Abstract
Estradiol facilitates the expression of male sexual behavior in Japanese quail within a few minutes. These rapid behavioral effects of estradiol could result from rapid changes in its local production in the preoptic area by aromatase, the enzyme converting testosterone into estradiol. Alternatively, aromatase activity may remain constant but fluctuations of local estradiol production could arise from rapid changes in the concentration of the enzymatic substrate, namely testosterone. Rapid increases of circulating testosterone levels have been observed in males of various species following social encounters. Surprisingly, in quail, the interaction with a female seems to result in a decrease in circulating testosterone levels. However, in that study conducted in quail, the samples were collected at intervals longer than the recently observed rapid effects of estradiol on sexual behavior. In the present study we investigated whether plasma testosterone concentrations fluctuate on a shorter time-frame. Eleven male were tested 5 min before and 5, 15 or 30 min after being allowed to have visual access to a female or to copulate with a female for 5 min. Both types of interactions resulted in a significant decline in circulating testosterone levels at latencies as short as 5 min. These data demonstrate that the decrease in testosterone levels is initiated shortly after sexual encounters. Because visual interactions with a female did not result in a rapid increase in testosterone concentrations, these findings rule out the possibility that a rapid rise in circulating testosterone levels participates in the rapid increase in brain estrogen synthesis and its facilitatory effects on copulatory behavior.
Keywords: testosterone, male sexual behavior, radioimmunoassay, bird
Introduction
Estrogens influence numerous physiological processes through two different, but complementary, modes of actions. The classical (genomic) mechanism involves the binding of the hormone to specific nuclear receptors (estrogen receptor α and β) that act as transcription factors and initiate the transcription of target genes. These genomic effects are relatively slow and develop with latencies ranging from 1 hour to several days. Estrogens also exerts non-genomic effects consisting of rapid and transient effects that are 1) initiated at the cell membrane or in the cytoplasm, 2) do not require protein synthesis and 3) have been implicated in the modulation of neural excitability, neuroprotection and neuronal plasticity (For review, Vasudevan and Pfaff, 2007). It is also the case that cellular events activated by estrogens via non-transcriptional membrane or cytoplasmic effects can result in the induction of genomic effects in some cases through, for example, phosphorylations of nuclear receptors, co-activators or transcription factors. For this reason, these genomic effects are designated indirect genomic effects (McEwen and Alves, 1999). Over the past decade, rapid effects of estrogens on behavior presumably involving non-genomic actions have been documented across a number of different taxa such as fish, birds and rodents (Becker, 1990; Cross and Roselli, 1999; Hayden-Hixson and Ferris, 1991; Kow and Pfaff, 2004; Remage-Healey and Bass, 2004). In Japanese quail, estradiol facilitates the expression of male-typical copulatory behavior within 15 minutes in castrated males chronically treated with a sub-optimal dose of testosterone that is unable to elicit by itself sexual activity in most subjects (Cornil et al., 2006b). Such a latency of action is considerably shorter than the several days commonly required for the activation of behavioral responses by steroid hormones via genomic mechanisms thus indicating that non-genomic processes are probably involved.
One question arising from these findings is that of the source of the estrogens for these relatively rapid effects (Cornil et al., 2006a). Estrogen effects initiated at the membrane appear to require estradiol concentrations higher than those circulating in the blood (Cornil et al., 2006a; Prange-Kiel and Rune, 2006; Woolley, 2007). Also, in order to benefit from the temporal resolution provided by the fast membrane response, it is assumed that the endogenous ligand’s availability must also change quickly to activate rapid actions of estrogens. Hence, it is important to investigate whether there exist mechanisms able to modulate rapidly the local concentration of estrogens and the subsequent rapid actions of estrogens. One mechanism that can rapidly regulate brain estrogen synthesis involves rapid changes in local estrogen production by the enzyme aromatase. This enzyme catalyzes the conversion of testosterone into estradiol (Simpson et al., 1994). One of the richest populations of aromatase-containing neurons is found in the preoptic area where these neurons play a critical role in the control of male sexual behavior (Balthazart et al., 1990; Balthazart and Surlemont, 1990; Foidart et al., 1995). Although it has long been assumed that the pivotal role of aromatase relied essentially on the transcriptional control of its concentration by testosterone, a process requiring hours to days to appear, recent data unravelled a new mode of control of estrogen synthesis that is expressed on a much shorter timescale (Balthazart et al., 2004). Indeed, in vitro experiments demonstrated that preoptic/hypothalamic aromatase activity is reversibly inhibited within 5 minutes by calcium dependent phosphorylations (Balthazart et al., 2001; Balthazart et al., 2005; 2006; Balthazart et al., 2003). Rapid changes in enzymatic activity are also observed following a sexual interaction of 1 or 5 min or the exposure to an acute stress thus indicating that similar processes take place in vivo (Cornil et al., 2005). The involvement of local estrogen production by aromatase in the initiation of male sexual behavior is supported by the demonstration that the injection of aromatase inhibitors rapidly inhibits the expression of appetitive and consummatory aspects of male sexual behavior in birds and rodents (Cornil et al., 2006c). Rapid modulations of estrogen synthesis by aromatase activity thus constitute one possible mechanism to rapidly change local estrogen concentration and acutely control male sexual behavior.
It is also possible that the rapid changes in local estrogen concentrations are driven by fluctuations in the concentration of the enzymatic substrate of aromatase, namely testosterone. Such a mechanism could facilitate rapid changes in estrogen synthesis regardless of the dynamics of aromatase activity. Rapid increases (in the range of minutes) of circulating hormone levels triggered by environmental/social stimuli have been reported. In particular, a rapid rise of plasma testosterone levels has been observed in males of various vertebrate species following the presentation of a female and/or after copulation with her (Batty, 1978; Cerda-Molina et al., 2006; Kamel et al., 1975; Kamel et al., 1977; Macrides et al., 1975; Wingfield, 1985). The fastest effects reported occurred within 5–15 min (Batty, 1978; Purvis and Haynes, 1974; Saginor and Horton, 1968). The bolus of testosterone secreted by the testes following a sexual encounter could rapidly be aromatized in the brain and the estrogen produced could then contribute to the modulation of behavior. Such a mechanism would provide anatomical specificity to the behavioral response since only aromatase-containing regions would react even though the entire body (and brain) would be exposed to increased testosterone concentrations. This mechanism could function in parallel to the rapid modulations of estrogen synthesis by changes of aromatase activity.
A previous study investigated the endocrine response of males to visual presentation of a female Japanese quail and found that such interactions result in a decrease in the concentration of plasma testosterone (Delville et al., 1984). However, the samples were collected at intervals of 1 hour, 4 hours to several days that are longer than the recently observed rapid effects of estradiol. The present study was designed to determine whether circulating testosterone concentration varies on a time scale compatible with the documented rapid effects of estrogens on male sexual behavior. For this purpose, sexually experienced males were allowed to see a female through a plexiglass barrier for 5 min (Experiment 1) or to copulate with a female for 5 min (Experiment 2). Appetitive and consummatory aspects of male sexual behavior were recorded and blood samples were taken before, immediately after, and 15 or 30 minutes after this interaction. In order to avoid the problem of repeated handling and bleeding of the same birds within the sampling period (a procedure that could result in elevated concentrations of corticosterone), blood samples were collected from the same birds once a week at the 4 different time points.
