Abstract
Background
Monoamine dysfunction, particularly of the serotonin system, has been the dominant hypothesis guiding research and treatment development in affective disorders. The majority of research has been performed in mid-life depressed adults. The importance of understanding the neurobiology of depression in older adults is underscored by increased rates of mortality and completed suicide and an increased risk of Alzheimer's dementia. To evaluate the dynamic response of the serotonin system, the acute effects of citalopram infusion on cerebral glucose metabolism was measured in depressed older adults and control subjects. The hypothesis was tested that smaller decreases in metabolism would be observed in cortical and limbic regions in depressed older adults relative to controls.
Methods
Sixteen depressed older adults and thirteen controls underwent two resting Positron Emission Tomography (PET) studies with the radiotracer [18F]-2-deoxy-2-fluoro-D-glucose after placebo and citalopram infusions.
Results
In controls compared to depressed older adults, greater citalopram induced decreases in cerebral metabolism were observed in the right anterior cingulate, middle temporal (bilaterally), left precuneus, and left parahippocampal gyri. Greater decreases in the depressed older adults than controls was observed in left superior and left middle frontal gyri and increases in left inferior parietal lobule, left cuneus, left thalamus and right putamen.
Conclusion
In depressed older adults relative to controls, the cerebral metabolic response to citalopram is blunted in cortico-cortico and cortico-limbic pathways and increased in the left hemisphere (greater decrease interiorly and increases posterior). These findings suggest both blunted and compensatory cerebral metabolic responses to citalopram in depressed older adults.
Keywords: selective serotonin reuptake inhibitors, citalopram, serotonin, Positron Emission Tomography (PET), glucose metabolism, depression, aging
Introduction
The primary neurobiological hypothesis in major depressive disorder is that of decreased monoaminergic function (norepinephrine, dopamine, and in particular, serotonin; 1,2). The monoamine hypothesis has been a major focus of post-mortem and neuroimaging research. However, these studies have been performed mainly in mid-life depressed patients. The striking decline in monoamine neurotransmission with the normal aging process may underlie the higher rates of relapse and suicidality associated with major depression in late life (3,4,5). Evidence from clinical, neuropsychological, and epidemiological studies demonstrates the substantial impact of depressive symptoms in late life with respect to cognitive impairment, apathy, functional disability, and mortality (6-9). Thus, there is a compelling need to understand the neurobiological substrates of depressive symptoms in late life (10).
To evaluate monoaminergic dysfunction in depression, neuroimaging studies have focused primarily on the serotonin system. Prior studies have measured the cerebral metabolic response to pharmacologic alterations in serotonin, as well as serotonin synthesis rates, serotonin transporter and serotonin receptor (5-HT1A, 5-HT2A) availability. These studies have provided evidence of reduced serotonin synthesis, modest or no reductions in serotonin transporter, 5-HT1A and 5-HT2A receptor availability. Studies of the effects of antidepressant treatment on serotonin receptor availability are controversial. (as reviewed by 10, 11). While not as neurochemically specific as using a serotonergic radiotracer, measures of regional cerebral blood flow (rCBF) or glucose metabolism have consistently demonstrated sensitivity in detecting the effects of illness and treatment that are correlated with severity of depressive symptoms at the time of scanning (12-15). The combination of rCBF or glucose metabolism measures with a pharmacologically selective serotonergic intervention may further enhance the information obtained from these neuroimaging measures, as shown by studies in mid-life depressed adults (16-18). A table summarizing representative studies is provided as supplemental data. As glucose metabolic activity represents the final common pathway of neurochemical activity in the brain, the functional neuroanatomic data would inform subsequent studies to characterize the neurochemical mechanisms underlying the glucose metabolic effect using PET and selective neuroreceptor radiotracers.
The impetus for the present study was that the integration of cerebral glucose metabolism measures with acute, intravenous administration of a pharmacologically selective antidepressant drug would be a unique opportunity to evaluate serotonin modulation of neural circuitry in geriatric depression. Positron Emission Tomography (PET) imaging was employed to measure the acute cerebral metabolic effects of the selective serotonin reuptake inhibitors (SSRI), citalopram (celexa) in depressed older adults and in non-depressed control subjects. In the absence of a radiotracer that could be used to directly measure serotonin function, the methodology used in this study represented the most direct and non-invasive method available to measure serotonergic function in vivo in human subjects (19,20). Prior neuroimaging studies to evaluate the effects of antidepressants in mid-life and depressed older adults, including total sleep deprivation (TSD) and pharmacologic treatments (12-17, 19-22) have shown reductions in cerebral glucose metabolism in anterior cingulate, middle and inferior frontal cortices and precuneus. Increases in inferior parietal lobule, occipital cortex and basal ganglia have been observed, as well (14). As these findings may be attributable in part to an increase in serotonin concentrations, it was hypothesized that acute administration of a selective serotonergic agent, citalopram, would be associated with a reduction in cerebral metabolism in cortical areas, including the anterior cingulate gyrus (BA 24), superior and middle prefrontal and parietal (precuneus) cortices and increases in basal ganglia (putamen) and occipital cortex. The acute decreases in metabolism were hypothesized to be greater in the control subjects compared to the depressed older adults, particularly in cortical and limbic structures (anterior cingulate gyrus, precuneus and parahippocampal gyrus), indicative of decreased functional serotonergic response in depressed older adults. Prolactin concentrations were measured to evaluate the effects of citalopram administration on the serotonin system independently of the glucose metabolism measures. The acute increase in prolactin after a pharmacologic increase in serotonin has been observed in numerous human studies and has been shown in preclinical studies to reflect an activation of post-synaptic, hypothalamic serotonin receptors (5-HT1A, 5-HT2A and 5-HT2C subtypes; as reviewed by 23). Thus, the decreased cerebral metabolic response to citalopram in depressed older adults relative to controls was hypothesized to be accompanied by a decreased neuroendocrine (prolactin) response.
Materials and Methods
Study Design
Depressed older adults and controls underwent two PET scans on two consecutive days to measure the effects of saline/citalopram administration on cerebral glucose metabolism, as described previously (19, 20, 24).
Subject Screening and Selection
Both depressed older adults and control subjects underwent psychiatric evaluation (including a structured clinical interview, [SCID25]), laboratory testing (including CBC and blood chemistry, glucose levels and thyroid function tests), toxicology screening and Magnetic Resonance (MR) imaging scans (GE 1.5T Magnetom Vision) prior to the PET scans. Depressed older adults were recruited from the Geriatric Psychiatry Outpatient Clinic at the Zucker Hillside Hospital and also from advertisements in the community. Control subjects were recruited from advertisements in the community.
