Abstract
Amyotrophic lateral sclerosis (ALS) is characterized by progressive degeneration of lower motor neurons resulting in paralysis and death. Epidemiological and clinical findings suggest that a decline in athletic performance may presage the clinical onset of ALS, but this possibility has not been tested in an animal model. By placing running wheels in each mouse’s cage to measure their exercise activity, we show that presymptomatic G93A SOD1 ALS mice are more active runners (15–20 km/day) than control mice (7–9 km/day). The ALS mice then exhibit a sharp decline in daily running distance 10–20 days prior to the onset of clinical disease. Within the group of ALS mice there were no significant correlations between cumulative lifetime running distance and age at clinical disease onset or age at death, suggesting that amount of exercise did not affect the course of the disease process. Our data show that presymptomatic ALS mice have a propensity for running long distances, and then dramatically reduce the amount they run prior to the appearance of clinical symptoms. The monitoring of voluntary running distance may provide a valuable biomarker to evaluate the efficacy of potential therapeutic interventions for ALS in preclinical studies.
Keywords: ALS, exercise, mutant SOD1
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder that primarily affects lower motor neurons resulting in progressive muscle atrophy that eventually leads to respiratory failure and death within 1–5 years (Traynor et al., 2000). Whereas most cases of ALS occur sporadically with no known cause, approximately 10% of ALS cases are inherited in an autosomal dominant manner. About 20% of these inherited cases are caused by missense mutations in the Cu/Zn superoxide dismutase gene resulting in an adverse gain of function of the SOD1 protein (Pasinelli and Brown, 2006). Epidemiological and experimental findings suggest that individuals with high levels of energy expenditure and/or low levels of energy intake may be at increased risk for ALS (Mattson et al., 2007). Beginning with the baseball player Lou Gehrig, publicized cases of prominent athletes developing ALS at relatively early ages suggested a possible association between energy expenditure and ALS (Kasarskis and Winslow, 1989). The results of several epidemiological studies (Desport et al., 1999; Scarmeas et al., 2003; Kurzke, 1982), but not others (Longstreth et al., 1998), were consistent with slender athletes being at increased risk for ALS. Studies of animal models of ALS suggest that a low energy intake exacerbates (Pedersen and Mattson, 1999) and a high energy intake retards (Mattson et al., 2007; Hamadeh et al., 2005; Dupuis et al., 2004) the disease process. However, it remains unclear whether exercise itself is harmful, beneficial or has no affect on ALS risk and/or disease progression (Liebetanz et al., 2004; Kaspar et al., 2005).
Transgenic mice that express mutant human SOD1 (mSOD1 mice) exhibit progressive clinical and histopathological phenotypes that are quite similar to those of ALS patients (Wong et al., 2002; Lobsiger and Cleveland, 2007). The first clinical signs observed in mSOD1 mice are hind limb clasping and wobbly gait, followed by progressive limb paralysis and death (Kieran et al., 2004). The ability to quantitatively measure the onset and progression of the disease in mice is important for elucidating underlying cellular and molecular pathogenic events, and for determining when treatments should be administered and if they are effective in treating the disease. Several approaches have been used to establish the symptom onset in ALS mice including rotarod performance, computerized gait analysis and observations of hind limb clasping and paralysis (Weydt et al., 2003; Wooley et al., 2005; Stam et al., 2008). However, all of these methods measure the animal’s motor function under forced conditions, subjecting the animals to unnecessary stress and requiring significant time to perform each test. In the present study, we have developed a method to non-invasively and objectively measure the motor function of each mouse by recording the distance they run in a home cage exercise wheel. We observed that presymtomatic ALS mice voluntarily run further each day than nontransgenic control mice. The ALS mice then exhibit a sharp decline in daily running distance 10–20 days before the onset of motor symptoms. Neither the age of the mice at clinical disease onset nor their progression toward death were associated with cumulative lifetime running distance, suggesting that amount of exercise did not affect the course of the disease process. Therefore, using an in cage running wheel to monitor the decline in spontaneous running can be used as a biomarker to complement traditional motor dysfunction signs in preclinical studies that evaluate the efficacy of therapeutic interventions for ALS.
