Abstract
Effector/memory T cells (Tem) are required to maintain successful immunity, while regulatory T cells (Treg) are required to prevent excessive/uncontrolled inflammation and/or autoimmunity. Although both Tem and Treg cells are increased during aging, the relationship between the increased proportion of Foxp3+ Treg cells and CD44+ Tem cells with aging is not clearly understood. Therefore, we investigated the role of Foxp3+ Treg cells and CD44+ Tem cells with aging. We found that Foxp3+ Treg cells are increased in parallel with CD44+ Tem cells in SJL/J mice with aging, and that all Foxp3+ Treg cells are of CD44+ Tem phenotype, suggesting that the increased Foxp3+ Treg cells originated from the expanded pool of CD44+ Tem cells with aging. Our in vitro kinetic studies further suggested that Foxp3+ Treg cells are converted through the CD44+ stage. Furthermore, we observed that although the balance between Foxp3+ Treg and CD44+Foxp3- Tem cells remained with aging, the aged mice have higher ratios of both Tem and Treg cells versus naïve T cells resulting in the “shrunken” naïve T cell pools. Our results suggest that an age-associated imbalance of T cell repertoire is a mechanism that contributes to spontaneous occurrence of Hodgkin’s-like lymphoma in aged SJL/J mice.
Keywords: regulatory T cell, effector/memory T cell, Foxp3, aging
Introduction
Aging is an inherent and irreversible complex event which gradually leads to changes in the body structure, decreased functional capacity and increased probability of death [1]. Aged people are prone to infections characterized by more severe symptoms, prolonged duration, and poor prognosis. Increased susceptibility to infections reflects the profound age-related changes in the immune system that are collectively termed as immune senescence [1]. Immune senescence may be the major predisposing factor contributing to increases in infections, and occurrence of cancer in aged people [2-3]. Several mechanisms contribute to the aging-related decline of immune responses, such as progressive reduction in naïve T cell output from thymus, and peripheral expansion of effector/memory T cells during the aging process [1, 4].
Effector/memory T cells (Tem) express different pattern of cell surface markers, and functionally they respond in several different ways from naive cells. In humans, naive T cells are CD45RA positive and CD45RO negative, while Tem cells are CD45RA negative and CD45RO positive. However, in mice, these surface markers are less reliable than that in human; instead the levels of CD44 and CD62L on the surface are preferred to be used to distinguish between naïve and effector/memory T cells, e.g., naive T cells are CD44-CD62L+, but Tem cells are CD44+CD62L- [5]. It has been noted that both activation of lymphocytes and transition to memory/effector phenotypes are associated with increased surface levels of CD44 [6, 7].
CD4+ T cells play an essential role in mediating both humoral and cellular immune responses. They act as helpers in assisting B cells to differentiate into antibody secreting plasma cells [8], helping CD8 T cells to develop into cytotoxic cells [9] and facilitating the activation of macrophages [10]. Accumulating evidences suggest an increase in the proportion of CD44+ Tem cells but a decrease in the proportion of CD44- naïve CD4+ T cells with aging [11-14].
More recently, attentions have been focused on the possibility that altered immunity with age may be partially attributed to the alterations of Foxp3+ regulatory T (Treg) cells which are now known to represent an essential component of the adaptive immune system [15]. A growing number of studies suggest that there is an increased frequency of Foxp3+ Treg cells in both mice and humans with aging [16-19].
CD44+ Tem cells are required to maintain the productive immunity, while Foxp3+ Treg cells are needed to keep the immune system from excessive inflammation and/or autoimmunity [20]. Thus, both of these cell types are extremely important for the maintenance of an immunological homeostasis in the host. It appears that both CD44+ Tem cells and Foxp3+ Treg cells are increased with senescence. However, the relationship between the increased proportion of Foxp3+ Treg cells and CD44+ Tem cells with aging is unclear. We explored this issue by investigating the immune senescence of SJL/J mice which are prone to Hodgkin’s-like lymphoma with aging [21]. We found that Foxp3+ Treg cells were increased in parallel with CD44+ Tem cells during aging. Particularly interesting, we observed that almost all Foxp3+ Treg cells are of Tem phenotype, suggesting that these increased Foxp3+ Treg cells are from the expanded pool of CD44+ Tem cells with aging. Our in vitro kinetic analysis supports this idea by showing that before conversion to Foxp3+ Treg cells, naïve T cells need to acquire a Tem-like phenotype (CD44+). Furthermore, we found that although the balance between Foxp3+ Treg cells and Foxp3-CD44+ Tem cells was not affected with aging, the increased ratios of both CD44+ Tem and Foxp3+ Treg relative to naïve T cells were observed in aged mice. Resultant “shrinkage” in naïve T cell pool correlates well with the increased occurrence of Hodgkin’s-like lymphoma in aged SJL/J mice. Taken together, these findings will contribute to a better understanding of the changes in the immune system during the aging process.
Materials and Methods
Mice
Healthy young (1-2 months old) and old (9-10 months old) gender-matched SJL/J mice (Jackson Laboratory) were used for the experiments. Old mice with gross pathology or lymphoma after dissection were excluded from immunophenotype assay. All animal studies were approved by the Louisiana State University Animal Care and Use Committee.
Reagents
Fluorescein isothiocyanate (FITC) labeled anti-mouse CD44, phycoerythrin-Cy5 (PE-Cy5) labeled anti-mouse CD4, allophycocyanin (APC) labeled anti-mouse CD62L, APC labeled anti-mouse CD25, FITC labeled anti-mouse IL-2, functional-grade anti-mouse CD3e (145-2C11) and CD28 (37.51), appropriate isotype control antibodies and mouse Foxp3 staining kit were purchased from eBioscience. Phorbol 12-myristate 13-actate (PMA), Ionomycin (ION) and Brefeldin A (BFA) were obtained from Sigma. Cell isolation kit for mouse naïve CD4 T cells (CD44-) was purchased from StemCell Technologies.