Sixteen depressed older adults (six male/ten females, mean age of 65.3±9.1 years) who met DSM-IV criteria for current major depressive episode (non-bipolar, non-psychotic) were enrolled. Thirteen control subjects (five males/eight females, mean age of 67.4±7.4 years) were enrolled who did not meet DSM-IV criteria for current or past Axis I psychiatric disorders in themselves or in their first degree relatives. Depression and anxiety symptoms were assessed by the following scales: Hamilton Depression Rating Scale-24 item [HDRS26], Hamilton Anxiety Rating Scale [HARS27], Beck Depression Inventory [BDI28], and Geriatric Depression Scale [GDS29]). The differences in age between the two groups were not statistically significant (F (1,27)=-0.47, p > 0.5). The controls and depressed older adults did not differ significantly in body weight (controls: 69.1 ± 13.3, depressed older adults: 73.1 ± 17.4; F (1,27)=-0.47, p > 0.5) Subjects were excluded who had a history of or current neurological or other Axis I psychiatric disorders (including substance abuse) or who were not medically stable (including a current diagnosis of insulin dependent diabetes and poorly controlled hypertension) or who had used prescription (including beta blockers), over the counter medications (e.g. antihistamines, cold medications) or herbal supplements with central nervous system effects within the past two weeks. The preliminary results of six of the depressed older adults and five of the controls in the present sample were described in an earlier report (20). The demographic characteristics between these subjects and the subjects recruited subsequently did not differ significantly. Twelve depressed older adults (75%) had never been treated with psychotropic drugs. These subjects experienced the first episode of depression for which medication was prescribed. Of the four depressed older adults previously treated, three depressed older adults were treated with Zoloft prior to study entry (treatment ended from six months to two years prior to study enrollment) and a fourth depressed older adults had been treated with nortriptyline until two weeks prior to the PET scan (at which time the plasma nortriptyline concentrations were undetectable). The clinical, neuroendocrine and neuroimaging data for this patient did not differ significantly from that of the rest of the patient group, despite the relatively recent exposure to nortriptyline. After a complete description of the study to the subjects, written informed consent was obtained according to procedures established by the Institutional Review Board and the Radiation Safety Committee of the North Shore-Long Island Jewish Health System.
Acute Citalopram Intervention
The subjects were scanned on two consecutive days after an infusion of either placebo (250ml of saline) or citalopram (40mg of the drug diluted in 250ml saline) over 60 minutes. The radiotracer was injected approximately 30 minutes after the end of the infusion, the time when the maximal effects on cerebral metabolism and neuroendocrine function have been observed (19). The study was single blind in that the subjects were told that they would receive either citalopram or placebo (saline) prior to each study, but the investigator knew the identity of the infusion. The order of placebo-drug administration was not randomized because if the drug were administered first, there may be carry over effects to the second scan. As pointed out by Kapitany et al. (30), a time interval of at least three weeks would be necessary between placebo and drug conditions due to the known carry-over effects of serotonergic drugs. It would not be possible for ethical reasons to maintain the depressed older adults unmedicated during such a long time interval.
On the days of the infusion studies, serum and plasma samples were obtained at pre-determined intervals (baseline, end of infusion, 15, 30, 60 90, 120 minutes post-infusion) to measure concentrations of citalopram and prolactin, respectively. The plasma samples were analyzed in the Geriatric Psychopharmacology Laboratory, Department of Psychiatry, University of Pittsburgh School of Medicine as described previously (19,20). The drug levels and neuroendocrine data were analyzed using repeated measures analysis of variance with diagnosis (comparison subject/ depressed older adult) as a between subject factor and condition (2 levels: placebo/citalopram) and time (7 levels: pre, end of infusion, 15, 30, 60, 90 and 120 minutes post-infusion) as within subject factors.
PET Imaging Procedures
The PET scans were performed using a GE Advance Tomograph in the Center for Neurosciences, Feinstein Institute for Medical Research, as described previously (19,20) All PET studies began at the same time of day (10 AM). Upon arrival at the PET Facility, one catheter was placed in a vein in the left arm for radiotracer and placebo/citalopram infusion and a second catheter was placed in a vein in the right arm for sampling of citalopram and neuroendocrine levels. Fifteen to thirty minutes after the end of the infusion of placebo/citalopram, 5 mCi of [18F]-2-deoxy-2-fluoro-D-glucose ([18F]-FDG) was injected as an intravenous bolus. During the uptake interval, the subjects sat in a darkened quiet room with eyes open and ears unoccluded. Twenty-five minutes after radiotracer injection, subjects were positioned in the GE Advance Tomograph. A ten-minute transmission scan and a five-minute two-dimensional emission scan were acquired first to perform photon attenuation correction. A three-dimensional emission scan began at 40 minutes after radiotracer injection and lasted for 10 minutes. At the end of the PET scan, the subjects were removed from the PET scanner and the intravenous lines were removed after completion of the blood sampling. The subjects were debriefed as to her/his perception of the study.
Data and Image Analysis
Glucose metabolic rates were calculated (in ml/100g/min) on a pixel by pixel basis according to validated methods (31). PET data processing was performed on the quantitative glucose metabolism images using the statistical parametric mapping program (SPM5, Institute of Neurology, London; 32). This is a data driven analytic approach that performs statistical tests on each voxel in the image. The images were smoothed with an isotropic Gaussian kernel (FWHM 8mm for all directions). The glucose metabolic rates were normalized by scaling to a common mean value (50) across all scans, after establishing that the global means did not differ significantly between groups and conditions (p > 0.05). The data were normalized to a global mean because of the greater test-retest variability for absolute compared to relative glucose metabolism observed in numerous studies (e.g. 33). For the PET data, two different analyses were performed using the flexible factorial option in SPM5. First, a within subject comparison for placebo and citalopram conditions was performed for the control and depressed older adult groups, separately. Secondly, a between group comparison (control subjects versus depressed older adults) of the differences in response (placebo/citalopram) was performed to evaluate whether the acute cerebral metabolic response to citalopram differed between depressed older adults and control subjects. To control for multiple comparisons, a more stringent probability level (p < 0.001) and cluster size (greater than 50 voxels) is used to limit the significant regional differences reported. The comparisons were considered significant at a t threshold greater than 3.51 (z > 2.98, p < 0.001; uncorrected for multiple independent comparisons).