Methods
Experimental animals
Eleven sexually mature and experienced male Japanese quail (Coturnix japonica) were used as subjects. Eighteen sexually mature and experienced females served as stimuli. All birds were purchased from a local breeder (CBT Farms, Chestertown, MD). Males were housed individually, while females were housed in common brooder cages. All animals were housed under a 14L:10D photoperiod regimen and were provided with food and water ad libitum. Birds were also periodically weighed to the nearest gram and the size of their cloacal gland was measured with calipers (greatest width × greatest length in mm2 = cloacal gland area). The size of the cloacal gland in male quail is positively correlated in a close manner with circulating testosterone concentrations (Sachs, 1967). All procedures were approved by the Johns Hopkins Animal Care and Use Committee.
Behavioral tests
Rhythmic cloacal sphincter movements (RCSM) are produced in response to the visual presentation of a female. These sphincter muscle movements are used by reproductively active males just before copulation to produce a stiff meringue-like foam that is transferred into the female’s cloaca during copulation and that is thought to enhance the male fertilization success (Seiwert and Adkins-Regan, 1998). This foam is produced by the cloacal gland, a large sexually dimorphic, androgen-sensitive, external protuberance of the caudal lip of the cloaca (Sachs, 1967). It has been shown previously that gonadally intact, sexually active males rapidly increase the rate of these movements when they are provided with visual access to a female (Seiwert and Adkins-Regan, 1998; Thompson et al., 1998). Interestingly, the RCSM rate is higher when a sexually mature male is presented with visual access to a female as compared to a male suggesting that these cloacal contractions are not simply triggered by visual cues of a general sort but are preferentially induced by visual access to a sexual partner (Seiwert and Adkins-Regan, 1998). Their expression depends on the endocrine status of the birds: the RCSM frequency decreases after castration but increases following a systemic treatment with exogenous testosterone (Balthazart et al., 1998). These movements are also inhibited by lesions of the preoptic area, a brain region that is known to control appetitive as well as consummatory aspects of sexual behavior (Balthazart and Ball, 1998). These movements thus provide an excellent measure of appetitive sexual behavior in quail. We argue that measurement of the RCSM response rate in the presence of a female is a good measure of the male’s propensity to engage in copulation per se (i.e. a measure of his underlying sexual motivation) (Seiwert and Adkins-Regan, 1998).
RCSM were quantified in an aquarium (40 cm long × 20 cm wide × 25 cm high) located on a raised platform. A mirror was placed under the aquarium at a 45° angle and provided the observer with an unobstructed view of the male’s cloacal area. At the beginning of each behavioral test, the aquarium was divided into two chambers by an opaque sliding panel and glass partition. One experimental male was placed in one of the chambers and a stimulus egg-laying female was placed in the other chamber. RCSMs occurring spontaneously or following defecation were directly counted for 5 min during which the male could not see the female (Basal RCSMs). The sliding panel was then removed so that the male and female were only separated by a glass partition. The male had visual access to the female although he could not physically interact with her. The RCSM were quantified by direct observation for an additional 5 min under these conditions (female elicited RCSMs).
Consummatory sexual behavior
Copulatory behavior was quantified in a wooden arena separate from the aquarium used to quantify RCSMs (60 × 40 × 50 cm). The experimental male was introduced into the empty arena and left undisturbed for 5 min for a period of habituation after which he was provided with a sexually mature female with which the male could freely interact during a 5 min period. During that time, the frequency and latency of the first occurrence of male sexual behaviors were directly recorded. The following behavior patterns were systematically noted: strut, neck-grab (NG), mount attempt (MA), mount (M) and cloacal contact movements (CCM) (For a detailed description, see (Adkins and Adler, 1972; Hutchison, 1978). These data provided a measure of the consummatory behavior of the birds.
Experimental procedure
Before the beginning of the study, all subjects received three pre-test copulatory trials. Birds were then ranked according to the CCM frequency they displayed on the last pre-test trial. Based on this ranking, they were assigned to four different groups to make sure that subjects showing high and low sexual activity were evenly distributed among the four groups. This was verified by one-way ANOVAs for CCM frequencies and latencies (CCM frequency: F3,8 = 0.129, p = 0.9399; CCM latency: F3,8 = 0.571, p = 0.6498). A one-way ANOVA (F3,8 = 0.912, p = 0.4771) was also performed to ensure that the cloacal gland area, which positively correlates with circulating testosterone concentrations (Sachs, 1967), did not differ between groups and would then not bias the statistical results of the order of testing since each group was tested for each condition in a different order. Body weight was also found not to differ between groups (F3,8 = 0.648, p = 0.6057). During the pre-test week, birds also received three habituation trials to the aquarium where they will receive visual access to the female.
Experiment 1 assessed the effect of visual access to a female on plasma testosterone concentrations, while Experiment 2 tested the effect of copulating with a female on plasma testosterone concentrations. Both experiments were conducted following the same experimental procedure (Fig. 1). Blood samples were taken from each animal once a week at one of the following time points in the experimental procedures each week based on each group the animals belonged to: immediately after the habituation period (−5 min) or 0, 15 or 30 min after the completion of the sexual interaction. Four birds were taken out of their home cage at a time and transported to the testing room in a bird carrier (widowhood box designed for pigeons with 6 compartments) to which they were already habituated. While the other subjects waited in the carrier, the first subject was tested for the condition “30min”. It was then returned to the bird carrier and left undisturbed until its blood was collected. In the meantime, the second subject was placed in the empty test arena for 5 min after which its blood was immediately collected (condition “−5min”). The third subject was then placed in the empty arena. After 5 min of habituation, it was allowed to (visually or physically) interact for 5 min with a female. Its blood was collected immediately after the end of the behavioral test (condition “0min”). After the blood of the first subject had been collected, the last subject was tested for the condition “15min”. It was then returned to the bird carrier and left undisturbed until its blood was collected. Subjects were then all returned to their respective home cages. The handling time was recorded throughout the experiments, and was defined as the interval from the moment the subjects were taken out of the home cage until the blood sample was collected.
Figure 1.