Flow cytometry assay
Cells were dispersed from the lymph nodes, spleen tissues or thymus by extrusion through a 70 μm Nylon Cell Strainer (BD Falcon). Following three washes in PBS, the resulting single cell suspension of lymphocytes was subjected to immunostaining with indicated FITC-, PE-, Cychrome-, or APC-conjugated antibodies for surface (CD44, CD4, and CD62L) or/and intracellular (Foxp3) staining. All samples were analyzed by FACSCalibur (Becton Dickinson).
Naive CD4+ CD44- T cell isolation and cell culture
Cells were obtained from young mice (1-2 months old), as their lymphocytes contain higher percentage of naïve CD4+CD44- T cells. CD4+CD44- naïve T cells were separated from pooled lymph nodes and spleens by the magnetic nanoparticle based isolation kit for mouse naive CD4+ T cells from StemCell Technologies. A purity of 95% of the naïve T cells (CD4+CD44-) was determined by FACS. For in vitro culture, CD4+CD44- naïve T cells (2×106/well) were cultured in 24-well flat-bottom tissue culture plates (Corning Costar) with 10 μg/ml-bound of anti-CD3e and 2 μg/ml of soluble anti-CD28 in culture medium at 37 °C in a humidified atmosphere of 5% CO2 in air for 24, 48, 72 and 96 hours. RPMI1640 was used as the culture medium supplemented with 1 mM sodium pyruvate, 1.2% sodium bicarbonate (BioWhittaker), 2 mM glutamine (Sigma), 25 μg/ml gentamicin (Sigma), and 10% heat-inactivated new cattle serum.
Intracellular detection of Foxp3 or IL-2
At the indicated time point, cells were harvested, washed with PBS and stained with FITC-CD44, PE-Cy5-CD4, and APC-CD62L or APC-CD25 for surface staining. Intracellular staining analysis was performed by the manufacturer’s instruction of anti-mouse Foxp3 staining kit. For intracellular IL-2 detection, purified naïve CD4+CD44- T cells were first stimulated with anti-CD3e and anti-CD28 for 72h, then re-stimulated with PMA (100 ng/ml) plus ionomycin (1μg/ml) for 4 h in the presence of Brefeldin A (BFA, 10 μg/ml). The cells were harvested, washed with PBS, and then stained on the surface with anti-CD4-PE-Cy5. After being fixed and permeabilized with Cytofix/Cytoperm buffer, the cells were further stained with anti-IL-2-FITC and anti-Foxp3-PE.
Statistical analysis
Results are expressed as mean ± standard deviation (SD). The level of significance was set at P < 0.05. Correlation analyses were quantified by the Pearson correlation test. All experiments were repeated more than three times.
Results
Increased frequency of peripheral Foxp3+ Treg cells with aging
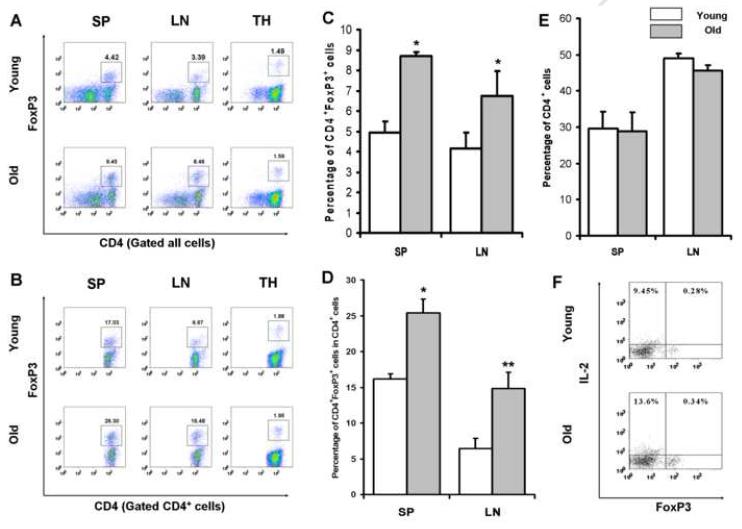
Accumulating evidence indicates increased pool of Foxp3+ Treg cells in aged BALB/c and C57BL/6 mice [17-19]. To determine whether the alteration of Foxp3+ Treg cells occurs in aged SJL/J mice which have a higher incidence of Hodgkin’s-like lymphoma, we evaluated the presence of Foxp3+CD4+ T cells in the spleen, lymph nodes and thymus from young (1-2 months) and healthy old (9-10 months) SJL/J mice. As shown in Fig. 1A and 1C, aged mice possessed a significantly increased proportion of Foxp3+ T cells among the total lymphocyte population in their spleen (old: 8.7 ± 0.2 %/young: 4.9 ± 0.5 %, P<0.05) and lymph nodes (old:7.1 ± 1.1 %/young: 4.1 ± 0.7 %, P<0.05). This increase is more appreciable when analyzed in gated CD4+ T cells (Fig. 1B and 1D): the percentage of Foxp3+ cells among the total CD4+ T cells was more evidently increased in aged spleen (old: 23.3 ± 1.9 %,/young: 17.1 ± 0.7 %, P<0.05) and lymph nodes (old: 14.7 ± 2.3 %/young: 6.4 ± 1.4 %, P<0.01). In both young and aged mice, relative to lymph nodes, the spleen contains a higher percentage of Foxp3+ T cells. However, the presence of an age-related rise in the frequency of Foxp3+CD4+ T cells was consistent between spleen and lymph nodes. Although there was a significant increase in the percentage of Foxp3+CD4+ T cells both in spleen and lymph nodes, we did not find any simultaneous increase in aged thymus. In addition, we did not observe significant difference in the percentage of total CD4+ T cells in both spleen and lymph nodes of aged and young animals (Fig. 1E). These results suggest that there is a significantly increased proportion of Foxp3+CD4+ T cells in peripheral lymphoid tissues of SJL/J mice with aging.