Results
The clinical characteristics of the groups, including the means and standard deviations for depressive and anxiety symptoms and global cognitive function for the controls and depressed older adults are shown in Table 1. The means and standard deviations for the citalopram and prolactin concentrations and the percentage change between placebo and citalopram infusions for the prolactin concentrations are shown in Table 2. For the citalopram concentrations, there was a significant effect of time (F=12.41, df=2,19, p < 0.001), but the effect of group (F=0.17, df=2,19, p > 0.1) and the group by time (F=0.43, df=2,19, p > 0.1) interaction was not-significant. This indicates that the citalopram concentrations and time course did not differ significantly between depressed older adults and controls. The citalopram concentration at the end of infusion was non-significantly higher in the controls than the depressed older adults. However, during the time of the [18F]-FDG radiotracer uptake (30-60 minutes post-end of infusion), the citalopram concentrations were similar between the two groups.
Table 1.
Mood and Anxiety Ratings
| Mean ± Standard Deviation | |||||
|---|---|---|---|---|---|
| Hamilton Depression Rating Scale | Geriatric Depression Scale | Beck Depression Inventory | Hamilton Anxiety Rating Scale | MMSE | |
| Controls | 0.6 ± 0.9 | 1.5 ± 1.6 | 0.55 ± 0.93 | 1.8 ± 2.1 | 28.8 ± 1 |
| Older Depressed Adults | 26.0 ± 3.5 | 20.1 ± 5.2 | 11.8 ± 3.0 | 13.1 ± 5.4 | 28.6 ± 1 |
Table 2.
Plasma Concentrations of Citalopram and Prolactin
| Citalopram Concentrations (ng/mL) Mean ± Standard Deviation | ||||||
|---|---|---|---|---|---|---|
| Controls | Depressed Older Adults | |||||
| End of Infusion (EOI) | 87.8 ± 51.9 | 76.2 ± 33.5 | ||||
| 15 minutes Post-EOI | 56.4 ± 12.0 | 52.5 ± 14.2 | ||||
| 30 minutes Post-EOI | 52.2 ± 15.8 | 51.1 ± 13.3 | ||||
| 60 minutes Post-EOI | 48.3 ± 10.9 | 46.2 ± 13.5 | ||||
| 90 minutes Post-EOI | 46.3 ± 8.4 | 42.5 ± 10.5 | ||||
| 120 minutes Post-EOI | 43.9 ± 11.4 | 44.1 ± 13.1 | ||||
| Prolactin Concentrations (ng/mL) Mean ± Standard Deviation | ||||||
| Controls | Depressed Older Adults | |||||
| Placebo | Citalopram | %Δ | Placebo | Citalopram | %Δ | |
| Pre-Infusion | 8.1 ± 3.2 | 9.0 ± 4.6 | 6.0 ± 25.5 | 7.1 ± 2.4 | 7.8 ± 3.0 | 13.1 ± 50.1 |
| End of Infusion (EOI) | 9.5 ± 4.6 | 16.3 ± 14.9 | 69.5 ± 142.0 | 7.4 ± 4.2 | 12.4 ± 7.6 | 47.3 ± 68.8 |
| 15 minutes Post-EOI | 9.1 ± 3.8 | 14.8 ± 13.7 | 59.8 ± 116.8 | 7.3 ± 3.7 | 11.5 ± 7.8 | 25.3 ± 52.9 |
| 30 minutes Post-EOI | 9.3 ± 3.9 | 15.6 ± 10.6 | 81.5 ± 125.3 | 6.9 ± 2.8 | 11.1 ± 6.1 | 39.8 ± 75.3 |
| 60 minutes Post-EOI | 8.7 ± 3.7 | 14.8 ± 8.5 | 81.5 ± 105.0 | 6.2 ± 2.1 | 10.5 ± 5.2 | 52.5 ± 77.2 |
| 90 minutes Post-EOI | 9.5 ± 4.4 | 14.1 ± 6.9 | 58.6 ± 64.0 | 6.6 ± 2.8 | 10.3 ± 4.6 | 50.6 ± 62.8 |
| 120 minutes Post-EOI | 10.5 ± 4.7 | 13.3 ± 6.3 | 31.5 ± 37.4 | 7.0 ± 2.6 | 11.3 ± 6.8 | 43.9 ± 47.9 |
For the prolactin concentrations, there was a significant effect of condition (F=13.8, df=1, p < 0.001) and time (F=3.9, df=6, p < 0.05), but the main effect of group (F=2.57, df=1,24, p > 0.05), group by condition (F=0.8, df=1,24; p > 0.1), group by time (F=0.573, df=6,144; p > 0.1) and group by condition by time (F=0.6, df=6,144; p > 0.1) interactions were not-significant. Thus, significantly higher prolactin concentrations were associated with citalopram administration. The magnitude of this effect did not differ significantly between depressed older adults and controls. The percentage differences in prolactin concentrations indicate that at most of the time points (except 120 minutes post-infusion), the magnitude change was non-significantly greater for controls than depressed older adults. The between group differences in citalopram or prolactin concentrations were not significantly different when the subjects' weight was included as a covariate in the analysis. The results of the SPM analysis of the cerebral metabolic data are shown in Tables 3A, B, and C. The results of the within group comparisons (citalopram versus placebo) are shown in Table 3A (controls) and 3B (depressed older adults). Table 3C shows the comparison of the citalopram response (citalopram versus placebo conditions) in control subjects relative to depressed older adults
Table 3.