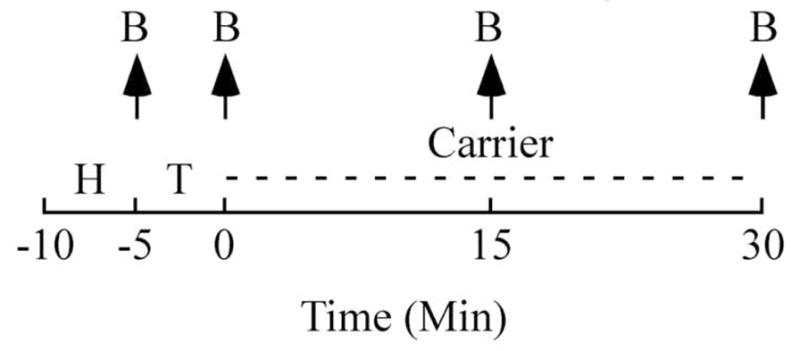
Time-line (minutes) illustrating the sampling schedule used for both experiments. Blood samples (B) were drawn at 4 time points indicated by the arrows point up. Birds were used as their own control so that their blood was collected once a week at a different time point. The collection was made in a different order for the 4 different groups in order to control for the order of testing. H: Habituation in the testing arena (5 min), T: test (5 min) consisting in visual presentation of a female (Experiment 1) or in copulating with a female (Experiment 2). After the sexual interaction, the birds tested for latencies of 15 or 30 min were returned to the carrier (as represented by the dashed line) and left undisturbed until their blood was collected.
In experiment 1, the animals were tested once a week between 2pm and 6pm. Experiment 1 was completed within 4 weeks after which subjects were left undisturbed for 1 month. One week prior to the start of experiment 2, blood was collected from each bird to get a baseline value of plasma testosterone concentration in birds who had not encountered a social interaction. For this purpose, the subjects were taken (6 at a time) in the transportation box and transported to the room where blood collection took place. One week later, birds were tested once a week between 3:30 pm and 7:30pm for 4 weeks to complete experiment 2. Two weeks after the completion of experiment 2, blood was collected immediately after each bird had been taken out of its home cage. These measurements were used as control values for potential anticipation of coming social interactions potentially signaled by transportation, time spent waiting in the carrier or the placement in the empty test arena.
Testosterone assay
Samples were taken by puncturing the alar (wing) vein with 25-gauge needle and 500 to 1000 μl of blood was collected into heparinized tubes. The blood samples were transferred into microfuge tubes and centrifuged at 9000 g for 9 min. The plasma was removed and stored in 1.5ml Eppendorf® vials at −20°C until assayed for testosterone. The plasma was analyzed in duplicate (50μl) using a commercially available 125I Coat-A-Count kit for total testosterone (Siemens Medical Solutions Diagnostics, Los Angeles, CA). In order to increase the sensitivity of the assay we extended the lower limit of the standard curve by diluting the lowest standard control to yield concentrations of 25, 50 and 100 pg/ml. As illustrated in figure 2, this extension does not affect the linearity of the curve up to 100pg/ml. The comparison of the raw data with the standard curve indicates that 98.4% of the samples (123 out of 125) were on its linear portion. The 2 samples found to be out the linear portion of the standard curve belong to the “carrier baseline” measurements. This assay was highly sensitive (i.e. 100 pg/ml) and is highly specific for testosterone and shows negligible cross reactivity with other steroids including dihydrotestosterone (<3.5%); 17β-estradiol (< 0.01%); corticosterone (< 0.01%) and this kit has been used in previous avian studies (Stevenson et al., 2008). All samples were run in a single assay and the intra-assay coefficient of variation averaged 12%.
Figure 2.
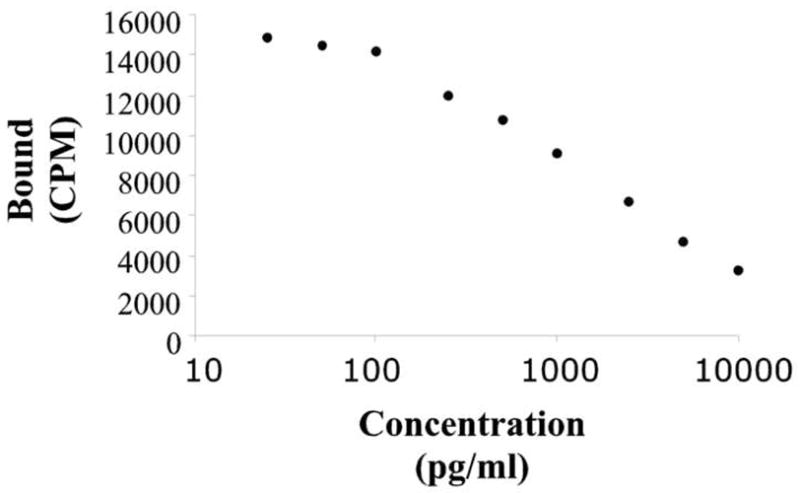
Standard curve employed in our assay system (125I Coat-A-Count kit for total testosterone [Siemens Medical Solutions Diagnostics, Los Angeles, CA]) to convert bound fraction to known testosterone concentrations. To increase the sensitivity of the assay, the lower limit of the standard curve was extended to concentrations of 25, 50 and 100 pg/ml. This extension did not affect the linearity of the curve up to 100pg/ml. Only 2 samples out of 125 were found to be beyond this linear portion. For more detail, see methods.
Data analysis
Unless stated otherwise, plasma testosterone concentrations were compared by mixed design ANOVAs with the latency from sexual interaction to bleeding as a repeated factor and groups as the independent factor. Pearson correlation analysis was used to determine the extent to which plasma concentrations of testosterone correlated with RCSM, MA and CCM frequencies as well as handling time.
Results
Experiment 1: Effects of visual exposure to a female
The analysis of the measures of the androgen-dependent cloacal gland revealed no effect of the repeated sexual interaction (F3,21 = 1.301, p = 0.3002) as well as no difference among groups and no interaction between the two factors (F3,7 = 0.637, p = 0.6147; F9,21 = 1.354, p = 0.2695). The body weight of the four groups of birds was similar at the start of the experiment (see methods) and it was not affected by repeated visual presentation of a female and did not change during the course of the experiment (no significant effect of main factors and no interaction in the ANOVA; Repetition, F3,21 = 2.330, p = 0.1036; F3,7 = 0.333, p = 0.8025; F9,21 = 1.070, p = 0.4231).
A mixed-design ANOVA with the latency to blood draw as the repeated factor and the experimental groups as the independent factor revealed a significant effect of latency from visual interaction with a female on circulating concentration of testosterone (F3,21 = 4.487, p = 0.0139) but no effect of test order (groups) and interaction between the two factors (F3,7 = 0.285, p = 0.8347; F9,21 = 0.922, p = 0.5262). Given that no group effect was identified and in order to determine the origin of the latency difference, a one-way ANOVA with the latency as the repeated measure followed by a Tukey post-hoc analysis was then run. As illustrated in figure 3, this analysis revealed that the significant effect of treatment (F3,30 = 4.930, p = 0.0067) results from a significant reduction of circulating testosterone levels measured 30 min after visual interaction as compared to the levels measured in the absence of female (−5 min) or immediately after her presentation (0 min) as indicated by the Tukey post-hoc analysis.
Figure 3.