Figure 1. Increased Foxp3+CD4+T cells in the peripheral lymphoid organs with aging.
Distribution of Foxp3+ cells from representative FACS analysis is shown in the total lymphocytes (A) or in the gated CD4+ cells (B) from the spleen (SP), lymph nodes (LN) and thymus (TH). The numbers shown in the graph are the percentages of Foxp3+ cells among total lymphocytes (A) or gated CD4+ cells (B), respectively. Statistical analysis of the distribution of Foxp3+ cells in the total lymphocytes (C) and the gated CD4+ cells (D) of young (n=6) and old (n=6) SJL/J mice. No statistical difference was found in the percentage of CD4+ T cells between young and old mice (E). Intracellular detection of IL-2 production in Foxp3+ cells from both young and old mice (F). Results are representative of six experiments in young or aged mice. *, P<0.05; **, P<0.01; compared with the corresponding groups.
Foxp3 expression does not always correlate with regulatory activity, as it is also induced on newly activated CD4+ effector population [22], thus, the examination of its inhibitory function is important to determine whether the Foxp3+ cells detected in our study are the “true” Treg cells. It is impossible to assess this inhibitory function directly due to the limitation of obtaining Foxp3+ T cells. However, we circumvented this obstacle by analyzing the intracellular cytokine production in Foxp3+ T cells. This assay is based on the findings that although they both express Foxp3, the newly activated T cells but not the “true” Treg cells are able to produce IL-2 [23, 24]. Therefore, we examined the intracellular cytokine IL-2 production after PMA and ionomycin stimulation, as shown in Fig. 1F, our results showed that in contrast to Foxp3- cells, Foxp3+ cells from both young and old mice were actually unable to produce IL-2 which is in agreement with the widely accepted view that Foxp3+ Treg cells do not produce IL-2 [25-28]. These results further provided the evidence that these Foxp3+ T cells we detected in both young and aged mice were of the true Treg cells. Taken together, these data indicate that the proportion of Foxp3+ Treg cells in the peripheral lymphoid tissue is enhanced markedly along with aging in SJL/J mice.
Increased proportion of peripheral CD44+ effector/memory T cells (Tem) with aging
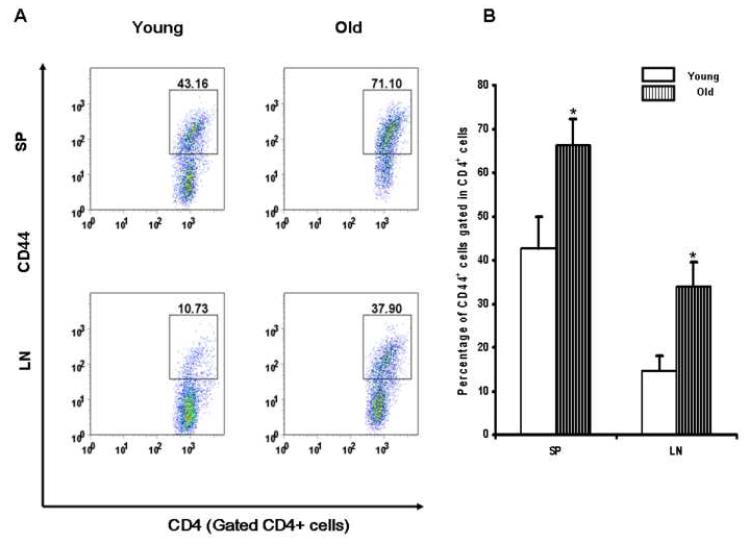
Expression of CD44 molecules on the surface has been used as a marker to identify effector/memory T cells [6, 7]. Since some earlier studies have shown that Tem increases with age [1-5], we analyzed the frequency of Tem both in young and aged SJL/J mice. As shown in Fig. 2A and 2B, aged SJL/J mice show a significantly larger population of CD44+ Tem cells both in their spleens (old: 66.3 ± 5.9 %/ young: 42.7 ± 7.1 %, P<0.05) and in their lymph nodes (old: 34 ± 5.4 %/ young:14.7 ± 3.4 %, P<0.01). Our results, therefore, demonstrated that CD44+ Tem cells are significantly increased in SJL/J mice with aging.
Figure 2. Increased CD44+CD4+ Tem cells in the peripheral lymphoid organs with aging.
Representative FACS samples of CD4 vs. CD44 expression are shown in the gated CD4+ cells from spleen (SP) and lymph nodes (LN) of young (n=6) and old (n=6) SJL/J mice (A). The numbers shown in the graph are the percentages of CD44+ cells among the CD4+ T cells. Statistical analysis of the percentage change of CD44+ T cells among the gated CD4+ T cells with aging (B). Results represent six experiments in young or aged mice, respectively. *, P<0.05 compared with the corresponding groups.
Foxp3+CD4+ Treg cells and CD44+ Tem cells are parallel increased with aging
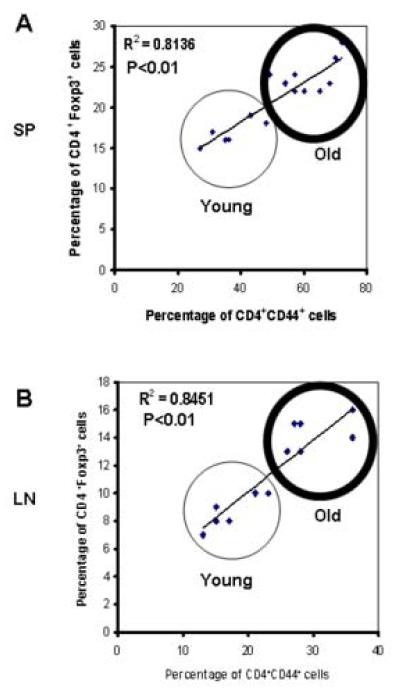
Since both Foxp3+ Treg cells and CD44+ Tem cells are increased along with aging in peripheral lymphoid organs, we next examined the correlation-ship between Foxp3+ Treg cells and CD44+ Tem cells by the statistical correlation efficiency analysis. We found that the increased percentage of Foxp3+ Treg cells has a strong positive correlation with the increased percentage of CD44+ Tem cells in spleen (Fig 3A, R2=0.81, P<0.01) as well as in lymph nodes (Fig 3B, R2=0.85, P<0.01), indicating that Foxp3+ Treg cells are increased in parallel with CD44+ Tem cells during aging in SJL/J mice.
