. Clin Exper Allergy. 1995;25:423–431. doi: 10.1111/j.1365-2222.1995.tb01073.x. [DOI] [PubMed] [Google Scholar]
- Blanc PD, Eisner MD, Katz PP, Yen IH, Archea C, Earnest G, et al. Impact of the home indoor environment on adult asthma and rhinitis. J Occup Environ Med. 2005;47:362–372. doi: 10.1097/01.jom.0000158708.32491.9d. [DOI] [PubMed] [Google Scholar]
- Bollinger ME, Eggleston PA, Flanagan E, Wood RA. Cat antigen in homes with and without cats may induce allergic symptoms. J Allergy Clin Immunol. 1996;97(4):907–914. doi: 10.1016/s0091-6749(96)80064-9. [DOI] [PubMed] [Google Scholar]
- Carswell F, Birmingham K, Oliver J, Crewes A, Weeks J. The respiratory effects of reduction of mite allergen in the bedrooms of asthmatic children - a double-blind controlled trial. Clin Exp Allergy. 1996;26:386–396. [PubMed] [Google Scholar]
- Chew GL, Higgins KM, Gold DR, Muilenberg ML, Burge HA. Monthly measurements of indoor allergens and the influence of housing type in a northeastern US city. Allergy. 1999;54:1058–1066. doi: 10.1034/j.1398-9995.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- Chinn S, Heinrich J, Anto JM, Janson C, Norback D, Olivieri M, et al. Bronchial responsiveness in atopic adults increases with exposure to cat allergen. Am J Respir Crit Care Med. 2007;176:20–26. doi: 10.1164/rccm.200612-1840OC. [DOI] [PubMed] [Google Scholar]
- Dharmage S, Bailey M, Raven J, Cheng A, Rolland J, Thien F, et al. Residential characteristics influence Der p 1 levels in homes in Melbourne, Australia. Clin Exp Allergy. 1999;29:461–469. doi: 10.1046/j.1365-2222.1999.00513.x. [DOI] [PubMed] [Google Scholar]
- Erwin EA, Wickens K, Curtis NJ, Siebers R, Woodfolk J, Barry D, et al. Cat and dust mite sensitivity and tolerance in relation to wheezing among children raised with high exposure to both allergens. J Allergy Clin Immunol. 2005;115:74–79. doi: 10.1016/j.jaci.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Fujisawa T, Katsumata H, Kato Y. House dust mite extract induces interleukin-9 expression in human eosinophils. Allergology International. 2008;57:141–146. doi: 10.2332/allergolint.O-07-498. [DOI] [PubMed] [Google Scholar]
- Gehring U, Heinrich J, Jacob B, Richter K, Fahlbusch B, Schlenvoigt G, et al. Respiratory symptoms in relation to indoor exposure to mite and cat allergens and endotoxins. Eur Respir J. 2001;18:555–563. doi: 10.1183/09031936.01.00096801. [DOI] [PubMed] [Google Scholar]
- Gehring U, Triche E, van Strien RT, Belanger K, Holford T, Gold DR, et al. Prediction of residential pet and cockroach allergen levels using questionnaire information. Environ Health Perspect. 2004;112(8):834–839. doi: 10.1289/ehp.6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gent J, Triche E, Holford T, Belanger K, Bracken M, Beckett W, et al. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA. 2003;290:1859–1867. doi: 10.1001/jama.290.14.1859. [DOI] [PubMed] [Google Scholar]
- Gotzsche PC, Johansen HK, Schmidt LM, Burr ML. House dust mite control measures for asthma (Review) Cochrane Database of Systematic Reviews. 2004;(4):CD001187. doi: 10.1002/14651858.CD001187.pub2. [DOI] [PubMed] [Google Scholar]
- Gruchalla RS, Pongracic J, Plaut M, Evans R, III, Visness CM, Walter M, et al. Inner city asthma study: Relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115:478–485. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Heinrich J, Holscher B, Douwes J, Richter K, Koch A, Bischof W, et al. Reproducibility of allergen, endotoxin and fungi measurements in the indoor environment. J Exp Anal Environ Epidemiol. 2003;13:152–160. doi: 10.1038/sj.jea.7500267. [DOI] [PubMed] [Google Scholar]
- Ingram JM, Sporik R, Rose G, Honsinger R, Chapman MD, Platts-Mills TAE. Quantitative assessment of exposure to dog (Can f 1) and cat (Fel d 1) allergens: Relation to sensitization and asthma among children living in Los Alamos, New Mexico. J Allergy Clin Immunol. 1995;96(4):449–456. doi: 10.1016/s0091-6749(95)70286-5. [DOI] [PubMed] [Google Scholar]
- Janson C, Anto J, Burney P, Chinn S, de Marco R, Heinrich J, et al. The European Community Respiratory Health Survey: what are the main results so far? Eur Respir J. 2001;18:598–611. doi: 10.1183/09031936.01.00205801. [DOI] [PubMed] [Google Scholar]
