Abstract
Background and Aim
Eosinophilic esophagitis (EoE) is characterized by medically/surgically-resistant gastroesophageal reflux symptoms and dense squamous eosinophilia. Studies suggest that histological assessment of esophageal eosinophilia alone cannot reliably separate patients with EoE from those with gastroesophageal reflux disease (GERD). Our goal was to develop an assay to identify EoE patients and perhaps differentiate EoE from other causes of esophageal eosinophilia.
Methods
A monoclonal antibody specific for an eosinophil secondary granule protein (eosinophil peroxidase (EPX)) was developed and shown to specifically identify intact eosinophils and detect eosinophil degranulation in formalin-fixed specimens. A histopathological scoring algorithm was developed to analyze data from patient evaluations; the utility of this algorithm was assessed using archived esophageal tissues from patients with known diagnoses of EoE and GERD as well as controls from two tertiary care centers.
Results
Intra/inter-observer blinded evaluations demonstrated a significant difference (P<0.001) between scores of samples taken from control subjects, from patients with esophageal eosinophilia who had a diagnosis of EoE, and from patients with GERD (P<0.001). This algorithm also was able to identify patients whose clinical course was suggestive of a diagnosis of EoE but that nonetheless failed to reach the critical threshold number of ≥15 eosinophils in a high-power (40×) microscopy field.
Conclusions
A novel immunohistochemical scoring system was developed to address an unmet medical need – to differentiate histological specimens from patients with EoE relative to those with GERD. The availability of a unique anti-EPX-specific monoclonal antibody, combined with the ease/rapidity of this staining method and scoring system, will provide a valuable strategy for the assessment of esophageal eosinophilia.
Keywords: Esophagus, Histopathology, Immunology, Pathology, eosinophil, degranulation
INTRODUCTION
Eosinophilic esophagitis (EoE) is one of the leading causes of dysphagia and food impaction in adults and is an important cause of vague symptoms previously associated with gastroesophageal reflux disease (GERD) in children 1. Despite increasing recognition of EoE, only recently have diagnostic criteria been published 1. Although these criteria set new standards for clinical care, the established diagnostic histological features focus solely on the numbers of intact eosinophils present in esophageal biopsies 2. In addition, the diagnosis of EoE hinges on the fact that GERD has been excluded, thus requiring either pre-treatment with a proton pump inhibitor or investigation with pH monitoring of the distal esophagus 1, 3, 4.
The underlying mechanisms associated with the pathogenesis in EoE remain largely unresolved. However, eosinophil granule proteins are thought to play a functional role in the gastrointestinal (GI) tract by increasing permeability and colonic inflammation 5, 6. In this regard, it is important to realize that extracellular deposition of eosinophil granule proteins may be the only evidence of eosinophil activation in esophagitis. Earlier studies of EoE patients suggested that a subset of patients have extensive eosinophilic degranulation with few intact eosinophils (see for example 7). However, because no studies have fully characterized degranulation patterns associated with EoE, the histological diagnosis of EoE is often solely based on the numbers of eosinophils infiltrating the epithelium. Thus, the aim of our study was to improve current abilities to identify EoE patients on the basis of additional features of eosinophilic inflammation, including the use of eosinophil degranulation as an important diagnostic criterion of EoE.
This retrospective study utilized a novel monoclonal antibody specific for the secondary granule protein eosinophil peroxidase (EPX) as part of immunohistochemical assessments of formalin-fixed and paraffin-embedded esophageal biopsies from patients with EoE, GERD, or controls. The results from these assessments demonstrated the utility of anti-eosinophil peroxidase monoclonal antibody (EPX-mAb) based immunohistochemistry to support clinical findings. Specifically, we report a quantitative scoring strategy based on the identification of infiltrating eosinophils and distinct patterns of degranulation. More importantly, this study showed that a diagnostic strategy using EPX-mAb led to more expedient assessments of patients with esophageal diseases. We anticipate that future clinical studies will validate EPX-mAb based evaluations, allowing distinctions between EoE, GERD, and other esophageal diseases. In turn, this will lead to more rapid diagnosis and treatment and thus provide a cost effective and accurate means of achieving a definitive diagnosis.