Methods
Seven-week-old male transgenic mice overexpressing mutant SOD1 (B6SJL-Tg(SOD1*G93A)1Gur/J) and control mice (B6SJL-Tg(SOD1)2Gur/J) were purchased from Jackson Laboratories (Bar Harbor, ME). At 9 weeks of age the mice were housed singly in cages with exercise wheels (Super Pet, IL) coupled to a bicycle computer (Sigma Sport USA, IL). Maximum speed, average speed and total running distance were recorded twice per week. The motor function of the mice was also scored twice per week using a 5 point clinical observation of the hind limbs with the following stages: 0, normal gait; 1, single leg limp; 2, single leg paralysis; 3, second leg limp; 4, second leg paralysis. Mice were euthanized when they reached stage 4. Disease onset was when the neurological score = 1, whereas disease progression was the rate (days) it took the mouse to go from a neurological score of 1 to a score of 4. Statistical analyses were performed using Statview software.
Results
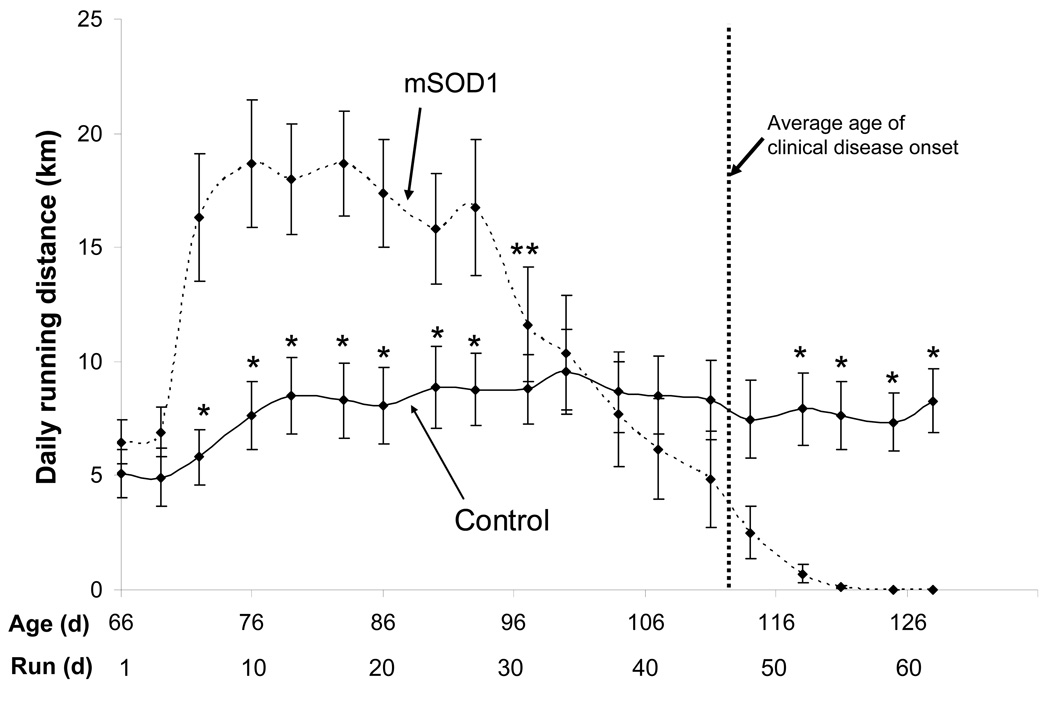
During the first three days of being housed in a cage with a running wheel, both mSOD1mice (n=15) and control mice (n=16) ran between 4 and 7 km/day (Fig. 1). Between days 3 and 6 the mSOD1 exhibited a large increase in their daily running distance to an average of more than 15 km/day, and then maintained this high level of running during the ensuing 3 weeks. In contrast, control mice increased their running distance only moderately during between days 3 and 10 to daily distances of 7–9 km/day and maintained this level throughout the course of the study (Fig. 1). The difference in voluntary daily running distance between mSOD1 and control mice during days 6–26 was highly significant (p=0.004). At approximately 86 days of age, the daily running distances of the mSOD1 mice began to plummet at a rate of approximately −0.6 km/day.