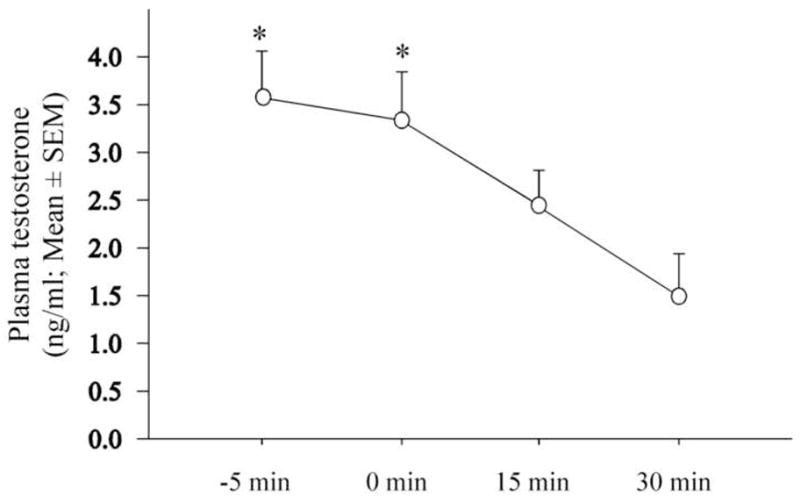
Effect of the exposure to a female on circulating testosterone concentration collected at different time points before (−5min) or after (0, 15 and 30 min) the sexual interaction. * p < 0.05 as compared to 30 min
The correlation analysis found no significant relationship between RCSM frequency and plasma testosterone levels at the 3 latencies following the female presentation (0 min: R = 0.483, F1,10 = 2.732, p = 0.1328; 15 min: R = 0.532, F1,10 = 3.454, p = 0.0924; 30 min: R = 0.202, F1,10 = 0.382, p = 0.5520).
The handling time varied by ± 15 min for each time point. Linear regression of plasma testosterone concentration (T) as a function of handling time (HT) revealed no significant correlation between the two variables at the various time points (−5 min: T = − 0.013 HT + 5.009, p = 0.8036; 0 min, T = − 0.011 HT + 3.289, p = 0.7793; 15 min, T = 0.052 HT + 0.838, p = 0.3375; 30 min, T = − 0.006 HT + 2.007, p = 0.9137) suggesting that there is no effect of handling on testosterone concentration within a period of 50 minutes.
Experiment 2: Effects of copulation
The analysis of the measures of the androgen-dependent cloacal gland revealed a significant effect of repeated copulations (F3,21 = 3.387, p = 0.0372) but no difference among groups and no interaction between the two factors (F3,7 = 1.212, p = 0.3738; F9,21 = 1.333, p = 0.2790; data not shown). A significant main effect of repetition was also found for the body weight (F3,21 = 25.670, p < 0.0001) as well as an interaction of the repetition (F9,21 = 4.058, p = 0.0039) and the group but no group difference was identified (F3,7 = 0.524, p = 0.6794; data not shown).
A mixed-design ANOVA with the latency to draw blood as the repeated factor and the experimental groups as the independent factor revealed a significant effect of latency from visual interaction with a female on the circulating concentration of testosterone (F3,21 = 9.610, p = 0.0003) but no effect of test order (groups) and the interaction between the two factors (F3,7 = 2.155, p = 0.1815; F9,21 = 1.038, p = 0.4440). Given that no group effect was identified, the data of the 4 groups were pooled to determine the origin of the latency difference. A one-way ANOVA with the latency as the repeated measure followed by a Tukey post-hoc analysis indicated a significant effect of treatment (F3,30 = 9.858, p = 0.0001) resulting from a significant reduction of circulating testosterone levels measured 15 and 30 min after copulation as compared to the levels measured immediately after (0 min) or before copulation (−5 min; Fig. 4).
Figure 4.
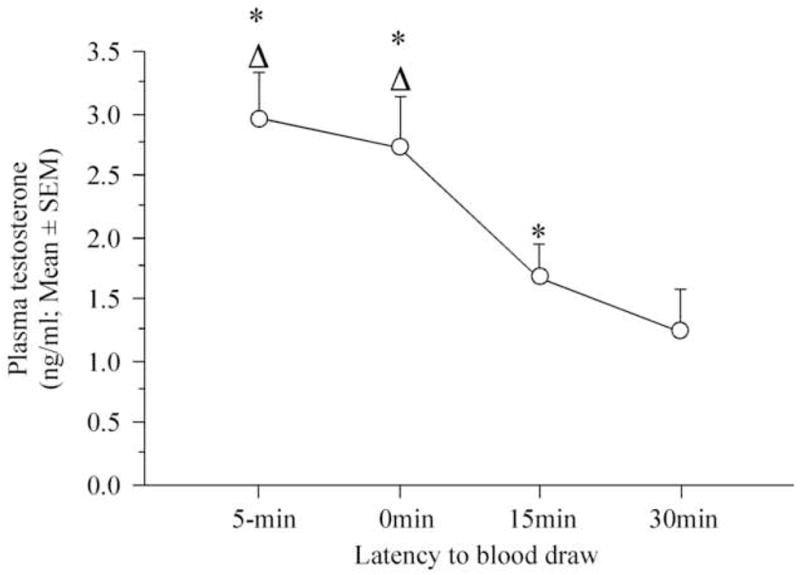
Effect of copulation on circulating testosterone concentration collected at different time points before (−5min) or after (0, 15 and 30 min) the sexual interaction. * p < 0.05 as compared to 30 min, Δp < 0.05 as compared to 15 min.
The correlation analysis revealed no significant relationship between MA and CCM frequency and plasma testosterone levels at the 3 latencies following the female presentation (MA: 0min, R = 0.463, F1,10 = 2.452, p = 0.1518; 15 min, R = 0.497, F1,9 = 2.954, p = 0.1198; 30 min, R = 0.163, F1,9 = 0.247, p = 0.6313; CCM: R = 0.436, F1,10 = 2.110, p = 0.1803; 15 min, R = 0.211, F1,10 = 0.421, p = 0.5327; 30 min, R = 0.170, F1,10 = 0.327, p = 0. 5814).
As in experiment 1, no correlation was found between handling time and testosterone levels (p > 0.1505). In average, handling time lasted 15, 30, 45 and 40 minutes for the 4 time points studied (−5, 0, 15 and 30 min, respectively).
Comparison of control conditions and baseline measurements
The analysis of testosterone levels obtained from birds taken out of the home cage and transported in the test room for the blood collection (“carrier control”) and from birds taken out of their home cage and immediately bled in the animal room (“home cage control”) as compared to the control condition (−5 min) of experiments 1 and 2 revealed no difference between these measures (F3,30 = 2.057, p = 0.1269; Figure 5).
Figure 5.