MATERIALS AND METHODS
The development of an EPX monoclonal antibody (EPX-mAb) and diagnostic scoring algorithm to evaluate esophageal patients
Human Subjects
Adult esophageal patients were identified retrospectively by gastroenterologists at Mayo Clinic Arizona. These patients were divided into four groups: Group I were patients diagnosed with EoE by virtue of (i) clinical symptoms at presentation (e.g., dysphagia, vomiting, and/or food impaction), (ii) GERD was ruled out with either pretreatment with proton pump inhibition, a normal pH/impedance monitor, and/or response to topical or systemic steroids, (iii) endoscopic findings (i.e., corrugations and/or furrows) were characteristic of EoE, (iv) histopathologic assessments of mid/proximal-esophageal biopsies demonstrating sclerosis of the lamina propria stroma, basal hyperplasia of the squamous epithelium, and/or intercellular epithelia edema, and, most importantly, (v) at least a single focus of ≥15 eosinophils/40× high power field (hpf) from mucosal biopsies. Group II patients were diagnosed with gastroesophageal reflux disease (GERD) based on symptoms which included response to proton pump inhibitors and epithelial biopsies that revealed <15 eosinophils/40× hpf with no evidence of intercellular edema, stromal fibrosis, or eosinophilic micro-abscesses. Control subjects (Group III) were obtained from autopsy and clinical specimens from patients whose esophageal epithelium was unremarkable and their medical records did not reveal history of either Inflammatory Bowel Disease (IBD), reflux, Barrett’s esophagus, adenocarcinoma of the esophagus, or eosinophilic disorders (e.g., hypereosinophilic syndrome (HES)). Group IV subjects were suspected EoE patients by virtue of displaying a clinical presentation and/or endoscopic observations compatible with EoE. Moreover, the middle or proximal esophageal biopsies of these patients revealed at most only two (2) ancillary histopathologies associated with EoE: sclerosis of the lamina propria stroma, basal hyperplasia of the squamous epithelium, intercellular epithelial edema, and/or the presence of eosinophilic micro-abscesses. Significantly, despite extensive reviews at high power (40×) of all sections of all biopsies taken from these patients, no foci of ≥15 eosinophils/40× hpf were identified, preventing an unambiguous diagnosis of EoE. Thus, these subsets of patients were specifically chosen based on their clinical, endoscopic, histopathological outcomes as a preliminary means of developing and then utilizing our EPX-mAb based algorithm.
We also performed a retrospective analysis of the esophageal tissues from children who received care at The Children’s Hospital, Denver and underwent upper endoscopy during the year 2006. Well documented clinical features, and in some cases the existence of follow-up assessments, allowed us to initially identify 48 children for potential study of which 14 were selected for analysis using EPX-mAb based immunohistochemistry based on the availability of an unambiguous diagnosis (Group I (EoE) - 7, Group II (GERD) - 3, Group IV (Control) - 4). Children with EoE (Group I) had symptoms referable to their esophagus, received at least 2 months of proton pump inhibition, ≥15 eosinophils/40× hpf in the esophageal epithelium with normal gastric and duodenal biopsies and demonstrated a clinical response to EoE treatment(s). GERD (Group II) was documented either by an abnormal pH/impedance monitoring of the distal esophagus or responsiveness to proton pump inhibitors, as well as a pathology report of <15 eosinophils/40× hpf in the esophageal epithelium. Control patients (Group III) showed no evidence of esophageal inflammation. In addition, 8 Indeterminate pediatric patients (Group IV) were identified within this cohort of children and subjected to EPX-mAb based immunohistochemistry.
Clinical descriptions of all patients included in this study, such as age, medical history/symptoms, and follow-up assessments (if available), are summarized either in the Results section and/or in the Supplemental On-Line Material (Supplemental Table 1 (S-Table 1)). This study was reviewed and performed in accordance with IRB approval at Mayo Clinic Arizona (IRB protocol number: 06-009236) and Colorado Multiple IRB (approval number 07-0888).
EPX-mAb based histopathologic scoring
Slides with biopsies (n ≥ 4 per patient) from the mid-proximal esophagus (i.e., >7cm from the esophageal-gastric junction) were coded by clinical histopathology laboratory personnel and subsequently stained by research lab-based personnel using EPX-mAb based immunohistochemistry.
Our assessments of patients using EPX-mAb based staining led to the identification of four independent diagnostic markers on the basis of their presence vs. absence in EoE relative to control subjects: (1) Presence or absence of infiltrating tissue eosinophils, (2) Evidence of eosinophil degranulation, (3) The extent of eosinophil infiltration and/or eosinophil degranulation in the maximally affected biopsy, and (4) The extent of eosinophil infiltration and/or eosinophil degranulation among the available patient biopsies. Detailed descriptions of each marker, including representative photomicrographs are presented in the Supplemental On-Line.