| Table 3A: Cerebral Metabolic Response to Acute Citalopram in Controls [Citalopram – Placebo Conditions] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DECREASE | Left Hemisphere | Right Hemisphere | ||||||||
| x (mm) | y (mm) | z (mm) | Z score | Cluster size | Structure | x (mm) | y (mm) | z (mm) | Z score | Cluster size |
| -1 | -16 | 42 | 2.8 | 1935 | Anterior Cingulate Gyrus | 8 | -32 | 35 | 2.88 | 1935 |
| -2 | 57 | -1 | 3.41 | 889 | Superior Frontal Gyrus | |||||
| -37 | 12 | 52 | 3.15 | 2278 | Middle Frontal Gyrus (BA 6) | |||||
| -55 | 4 | 29 | 2.98 | 1666 | Inferior Frontal Gyrus | |||||
| -51 | -1 | -16 | 3.39 | 3045 | Middle Temporal Gyrus | |||||
| -25 | -9 | -29 | 3.8 | 625 | Parahippocampal gyrus | |||||
| -15 | -30 | 9 | 3.02 | 576 | Thalamus (Pulvinar) | 21 | -32 | 3 | 3.13 | 705 |
| INCREASE | Left Hemisphere | Right Hemisphere | ||||||||
| x (mm) | y (mm) | z (mm) | Z score | Cluster size | Structure | x (mm) | y (mm) | z (mm) | Z score | Cluster size |
| Superior Frontal Gyrus | 30 | 51 | 24 | 3.06 | 5794 | |||||
| Middle Frontal Gyrus (BA 6) | 37 | 11 | 55 | 3.48 | 756 | |||||
| Inferior Frontal Gyrus | 54 | 26 | 8 | 3.03 | 5794 | |||||
| -15 | -67 | 42 | 3 | 2661 | Precuneus | |||||
| -39 | -85 | 23 | 3.1 | 143 | Superior Occipital Gyrus (BA 19) | |||||
| -29 | -33 | -26 | 3.02 | 324 | Cerebellum | 10 | -41 | -17 | 3.07 | 1138 |
| Table 3B: Cerebral Metabolic Response to Acute Citalopram in Depressed Older Adults [Citalopram – Placebo Conditions] | ||||||||||
| DECREASE | Left Hemisphere | Right Hemisphere | ||||||||
| x (mm) | y (mm) | z (mm) | Z score | Cluster size | Structure | x (mm) | y (mm) | z (mm) | Z score | Cluster size |
| Cingulate Gyrus | 5 | -39 | 29 | 3.13 | 1629 | |||||
| -15 | 24 | 48 | 4.21 | 1817 | Superior Frontal Gyrus | 15 | 52 | 18 | 3.10 | 1682 |
| -26 | 49 | 7 | 2.98 | 672 | Middle Frontal Gyrus | 46 | 27 | 30 | 3.17 | 212 |
| Superior Temporal Gyrus | 50 | -26 | 3 | 3.67 | 692 | |||||
| -31 | -19 | -19 | 3.14 | 438 | Parahippocampal Gyrus | 31 | -14 | -15 | 3.05 | 96 |
| INCREASE | Left Hemisphere | Right Hemisphere | ||||||||
| x (mm) | y (mm) | z (mm) | Z score | Cluster size | Structure | x (mm) | y (mm) | z (mm) | Z score | Cluster size |
| -22 | -55 | 61 | 3.16 | 694 | Superior Parietal Lobule | |||||
| -63 | -40 | 27 | 3.59 | 808 | Inferior Parietal Lobule (BA 40) | 5 | -39 | 29 | 3.13 | 1629 |
| Inferior Occipital Gyrus | 34 | -91 | -3 | 3.25 | 577 | |||||
| -6 | -14 | -10 | 3.21 | 553 | Brainstem | |||||
| -24 | -38 | -29 | 3.19 | 1641 | Cerebellum | 8 | -67 | -20 | 3.73 | 1695 |
| Table 3C: Comparison of Cerebral Metabolic Response to Acute Citalopram in Controls versus Depressed Older Adults [Citalopram – Placebo] | ||||||||||
| Greater Decreases in Controls than Depressed Older Adults | ||||||||||
| Left Hemisphere | Right Hemisphere | |||||||||
| x (mm) | y (mm) | z (mm) | Z score | Cluster size | Structure | x (mm) | y (mm) | z (mm) | Z score | Cluster size |
| Cingulate Gyrus | 10 | -29 | 37 | 3.23 | 447 | |||||
| -43 | -5 | 54 | 3.53 | 3432 | Pre-Central Gyrus (BA6) | |||||
| -54 | -43 | 6 | 3.03 | 1714 | Middle Temporal Gyrus | 62 | -6 | -9 | 3.42 | 308 |
| -25 | -11 | -27 | 3.2 | 295 | Parahippocampal Gyrus (BA 28) | |||||
| -54 | -25 | 47 | 3.43 | 3884 | Post-central Gyrus (BA2) | |||||
| -41 | -47 | 32 | 3.08 | 1520 | Supramarginal Gyrus | |||||
| -5 | -55 | 48 | 3.32 | 467 | Precuneus (BA7) | |||||
| Greater Increases in Controls than Depressed Older Adults | ||||||||||
| Left Hemisphere | Right Hemisphere | |||||||||
| x (mm) | y (mm) | z (mm) | Z score | Cluster size | Structure | x (mm) | y (mm) | z (mm) | Z score | Cluster Size |
| Superior Frontal Gyrus | 31 | 48 | 26 | 3.45 | 8781 | |||||
| Middle Frontal Gyrus | 37 | 40 | 22 | 3.51 | 8781 | |||||
| Greater Decreases in Depressed Older Adults than Controls | ||||||||||
| Left Hemisphere | Right Hemisphere | |||||||||
| x (mm) | y (mm) | z (mm) | Z score | Cluster size | Structure | x (mm) | y (mm) | z (mm) | Z score | Cluster size |
| -24 | 52 | 24 | 3.30 | 707 | Superior Frontal Gyrus (BA10) | |||||
| -37 | 12 | 53 | 3.49 | 2863 | Middle Frontal Gyrus (BA6) | |||||
| Greater Increases in Depressed Older Adults than Controls | ||||||||||
| Left Hemisphere | Right Hemisphere | |||||||||
| x (mm) | y (mm) | z (mm) | Z score | Cluster size | Structure | x (mm) | y (mm) | z (mm) | Z score | Cluster size |
| -21 | -85 | 46 | 3.76 | 491 | Cuneus | |||||
| -63 | -41 | 30 | 3.69 | 3884 | Inferior Parietal Lobule | |||||
| -16 | -30 | 9 | 2.79 | 254 | Thalamus | |||||
| Putamen | 28 | -17 | 4 | 2.61 | 185 | |||||
Cerebral Metabolic Response to Acute Citalopram in Control Subjects (Table 3A)
Decreases in metabolism were observed in right and left cingulate gyrus, left superior, middle and inferior frontal gyrus, left middle temporal gyrus, right and left thalamus and left parahippocampal gyrus. Increases in metabolism were observed in the right superior, middle and inferior frontal gyri, left precuneus, left superior occipital gyri and cerebellum (bilaterally).