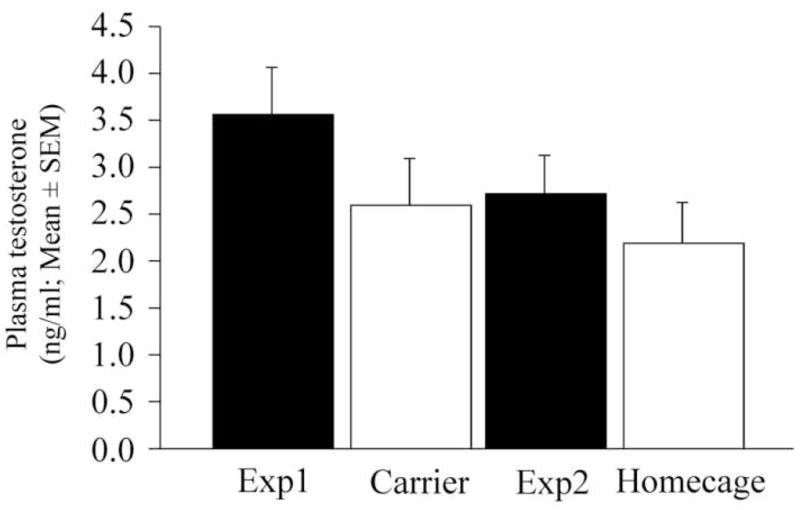
Comparison of the control conditions of experiments 1 and 2 with the two baseline measures of circulating levels of testosterone. See text for more details.
Discussion
It is well known that intersexual behavioral interactions can result in dramatic changes in male reproductive physiology (For review, see (Ball and Balthazart, 2002; Crews and Silver, 1985; Wingfield, 2006). For example, in one of the first studies of this sort Burger demonstrated that the presence of a female starling (Sturnus vulgaris) significantly enhances seasonal recrudescence in testicular volume (Burger, 1953). A series of detailed experimental studies in ring doves (Streptopelia risoria) (Lehrman, 1965) and canaries (Serinus canaria) (Hinde, 1965) provided strong support for the importance of behavioral synchronizing cues in coordinating the timing of key reproductive events between sexes. However, the vast majority of research on synchronizing cues have primarily investigated such effects over prolonged time periods (i.e., over intervals of at least four hours to one day; Feder et al., 1977; Gwinner et al., 1988; Moore, 1983; O’Connell et al., 1981a; O’Connell et al., 1981b; Pinxten et al., 2003; Rundfedlt and Wingfield, 1985). As detailed in the introduction, social encounters have been found to result in rapid increases of circulating hormone levels in males of various species. The present experiment provides evidence demonstrating that behavioral interactions between conspecifics can also result in a rapid decrease in hormone concentrations. These data indicate that visual interactions as well as copulation with a female result in a significant decline in circulating testosterone concentration after latencies as short as 20 minutes after the beginning of the sexual interaction. This confirms the observations made at longer latencies and demonstrates that the decline in plasma testosterone levels observed previously (Delville et al., 1984) is probably initiated shortly after sexual encounters.
Mechanism of the rapid decline of plasma testosterone
Previous data collected in quail also failed to establish that the presence of a hen can enhance testicular secretion in the male (Delville et al., 1984; Meddle et al., 1997). The depressed testosterone concentrations reported in some of these experiments were generally associated with elevated concentrations of corticosterone suggesting that the stress of manipulation is a possible cause of the plasma testosterone decline. In quail as in other species, exposure to a stressor results in a rapid increase in plasma corticosterone concentrations persisting as long as the stressor is present. Corticosterone drops within 15 to 30 min after stress cessation (Hazard et al., 2008; Hull et al., 2007). Evidence collected in mammals suggest that such a rise in corticosterone is responsible for stress-induced plasma testosterone fall through an acute action of corticosterone on testis physiology associated with the short half-life of testosterone in circulation (Dong et al., 2004). In the present experiments, handling time included several types of manipulation such as the transfer from the home cage to the carrier, the transportation, etc. Therefore it could be argued that stress encompassed the entire handling period. However, since most of the handling time was relatively similar for all collection times, it is assumed that subjects were submitted to a comparable level of stress in the various conditions tested. In support of this, correlation analyses failed to establish a relationship between time of handling and testosterone concentration. Also, although the animals submitted to the −5 or 0 min condition had already been manipulated for more than 10–15 min (latency at which stress effects are detected on T levels) by the time they reached blood collection, their testosterone titer did not differ from control levels of testosterone taken from the subjects taken out of the home cage and transported in the carrier but which were neither placed in the test arena nor allowed to experience a social interaction. Finally, if all these manipulations cause such a high degree of stress that it results in a decline in circulating testosterone, why is it that such a decline was not observed in other species? In ring doves, a slight testosterone rise is observed 4 hours after pairing with a courting female and testosterone peaks after one day of pairing (Feder et al., 1977). Previous data on quail indicate that the decline in circulating testosterone concentrations observed here only a few minutes after an interaction with a female lasts for at least a day and has disappeared after 3 days (Delville et al., 1984). This seems too long for a response to an acute stress. If anything, such response resembles this of male ring doves paired with females but with an opposite direction. Together, these data tend to eliminate the stress of manipulation as the primary source of the rapid decline in testosterone levels observed here and suggest that this decline is specifically associated to the interval of time separating blood collection from the sexual interaction. The underlying mechanism of such rapid decline is unknown at present. In ring doves, the secretion of testosterone is facilitated by auditory and visual cues from the courting female, similarly it is possible that female cues depress circulating testosterone in quail (O’Connell et al., 1981a). Further experiments should be carried out to confirm this hypothesis.
Contribution to the rapid changes in E2 concentrations and rapid effects of E2 on behavior?
The working hypothesis tested in this study was that, based on previous studies conducted in a variety of vertebrate species, it was possible that female cues induce a rapid elevation in plasma testosterone levels in males that could contribute to the rapid increase of local estrogen formation thought to play a role in the initiation of male sexual behavior. Indeed, peripheral injections of high doses of 17β-estradiol rapidly stimulate the expression of copulatory behavior, a significant behavioral stimulation is observed after only 5 minutes and reach a maximum after 15 minutes, while the acute blockade of estrogen synthesis by aromatase results in a rapid inhibition of both appetitive and consummatory aspects of male sexual behavior in quail as well as in mice. Such a rapidity of action is quite striking given that these effects were observed following systemic injections so that the hormone had first to reach the target tissue and accumulate above a certain threshold before it could activate a cellular response and in turn trigger the neuronal circuits involved in the behavioral activation. It is thus very likely that the effects would be even faster if the injections could have been delivered immediately to its targets in effective concentrations or if the modulation of the concentration of substrate for aromatization was provided endogenously. These pharmacological data thus suggest that a rise of estrogen synthesis is involved in the initiation of male sexual behavior. There is evidence that aromatase activity can rapidly change (within 5 minutes) in vitro and rapid changes of estrogen production have also been reported in vivo following male sexual behavior. The present study tested the possibility that rapid changes in circulating levels of testosterone could also contribute to the rapid rise of estrogen synthesis assumed to play a critical role in the activation of male sexual behavior. The results show that plasma testosterone concentrations of samples collected immediately after 5 minutes of visual access to a female or copulation with her were unchanged as compared to the samples collected right before the sexual interaction. The absence of a rise of testosterone in the plasma of birds displaying appetitive or consummatory sexual behavior indicates that, in quail at least, it is unlikely that rapid changes of estrogen production in the preoptic area are driven by a rapid increase of circulating testosterone concentration converted into estrogens through a steady-state aromatase activity. This observation appears to rule out the possibility that the initiation of sexual behavior relies on a rapid rise of circulating testosterone.