Statistical Analyses
Data were analyzed and graphed using GraphPad Prism statistics program (GraphPad Prism Software, San Diego, CA). Results are presented as means ± SEM. Statistical analysis was performed using ANOVA with Tukey. Differences between means considered significant when P<0.01.
RESULTS
EPX-mAb based immunohistochemistry: pathology assessments of EoE patients, including the detection of eosinophil activation (i.e., the release of granule proteins (degranulation))
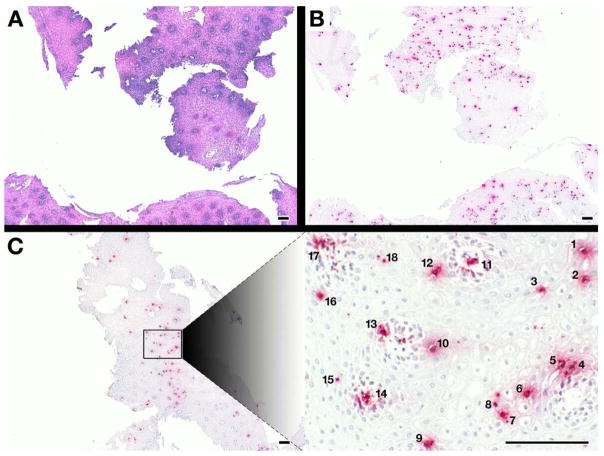
EPX-mAb based immunohistochemistry provided a strategy for the rapid detection of infiltrating eosinophils in esophageal biopsies. More importantly, this strategy also provided a method with which to identify quickly areas of biopsies that are likely to contain the focus of ≥15 eosinophils/40× hpf needed to meet the histological guidelines for a diagnosis of EoE. The photomicrographs of Figure 1 demonstrated the ease of identifying eosinophils within tissue sections using EPX-mAb based immunohistochemistry compared to H&E stained esophagus sections at low (5×, 20mm2 field of view) power (Figure 1(A, B)). Immunohistochemistry with EPX-mAb also allowed rapid identification of areas within the sections with a density of infiltrating eosinophils likely to achieve the ≥15 eosinophil/40× hpf needed for a guideline-driven EoE diagnosis (Figure 1(C)). More importantly, these photomicrographs highlighted the ease and utility of locating focal accumulation of eosinophils at low power prior to more detailed assessments at a higher magnification.
Figure 1. EPX-mAb based immunohistochemistry provides an efficient and rapid strategy to identify intact eosinophils infiltrating biopsies from eosinophil esophagitis patients.
A comparison of low (5×, 20mm2 field of view) power microscopy of (A) hematoxylin-eosin stained sections and (B) serial sections of the same patient following EPX-mAb based immunohistochemistry demonstrated that this immunohistochemical strategy easily permits the identification of infiltrating eosinophils in multiple biopsies within the field. (C) EPX-mAb based immunohistochemistry permits a rapid evaluation for the presence of intact infiltrating eosinophils of entire esophageal biopsies and the location of focal areas of eosinophil accumulation. The insert photograph in this panel is a high (40×, 0.29mm2 field of view) power field that was quickly/efficiently identified as a focal area of eosinophil accumulation (identified eosinophils are numbered 1–18) without the need of laborious time-consuming cell differential analyses. Scale bar = 100 μm.
A unique observation from our studies of EoE patients using EPX-mAb based immunohistochemistry was that degranulation (i.e., extracellular matrix deposition of EPX) was common (Figure 2). The importance of this observation is hard to overestimate because EPX-mAb based immunohistochemistry detected not only intact eosinophils but also eosinophil degranulation in areas with nominal numbers of intact eosinophils. Moreover, our examination of both adult and pediatric patients showed that extensive degranulation in the biopsies was nearly always associated with EoE, whereas GERD patients displayed lower levels of degranulation.
Figure 2. EPX-mAb based immunohistochemistry represents a novel strategy to detect eosinophil degranulation and the presence of released eosinophil peroxidase bound to tissue extracellular matrix.