Figure 3. Positive correlation of Foxp3+ Treg cells with CD44+ Tem cells in peripheral lymphoid organs with aging.
Significant correlation was found in the percentage of Foxp3+CD4+ Treg cells and CD44+CD4+ Tem cells from young (n=6) and old (n=6) SJL/J mice. The proportion of Foxp3+CD4+ Treg cells is in strong positive association with that of CD44+CD4+ Tem cells in both spleen (A, R2=0.81, P<0.01) and in lymph nodes (B, R2=0.85, P<0.01). Each square on the graph represents an individual animal.
Foxp3+ Treg cells are of CD44+ Tem phenotype
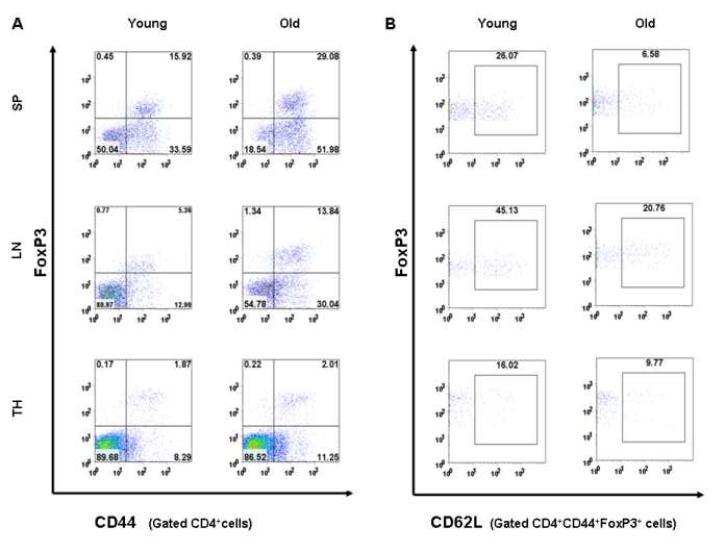
We next explored the relationship of between Foxp3+ Treg cells and CD44+ Tem cells by analyzing the surface expression of CD44 on the Foxp3+ Treg cells. Interestingly, as shown in Fig 4A, almost all Foxp3+ Treg cells from thymus, spleen or lymph nodes were shown to express high levels of CD44 on their surface. Furthermore, according to the expression level of CD62L, these CD44+Foxp3+ Treg cells can be sub-divided into CD62L+ and CD62L- type of Treg cells (Fig. 4B). These results indicate that Foxp3+ Treg cells in nature are of CD44+ Tem cells in both aged and young mice.
Figure 4. Foxp3+CD4+ Treg cells are of CD44+ Tem phenotype in both old and young SJL mice.
Representative FACS samples of CD44 vs. Foxp3 expression are shown in the gated CD4+ T cells from spleen (SP), lymph nodes (LN) and thymus (TH) of young (n=6) and old (n=6) SJL/J mice (A). Percentages of CD44+Foxp3+, CD44+Foxp3-, CD44-Foxp3+, CD44-Foxp3+ cells are indicated. Representative FACS samples of CD62L vs. Foxp3 expression are shown in the gated CD4+CD44+Foxp3+ Treg cells (B). Percentage of CD44+Foxp3+CD62L+ cells is indicated. Results represent six independent experiments.
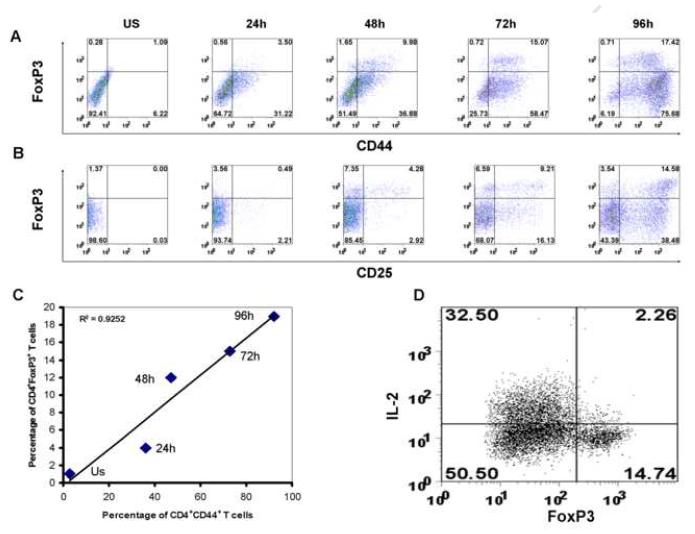
Naïve CD4+CD44- T cells acquire effector/memory-like phenotype (CD44+) before converting to Foxp3+ Treg cells
Since almost all Foxp3+ Treg cells are of CD44+ T cells, there are two possible models for naïve CD4+CD44- T cells to be converted to CD44+Foxp3+ Treg cells. One possibility is that naïve CD4+CD44- T cells could express Foxp3 first, then acquire CD44 on their surface; or the other possibility is that naïve CD4+CD44- T cells would have to express CD44+ first, which are then converted Foxp3+ cells. To evaluate these two possibilities, the naïve CD4+CD44- T cells were isolated from pooled spleens and lymph nodes of young SJL/J mice, and were stimulated with anti-CD3 and anti-CD28 for various periods of time. We then analyzed kinetically the surface expression of CD44 and intracellular expression of Foxp3 by FACS. As shown in Fig. 5A, while no Foxp3+ cells were present at the time of initial culture, they started to appear as early as 24h following in vitro T cell activation, and gradually increase their percentage along with the time of stimulation. Interestingly, even at the early time points (24-48h), almost ∼90% of the in vitro generated Foxp3+ T cells started to express CD44 (Fig 5A), whereas only ∼18-36% of them express CD25 (Fig. 5B), suggesting that the appearance of Foxp3+ cells is more mainly dependent on CD44 expression rather than CD25 expression. This was further supported by the statistical correlation assay that showed the proportion of Foxp3+ T cells had a strong positive correlation with that of CD44+ T cells in vitro(R2=0.92)(Fig. 5C). The kinetic analysis of CD44 and CD25 expression in relation to the expression of Foxp3 revealed that the naïve CD4+CD44- T cells were first turned into CD44+ which then became CD44+Foxp3+ Treg cells. In addition, as shown by intracellular cytokine staining in Fig. 5D, in contrast to the Foxp3- population, Foxp3+ cells generated from our culture condition produce very few IL-2 after restimulation, an indication of Treg nature of these cells. Taken together, these data indicate that before conversion to the Foxp3+ Treg cells, naïve CD44- T cells first need to acquire a CD44+ phenotype in vitro.
