Abstract
2-Amino-4,6-bis[(phosphonomethoxy)alkoxy]pyrimidines bearing two equal or different achiral or chiral phosphonoalkoxy chains have been prepared either by aromatic nucleophilic substitution of 2-amino-4,6-dichloropyrimidine or by alkylation of 4,6-dihydroxy-2-(methylsulfanyl)pyrimidine with appropriate phosphonate–bearing building block. Alkylation of 4,6-dihydroxy-2-(methylsulfanyl)pyrimidine proved to be the method of choice for efficient preparation of variety of bisphosphonates. The enantiomerical purity of selected compounds was determined by capillary electrophoresis. Antiviral activity of bisphosphonates is discussed.
Keywords: Acyclic Nucleoside Phosphonates, Pyrimidine, Bisphosphonates, Alkylation
1. Introduction
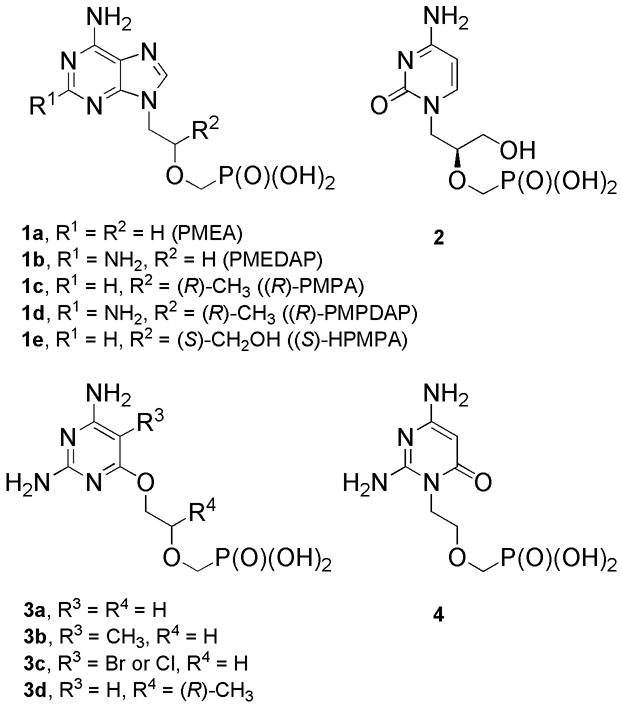
Acyclic nucleoside phosphonates [1] (ANPs) represent a key class of nucleotide analogs with a broad spectrum of antiviral and cytostatic activity. Among ANPs, particularly 9-[2-(phosphonomethoxy)ethyl]adenine (PMEA, adefovir, 1a, Figure 1) is active against DNA and retroviruses [2]; its prodrug, adefovir dipivoxil [3], was approved for hepatitis B therapy (Hepsera) [4]. 9-(R)-[2-(Phosphonomethoxy)propyl]adenine (PMPA, tenofovir, 1c), is a promising anti-HIV drug, its prodrug Viread was approved for treatment of AIDS [5]. A third type of antiviral compounds is represented by 9-(S)-[3-hydroxy-2-(phosphonometoxy)propyl]cytosine (HPMPC, cidofovir, Vistide, 2) which possesses general anti-DNA-viral activity [6]. Cidofovir was approved for treatment of cytomegalovirus retinitis in AIDS patients. The 2,6-diaminopurine derivatives (1b, 1d) and their guanine counterparts are potent antivirals and exhibit powerful antitumor activity [7].
Figure 1.
Acyclic nucleoside phosphonates.
We have recently described a new type of antiviral acyclic nucleoside phosphonates originating from 2-substituted 4-amino-6-hydroxypyrimidines [8]. Alkylation of 6-hydroxypyrimidines by phosphonate-bearing building block afforded a mixture of O6- and N1-regioisomers. While none of the isomeric 1-[2-(phosphonomethoxy)ethyl]pyrimidin-6-one derivatives 4 was antivirally active, compounds derived from 2,4-diamino-6-hydroxypyrimidine (3a–3d) and 2-amino-4,6-dihydroxypyrimidine significantly inhibited replication of retroviruses and herpes viruses in cell culture. Compounds 3 can be considered as analogues of 2,6-diaminopurine with an open imidazole ring of the purine moiety. This structural relation is strongly supported by the finding that the corresponding analogue of PMPDAP, i.e. 2,4-diamino-6-[2-(phosphonomethoxy)propoxy]pyrimidine (3d), has the same selective antiretroviral activity as (R)-PMPDAP (1d). This activity is also limited only to the (R)-enantiomer, while the (S)-enantiomer is similarly devoid of antiviral activity as is (S)-PMPDAP. Further SAR studies demonstrated that 5-substituted derivatives of 2,4-diamino-6-[2-(phosphonomethoxy)ethoxy]pyrimidine (3a) markedly inhibited retrovirus replication in cell culture [9]. The 5-methyl derivative 3b was exquisitely inhibitory to human immunodeficiency virus and Moloney murine sarcoma virus-induced cytopathicity in cell culture but also cytostatic to CEM cell cultures. The 5-halogen-substituted derivatives (3c) showed pronounced antiretroviral activity, comparable to that of the reference drugs adefovir and tenofovir, but were devoid of any measurable toxicity.