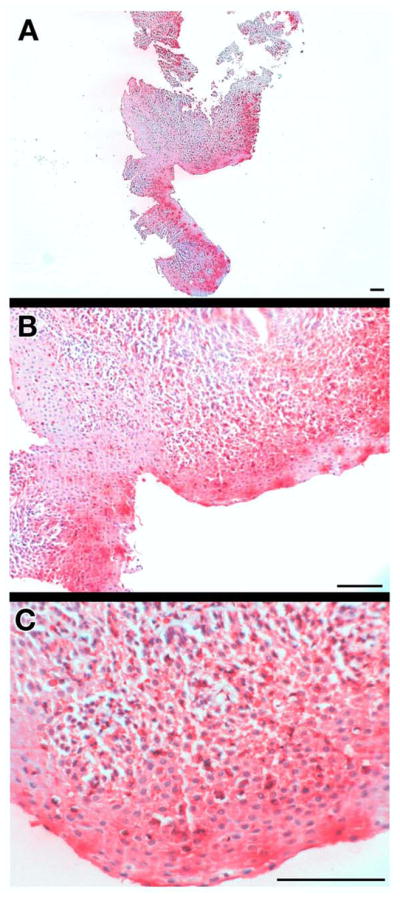
(A) Low (5×, 20mm2 field of view), (B) medium (16×, 1.8mm2 field of view), and (C) high (40×, 0.29mm2 field of view) microscopic fields of a proximal esophageal biopsy from an EoE patient demonstrate the utility of EPX-mAb based immunohistochemistry to detect eosinophil degranulation (i.e., EPX bound to extracellular matrix) in these biopsies. The results with this biopsy are representative of EoE patients which often display significant eosinophil degranulation. Scale bar = 100 μm.
EPX-mAb based immunohistochemistry and the development of a strategy to evaluate EoE vs. GERD patients
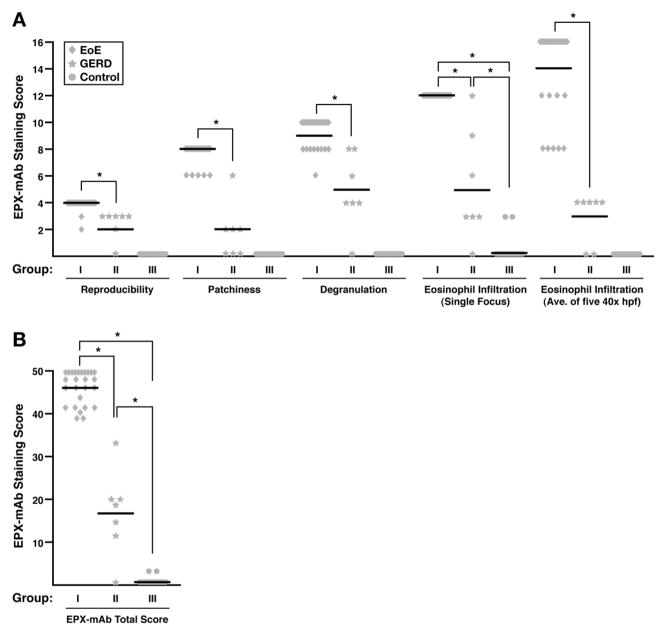
EPX-mAb based immunohistochemistry of mid-proximal biopsies (>7cm from the esophageal-gastric junction) from EoE patients (Group I), GERD patients (Group II), and control subjects (Group III) allowed for the identification of several EPX-mAb based histopathological markers that correlated with disease pathologies (see Methods and Supplemental On-Line Material). No observer to observer variations were observed in three of the four identified EPX-mAb based histopathological markers with only minor variations in the counting of intact eosinophils (<5%) observed among the four evaluators; in no case did these variations lead to different EPX-mAb based scores for given patient. However, the significantly increased sensitivity afforded by EPX-mAb based staining is noteworthy and represents a significant improvement over existing abilities. Specifically, two board-certified pathologists each counted eosinophils from serial sections of biopsies, one of which was stained with H&E to determined an average eosinophil count/40× hpf (average of 10–20 hpfs). The paired serial section was stained via EPX-mAb based immunohistochemistry and, in a blinded fashion, the same pathologists determined an average eosinophil count/40× hpf (average of 10–20 hpfs). These assessments showed that EPX-mAb based immunohistochemistry was able to detect >4 fold more eosinophils relative to inspection of H&E stained slides. The EPX-mAb based assessments of adult and pediatric patients are shown in S-Table 3 and summations of these scores are presented in Figure 3. These results showed that with one exception, the EPX-mAb based assessments were consistent with the previously established clinicopathological diagnosis based on tissue histopathology. In addition, statistically different EPX-mAb based scores (P<0.001) were observed between EoE, GERD, and control patients for each of the markers comprising the numerical algorithm (Figure 3(A, B)). EPX-mAb scores showed that most control subjects displayed total scores of zero with no control patients exceeding a score of 4. In contrast, EoE patients were uniformly identified (P<0.001) on the basis of scores at the other end of the scale within the range of 36–50. The EPX-mAb based scores also permitted identification of GERD patients (P<0.001) relative to either control or EoE patients with each of these GERD subjects scoring in the range of 5–35 (S-Table 3 and Figure 3(B)). Thus, EPX-mAb based histopathologic scoring not only identified EoE patients, but also provided qualitative measures and a quantitatively significant (P<0.001) means to distinguish EoE patients from patients with GERD. Significantly, our assessments of a well characterized cohort of children with histological evidence of EoE, GERD and otherwise unremarkable control tissues, demonstrated that EPX-mAb based immunohistochemical staining patterns replicated those observed in adults.