Figure 1.
Presymptomatic ALS mice exhibit a propensity to run long distances, and then undergo a precipitous fall in daily running distance prior to the onset of observable motor impairment. Comparison of average daily running distances of mSOD1 mice (n=15) and control mice (n=16). Values are the mean +/− SE. * p<0.05 compared to the corresponding value for mSOD1 mice (paired t-tests). **significant drop from peak running distance of mSOD1 mice (repeated measures ANOVA, p<0.05).
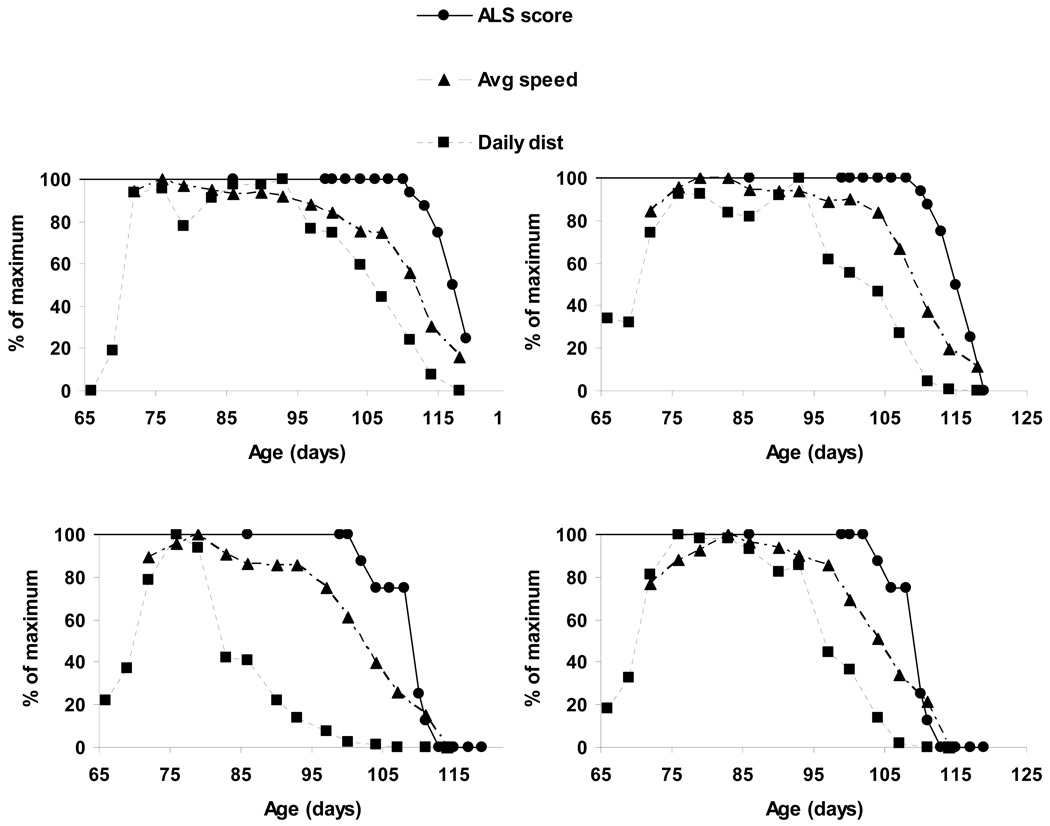
We next compared the time courses of changes in daily running distance and average running speed with disease onset and progression (clinical score) in individual mSOD1 mice. Figure 2 shows the normalized measurements for four representative mice. Average daily running began to decline when the mSOD1 mice were 85–95 days old, which is 10–20 days before any clinical symptoms were observed. A decrease in average running speed occurred after the decline in daily running distance and 3–6 days before the onset of motor symptoms (Fig. 2). These findings reveal a previously unknown adverse effect of mSOD1 on voluntary running distance that presages subsequent deficits in motor function.