Alternatively one could argue that the testosterone levels measured during the habituation period (5 min condition) are in fact elevated as compared to the home cage situation as a result of the anticipation of the social interaction to come. There is indeed evidence in rodents that the presentation of a neutral stimulus previously paired with copulation with a female can result in a rapid increase in the concentration of circulating testosterone (Graham and Desjardins, 1980). It is thus possible that the transportation, the time spent in the carrier and the habituation to the empty test arena might serve as conditioned stimuli predicting the social encounter and thus result in a modulation of testosterone release. In contrast, since they were also regularly taken out their home cage to be weighted or to measure their cloacal gland, it is unlikely that taking the animals out of their home cage could serve as a signal of a future social encounter. The comparison of the control levels of both experiments with the baseline levels obtained from birds taken out of their home cage (“home cage baseline” in fig. 5) indicates that these levels are not significantly different. This is especially true when compared with the levels of experiment 2 that have been obtained in a comparable interval of time. It thus seems unlikely that the anticipation of the social encounter resulted in an elevated testosterone titer in the control condition as compared to the blood collected from resting animals taken from their home cage.
Testosterone is not the only substrate of aromatase. Other androgens, such as androstenedione and dehydroepiandrosterone (DHEA), are also present in the plasma (Balthazart et al., 1986; Hutchison et al., 1984; Soma et al., 2008). DHEA is not directly aromatizable, it first needs to be converted into androstenedione by the 3β-hydroxysteroid dehydrogenase/isomerase (3β-HSD). Androstenedione can then be aromatized into estrone, which is reversibly transformed into estradiol by the enzyme 17β-hydroxysteroid dehydrogenase (17β-HSD), an enzyme also responsible for the reversible conversion of androstenedione into testosterone. 3β-HSD and 17β-HSD are both present in the avian brain and highly expressed in the preoptic area, in particular (London et al., 2003; London et al., 2006). These other androgens found in the circulation could thus also serve as substrates for estrogen synthesis, but whether their blood concentration fluctuates during or following sexual encounters is not known. Future studies should assess this possibility.
Altogether, the present results thus suggest that the rapid modulations of estrogen production by changes of aromatase kinetics within a few minutes identified in vitro (Balthazart et al., 2001; Balthazart et al., 2005; 2006; Balthazart et al., 2003; Remage-Healey et al., 2008) currently appear as the only mechanism able to rapidly change local estrogen concentration.
Functional significance of such reduced circulating testosterone levels following social interactions
In most mammalian and avian species studied to date, the presence of a female tends to result in an increase in testosterone release (Ball and Balthazart, 2002; Wingfield, 1994; 2006). It is therefore somewhat surprising that, in quail, sexual interaction with a female results in a reduction of circulating androgen levels. Obviously such a drop of testosterone is not necessary for copulation since it occurs after copulation is complete. But it is possible that it has something to do with testicular function. In ring doves, it was proposed that the testosterone rise induced by the female might influence sperm maturation such that the spermatogenic activity of males would be enhanced during courtship, which last for several days in this species, thus increasing their chance to fertilize eggs (O’Connell et al., 1981b). However, as opposed to doves, courtship behavior in quail is quite brief. Under lab conditions, experienced males achieve cloacal contact and sperm transfer within a few seconds. This might explain why there is no rise in testosterone but it does not explain why it drops. It could be argued that this decline reflects the termination of the behavioral sequence. Yet, testosterone also drops after the male has only been visually exposed to the female so that seems to be an unlikely explanation. Given the findings of our study and the previous study by Delville et al (1984) it does seem to be the case that the presence of a female can lead to a decrease in testosterone concentrations in some species. The reasons for such a pattern have not been systematically investigated.
In conclusion, although the origin and the function of the fall of circulating testosterone concentrations identified 15 and 30 minutes after sexual interaction remains to be elucidated, the present data indicate the rapid changes in blood concentrations of testosterone described here are probably not involved in rapid fluctuations of estrogen concentrations in the preoptic area and in the subsequent promoting effects of locally produced estrogens on male sexual behavior. Indeed, circulating concentrations of testosterone do not appear to fluctuate within a time scale compatible with the rapid behavioral effects of estrogens which have been shown to stimulate behavior within 5 minutes (Cornil et al., 2006b). Moreover, pharmacological manipulations suggest that the initiation of male sexual behavior by estrogens should be preceded by a rapid increase in local estrogen concentration (Cornil et al., 2006b; Cornil et al., 2006c). The decline of peripheral levels of testosterone shown in this study makes it even less likely to result in such an increased estrogen synthesis. Key questions remain to be resolved in order to understand definitively the relationships between circulating testosterone and changes in brain concentrations of estrogens. For example, how efficiently can peripheral steroids reach particular brain sites? Are steroids produced locally in brain sites such as the preoptic area in addition to steroids locally metabolized based on substrates produced in the periphery? A new generation of studies precisely measuring steroid concentrations in particular brain areas is emerging (Meffre et al., 2007; Remage-Healey et al., 2008) and will be important in resolving these issues.