Figure 3.
EPX-mAb based immunohistochemistry provides a quantitatively significant strategy (Supplemental Table 2 (S-Table 2)) to distinguish EoE vs. GERD patients. (A) Examination of the scores for individual EPX-mAb based parameters associated with the EoE (Group I), GERD (Group II), and control patients (Group III) found in Supplemental Table 3 (S-Table 3) demonstrated statistical differences (*P<0.001) for each of the parameters comprising the EPX-mAb based algorithm. (B) Statistical assessments (ANOVA with Tukey) of the average total EPX-mAb based staining scores (means ± SEM) for the EoE, GERD, and control patients from Supplemental Table 3 (S-Table 3) demonstrated the utility of this algorithm to distinguish between these patient populations (*P<0.001).
EPX-mAb based immunohistochemistry provides a unique ability to identify Indeterminate patients who fail to achieve an unambiguous diagnosis using current clinicopathological guidelines
The utility of these quantitative assessments with difficult to diagnose patients were demonstrated in subjects with a likely diagnosis of EoE but who nonetheless failed to achieve the prerequisite guideline of at least a single focus of ≥15 eosinophils/40× hpf (Group IV). Histological assessment of these patients’ epithelium using EPX-mAb based immunohistochemistry in some cases revealed scores within the range of subjects with EoE as identified in Group I (S-Tables 1 and 3) or scores less than 36 (but ≥5) and were thus within the diagnostic range of GERD patients (Group II (STables 1 and 3)). In particular, assessments with EPX-mAb based scoring of a cohort of Indeterminate pediatric patients (S-Tables 1 and 3) demonstrated that most of these patients (75%) achieved scores supporting a diagnosis of EoE (i.e., an EPX score of 36–50). However, the observations that perhaps as many as 25% of these children had an EPX-mAb based score consistent of a GERD diagnosis (i.e., EPX-mAb score of 5–35). Unfortunately, clinical follow-up assessments were not available, preventing us from correlating final clinical outcome of these patients with their initial EPX-mAb based diagnosis.
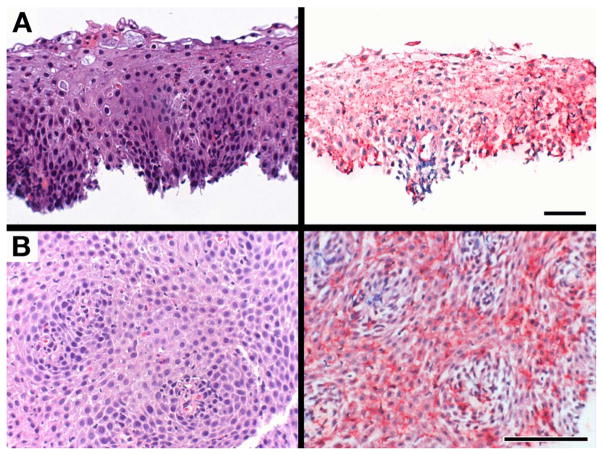
The availability of clinical follow-up assessments of many of our adult Indeterminate patients demonstrated the difficult-to-diagnose character of these esophageal patients based exclusively on existing clinicopathological criteria. Figure 4 represents serial slides (H&E vs. EPX-mAb based immunohistochemistry stained slides) from two problematic adult patients (i.e., patients #44 and #46 from S-Tables 1 and 3). These data demonstrate that while both traditional pathological assessment and EPX-mAb based immunohistochemistry each failed to detect the guideline required focus of ≥15 eosinophils/40× hpf, EPX-mAb based immunohistochemistry revealed significant areas of eosinophil degranulation. Thus, as noted earlier the EPX-mAb scoring provided additional histological support for the diagnosis of EoE (i.e., an EPX-mAb score of 36–50). Indeed, follow-up assessments of these patients revealed that patient #44 responded to corticosteroid treatment whereas patient #46 has thus far failed to display symptomatic improvement during an extended period of treatment with proton pump inhibitors. In contrast, another Indeterminate adult patient (patient #45) was identified as a GERD patient based on this subject’s EPX-mAb based score and follow-up assessments demonstrated that this patient became symptom-free following treatment with a proton pump inhibitor. Moreover, follow-up EPX-mAb based immunohistochemistry on biopsies on several other GERD patients responding to proton pump inhibitor based therapies demonstrated that the EPX-mAb scores of these patients were reduced to the control range of <5 (data not shown). These data highlight both the difficulties associated with identifying some esophageal patients based solely on existing diagnostic guidelines and the utility of EPX-mAb based immunohistochemistry as a strategy to resolve difficult and/or ambiguous cases.