Cerebral Metabolic Response to Acute Citalopram in Depressed Older Adults (Table 3B)
Decreases in metabolism were observed in the right cingulate gyrus, superior and middle frontal gyrus (bilaterally), right superior temporal gyrus and parahippocampal gyrus (bilaterally). Increases in metabolism were observed in the left superior and left inferior parietal lobule, right inferior occipital gyrus, left brainstem and cerebellum (bilaterally).
Comparison of Cerebral Metabolic Response to Acute Citalopram in Control Subjects versus Depressed Older Adults [Citalopram versus Placebo Conditions] (Table 3C)
Greater decreases in the control subjects relative to the depressed older adults were observed in the right cingulate gyrus, left pre and post-central, left middle temporal, left parahippocampal, left supramarginal gyri and left precuneus. Greater increases in the control subjects than depressed older adults were observed in right superior and right middle frontal gyri. Greater decreases in depressed older adults than control subjects were noted in the left superior and left middle frontal gyri. Greater increases in the depressed older adults relative to control subjects were observed in the left cuneus, left inferior parietal lobule and left thalamus, as well as the right putamen.
Discussion
The present study demonstrated differences in the acute cerebral metabolic response to citalopram between depressed older adults and control subjects. Greater metabolic decreases in the depressed older adults relative to the control subjects were observed in left frontal cortex, whereas the control subjects demonstrated greater right anterior cingulate and left posterior metabolic decreases than the depressed older adults. Control subjects showed greater metabolic increases than depressed older adults in right superior and middle frontal gyrus. The findings of greater metabolic increases in frontal cortex of control subjects relative to depressed older adults is consistent with the findings of Anderson et al.(17) who made a similar observation with respect to the acute cerebral metabolic effects of the serotonin releasing agent and reuptake inhibitor, fenfluramine, in younger depressed adults and control subjects. The magnitude of the neuroendocrine response (prolactin) to citalopram was also decreased in depressed older adults relative to controls, although the differences were not statistically significant.
In interpreting the results of the present study it is important to note that after completion of the acute citalopram study, the depressed older adults underwent a twelve week treatment trial with oral citalopram. 75% met criteria for treatment response (greater than 50% reduction in HDRS score and HDRS score less than ten after twelve weeks of treatment). Thus, the results observed in the present study largely reflect changes in the functional serotonergic response in treatment responders. This issue is raised because the cerebral metabolic response to citalopram in treatment resistant patients may be different. Neurobiological predictors associated with treatment response in geriatric depression involve similar neural pathways as affected by acute citalopram in the present study including observations of altered white matter functional connectivity and anterior cingulate activation during conflict processing (34,35).
The regional cerebral metabolic effects observed comprise several networks implicated in affective and cognitive function. Decreases in a fronto-temporal-parietal network (including the anterior cingulate gyrus, superior temporal gyrus and precuneus) are observed. This network is activated in tasks involving visuo-spatial imagery, episodic memory retrieval and self processing (as reviewed by 36), aspects of cognition that are affected in depression (37, 38). This network also includes the regions that comprise the ‘default network’, regions that are more active during the “resting” state than under conditions of cognitive activation (39, 40-41). The metabolic decreases in limbic structures that have a high concentration of serotonin and connectivity to neocortical regions (parahippocampal gyrus and amygdala) are observed uniquely in the present study compared to previous cerebral metabolism studies in geriatric depression (14, 15, 20). Afferent projections from the parahippocampal gyrus to the anterior cortical regions (anterior medial frontal, anterior cingulate cortices, insular cortex) have been characterized (42), as well as reciprocal projections between the amygdala (lateral, basal and accessory nuclei) and cortical regions including the medial prefrontal and anterior cingulate gyrus, superior temporal and insula (43). In contrast to the decreases in metabolism observed in these regions, the inferior parietal lobule, which integrates sensory and motor inputs, in addition to sensory (cuneus) and motor (putamen, cerebellum) show increased metabolism with treatment. Theses structures comprise a network that is activated during the visual information processing (e.g. visually guided reaching and voluntary saccadic eye movements 44,45).
Decreased cortical responsiveness measured with functional magnetic resonance imaging (fMRI) in several cognitive activation paradigms has been observed in depressed older adults relative to controls. In an executive function task, decreased right middle frontal, cingulate and inferior parietal hypoactivation was observed in symptomatic depressed older adults and cingulate and inferior parietal hypoactivation in remitted depressed older adults (46). Bilateral hypoactivation of dorsal anterior cingulate and hippocampus was observed during a verbal fluency task (47) and decreased prefrontal and increased caudate activation to an explicit sequence learning task has also been observed (48). Older depressed adults demonstrated a decreased response to negatively valenced stimuli in ventromedial prefrontal cortex (orbitofrontal cortex, rostral cingulate cortex and ventral medial frontal gyrus) which was correlated with depression severity and normalized with antidepressant treatment (49). With respect to episodic memory, older depressed adults demonstrated decreased activation of temporo-limbic structures relative to control subjects (50). These data suggest that the emotional circuitry that is overactive in younger depressed adults is underactive in older depressed adults. The circuitry associated with cognitive processing, especially executive function, is also underactive.
The acute metabolic alterations observed in the cortical and limbic regions are likely to be involve a serotonergic effect. Studies in animals and humans have shown 80% serotonin transporter occupancy, as well as increases in endogenous serotonin concentrations after acute citalopram administration (51-53). As has been reviewed previously, given that the regional pattern of metabolic alterations extends beyond the primary serotonergic projections, the metabolic alterations are likely to reflect secondary changes in other neurochemical systems known to be modulated by serotonin and located within the cortical and limbic regions affected (including glutamate, dopamine and acetylcholine 19, 54-56). For example, the same dose, route and time course of administration of citalopram has been shown to produce an increase in endogenous dopamine in healthy control subjects (as shown by an decrease in dopamine D2 receptor availability measured with PET and [11C]-raclopride) as the metabolic alterations reported in the present study (57).