Figure 5. In vitro conversion of Foxp3+ Treg cells from naïve CD4+ T cells needs to acquire a Tem-like phenotype (CD44+).
Naïve CD4+ T cells (CD44-) isolated from the pooled spleen and lymph nodes of young SJL/J mice were stimulated with coated anti-CD3 (10ug/ml) and anti-CD28 (1ug/ml) in a natural differentiation condition (no any additional cytokines supplemented). Representative FACS samples of CD44 (in A) or CD25 (in B) vs. Foxp3 expression are shown for the indicated time after stimulation. Percentages of CD44+Foxp3+, CD44+Foxp3-, CD44-Foxp3+ and CD44-Foxp3+ cells are indicated. As summarized in (C), the appearance (%) of Foxp3+ Treg cells with time is in strong association with that of CD44+ cells in vitro (R2=0.92). Intracellular detection of IL-2 production in Foxp3+ cells generated in vitro (D). After 72h stimulation, the cells were re-stimulated with PMA plus ionomycin in the presence of BFA for intracellular detection of IL-2 in Foxp3+ cells. Percentages of IL-2+Foxp3+, IL-2+Foxp3-, IL-2-Foxp3+ and IL-2-Foxp3+ cells are indicated. Results represent three independent experiments.
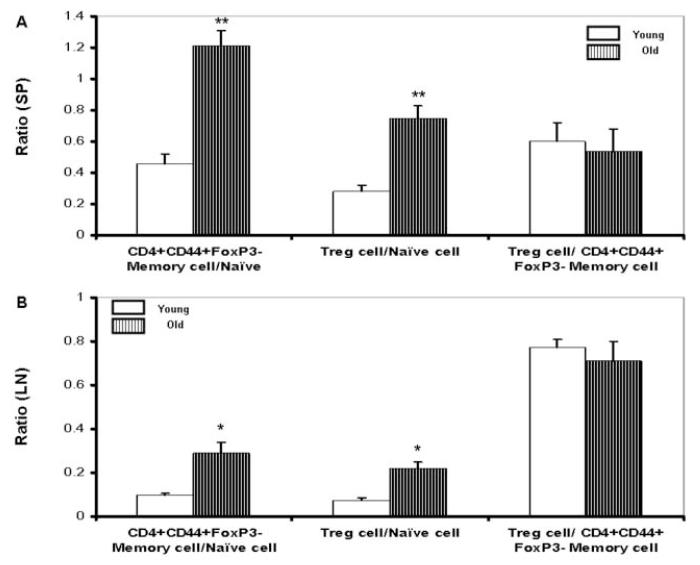
Imbalanced homeostasis of naïve versus CD44+Foxp3- Tem cells, and naïve versus CD44+Foxp3+ Treg cells
Maintenance of CD4+ T cell homeostasis is critical for normal functioning of the immune system. CD4+ T cell homeostasis is ultimately achieved through maintaining the balance of distinct T cell populations (naive, Tem and Treg cells), although the mechanisms that maintain homeostasis in each population are not fully understood. To gain a clinically relevant insight into the homeostasis of aged mice, we analyzed the ratios of Tem and Treg cells versus naïve T cells, respectively. Almost all Foxp3+ Treg cells in our study are of CD44+ phenotype, and to make the description easier, we defined here the Tem cells as CD44+Foxp3-, the Treg cells as CD44+Foxp3+ and the naïve T cell as CD44-. As shown in Fig. 6A and 6B, we found that there was a significantly increased ratio of Tem cells relative to naïve T cells in both aged spleen (old:1.21 ± 0.1/young: 0.46 ± 0.06, P<0.01) and lymph nodes (old: 0.29 ± 0.05/young: 0.097 ± 0.01, P<0.05). We also observed significant increase in the ratio of Foxp3+ Treg cells relative to naïve T cells in the aged spleen (old: 0.75 ± 0.08/young: 0.28 ± 0.04, P<0.01) and lymph nodes (old: 0.22 ± 0.03/young: 0.075 ± 0.01, P<0.05). While the ratio of Foxp3+ Treg cells relative to CD44+Foxp3- Tem cells maintained stable along with aging (old: 0.54 ± 0.14/ young: 0.6 ± 0.12 in spleen; old: 0.71 ± 0.09/ young: 0.77 ± 0.04 in lymph nodes). These data strongly suggest an aging-related imbalance between Foxp3+ Treg cells, CD44+Foxp3- Tem cells and naïve T cells in SJL/J mice.