Figure 4.
EPX-mAb based immunohistochemistry permits a diagnosis of eosinophilic esophagitis among patients with appropriate clinical symptoms and borderline endoscopic/histological results but who fail to achieve the current guideline recommendations of at least a single focus of ≥15 eosinophils/40× hpf among the available biopsies. Serial sections of proximal esophageal biopsies from (A) patient #44 and (B) patient #46 (see Supplemental Tables 1 and 3 (S-Tables 1 and 3)) were either stained with hematoxylin/eosin (left panels) or subjected to EPX-mAb based immunohistochemistry (right panels) and photographed at high (40×, 0.29mm2 field of view) power. Although both patients had failed to meet traditional pathology guidelines for a diagnosis of EoE, EPX-mAb based immunohistochemistry detected the presence of extensive eosinophil degranulation in the absence of ≥15 intact eosinophils/40× hpf (0.29mm2 field of view), elevating their EPX-mAb based total score within the range indicating an EoE diagnosis. Scale bar = 50 μm.
DISCUSSION
The EPX-mAb based immunohistochemical assay described in this report represents a novel tool and systematic method to assess esophageal tissues for evidence of eosinophilic inflammation in both children and adults. That is, the previously limited availability of eosinophil-specific antibody-staining options for immunohistochemical assays that are sensitive, reproducible, and useful in the most commonly available format (i.e., archived formalin-fixed paraffin-embedded tissues) had prevented the development and use of this strategy to evaluate patients. However, development of an eosinophil granule protein-specific monoclonal antibody, together with a novel histological scoring system, allowed for not only the specific detection of eosinophils and eosinophil degranulation but also provided a sensitive and cost effective method that specifically identified patients with EoE vs. GERD on the basis of a single histological evaluation. In addition, it allowed for the differentiation of “Indeterminate” patients in whom the diagnosis of EoE was not certain.
This study assessed children and adults with previously established diagnoses and not consecutive patients. In turn, this may have led to a selection bias ultimately leading to either higher EPX-mAb based scores for patients with EoE or lower EPX-mAb based scores for GERD and/or control subjects. However, in this rapidly evolving field, the utility of our retrospective analysis of very well defined patients has proven to be quite valuable, especially in light of the fact that a number of challenges have led to difficulty in obtaining a true diagnosis of EoE 4, 8. As a consequence, we propose the current scoring system as an initial effort to evaluate tissues that can now be used to prospectively study consecutive patients with esophageal diseases.
These findings address several critical and timely unmet needs in the care of patients which display eosinophilic esophageal inflammation: (i) The use of this EPX-mAb based scoring system allowed for the rapid identification of patients with EoE. (ii) Esophageal eosinophilia as a pure numerical value of intact eosinophils was improved by the additional sensitivity of EPX-mAb based immunohistochemical detection of tissue infiltrating eosinophils. This issue was highlighted in our assessments of Indeterminate pediatric patients where we found that although traditional H&E histopathological assessments failed to reveal a focus of ≥15 eosinophils/40× hpf, 75% of these cases displayed this guideline prerequisite as determined by EPX-mAb based immunohistochemistry. The sensitivity of this strategy was further enhanced by expanding the term “eosinophilia” to include assessment and quantification of eosinophil degranulation. (iii) Because an increasing body of evidence suggests that tissues from patients with GERD may have similar number of esophageal eosinophils as with EoE, this system allowed for differentiation between these two diseases 1, 3. The inclusion of multiple EPX-mAb based histological scoring parameters provided not only a statistically-significant quantitative means by which to identify EoE patients but also qualitative measures that appear to reliably differentiate these subjects from patients with GERD. In particular, a subset of EPX-mAb based parameters may be sufficient to achieve a quick, but accurate, differential diagnosis between these esophageal diseases. For example, 24 of 26 EoE patients displayed extensive degranulation in multiple biopsies compared to only 2 of 7 biopsies from GERD patients showing a similar level of degranulation. In addition, low power assessments of the fraction of patient biopsies displaying eosinophil degranulation (Reproducibility) and the fractional area of the maximally affected tissue fragment that displayed evidence of degranulation (Patchiness) together were alone sufficient to accurately identify EoE patients relative to subjects with GERD. (iv) Indeterminate diagnoses were clarified with EPX-mAb based immunohistochemistry. That is, this strategy allowed for a diagnosis of EoE and GERD in patients who achieved many, but not all, of the clinical, endoscopic, and histopathologic features of these diseases. Indeed, our study provides examples of indeterminate EoE patients who nonetheless failed to achieve the prerequisite ≥15 eosinophils/40× hpf.