Figure 2.
A decline of daily running distance, and to a lesser extent running speed, occurs prior to the onset of clinical symptoms in ALS mice. Examples of the time course of changes in daily running distance and average running speed (each expressed as a percentage of the maximum), in relation to the onset of clinical symptoms (ALS score) in four different mSOD1 mice. Note that a decline in daily running distance presaged the onset of symptoms by 10–20 days, and that a decrease in average running speed occurred prior to the onset of symptoms but after the decline in running distance. These four examples are representative of the results in all 15 mice examined.
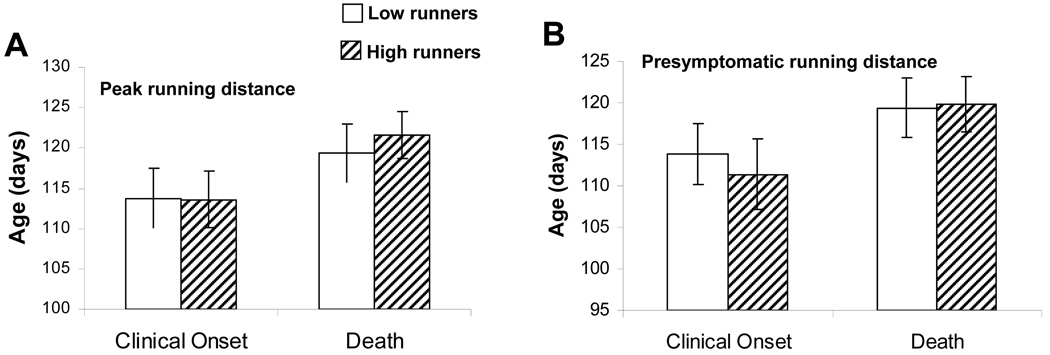
Previous studies have suggested that exercise either has no effect or delays disease onset and increases survival in mSOD1 mice (Kaspar et al., 2005; Veldink et al., 2003; Kirkiezos et al., 2003). First, we separated the running data for mSOD1 mice into two groups corresponding to the five mice with the highest peak daily running distances (>25 km/day) and the five mice with the lowest peak daily running distances (<15 km/day). When these two groups were compared with regards to their ages at disease onset and at death, there were no significant differences between low and high runners (Fig. 3A). Similarly, there was no significant effect of total presymptomatic running distance (top five mice compared to bottom five mice) on age at disease onset or age at death (Fig. 3B). Thus, neither age at the onset of clinical disease nor age at death were correlated with total running distance or peak daily running distance prior to disease onset in mSOD1 mice.
Figure 3.
Neither age at the onset of clinical disease nor age at death are correlated with total running distance or peak daily running distance prior to disease onset in mSOD1 mice. The 5 mice that ran the farthest total presymptomatic distance or that had the greatest peak daily running distance were considered high runners and the 5 mice that ran the least total distance or had the lowest peak daily running distance were considered low runners. There were no significant differences between low and high runners in either age at clinical onset or age at death.