Acknowledgments
We would like to thank Drs Jacques Balthazart and Mélanie Taziaux for their helpful discussions and advices throughout this study as well as Dr Thierry D. Charlier and two anonymous reviewers for their thorough reading and comments on a previous version of this manuscript. This work is supported by a grant from the NIH (R01 MH 50388). CAC is a F.N.R.S. Postdoctoral Researcher and TJS is supported by an NSERC PGS-D 334570.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins EK, Adler NT. Hormonal control of behavior in the japanese quail. J Comp Physiol Psychol. 1972 ;81:27–36. doi: 10.1037/h0033315. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Neuroendocrine mechanisms regulating reproductive cycles and reproductive behavior in birds. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, brain and behavior. Academic Press; San Diego, CA: 2002. pp. 649–798. [Google Scholar]
- Balthazart J, Absil P, Gérard M, Appeltants D, Ball GF. Appetitive and consummatory male sexual behavior in japanese quail are differentialy regulated by subregions of the preoptic medial nucleus. Journal of Neuroscience. 1998;18:6512–6527. doi: 10.1523/JNEUROSCI.18-16-06512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF. Rapid and reversible inhibition of brain aromatase activity. J Neuroendocrinol. 2001;13:63–73. doi: 10.1046/j.1365-2826.2001.00598.x. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF. Interactions between kinases and phosphatases in the rapid control of brain aromatase. J Neuroendocrinol. 2005;17:553–559. doi: 10.1111/j.1365-2826.2005.01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology. 2006;147:359–366. doi: 10.1210/en.2005-0845. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Charlier TD, Ball GF. Calcium-dependent phosphorylation processes control brain aromatase in quail. European Journal of Neuroscience. 2003;17:1591–1606. doi: 10.1046/j.1460-9568.2003.02598.x. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Cornil CA, Ball GF. Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action. Physiology and Behavior. 2004;83:247–270. doi: 10.1016/j.physbeh.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. The japanese quail as a model system for the investigation of steroid-catecholamine interactions mediating appetitive and consummatory aspects of male sexual behavior. Annual Review of Sex Research. 1998;9:96–176. [PubMed] [Google Scholar]
- Balthazart J, Delville Y, Sulon Y, Hendrick JC. Plasma levels of luteinizing hormone and of five steroids in photostimulated, castrated and testosterone-treated male and female Japanese quail (Coturnix coturnix japonica) General Endocrinol (Life SciAdv) 1986;5:31–36. [Google Scholar]
- Balthazart J, Evrard L, Surlemont C. Effects of the non-steroidal inhibitor R76713 on testosterone-induced sexual behavior in the japanese quail (Coturnix coturnix japonica) Hormones and Behavior. 1990;24:510–531. doi: 10.1016/0018-506x(90)90039-z. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Surlemont C. Androgen and estrogen action in the preoptic area and activation of copulatory behavior in quail. Physiol Behav. 1990;48:599–609. doi: 10.1016/0031-9384(90)90198-d. [DOI] [PubMed] [Google Scholar]
- Batty J. Acute changes in plasma testosterone levels and their relation to measures of sexual behaviour in the male house mouse (Mus musculus) Anim Behav. 1978;26:349–357. doi: 10.1016/0003-3472(78)90053-2. [DOI] [PubMed] [Google Scholar]
- Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neuroscience Letters. 1990;118:169–171. doi: 10.1016/0304-3940(90)90618-j. [DOI] [PubMed] [Google Scholar]
- Burger JW. The effect of photic and psychic stimuli on the reproductive cycle of the male starling, Sturnus vulgaris. Journal of Experimental Zoology. 1953:124. [Google Scholar]
- Cerda-Molina AL, Hernandez-Lopez L, Chavira R, Cardenas M, Paez-Ponce D, Cervantes-De la Luz H, Mondragon-Ceballos R. Endocrine changes in male stumptailed macaques (Macaca arctoides) as a response to odor stimulation with vaginal secretions. Horm Behav. 2006;49:81–87. doi: 10.1016/j.yhbeh.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Ball GF, Balthazart J. Functional significance of the rapid regulation of brain estrogen action: Where do the estrogens come from? Brain Res. 2006a;1126:2–26. doi: 10.1016/j.brainres.2006.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Balthazart J. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behavioural Brain Research. 2006b;66:110–123. doi: 10.1016/j.bbr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, Balthazart J. Sexual behavior affects preoptic aromatase activity and brain monoamines’ levels. Endocrinology. 2005;146:3809–3820. doi: 10.1210/en.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Taziaux M, Baillien M, Ball GF, Balthazart J. Rapid effects of aromatase inhibition on male reproductive behaviors in Japanese quail. Horm Behav. 2006c;49:45–67. doi: 10.1016/j.yhbeh.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Silver R. In: Reproductive physiology and behavior interactions in nonmammalian vertebrates Handbook of behavioral Neurobiology. Adler N, Pfaff D, Goy RW, editors. Vol. 7. Plenum Publishing Co; New York: 1985. pp. 101–182. [Google Scholar]
- Cross E, Roselli CE. 17β-estradiol rapidly facilitates chemoinvestigation and mounting in castrated male rats. American Journal of Physiology. 1999;276:R1346–R1350. doi: 10.1152/ajpregu.1999.276.5.R1346. [DOI] [PubMed] [Google Scholar]
- Delville Y, Sulon J, Hendrick JC, Balthazart J. Effect of the presence of females on the pituitary-testicular activity in male Japanese quail (Coturnix coturnix japonica) Gen Comp Endocrinol. 1984;55:295–305. doi: 10.1016/0016-6480(84)90115-1. [DOI] [PubMed] [Google Scholar]
- Dong Q, Salva A, Sottas CM, Niu E, Holmes M, Hardy MP. Rapid glucocorticoid mediation of suppressed testosterone biosynthesis in male mice subjected to immobilization stress. J Androl. 2004;25:973–981. doi: 10.1002/j.1939-4640.2004.tb03170.x. [DOI] [PubMed] [Google Scholar]
- Feder HH, Storey A, Goodwin D, Reboulleau C, Silver R. Testosterone and “5alpha-dihydrotestosterone” levels in peripheral plasma of male and female ring doves (Streptopelia risoria) during and reproductive cycle. Biol Reprod. 1977;16:666–677. doi: 10.1095/biolreprod16.5.666. [DOI] [PubMed] [Google Scholar]
- Foidart A, Reid J, Absil P, Yoshimura N, Harada N, Balthazart J. Critical re-examination of the distribution of aromatase-immunoreactive cells in the quail forebrain using antibodies raised against human placental aromatase and against the recombinant quail, mouse or human enzyme. J Chem Neuroanat. 1995;8:267–282. doi: 10.1016/0891-0618(95)00054-b. [DOI] [PubMed] [Google Scholar]
- Graham JM, Desjardins C. Classical conditioning: induction of luteinizing hormone and testosterone secretion in anticipation of sexual activity. Science. 1980;210:1039–1041. doi: 10.1126/science.7434016. [DOI] [PubMed] [Google Scholar]
- Gwinner H, Gwinner E, Dittami J. Effect of nestboxes on LH, testosterone, testicular size and the reproductive behavior of male European starlings in spring. Behavior. 1988;103:68–81. [Google Scholar]
- Hayden-Hixson DM, Ferris CF. Steroid-specific regulation of agonistic responding in the anterior hypothalamus of male hamsters. Physiology & Behavior. 1991;50:793–799. doi: 10.1016/0031-9384(91)90020-o. [DOI] [PubMed] [Google Scholar]
- Hazard D, Couty M, Richard S, Guemene D. Intensity and duration of corticosterone response to stressful situations in Japanese quail divergently selected for tonic immobility. Gen Comp Endocrinol. 2008;155:288–297. doi: 10.1016/j.ygcen.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Hinde RA. Interaction of internal and external factors in integration of canary reproduction. In: Beach FA, editor. Sex and Behavior. John Wiley and Sons; New York: 1965. pp. 381–415. [Google Scholar]