In summary, differences in serotonin modulation of cerebral glucose metabolism were observed in controls relative to older depressed adults. Future studies to compare younger to younger depressed adults are important to understand the neurobiological mechanism underlying the greater impact of depression in late life with respect to disease course and cognitive impairment. While the majority of depressed older adults enrolled in the present study had not been treated previously with antidepressant medication and demonstrated significant clinical improvement with citalopram, a critical next step for this research is the investigation of depressed older adults who fail to respond despite an adequate course of antidepressant treatment. Early biomarkers of treatment response in geriatric depression, as we have shown previously with TSD (15), would be useful to identify patients who might have a greater clinical benefit from a different class of antidepressants (e.g. selective noradrenergic reuptake inhibitors) or require more intensive treatment. The functional neuroanatomic differences observed between depressed older adults and controls inform the design of future mechanistic studies to understand the neurochemical and cognitive mechanisms underlying the metabolic effects. Such information would provide a more comprehensive understanding of the neurobiology of geriatric depression, which may have implications for the development of more effective prevention and intervention strategies.
Supplementary Material
Supplemental Data: Neuroimaging Studies of Pharmacologic and Antidepressant Interventions in Depression
Figure 1.
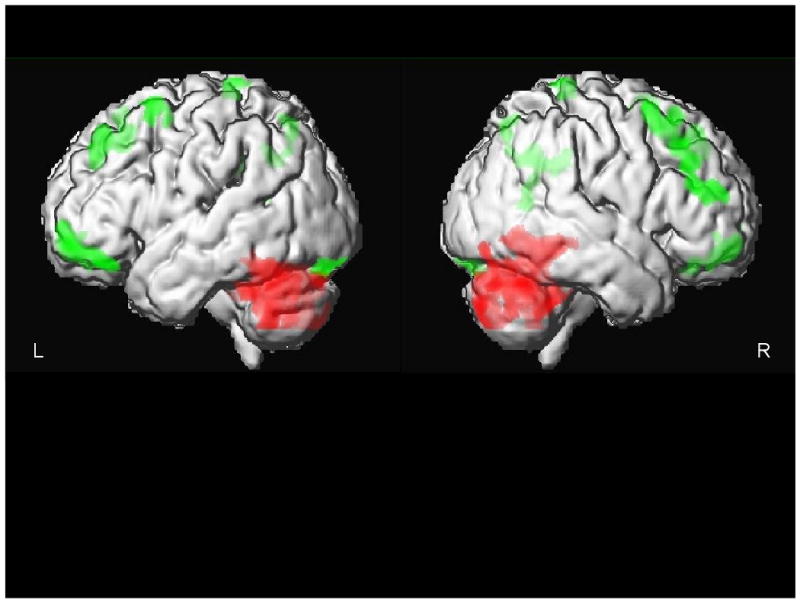
The Effects of Acute Citalopram Treatment on Cerebral Glucose Metabolism in Geriatric Depression. Regions of metabolic decrease (green) and increase (red) are superimposed on an MR rendering from a representative subject.
Acknowledgments
The present study was supported in part by National Institute of Health: MH 01621 (GSS), MH 49936 (GSS), MH 57078 (GSS), MH 64823 (GSS), M01 RR 018535 (Chiorazzi). Citalopram medication was provided by Forest Laboratories. David Bjelke, CNMT and Claude Margouleff, B.S. are gratefully acknowledged for their contribution to the conduct of the PET studies. Bruce G. Pollock, M. D., Ph.D., Margaret Kirshner, B.S., Denise Soriso, B. S. and Kimberly A. Huber, M.P.H., Geriatric Psychopharmacology Laboratory, Department of Psychiatry, University of Pittsburgh School of Medicine are gratefully acknowledged for providing the intravenous formulation of citalopram and performing the analyses of the citalopram and neuroendocrine samples. Dr Smith received research funding from the National Institute of Mental Health and the National Alliance for Research in Schizophrenia and Depression.
Footnotes
Financial Disclosures: Citalopram medication in pill form for chronic administration was provided by Forest Laboratories. The authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. American Journal of Psychiatry. 1965;122(5):509–22. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- 2.Lapin IP, Oxenkrug GF. Intensification of the central serotonergic processes as a possible determinant of the thymoleptic effect. Lancet. 1969;1(7586):132–6. doi: 10.1016/s0140-6736(69)91140-4. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds CF, 3rd, Dew MA, Frank E, Begley AE, Miller MD, Cornes C, et al. Effects of age at onset of first lifetime episode of recurrent major depression on treatment response and illness course in elderly patients. Am J Psychiatry. 1998;155:795–799. doi: 10.1176/ajp.155.6.795. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds CF, 3rd, Frank E, Dew MA, Houck PR, Miller M, Mazumdar S, et al. Treatment of 70(+)-year-olds with recurrent major depression. Excellent short-term but brittle long-term response. Am J Geriatr Psychiatry. 1999;7:64–69. [PubMed] [Google Scholar]
- 5.Conwell Y, Duberstein P, Cox C. Relationships of age and axis I diagnoses in victims of completed suicide: a psychological autopsy study. American Journal of Psychiatry. 1996;153:1001–1008. doi: 10.1176/ajp.153.8.1001. [DOI] [PubMed] [Google Scholar]
- 6.Thomas AJ, Gallagher P, Robinson LJ, Porter RJ, Young AH, Ferrier IN, O'Brien JT. A comparison of neurocognitive impairment in younger and older adults with major depression. Psychological Medicine. 2008;30:1–9. doi: 10.1017/S0033291708004042. 2008. [DOI] [PubMed] [Google Scholar]
- 7.Krishnan KR, Hays JC, Tupler LA, George LK, Blazer DG. Clinical and phenomenological comparisons of late-onset and early-onset depression. Am J Psychiatry. 1995;152(5):785–8. doi: 10.1176/ajp.152.5.785. [DOI] [PubMed] [Google Scholar]
- 8.Alexopoulos GS, Vrontou C, Kakuma T, Meyers BS, Young RC, Klausner E, et al. Disability in geriatric depression. Am J Psychiatry. 1996;153:877–885. doi: 10.1176/ajp.153.7.877. [DOI] [PubMed] [Google Scholar]
- 9.Bruce ML, Leaf PJ. Psychiatric disorders and 15-month mortality in a community sample of older adults. Am J Public Health. 1989;79:727–730. doi: 10.2105/ajph.79.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith GS, Gunning-Dixon FM, Lotrich FE, Taylor WD, Evans JD. Translational research in late-life mood disorders: implications for future intervention and prevention research. Neuropsychopharmacology. 2007;32(9):1857–75. doi: 10.1038/sj.npp.1301333. [DOI] [PubMed] [Google Scholar]
- 11.Smith GS, Koppel J, Goldberg S. Applications of neuroreceptor imaging to psychiatry research. Psychopharmacol Bull. 2003;37(4):26–65. [PubMed] [Google Scholar]