Discussion
By monitoring voluntary wheel running of mSOD1 mice, we are able to objectively measure the motor function of the mice without forcing them to perform motor tasks or causing stress. We found that mSOD1 mice progressively reduce the distance they run beginning approximately 2–3 weeks prior to the onset of clinically discernable motor impairment. Running speed also declined prior to the onset of clinical symptoms, but only after a time lag of approximately 1 week after the decline in daily running distance. These findings suggest that the endurance of the mSOD1 mice is first affected resulting in a decreased duration of individual bouts of running (without a reduction in running speed). Previous case studies of human subjects with ALS have suggested that a decline in athletic performance presages the onset of the typical discernable ALS symptoms of muscle weakness and fatigue. The best-known example is that of Lou Gehrig whose batting average declined precipitously in the 2 years prior to his being diagnosed with ALS (Kasarskis and Winslow, 1989). Our findings therefore suggest that the monitoring of daily running distance in rodent models of ALS can provide a quantitative measure of disease onset and progression. The ability to detect and quantify a pre-symptomatic indicator of impending disease onset should provide a valuable experimental tool for future studies of cellular and molecular mechanisms of ALS disease pathogenesis, as well as a marker to evaluate the efficacy of potential therapeutic interventions in preclinical trials.
Interestingly, the distances that presymptomatic mSOD1 mice ran (15–20 km/day) were much greater than the distances that nontransgenic control mice of the same genetic background ran (7–9 km/day). This distance is also much greater than the distances that mSOD1 mice have been forced to run in previous studies (Liebetanz et al., 2004; Veldink et al., 2003; Kirkinezos et al., 2003). The mechanism by which mutant SOD1 promotes increased voluntary running is unknown, but might be related to an increase in motor neuron excitability. Previous studies have shown that motor neurons from presymptomatic mSOD1 mice exhibit increased excitability resulting from increased persistent Na+ currents (Kuo et al., 2005). Similar increases in the excitability of motor neuron axons have been documented in ALS patients (Vucic and Kiernan, 2006; Kanai et al., 2006). Hyper-excitability of upper motor neurons in the cerebral cortex of ALS patients has also been documented (Zanette et al., 2002), an alteration that could conceivably promote increased amounts of voluntary exercise and exercise performance. Whether the increased excitability of upper and lower motor neurons contributes to their degeneration in ALS remains unclear. On the one hand the drug riluzole, which reduces the excitability of motor neurons, slows disease progression in mSOD1 mice and in ALS patients (Gurney et al., 1998; Miller et al., 2007). On the other hand exercise either delays, or has no effect on, disease onset in mSOD1 mice (Kaspar et al., 2005; Stam et al., 2008; Veldink et al., 2003; Kirkinezos et al., 2003). In the present study there were no significant effects of total running distance or peak running distance on the clinical onset or survival of mSOD1 mice, suggesting that voluntary running does not exacerbate the ALS disease process. Several studies of humans have suggested that intense physical activity may increase the risk of ALS (Mattson et al., 2006); for example, high performing soccer players may be at increased risk for ALS (Chio et al., 2005; Wicks et al., 2007). Whereas moderate exercise may benefit ALS patients by reducing muscle atrophy (Bello-Haas et al., 2008; Drory et al., 2001), exercising to exhaustion may promote excitotoxic degeneration of motor neurons (Cleveland et al., 2001).
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Aging, NIH.
Footnotes
Potential conflicts of interest: nothing to report.