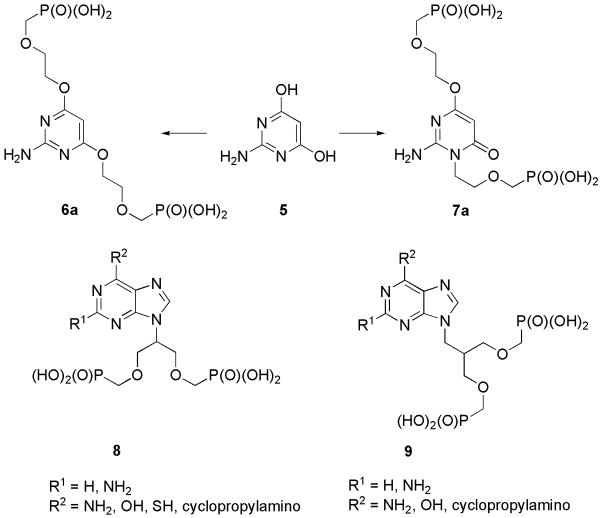
The isomeric compounds 6a and 7a (Figure 2) bearing two phosphonoalkoxy chains [8a] may be considered as a second group of “open-ring” ANPs and potential antivirals. Direct alkylation of 2-amino-4,6-dihydroxypyrimidine (5) with 2-(diisopropoxyphosphorylmethoxy)ethylchloride gave a mixture of disubstituted regioisomers 6a and 7a approximately in the same ratio. While 2-amino-4-[2-(phosphonomethoxy)ethoxy]-1-[2-(phosphonomethoxy)ethyl]pyrimidin-6(1H)-one (7a) was not antivirally active, 2-amino-4,6-bis[2-(phosphonomethoxy)ethoxy]pyrimidine (6a) was reported to show antiretroviral activity [8a]. Another study concerning bisphosphonate derivatives 8 and 9 [10] was also performed in our laboratory.
Figure 2.
Acyclic nucleoside bisphosphonates.
In this paper, we describe efficient synthesis of series of bisphosphonates derived from 4,6-(dihydroxy)pyrimidine. We were interested in regioselective synthesis of O-alkylated pyrimidine derivatives bearing two equal or different chiral phosphonoalkoxy chains as potentially biologically active compounds from a large family of phosphonate antivirals.
2. Results and discussion
2.1. Stepwise synthesis of bisphosphonates from 2-amino-4,6-dichloropyrimidine
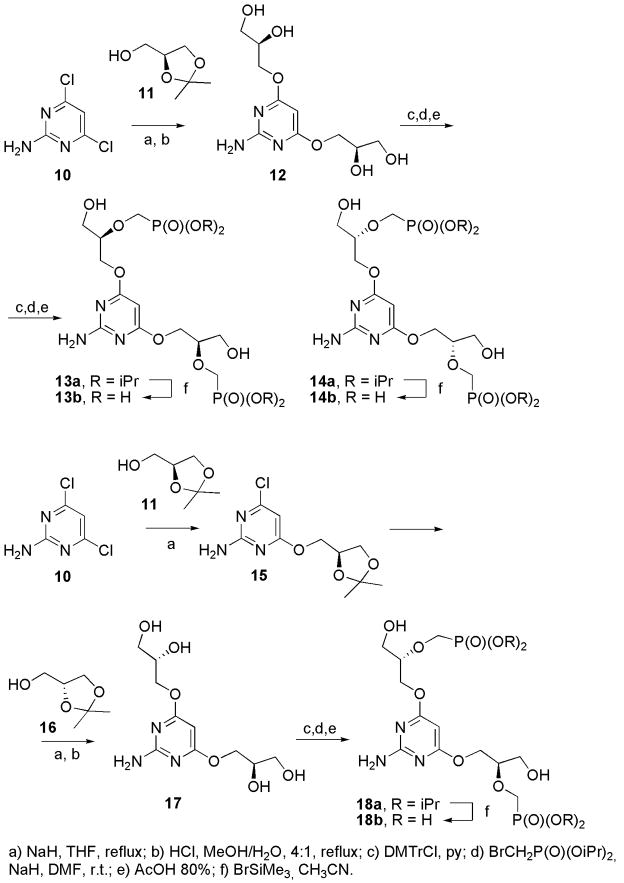
Synthesis of bisphosphonates bearing two 3-hydroxy-2-(phosphonometoxy)propoxy (HPMPO) chains at positions 4 and 6 of the pyrimidine moiety started from 2-amino-4,6-dichloropyrimidine (10) (Scheme 1). While alkylation of 2-amino-4,6-dihydroxypyrimidine (5) afforded a mixture of O– and N–alkylated products 6a and 7a [8a], nucleophilic aromatic substitution of 10 with appropriate alkylating agent can give only O–regioisomer. Reaction of 10 with 2 equivalents of (S)-1,2-isopropylideneglycerol (11) was performed in THF in the presence of NaH as a base; subsequent deprotection by diluted hydrochloric acid gave 2,3-dihydroxypropoxy derivative 12 in 64% yield. Primary hydroxyl groups were protected by treatment with 4,4′-dimethoxytrityl chloride and alkylation of secondary hydroxy groups by diisopropoxyphosphorylmethyl bromide followed by deprotection with acetic acid afforded bis-HPMPO derivative 13a. Diisopropyl esters were cleaved under standard conditions (bromotrimethylsilane in acetonitrile, followed by hydrolysis) to afford free phosphonic acid 13b. The enantiomer 14b was prepared by the same procedure as compound 13b from pyrimidine 10 and (R)-1,2-isopropylideneglycerol (16). The diastereosisomer 