- 12.Wu JC, Gillin JC, Buchsbaum MS, Schachat C, Darnall LA, Keator DB, Fallon JH, Bunney WE. Sleep deprivation PET correlations of Hamilton symptom improvement ratings with changes in relative glucose metabolism in patients with depression. J Affect Disord. 2008;107(13):181–6. doi: 10.1016/j.jad.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 13.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 14.Smith G, Reynolds C, Pollock B, Derbyshire S, Nofzinger E, Dew M, Milko D, Meltzer C, Kupfer D. Acceleration of the cerebral glucose metabolic response to antidepressant treatment by total sleep deprivation in geriatric depression. American Journal of Psychiatry. 1999;156:683–689. doi: 10.1176/ajp.156.5.683. [DOI] [PubMed] [Google Scholar]
- 15.Smith GS, Reynolds CF, 3rd, Houck PR, Dew MA, Ma Y, Mulsant BH, Pollock BG. Glucose metabolic response to total sleep deprivation, recovery sleep, and acute antidepressant treatment as functional neuroanatomic correlates of treatment outcome in geriatric depression. Am J Geriatr Psychiatry. 2002;10(5):561–7. [PubMed] [Google Scholar]
- 16.Mann JJ, Malone KM, Diehl DJ, Perel J, Cooper TB, Mintun MA. Demonstration in vivo of reduced serotonin responsivity in the brain of untreated depressed patients. Am J Psychiatry. 1996;153(2):174–82. doi: 10.1176/ajp.153.2.174. [DOI] [PubMed] [Google Scholar]
- 17.Anderson AD, Oquendo MA, Parsey RV, Milak MS, Campbell C, Mann JJ. Regional brain responses to serotonin in major depressive disorder. J Affect Disord. 2004;82(3):411–7. doi: 10.1016/j.jad.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Bremner JD, Vythilingam M, Ng CK, Vermetten E, Nazeer A, Oren DA, Berman RM, Charney DS. Regional brain metabolic correlates of alpha-methylparatyrosine-induced depressive symptoms: implications for the neural circuitry of depression. JAMA. 2003;289(23):3125–34. doi: 10.1001/jama.289.23.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith G, Ma Y, Dhawan V, et al. Serotonin Modulation of Cerebral Glucose Metabolism measured with Positron Emission Tomography (PET) in Human Subjects. Synapse. 2002a;45(2):105–112. doi: 10.1002/syn.10088. [DOI] [PubMed] [Google Scholar]
- 20.Smith GS, Kramer E, Hermann CR, Goldberg S, Ma Y, Dhawan V, et al. Acute and chronic effects of citalopram on cerebral glucose metabolism in geriatric depression. Am J Geriatr Psychiatry. 2002;10:715–723. [PubMed] [Google Scholar]
- 21.Mayberg HS, Brannan SK, Tekell JL, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 22.Buchsbaum MS, Wu J, Siegel BV, et al. Effect of sertraline on regional metabolic rate in patients with affective disorder. Biol Psychiatry. 1997;41:15–22. doi: 10.1016/s0006-3223(96)00097-2. [DOI] [PubMed] [Google Scholar]
- 23.Raap DK, Van de Kar LD. Selective serotonin reuptake inhibitors and neuroendocrine function. Life Sci. 1999;65(12):1217–35. doi: 10.1016/s0024-3205(99)00169-1. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg S, Smith G, Ma Y, Kramer E, Pollock B, Eidelberg D. Serotonin modulation of cerebral glucose metabolism in normal aging. Neurobiology of Aging. 2004;25:167–174. doi: 10.1016/s0197-4580(03)00088-5. [DOI] [PubMed] [Google Scholar]
- 25.First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV axis 1 disorders-patient edition (SCID-I/P) New York: New York Psychiatric Institute; 1995. [Google Scholar]
- 26.Hamilton M. A rating scale for depression. J Neurol, Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamilton M. The assessment of anxiety states by rating. British J Med Psychology. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 28.Beck AT, Steer RA, Ball R, et al. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 29.Yesavage JA. Geriatric Depression Scale. Psychopharm Bull. 1988;24(4):709–11. [PubMed] [Google Scholar]
- 30.Kapitany T, Schindl M, Schindler S, Henelmann B, Fureder T, Barnas C, Sieghart, Kasper S. The citalopram challenge test in patients with major depression and in healthy controls. Psychiatry Res. 1999;88:75–88. doi: 10.1016/s0165-1781(99)00082-7. [DOI] [PubMed] [Google Scholar]
- 31.Takikawa S, Dhawan V, Spetsieris P, Robeson W, Chaly T, Dahl R, Margouleff D, Eidelberg D. Noninvasive quantitative fluorodeoxyglucose PET studies with an estimated input function derived from a population-based arterial blood curve. Radiology. 1993;188(1):131–6. doi: 10.1148/radiology.188.1.8511286. [DOI] [PubMed] [Google Scholar]
- 32.Friston KJ, Ashburner J, Kiebel SJ, Nichols TE, Penny WD, editors. Statistical Parametric Mapping: The Analysis of Functional Brain Images. Academic Press; 2007. [Google Scholar]
- 33.Bartlett EJ, Brodie JD, Wolf AP, Christman DR, Laska E, Meissner M. Reproducibility of cerebral glucose metabolic measurements in resting human subjects. J Cereb Blood Flow Metab. 1988;8(4):502–12. doi: 10.1038/jcbfm.1988.91. [DOI] [PubMed] [Google Scholar]
- 34.Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Latoussakis V, Kanellopoulos D, Klimstra S, Lim KO, Hoptman MJ. Microstructural White Matter Abnormalities and Remission of Geriatric Depression. Am J Psychiatry. 2008 Jan 2; doi: 10.1176/appi.ajp.2007.07050744. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 35.Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Kalayam B, Katz R, Kanellopoulos D, Etwaroo GR, Klimstra S, Foxe JJ. Event-related potentials in an emotional go/no-go task and remission of geriatric depression. Neuroreport. 2007;18(3):217–21. doi: 10.1097/WNR.0b013e328013ceda. [DOI] [PubMed] [Google Scholar]