References
- Bello-Haas VD, Florence JM, Kloos AD, Scheirbecker J, Lopate G, Hayes SM, Pior EP, Mitsumoto H. A randomized controlled trial of resistance exercise in individuals with ALS. Neurology. 2008;71:864–865. doi: 10.1212/01.wnl.0000264418.92308.a4. [DOI] [PubMed] [Google Scholar]
- Chiò A, Benzi G, Dossena M, Mutani R, Mora G. Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain. 2005;128:472–476. doi: 10.1093/brain/awh373. [DOI] [PubMed] [Google Scholar]
- Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- Desport JC, Preux PM, Truong TC, Vallat JM, Sautereau D, Couratier P. Nutritional status is a prognostic factor for survival in ALS patients. Neurology. 1999;53:1059–1063. doi: 10.1212/wnl.53.5.1059. [DOI] [PubMed] [Google Scholar]
- Drory VE, Goltsman E, Reznik JG, Mosek A, Korczyn AD. The value of muscle exercise in patients with amyotrophic lateral sclerosis. J Neurol Sci. 2001;191:133–137. doi: 10.1016/s0022-510x(01)00610-4. [DOI] [PubMed] [Google Scholar]
- Dupuis L, Oudart H, Rene F, Gonzalez de Aguilar JL, Loeffler JP. Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: benefit of a high-energy diet in a transgenic mouse model. Proc Natl Acad Sci USA. 2004;101:11159–11164. doi: 10.1073/pnas.0402026101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney ME, Fleck TJ, Himes CS, Hall ED. Riluzole preserves motor function in a transgenic model of familial amyotrophic lateral sclerosis. Neurology. 1998;50:62–66. doi: 10.1212/wnl.50.1.62. [DOI] [PubMed] [Google Scholar]
- Hamadeh MJ, Rodriquez MC, Kaczor JJ, Tarnopolsky MA. Caloric restriction transiently improves motor performance but hastens clinical onset of disease in the Cu/Zn-superoxide dismutase mutant G93A mouse. Muscle Nerve. 2005;31:214–220. doi: 10.1002/mus.20255. [DOI] [PubMed] [Google Scholar]
- Kanai K, Kuwabara S, Misawa S, Tamura N, Ogawara K, Nakata M, Sawai S, Hattori T, Bostock H. Altered axonal excitability properties in amyotrophic lateral sclerosis: impaired potassium channel function related to disease stage. Brain. 2006;129:953–962. doi: 10.1093/brain/awl024. [DOI] [PubMed] [Google Scholar]
- Kasarskis EJ, Winslow M. When did Lou Gehrig's personal illness begin? Neurology. 1989;39:1243–1245. doi: 10.1212/wnl.39.9.1243. [DOI] [PubMed] [Google Scholar]
- Kaspar BK, Frost LM, Christian L, Umapathi P, Gage FH. Synergy of insulin-like growth factor-1 and exercise in amyotrophic lateral sclerosis. Ann Neurol. 2005;57:649–655. doi: 10.1002/ana.20451. [DOI] [PubMed] [Google Scholar]
- Kieran D, Kalmar B, Dick JR, Riddoch-Contreras J, Burnstock G, Greensmith L. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat Med. 2004;10:402–405. doi: 10.1038/nm1021. [DOI] [PubMed] [Google Scholar]
- Kirkinezos IG, Hernandez D, Bradley WG, Moraes CT. Regular exercise is beneficial to a mouse model of amyotrophic lateral sclerosis. Ann Neurol. 2003;53:804–807. doi: 10.1002/ana.10597. [DOI] [PubMed] [Google Scholar]
- Kuo JJ, Siddique T, Fu R, Heckman CJ. Increased persistent Na(+) current and its effect on excitability in motoneurones cultured from mutant SOD1 mice. J Physiol. 2005;563:843–854. doi: 10.1113/jphysiol.2004.074138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzke JF. Epidemiology of amyotrophic lateral sclerosis. Adv. Neurol. 1982;36:281–302. [PubMed] [Google Scholar]
- Liebetanz D, Hagemann K, von Lewinski F, Kahler E, Paulus W. Extensive exercise is not harmful in amyotrophic lateral sclerosis. Eur J Neurosci. 2004;20:3115–3120. doi: 10.1111/j.1460-9568.2004.03769.x. [DOI] [PubMed] [Google Scholar]