18b was prepared by reaction of pyrimidine 10 with one equivalent of 11 and one equivalent of NaH in THF; the monoalkylated product 15 was treated with 16 under the same conditions to afford dihydroxypropoxy derivative 17. Further procedure was identical with that described for the compound 13b. Compounds 13b, 14b and 18b were purified by preparative HPLC; triethylammonium salts of phosphonic acids were converted to the free phosphonic acids on a column of Dowex 50 × 8 in H+ form.
Scheme 1.
Synthesis of bis-HPMPO derivatives.
Synthesis of bisphosphonates bearing two 2-(phosphonomethoxy)propoxy (PMPO) chains or two different phosphonoalkoxy chains by this method failed. Reaction of 10 with phosphonoalkoxyalkanol gives only product of monosubstitution. 2-Amino-4-chloro-6-[2-(diisopropoxyphosphorylmethoxy)ethoxy]pyrimidine is further unreactive towards nucleophilic aromatic substitution. The ether bond at position 6 of the pyrimidine moiety is relatively labile and under harsh reaction conditions (basic, acidic or high temperature) decomposes. For details see the supporting information.
2.2. Synthesis of bisphosphonates from 4,6-dihydroxy-2-(methylsulfanyl)pyrimidine
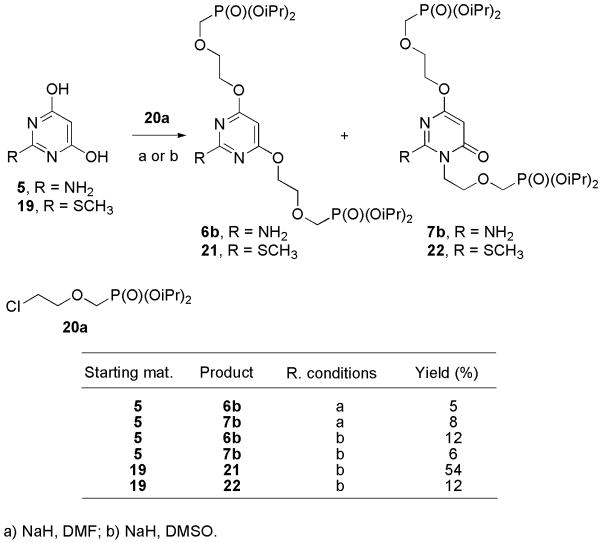
Since synthesis of bisphosphonates bearing two different chains from 10 failed, we focused on alkylation of dihydroxypyrimidine 5 and 19 with phosphonate 20a [11] (Table 1). Previously described [8a] alkylation of 2-amino-4,6-dihydroxypyrimidine (5) in the presence of NaH as a base in DMF afforded a mixture of compounds 6b and 7b in approximately 1:1 ration in a very low yield. Alkylation of 5 in DMSO slightly increased the yield. Alkylation of commercially available 4,6-dihydroxy-2-(methylsulfanyl)pyrimidine (19) in DMSO gave O–alkylated regioisomer 21 as a major product in 54% yield; regioisomer 22 was formed in 12% yield (Scheme 2). Alkylation of 19 in DMF did not proceed at all because of poor solubility of disodium salt of pyrimidine 19 in DMF; this might be also a reason of a low yield in the reaction described in literature [8a]. Thus, the alkylation of pyrimidine 19 was the method of choice for the synthesis of bisphosphonates. This method afforded compound 6a in 18% overall yield compared to the previously reported 3.5% yield; and moreover, formation of N–alkylated regioisomers was suppressed. The reaction sequence further allows introduction of modifications at positions 2, 4 and 6 of the 4,6-(dihydroxy)pyrimidine moiety in satisfactory yields.