- 36.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–8. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 37.Bhalla RK, Butters MA, Mulsant BH, Begley AE, Zmuda MD, Schoderbek B, Pollock BG, Reynolds CF, 3rd, Becker JT. Persistence of neuropsychologic deficits in the remitted state of late-life depression. Am J Geriatr Psychiatry. 2006;14(5):419–27. doi: 10.1097/01.JGP.0000203130.45421.69. [DOI] [PubMed] [Google Scholar]
- 38.Nebes RD, Pollock BG, Houck PR, Butters MA, Mulsant BH, Zmuda MD, Reynolds CF., 3rd Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized, double-blind clinical trial with nortriptyline and paroxetine. J Psychiatr Res. 2003;37(2):99–108. doi: 10.1016/s0022-3956(02)00085-7. [DOI] [PubMed] [Google Scholar]
- 39.Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage. 2006;29(2):452–66. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buckner RL, Vincent JL. Unrest at rest: default activity and spontaneous network correlations. Neuroimage. 2007;37(4):1091–6. doi: 10.1016/j.neuroimage.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 42.Mohedano-Moriano A, Pro-Sistiaga P, Arroyo-Jimenez MM, Artacho-Pérula E, Insausti AM, Marcos P, Cebada-Sánchez S, Martínez-Ruiz J, Muñoz M, Blaizot X, Martinez-Marcos A, Amaral DG, Insausti R. Topographical and laminar distribution of cortical input to the monkey entorhinal cortex. J Anat. 2007;211(2):250–60. doi: 10.1111/j.1469-7580.2007.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. J Comp Neurol. 2002;451(4):301–23. doi: 10.1002/cne.10339. [DOI] [PubMed] [Google Scholar]
- 44.Dejardin S, Dubois S, Bodart JM, Schiltz C, Delinte A, Michel C, Roucoux A, Crommelinck M. PET study of human voluntary saccadic eye movements in darkness: effect of task repetition on the activation pattern. Eur J Neurosci. 1998;10(7):2328–36. doi: 10.1046/j.1460-9568.1998.00245.x. [DOI] [PubMed] [Google Scholar]
- 45.Kertzman C, Schwarz U, Zeffiro TA, Hallett M. The role of posterior parietal cortex in visually guided reaching movements in humans. Exp Brain Res. 1997;114(1):170–83. doi: 10.1007/pl00005617. [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Krishnan KR, Steffens DC, Potter GG, Dolcos F, McCarthy G. Depressive state- and disease-related alterations in neural responses to affective and executive challenges in geriatric depression. Am J Psychiatry. 2008;165(7):863–71. doi: 10.1176/appi.ajp.2008.07101590. [DOI] [PubMed] [Google Scholar]
- 47.de Asis JM, Stern E, Alexopoulos GS, Pan H, Van Gorp W, Blumberg H, Kalayam B, Eidelberg D, Kiosses D, Silbersweig DA. Hippocampal and anterior cingulate activation deficits in patients with geriatric depression. Am J Psychiatry. 2001;158(8):1321–3. doi: 10.1176/appi.ajp.158.8.1321. [DOI] [PubMed] [Google Scholar]
- 48.Aizenstein HJ, Butters MA, Figurski JL, Stenger VA, Reynolds CF, 3rd, Carter CS. Prefrontal and striatal activation during sequence learning in geriatric depression. Biol Psychiatry. 2005;58(4):290–6. doi: 10.1016/j.biopsych.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 49.Brassen S, Kalisch R, Weber-Fahr W, Braus DF, Büchel C. Ventromedial prefrontal cortex processing during emotional evaluation in late-life depression: a longitudinal functional magnetic resonance imaging study. Biol Psychiatry. 2008;64(4):349–55. doi: 10.1016/j.biopsych.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 50.Grön G, Bittner D, Schmitz B, Wunderlich AP, Riepe MW. Subjective memory complaints: objective neural markers in patients with Alzheimer's disease and major depressive disorder. Ann Neurol. 2002;51(4):491–8. doi: 10.1002/ana.10157. [DOI] [PubMed] [Google Scholar]
- 51.Hinz R, Selvaraj S, Murthy NV, Bhagwagar Z, Taylor M, Cowen PJ, Grasby PM. Effects of citalopram infusion on the serotonin transporter binding of [(11)C]DASB in healthy controls. J Cereb Blood Flow Metab. 2008;28(8):1478–90. doi: 10.1038/jcbfm.2008.41. [DOI] [PubMed] [Google Scholar]
- 52.Kreiss DS, Wieland S, Lucki I. The presence of a serotonin uptake inhibitor alters pharmacological manipulations of serotonin release. Neuroscience. 1993;52(2):295–301. doi: 10.1016/0306-4522(93)90157-b. [DOI] [PubMed] [Google Scholar]
- 53.Invernizzi R, Velasco C, Bramante M, Longo A, Samanin R. Effect of 5-HT1A receptor antagonists on citalopram-induced increase in extracellular serotonin in the frontal cortex, striatum and dorsal hippocampus. Neuropharm. 1997;36:467–473. doi: 10.1016/s0028-3908(97)00060-9. [DOI] [PubMed] [Google Scholar]
- 54.Golembiowska K, Dziubina A. Effect of acute and chronic administration of citalopram on glutamate and aspartate release in the rat prefrontal cortex. Polish Journal of Pharmacology. 2000;52(6):441–8. [PubMed] [Google Scholar]
- 55.Lucas G, De Deurwaerdere P, Porras G, Spampinato U. Endogenous serotonin enhances the release of dopamine in the striatum only when nigro-striatal dopaminergic transmission is activated. Neuropharm. 2000;39(11):1984–1995. doi: 10.1016/s0028-3908(00)00020-4. [DOI] [PubMed] [Google Scholar]
- 56.Hilgert M, Buchholzer M, Jeltsch H, Kelche C, Cassel JC, Klein J. Serotonergic modulation of hippocampal acetylcholine release after long-term neuronal grafting. Neuroreport. 2000;11(14):3063–3065. doi: 10.1097/00001756-200009280-00006. [DOI] [PubMed] [Google Scholar]
- 57.Smith G, Ma Y, Dhawan V, Chaly T, Belakhleff A, Eidelberg D. Selective Serotonin Reuptake Inhibitor (SSRI) modulation of striatal dopamine measured with [11C]-raclopride and positron emission tomography (PET) Synapse. 2009;63(1):1–6. doi: 10.1002/syn.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data: Neuroimaging Studies of Pharmacologic and Antidepressant Interventions in Depression