- Lobsiger CS, Cleveland DW. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat Neurosci. 2007;10:1355–1360. doi: 10.1038/nn1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstreth WT, McGuire V, Koepsell TD, Wang Y, van Belle G. Risk of amyotrophic lateral sclerosis and history of physical activity: a population-based case-control study. Arch Neurol. 1998;55:201–206. doi: 10.1001/archneur.55.2.201. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Cutler RG, Camandola S. Energy intake and amyotrophic lateral sclerosis. Neuromolecular Med. 2007;9:17–20. doi: 10.1385/nmm:9:1:17. [DOI] [PubMed] [Google Scholar]
- Miller RG, Mitchell JD, Lyon M, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Cochrane Database Syst Rev. 2007 Jan;24(1) doi: 10.1002/14651858.CD001447.pub2. CD001447. [DOI] [PubMed] [Google Scholar]
- Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- Pedersen WA, Mattson MP. No benefit of dietary restriction on disease onset or progression in amyotrophic lateral sclerosis Cu/Zn-superoxide dismutase mutant mice. Brain Res. 1999;833:117–120. doi: 10.1016/s0006-8993(99)01471-7. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Shih T, Stern Y, Ottman R, Rowland LP. Premorbid weight, body mass, and varsity athletics in ALS. Neurology. 2003;59:773–775. doi: 10.1212/wnl.59.5.773. [DOI] [PubMed] [Google Scholar]
- Stam NC, Nithianantharajah J, Howard ML, Atkin JD, Cheema SS, Hannan AJ. Sex-specific behavioural effects of environmental enrichment in a transgenic mouse model of amyotrophic lateral sclerosis. Eur J Neurosci. 2008;28:717–723. doi: 10.1111/j.1460-9568.2008.06374.x. [DOI] [PubMed] [Google Scholar]
- Traynor BJ, Codd MB, Corr B, Forde C, Frost E, Hardiman OM. Clinical features of amyotrophic lateral sclerosis according to the El Escorial and Airlie House diagnostic criteria: A population-based study. Arch Neurol. 2000;57:1171–1176. doi: 10.1001/archneur.57.8.1171. [DOI] [PubMed] [Google Scholar]
- Veldink JH, Bär PR, Joosten EA, Otten M, Wokke JH, van den Berg LH. Sexual differences in onset of disease and response to exercise in a transgenic model of ALS. Neuromuscul Disord. 2003;13:737–743. doi: 10.1016/s0960-8966(03)00104-4. [DOI] [PubMed] [Google Scholar]
- Vucic S, Kiernan MC. Axonal excitability properties in amyotrophic lateral sclerosis. Clin Neurophysiol. 2006;117:1458–1466. doi: 10.1016/j.clinph.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Weydt P, Hong SY, Kliot M, Möller T. Assessing disease onset and progression in the SOD1 mouse model of ALS. Neuroreport. 2003;14:1051–1054. doi: 10.1097/01.wnr.0000073685.00308.89. [DOI] [PubMed] [Google Scholar]
- Wicks P, Ganesalingham J, Collin C, Prevett M, Leigh NP, Al-Chalabi A. Three soccer playing friends with simultaneous amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2007;8:177–179. doi: 10.1080/17482960701195220. [DOI] [PubMed] [Google Scholar]
- Wong PC, Cai H, Borchelt DR, Price DL. Genetically engineered mouse models of neurodegenerative diseases. Nat Neurosci. 2002;5:633–639. doi: 10.1038/nn0702-633. [DOI] [PubMed] [Google Scholar]
- Wooley CM, Sher RB, Kale A, Frankel WN, Cox GA, Seburn KL. Gait analysis detects early changes in transgenic SOD1(G93A) mice. Muscle Nerve. 2005;32:43–50. doi: 10.1002/mus.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanette G, Tamburin S, Manganotti P, Refatti N, Forgione A, Rizzuto N. Different mechanisms contribute to motor cortex hyperexcitability in amyotrophic lateral sclerosis. Clin Neurophysiol. 2002;113:1688–1697. doi: 10.1016/s1388-2457(02)00288-2. [DOI] [PubMed] [Google Scholar]