It is interesting that some EoE and GERD patients had biopsies with many intact eosinophils but minimal levels of degranulation (e.g., patient #6 and #10 and patient #27 vs. #29 (S-Table 3), respectively), whereas other patients displayed widespread degranulation in the presence of very few intact eosinophils (e.g., patient #44, #46, and #47). These observations suggest that the degranulation detected in the tissue sections of this study was specific/unique to individual patient biopsies and unlikely to be a consequence of artefactual events associated with tissue processing and/or handling 9. Thus, while current guidelines propose that identification of ≥15 eosinophils/40× hpf (in the proper clinical/endoscopic context) is required for the diagnosis of EoE, the EPX-mAb based scoring system’s utilization of multiple parameters such as eosinophil degranulation, provides an alternative diagnostic strategy for pathologists/clinicians.
EPX-mAb based immunohistochemical evaluations address long held technical issues related to assessment of eosinophil degranulation. Previous studies of a limited number of subjects identified that the mucosa affected by EoE displayed enhanced eosinophil degranulation (i.e., deposition of eosinophil derived neurotoxin (EDN 10) or major basic protein (MBP 9) relative to GERD patients. However, issues arise with the use of antibodies reactive to each of these granule protein constituents. For example, although EDN is a prominent eosinophil secondary granule protein, several studies have demonstrated that unlike eosinophil peroxidase, EDN is not eosinophil specific and is expressed in both other leukocytes (e.g., neutrophils 11) and tissue/organs (e.g., liver 12). In addition, the cationic character of MBP, together with its propensity to “stick” to virtually any substratum as well as its near insolubility in environments at neutral pH, have been problematic issues that may have biased earlier studies by artefactually limiting the extent of observable areas of degranulation. Moreover, these intensely staining local aggregates may also have been responsible for the perception that tissue handling/processing in earlier studies led to eosinophil degranulation 9. In contrast, the nominal cationic character of eosinophil peroxidase (pI ~8.9 13) together with its greater solubility at neutral pH would prevent aggregation and allow this granule protein to disperse to a greater extent.
The practical utility of this method lies in its simplicity, durability and cost-saving features. Immunohistochemical staining with EPX-mAb is straightforward, can be performed on archived formalin-fixed paraffin-embedded tissues and requires no unique technology. The assessment and scoring evaluations of the EPX-mAb based staining patterns in children and adults were reproducible as evidenced by negligible inter-observer variability. The added time and cost for this assessment will be minimal when taken in the context of achieving the correct diagnosis in a rapid time frame. For example, current diagnostic guidelines support the strategy that a diagnosis of GERD must be ruled out as a cause for esophageal eosinophilia before assigning a diagnosis of EoE. In this light, a patient would need to undergo either two (2) months of proton pump inhibition or a pH/impedance monitoring of the distal esophagus both of which can be time consuming, costly and potentially uncomfortable. However, in the context of a preponderance of clinical symptoms, endoscopic results, and evaluations of histopathology, the EPX-mAb based scoring algorithm could provide a quantitatively definitive evaluation of initial biopsies permitting an immediate diagnosis of EoE, including potentially the differentiation between difficult to diagnose EoE vs. GERD patients (i.e., Indeterminate cases). That is, despite guideline driven diagnoses that had been unclear, EPX-mAb based immunohistochemistry appears capable of identifying subsets of patients as either EoE or GERD. These observations suggest either an overlap between these two types of esophageal patients based on clinicopathologic findings or that a unique subset of EoE patients are instead simply difficult to diagnose GERD patients. In summary, we anticipate that validation and future use of this system will improve care of children and adults and allow for greater understanding of esophageal inflammatory diseases.
Supplementary Material
Acknowledgments
Grant Support and other assistance: The studies presented regarding the use of these antibodies in the diagnosis of human disease was supported by the Mayo Foundation and research grants from the NIH to JJL (HL065228, CA112442. and K26-RR019709), NAL (HL058723), and GTF (DK62245 and CURED). The creation/production of EPX-reactive mouse monoclonal antibodies was supported, in part, by a sponsored research grant from Schering-Plough.