Figure 6. Imbalanced homeostasis of naïve versus CD44+Foxp3- Tem and CD44+Foxp3+ Treg cells with aging.
In spleen (A) and lymph nodes (B), the ratios of both CD44+Foxp3- Tem cells and Foxp3+ Treg cells vs. CD44- naïve cells are shown the significant increases in old (n=6) as compared with young (n=6) mice. But the ratio of Foxp3+ Treg cells vs. CD44+Foxp3- Tem cells remains stable with age. Data were obtained from the gated CD4+ cells. Results represent six experiments. *, P<0.05; **, P<0.01; compared with corresponding groups.
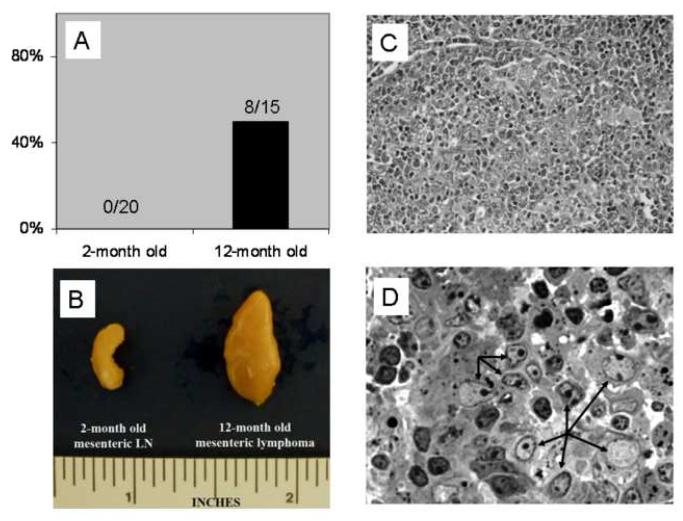
High incidence of spontaneous Hodgkin’s-like lymphoma with aging in SJL/J mice
Both Treg and Tem cells are of antigen-experienced cells, the increased Treg and Tem cells with aging consequently results in a shrunken pool of naïve T cells which are the most important cells available against those emerging antigens derived from new infections or spontaneous tumors. It has been known for years that compared with other inbred strains, SJL/J mice tend to have higher incidence of Hodgkin’s-like lymphomas [21]. An interesting question is whether the age-related immune imbalance plays a role in the pathology of the aged SJL/J mice. We examined this issue by comparing the occurrence of spontaneous lymphoma in young (1-2- month old) and aged (12-month old) SJL/J mice. All of the mice used in this survey were autopsied for the presence of neoplasms in the immune organs after euthanization. As shown in Fig.7A, no tumorous lymph nodes or other organs were found in the 20 young mice we examined, but 53% of aged mice (8 out 15) were shown to have large-sized tumors at the mesenteric lymph nodes (Fig. 7B). Histopathological examination of these tumors revealed that tumor cells have totally effaced the normal architecture of lymph node, under low magnification no intact lymphoid follicles could be found (Fig. 7C). Under higher magnification (Fig. 7D), the tumor cell was shown to have a large nucleus with one or two prominent nucleoli and abundant cytoplasm resembling the mononuclear variant of Hodgkin’s cells. These tumor cells are admixed with numerous lymphocytes similar to the mixed type of Hodgkin’s lymphoma. Hence, the immune imbalance with aging correlates with the higher incidence of Hodgkin’s-like lymphoma in SJL/J mice.
Figure 7. Higher incidence of spontaneous Hodgkin’s-like lymphoma in aged SJL/J mice.
The incidence of spontaneous lymphoma at the mesenteric lymph nodes in young (1-2 month old, n=20) and aged (12-month old, n=15) SJL/J mice (A). Gross appearance of a mesenteric lymphoma from an examplary aged mouse compared with a normal mesenteric lymph node from a young mouse (B). Histopathological examination of the mesenteric lymphoma at low (C) and high (D) magnification, HE staining. The arrows in (D) indicate the tumor cells that resemble the mononuclear variants of Hodgkin’s cells.
Discussion
In this study, we have investigated the relationship between Foxp3+ Treg cells and CD44+ Tem cells in SJL/J mice with aging. Our study shows a parallel increase of Foxp3+ Treg cells and CD44+ Tem cells in SJL/J mice with aging. Of particular interest, we found that almost all Foxp3+ Treg cells are of effector/memory phenotype (expressing CD44). Therefore, the Foxp3+ Treg cells are included within the group of traditionally regarded effector/memory CD44+ T cells. Furthermore, although the balance between Treg and Tem cells is maintained with aging, the aged mice lose the balance between Tem and naïve T cells, and also between Treg and naïve T cells.
The increased percentage of Foxp3+ Treg cells in peripheral lymphoid organs in aged SJL/J mice is in agreement with the findings reported in aged BALB/c mice [18,19] and C57BL/6 mice [17,29], suggesting that an age-related increase of Treg cells is a common phenomenon among different strains. It has been proposed that the percentages of effector/memory T cells increase with age, which is supported by the markedly increased expression of CD44 on T cells in aged mice [1-5]. In agreement with these reports [1-5], we showed a significantly increased percentage of CD44+ T cells in peripheral lymphoid organs in aged SJL/J mice. Of particular interest, we found that CD44+ T cells in SJL/J mice are heterogenous populations which include both traditional effector/memory T cells (CD44+Foxp3-) and Treg cells (CD44+Foxp3+).