- Hull KL, Cockrem JF, Bridges JP, Candy EJ, Davidson CM. Effects of corticosterone treatment on growth, development, and the corticosterone response to handling in young Japanese quail (Coturnix coturnix japonica) Comp Biochem Physiol A Mol Integr Physiol. 2007;148:531–543. doi: 10.1016/j.cbpa.2007.06.423. [DOI] [PubMed] [Google Scholar]
- Hutchison JB, Wingfield JC, Hutchison RE. Sex differences in plasma concentrations of steroids during the sensitive period for brain differentiation in the zebra finch. J Endocrinol. 1984;103:363–369. doi: 10.1677/joe.0.1030363. [DOI] [PubMed] [Google Scholar]
- Hutchison RE. Hormonal differentiation of sexual behavior in Japanese quail. Hormones and Behavior. 1978;11:363–387. doi: 10.1016/0018-506x(78)90038-7. [DOI] [PubMed] [Google Scholar]
- Kamel F, Mock EJ, Wright WW, Frankel AI. Alterations in plasma concentrations of testosterone, LH, and prolactin associated with mating in the male rat. Horm Behav. 1975;6:277–288. doi: 10.1016/0018-506x(75)90014-8. [DOI] [PubMed] [Google Scholar]
- Kamel F, Wright WW, Mock EJ, Frankel AI. The influence of Mating and Related Stimuli on Plasma-Levels of Luteinizing-Hormone, Follicle-Stimulating Hormone, Prolactin, and Testosterone in Male Rat. Endocrinology. 1977;101:421–429. doi: 10.1210/endo-101-2-421. [DOI] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. PNAS. 2004;101:12354–11235. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman D. Interaction between internal and external environments in the regulation of the reproductive cycle of the ring dove. In: Beach FA, editor. Sex and Behavior. John Wiley and Sons; New York: 1965. pp. 355–379. [Google Scholar]
- London SE, Boulter J, Schlinger BA. Cloning of the zebra finch androgen synthetic enzyme CYP17: a study of its neural expression throughout posthatch development. J Comp Neurol. 2003;467:496–508. doi: 10.1002/cne.10936. [DOI] [PubMed] [Google Scholar]
- London SE, Monks DA, Wade J, Schlinger BA. Widespread capacity for steroid synthesis in the avian brain and song system. Endocrinology. 2006;147:5975–5987. doi: 10.1210/en.2006-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrides F, Bartke A, Dalterio S. Strange females increase plasma testosterone levels in male mice. Science. 1975;189:1104–1106. doi: 10.1126/science.1162363. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocrine reviews. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- Meddle SL, King VM, Follett BK, Wingfield JC, Ramenofsky M, Foidart A, Balthazart J. Copulation activates Fos-like immunoreactivity in the male quail forebrain. Behav Brain Res. 1997;85:143–159. doi: 10.1016/s0166-4328(97)87581-x. [DOI] [PubMed] [Google Scholar]
- Meffre D, Pianos A, Liere P, Eychenne B, Cambourg A, Schumacher M, Stein DG, Guennoun R. Steroid profiling in brain and plasma of male and pseudopregnant female rats after traumatic brain injury: analysis by gas chromatography/mass spectrometry. Endocrinology. 2007;148:2505–2517. doi: 10.1210/en.2006-1678. [DOI] [PubMed] [Google Scholar]
- Moore MC. Effect of female sexual displays on the endocrine physiology and behavior of male white crowned sparrows, Zonotrichia leucophrys. Journal of Zoology. 1983;199:137–148. [Google Scholar]
- O’Connell ME, Reboulleau C, Feder HH, Silver R. Social interactions and androgen levels in birds. I. Female characteristics asociated with increased plasam androgen levels in the male ring dove (Streptopelia risoria) Gen Comp Endocrinol. 1981a:44. doi: 10.1016/0016-6480(81)90332-4. [DOI] [PubMed] [Google Scholar]
- O’Connell ME, Silver R, Feder HH, Reboulleau C. Social interactions and androgen levels in birds. II. Social factors associated with a decline in plasma androgen levels in male ring doves (Streptopelia risoria) Gen Comp Endocrinol. 1981b;44:464–469. doi: 10.1016/0016-6480(81)90333-6. [DOI] [PubMed] [Google Scholar]
- Pinxten R, Ridder E, Eens M. Female presence effects male behavior and testosterone levels in the European starling (Sturnus vulgaris) Hormones and Behavior. 2003;44:103–109. doi: 10.1016/s0018-506x(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Rune GM. Direct and indirect effects of estrogen on rat hippocampus. Neuroscience. 2006;138:765–772. doi: 10.1016/j.neuroscience.2005.05.061. [DOI] [PubMed] [Google Scholar]
- Purvis K, Haynes NB. Short-term effects of copulation, human chorioninc gonadotrophin injection and non-tactile association with a female on testosterone levels in the male rat. Journal of Endocrinology. 1974;60:429–439. doi: 10.1677/joe.0.0600429. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Rapid, hierarchical modulation of vocal patterning by steroid hormones. Journal of Neuroscience. 2004;24:5892–5900. doi: 10.1523/JNEUROSCI.1220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels uctuate rapidly during social interactions. Nature Neuroscience. 2008 doi: 10.1038/nn.2200. Advance oline publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundfedlt S, Wingfield JC. Experimentally prolonged sexual activity in female sparrows delays termination of reproductive activity in their untreated mates. Animal Behaviour. 1985;33:403–410. [Google Scholar]
- Sachs BD. Photoperiodic control of the cloacal gland of the Japanese quail. Science. 1967;157:201–203. doi: 10.1126/science.157.3785.201. [DOI] [PubMed] [Google Scholar]
- Saginor M, Horton R. Reflex Release of Gonadotropin and Increased Plasma Testosterone Concentration in Male Rabbits during Copulation . Endocrinology. 1968;82:627. doi: 10.1210/endo-82-3-627. [DOI] [PubMed] [Google Scholar]
- Seiwert CM, Adkins-Regan E. The foam production system of the male japanese quail: characterization of structure and function. Brain BehavEvol. 1998;52:61–80. doi: 10.1159/000006553. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD, Mendelson CR, Bulun SE. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. EndocrRev. 1994;15:342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- Soma KK, Scotti MA, Newman AE, Charlier TD, Demas GE. Novel mechanisms for neuroendocrine regulation of aggression. Front Neuroendocrinol. 2008;29:476–489. doi: 10.1016/j.yfrne.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Stevenson TJ, Bentley GE, Ubuka T, Arckens L, Hampson E, MacDougall-Shackleton SA. Effects of social cues on GnRH-I, GnRH-II, and reproductive physiology in female house sparrows (Passer domesticus) Gen Comp Endocrinol. 2008;156:385–394. doi: 10.1016/j.ygcen.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Thompson RR, Goodson JL, Ruscio MG, Adkins-Regan E. Role of the archistriatum nucleus taeniae in the sexual behavior of male japanese quail (Coturnix japonica): a comparison of function with the medial nucleus of the amygdala in mammals. Brain Behav Evol. 1998;51:215–229. doi: 10.1159/000006539. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Membrane Initiated Actions of Estrogens in Neuroendocrinology: Emerging Principles. Endocr Rev. 2007;28:1–19. doi: 10.1210/er.2005-0021. [DOI] [PubMed] [Google Scholar]
- Wingfield JC. Short-term changes in plasma levels of hormones during establishment and defense of a breeding territory in male song sparrows, Melospiza melodia. Horm Behav. 1985;19:174–187. doi: 10.1016/0018-506x(85)90017-0. [DOI] [PubMed] [Google Scholar]
- Wingfield JC. Hormone-behavior interactions and mating systems in male and female birds. In: Short RV, Balaban E, editors. The differences between sexes. Cambridge University Press; Cambridge: 1994. pp. 303–330. [Google Scholar]
- Wingfield JC. Communication behaviors, hormone-behavioral interactions and reproduction in vertebrates. In: Neil JD, editor. Knobil and Neil’s Physiology of Reproduction. Academic Press- Elsevier; San DIego, CA: 2006. pp. 1995–2040. [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]