We are grateful to all our Mayo Clinic Arizona (MCA) and Children’s Hospital Denver (CHD) clinical colleagues and their patients. We are grateful for the invaluable insight provided by our pathology colleagues, including Drs. K. Leslie (MCA), R. Valdez (MCA), C. Conley (MCA), J. Sweeney (MCA), Mark Lovell (CHD), and Kelley Capocelli (CHD). We also would like to thank Wendy Moore (CHD), S. Montgomery (MCA), and C. Sinclair (MCA). We are indebted to Media Support Services (M. Ruona (MCA) and N. Boruff (MCA)) and to our administrative assistants L. Mardel (MCA), P. McGarry (MCA), and Shirley (“Charlie”) Kern (MCA). In addition, we wish to express our thanks to Mayo Clinic Arizona Immunology Core Facility (Director, Tammy Brehm-Gibson). The creation/production of EPX-reactive mouse monoclonal antibodies was supported, in part, by a sponsored research grant from Schering-Plough.
Abbreviations
- EoE
eosinophilic esophagitis
- GERD
gastroesophageal reflux disease
- EPX-mAb
anti-eosinophil peroxidase monoclonal antibody
Footnotes
Writing assistance: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: A systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342–63. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Collins MH. Histopathologic features of eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:59–71. viii–ix. doi: 10.1016/j.giec.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigo S, Abboud G, Oh D, et al. High intraepithelial eosinophil counts in esophageal squamous epithelium are not specific for eosinophilic esophagitis in adults. Am J Gastroenterol. 2008;103:435–42. doi: 10.1111/j.1572-0241.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- 4.Odze RD. Pathology of eosinophilic esophagitis: What the clinician needs to know. Am J Gastroenterol. 2009;104:485–90. doi: 10.1038/ajg.2008.40. [DOI] [PubMed] [Google Scholar]
- 5.Furuta GT, Nieuwenhuis EE, Karhausen J, et al. Eosinophils alter colonic epithelial barrier function: Role for major basic protein. Am J Physiol Gastrointest Liver Physiol. 2005;289:G890–7. doi: 10.1152/ajpgi.00015.2005. [DOI] [PubMed] [Google Scholar]
- 6.Forbes E, Murase T, Yang M, et al. Immunopathogenesis of experimental ulcerative colitis is mediated by eosinophil peroxidase. J Immunol. 2004;172:5664–5675. doi: 10.4049/jimmunol.172.9.5664. [DOI] [PubMed] [Google Scholar]
- 7.Mueller S, Aigner T, Neureiter D, et al. Eosinophil infiltration and degranulation in oesophageal mucosa from adult patients with eosinophilic oesophagitis: A retrospective and comparative study on pathological biopsy. J Clin Pathol. 2006;59:1175–80. doi: 10.1136/jcp.2005.031922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dellon ES, Aderoju A, Woosley JT, et al. Variability in diagnostic criteria for eosinophilic esophagitis: A systematic review. Am J Gastroenterol. 2007;102:2300–13. doi: 10.1111/j.1572-0241.2007.01396.x. [DOI] [PubMed] [Google Scholar]
- 9.Kato M, Kephart GM, Talley NJ, et al. Eosinophil infiltration and degranulation in normal human tissue. Anat Rec. 1998;252:418–25. doi: 10.1002/(SICI)1097-0185(199811)252:3<418::AID-AR10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 10.Kephart G, Alexander J, Arora A, et al. Localization of eosinophil-derived neurotoxin in esophageal tissues: A potential biomarker for eosinophilic esophagitis. J Allergy Clin Immunol. 2008;121:S44–S45. [Google Scholar]
- 11.Sur S, Glitz DG, Kita H, et al. Localization of eosinophil-derived neurotoxin and eosinophil cationic protein in neutrophilic leukocytes. J Leukoc Biol. 1998;63:715–22. doi: 10.1002/jlb.63.6.715. [DOI] [PubMed] [Google Scholar]
- 12.Sorrentino S, Glitz DG, Hamann KJ, et al. Eosinophil-derived neurotoxin and human liver ribonuclease. Identity of structure and linkage of neurotoxicity to nuclease activity. J Biol Chem. 1992;267:14859–65. [PubMed] [Google Scholar]
- 13.Ten RM, Pease LR, McKean DJ, et al. Molecular cloning of the human eosinophil peroxidase. J Exp Med. 1989;169:1757–1769. doi: 10.1084/jem.169.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.