Although some recent reports have shown that Foxp3+ Treg cells in aged hosts are of CD44+ effector/memory T cell phenotype [19, 29, 30], our results showed that even in young mice (4-8 weeks), Foxp3+ Treg cells are of CD44+ phenotype suggesting that the increased Foxp3+ Treg cells with aging are originated from CD44+ T cells. Our in vitro kinetics analysis further demonstrated that before converting to Foxp3+ Treg cells, naïve T cells need to acquire an effector/memory-like phenotype (CD44+), and thereafter convert to Foxp3+ Treg cells. Based on our observations, we propose that after activation by antigens, CD44 up-regulation is the initial step for the induction of Foxp3+ Treg cells; then some CD44+ T cells develop into CD44+Foxp3- effector/memory T cells, while the others differentiate into CD44+Foxp3+ Treg cells.
It is unlikely that the increased Foxp3+ Treg cells in aged mice are derived from thymus. One of the reasons for this is that aging-related thymic involution would dramatically reduce rather than increase the total output of all new T cells, which has long been considered the most reasonable cause of the age-related decline in T cell function [31-34]. In addition, our data showed that in the aged thymus the percentage of Foxp3+ Treg cells did not increase at all, indicating that it is impossible for the aged thymus to contribute to the peripheral increase of Foxp3+ Treg cells with aging. Therefore, the increased occurrence of peripheral Foxp3+ Treg cells through an increased pool of CD44+ T cells is a more reasonable explanation for the increased proportion of Foxp3+ Treg cells with aging [20, 35, and 36]. That means during aging the expansion of CD44+ effector/memory pools is the prerequisite for Foxp3+ Treg cells to expand.
According to their distinct functions, CD4+ T cells can be divided into three functional groups: 1) naïve CD4+ T cells, which are the reserves needed for protecting against new or emerging antigens derived from either pathogens or spontaneous tumors; 2) effector/memory CD4+ T cells, which are required to maintain the immunity for the previous pathogen exposure; and 3) regulatory CD4+ T cells, which have the ability to keep the immune system in check to avoid excessive inflammation and/or autoimmunity. A balanced homeostasis of these three populations is important for an individual to maximally achieve the benefit provided by the immune system. In aged SJL/J mice, we showed that although the balance remained the same between Foxp3+ Treg cells and effector/memory cells, an imbalance between naïve T cells and effector/memory T cells or Foxp3+ Treg cells was detected. These imbalances of homeostasis results in a shrunk or reduced pool of naïve CD4+ T cells by the involvement of compromised thymic replenishment and reduction of a naïve CD4+ T cells repertoire due to chronic activation [37, 38]. Age-related immune imbalance may contribute to increased susceptibility to emerging infections and neoplasms in animals and human. In support of our view, the aged C57BL/6 mice have been shown to be more susceptible to Leishmania major infection [29]. In our present study, we found that the immune imbalance in SJL/J mice with aging is associated with the higher incidence of Hodgkin’s-like lymphoma. Although a direct link between the age-related immune imbalance and the higher risk of tumors or infections in aged mice has so far been inconclusive, our data highlight that aging-associated imbalance of CD4+ T cells plays an important role in tumor pathogenesis in aged animals.
Acknowledgments
The authors thank Ms. Marilyn Dietrich at the Immunology Core of LSU for the FACS data analysis, and Leah K. Canaday for helping the manuscript preparation. This work was supported by a Research Grant (RG3855A2/T) from the National Multiple Sclerosis Society (to JMF) and an intramural CORP fund by the School of Veterinary Medicine at LSU (to JMF). SJ was supported by a Research Grant from the American Lung Association (RG-22442-N), a Scientist Award from the Flight Attendant Medical Research Institute (YCSA-062466); and a grant from the NIH (R01 HL-091958).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kovaiou RD, Grubeck-Loebenstein B. Age-associated changes within CD4+ T cells. Immunol. Lett. 2006;107:8–14. doi: 10.1016/j.imlet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Pawelec G, Koch S, Franceschi C, Wikby A. Human immunosenescence: does it have an infectious component? Ann. N. Y. Acad Sci. 2006;1067:56–65. doi: 10.1196/annals.1354.009. [DOI] [PubMed] [Google Scholar]
- 3.Hakim FT, Flomerfelt FA, Boyiadzis M, Gress RE. Aging, immunity and cancer. Curr. Opin. Immunol. 2004;16:151–6. doi: 10.1016/j.coi.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of aging. J. Pathol. 2007;211:144–56. doi: 10.1002/path.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu. Rev. Immunol. 1998;16:201–23. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 6.Budd RC, Cerottini JC, Horvath C, Bron C, Pedrazzini T, Howe RC, MacDonald HR. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J. Immunol. 1987;138:3120–9. [PubMed] [Google Scholar]
- 7.Lee WT, Vitetta ES. The differential expression of homing and adhesion molecules on virgin and memory T cells in the mouse. Cell. Immunol. 1991;132:215–22. doi: 10.1016/0008-8749(91)90020-c. [DOI] [PubMed] [Google Scholar]
- 8.Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand GP39. Annu. Rev. Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 9.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 10.Roman E, Miller E, Harmsen A, Wiley J, Von Andrian UH, Huston G. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J. Exp. Med. 2002;196:957–968. doi: 10.1084/jem.20021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lerner A, Yamada T, Miller RA. Pgp-1hi T lymphocytes accumulate with age in mice and respond poorly to concanavalin A. Eur. J. Immunol. 1989;19:977–82. doi: 10.1002/eji.1830190604. [DOI] [PubMed] [Google Scholar]