- Kercsmar CM, Dearborn DG, Schluchter M, Xue L, Kirchner HL, Sobolewski J, et al. Reduction in asthma morbidity in children as a result of home remediation aimed at moisture sources. Environ Health Perspect. 2006;114:1574–1580. doi: 10.1289/ehp.8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley SJ, Goldthorpe S, Craven M, Woodcock A, Custovic A. Relationship between exposure to domestic allergens and bronchial hyperresponsiveness in non-sensitised, atopic asthmatic subjects. Thorax. 2005;60:17–21. doi: 10.1136/thx.2004.027839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaderer BP, Belanger K, Triche E, Holford T, Gold DR, Kim Y, et al. Dust mite, cockroach, cat, and dog allergen concentrations in homes of asthmatic children in Northeastern United States: Impact of socioeconomic factors and population density. Environ Health Perspect. 2002;110:419–425. doi: 10.1289/ehp.02110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I-S. Effect of bedding control on amount of house dust mite allergens, asthma symptoms, and peak expiratory flow rate. Yonsei Med J. 2003;44(2):313–322. doi: 10.3349/ymj.2003.44.2.313. [DOI] [PubMed] [Google Scholar]
- Lewis SA, Weiss ST, Platts-Mills TAE, Burge H, Gold DR. The role of indoor allergen sensitization and exposure in causing morbidity in women with asthma. Am J Respir Crit Care Med. 2002;165:961–966. doi: 10.1164/ajrccm.165.7.2103044. [DOI] [PubMed] [Google Scholar]
- Marks GB, Tovey ER, Toelle BG, Wachinger S, Peat JK, Woolcock AJ. Mite allergen (Der p 1) concentration in houses and its relation to the presence and severity of asthma in a population of Sydney schoolchildren. J Allergy Clin Immunol. 1995;96:441–448. doi: 10.1016/s0091-6749(95)70285-7. [DOI] [PubMed] [Google Scholar]
- Morgan WJ, Crain EF, Gruchalla RS, O’Connor GT, Kattan M, Evans R, III, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351:1068–1080. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- Nitschke M, Pilotto LS, Attewell RG, Smith BJ, Pisaniello D, Martin J, et al. A cohort study of indoor nitrogen dioxide and house dust mite exposure in asthmatic children. J Occup Environ Med. 2006;48:462–469. doi: 10.1097/01.jom.0000215802.43229.62. [DOI] [PubMed] [Google Scholar]
- Noertjojo K, Dimich-Ward H, Obata H, Manfreda J, Chan-Yeung MB. Exposure and sensitization to cat dander: Asthma and asthma-like symptoms among adults. J Allergy Clin Immunol. 1999;103:60–65. doi: 10.1016/s0091-6749(99)70526-9. [DOI] [PubMed] [Google Scholar]
- Platts-Mills TAE, Vervloet D, Thomas WR, Aalberse RC, Chapman MD. Indoor allergens and asthma: Report of the Third International Workshop. J Allergy Clin Immunol. 1997;100:S1–S24. doi: 10.1016/s0091-6749(97)70292-6. [DOI] [PubMed] [Google Scholar]
- Salo PM, Arbes SJ, Zeldin DC. Exposure to multiple indoor allergens in US homes: Results from the National Survey of Lead and Allergens. Am J Respir Crit Care Med. 2007;175:A428. [Google Scholar]
- Simpson A, Custovic A, Pipis S, Adisesh A, Faragher B, Woodcock A. Exhaled nitric oxide, sensitization, and exposure to allergens in patients with asthma who are not taking inhaled steroids. Am J Respir Crit Care Med. 1999;160:45–49. doi: 10.1164/ajrccm.160.1.9809091. [DOI] [PubMed] [Google Scholar]
- Sporik R, Squillace SP, Ingram JM, Rakes G, Honsinger RW, Platts-Mills TAE. Mite, cat, and cockroach exposure, allergen sensitisation, and asthma in children: A case-control study of three schools. Thorax. 1999;54:675–680. doi: 10.1136/thx.54.8.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunnicliffe WS, Fletcher TJ, Hammond K, Roberts K, Custovic A, Simpson A, et al. Sensitivity and exposure to indoor allergens in adults with differing asthma severity. Eur Respir J. 1999;13:654–659. doi: 10.1183/09031936.99.13365499. [DOI] [PubMed] [Google Scholar]
- United States Department of Health and Human Services. Global Initiative for Asthma, Global Strategy for Asthma Management and Prevention 02-3659. Washington, DC: National Institutes of Health, National Heart, Lung, and Blood Institute; Feb, 2002. [Google Scholar]
- van Strien RT, Gehring U, Belanger K, Triche E, Gent J, Bracken MB, et al. The influence of air conditioning, humidity, temperature and other household characteristics on mite allergen concentrations in the Northeastern United States. Allergy. 2004;59(6):645–652. doi: 10.1111/j.1398-9995.2004.00470.x. [DOI] [PubMed] [Google Scholar]
- Zock JP, Brunekreef B, Hazebroek-Kampschreur AAJM, Roosjen CW. House dust mite allergen in bedroom floor dust and respiratory health of children with asthmatic symptoms. Eur Respir J. 1994;7:1254–1259. doi: 10.1183/09031936.94.07071254. [DOI] [PubMed] [Google Scholar]
- Zock JP, Heinrich J, Jarvis D, Verlato G, Norback D, Plana E, et al. Distribution and determinants of house dust mite allergens in Europe: The European Community Respiratory Health Survey II. J Allergy Clin Immunol. 2006;118:682–690. doi: 10.1016/j.jaci.2006.04.060. [DOI] [PubMed] [Google Scholar]