- 12.Ernst DN, Hobbs MV, Torbett BE, Glasebrook AL, Rehse MA, Bottomly K, Hayakawa K, Hardy RR, Weigle WO. Differences in the expression profiles of CD45RB, Pgp-1, and 3G11 membrane antigens and in the patterns of lymphokine secretion by splenic CD4+ T cells from young and aged mice. J. Immunol. 1990;145:1295–302. [PubMed] [Google Scholar]
- 13.Miller RA. Biomarkers of aging: prediction of longevity by using age-sensitive T-cell subset determinations in a middle-aged, genetically heterogeneous mouse population. J. Gerontol. A. Biol. Sci. Med. Sci. 2001;56:B180–6. doi: 10.1093/gerona/56.4.B180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovaiou RD, Weiskirchner I, Keller M, Pfister G, Cioca DP, Grubeck-Loebenstein B. Age-related differences in phenotype and function of CD4+ T cells are due to a phenotypic shift from naive to memory effector CD4+ T cells. Int. Immunol. 2005;17:1359–66. doi: 10.1093/intimm/dxh314. [DOI] [PubMed] [Google Scholar]
- 15.Dejaco C, Duftner C, Schirmer M. Are regulatory T-cells linked with aging? Exp. Gerontol. 2006;41:339–45. doi: 10.1016/j.exger.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, Nayak L, Moss PA. The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin. Exp. Immunol. 2005;140:540–6. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S. CD4+CD25+Foxp3+ T cells and CD4+CD25-Foxp3+ T cells in aged mice. J. Immunol. 2006;176:6586–93. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- 18.Sharma S, Dominguez AL, Lustgarten J. High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J. Immunol. 2006;177:8348–55. doi: 10.4049/jimmunol.177.12.8348. [DOI] [PubMed] [Google Scholar]
- 19.Zhao L, Sun L, Wang H, Ma H, Liu G, Zhao Y. Changes of CD4+CD25+Foxp3+ regulatory T cells in aged Balb/c mice. J. Leukoc. Biol. 2007;81:1386–94. doi: 10.1189/jlb.0506364. [DOI] [PubMed] [Google Scholar]
- 20.Akbar AN, Vukmanovic-Stejic M, Taams LS, Macallan DC. The dynamic co-evolution of memory and regulatory CD4+ T cells in the periphery. Nat. Rev. Immunol. 2007;7:231–7. doi: 10.1038/nri2037. [DOI] [PubMed] [Google Scholar]
- 21.Kumar RK. Animal model of human disease — Hodgkin’s disease: SJL murine lymphoma. Amer. J. Pathol. 1983;110:393–396. [PMC free article] [PubMed] [Google Scholar]
- 22.Walker MR, Carson BD, Nepom GT, Ziegler SF, Buckner JH. De novo generation of antigen-specific CD4+CD25+ regulatory T cells from human CD4+CD25- cells. Proc. Natl. Acad. Sci. U. S. A. 2005;102:4103–8. doi: 10.1073/pnas.0407691102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int. Immunol. 2007;19:345–54. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 24.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–90. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat. Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 26.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc. Natl. Acad. Sci. U. S. A. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott-Browne JP, Shafiani S, Tucker-Heard G, Ishida-Tsubota K, Fontenot JD, Rudensky AY, Bevan MJ, Urdahl KB. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J. Exp. Med. 2007;204:2159–2169. doi: 10.1084/jem.20062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levings MK, Sangregorio R, Roncarolo MG. Human CD25(+)CD4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J. Exp. Med. 2001;193:1295–1302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA, Belkaid Y, Chougnet C. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J. Immunol. 2008;181:1835–48. doi: 10.4049/jimmunol.181.3.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santner-Nanan B, Seddiki N, Zhu E, Quent V, Kelleher A, de St. Groth BF, Nanan R. Accelerated age-dependent transition of human regulatory T cells to effector memory phenotype. Int. Immunol. 2008;20:375–383. doi: 10.1093/intimm/dxm151. [DOI] [PubMed] [Google Scholar]
- 31.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–4. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 32.Hirokawa K, Utsuyama M, Kasai M, Kurashima C, Ishijima S, Zeng YX. Understanding the mechanism of the age-change of thymic function to promote T cell differentiation. Immunol, Lett. 1994;40:269–77. doi: 10.1016/0165-2478(94)00065-4. [DOI] [PubMed] [Google Scholar]
- 33.Haynes L, Eaton SM, Swain SL. Effect of age on naive CD4 responses: impact on effector generation and memory development. Springer, Semin, Immunopathol. 2002;24:53–60. doi: 10.1007/s00281-001-0095-2. [DOI] [PubMed] [Google Scholar]
- 34.Tuovinen H, Laurinolli TT, Rossi LH, Pekkarinen PT, Mattila I, Arstila TP. Thymic production of human FOXP3(+) regulatory T cells is stable but does not correlate with peripheral FOXP3 expression. Immunol, Lett. 2008;117:146–53. doi: 10.1016/j.imlet.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Vukmanovic-Stejic M, Zhang Y, Cook JE, Fletcher JM, McQuaid A, Masters JE, Rustin MH, Taams LS, Beverley PC, Macallan DC, Akbar AN. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J. Clin. Invest. 2006;116:2423–33. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vukmanovic-Stejic M, Agius E, Booth N, Dunne PJ, Lacy KE, Reed JR, Sobande TO, Kissane S, Salmon M, Rustin MH, Akbar AN. The kinetics of CD4Foxp3 T cell accumulation during a human cutaneous antigen-specific memory response in vivo. J. Clin. Invest. 2008;118:3639–3650. doi: 10.1172/JCI35834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cicin-Sain L, Messaoudi I, Park B, Currier N, Planer S, Fischer M, Tackitt S, Nikolich-Zugich D, Legasse A, Axthelm MK, Picker LJ, Mori M, Nikolich-Zugich J. Dramatic increase in naive T cell turnover is linked to loss of naive T cells from old primates. Proc. Natl. Acad. Sci. U. S. A. 2007;104:19960–5. doi: 10.1073/pnas.0705905104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourgeois C, Hao Z, Rajewsky K, Potocnik AJ, Stockinger B. Ablation of thymic export causes accelerated decay of naive CD4 T cells in the periphery because of activation by environmental antigen. Proc. Natl. Acad. Sci. U. S. A. 2008;105:8691–6. doi: 10.1073/pnas.0803732105. [DOI] [PMC free article] [PubMed] [Google Scholar]