Table 1.
Substitution pattern of compounds 20, 23 and 24.
| Compd. 20 | R1, R2 | Y | Compd. 23, 24 | R1 |
|---|---|---|---|---|
| a | H | Cl | 23a | H |
| b | (S)-CH3 | OTs | 23b | (S)-CH3 |
| c | (R)-CH3 | OTs | 23c | (R)-CH3 |
| d | (S)-CH2OH | OTs | 24a | (S)-CH3 |
| e | (R)-CH2OH | OTs | 24b | (R)-CH3 |
Scheme 2.
Alkylation of 4,6-(dihydroxy)pyrimidines.
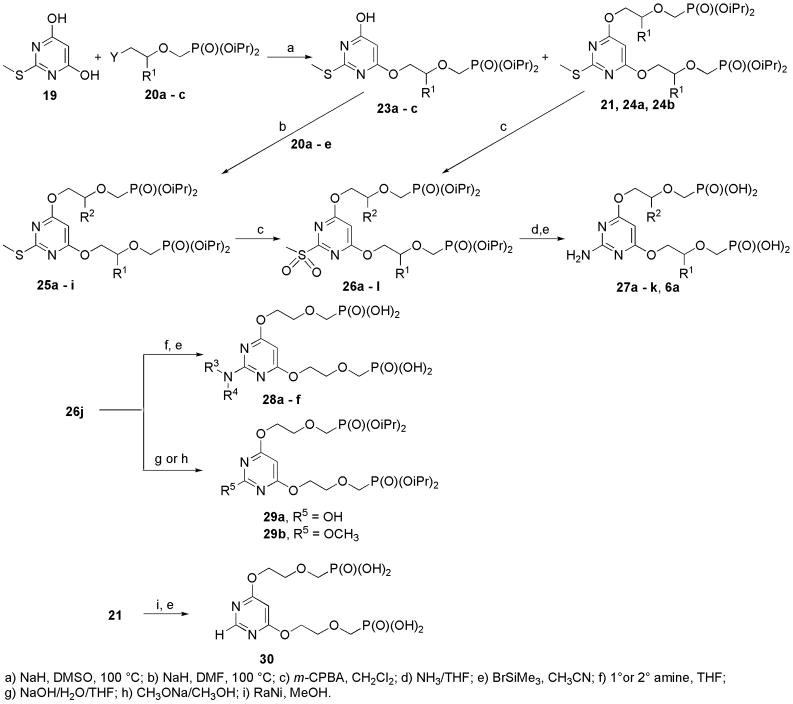
Alkylation of pyrimidine 19 with one equivalent of appropriate phosphonate 20 (Table 1) in DMSO afforded a mixture of mono- and dialkylated product 23 and 24 (Scheme 3, Table 1), respectively. Monoalkylated product 23 was subsequently alkylated by 20 in DMF to afford bisderivative bearing two different substituents 25a–i (Table 2). 2-Methylsulfanyl group of compounds 24 and 25 (Table 2) was oxidized by m-CPBA in dichloromethane [12] to give 2-methylsulfonyl derivatives 26, which were further converted to 2-amino congeners using liquid ammonia in THF at r.t. [13a]. Final deprotection of diisopropyl esters by bromotrimethylsilane afforded free phosphonic acids 6a and 27a–k.
Scheme 3.
Synthesis of bisphosphonates by alkylation of 4,6-(dihydroxy)-2-(methylsulfanyl)pyrimidine.
Table 2.
Substitution pattern of compounds 25, 26 and 27.
| Compd. 25, 26 | R1 | R2 | Product | R1 | R2 |
|---|---|---|---|---|---|
| 25a, 26a | H | (S)-CH3 | 27a | H | (S)-CH3 |
| 25b, 26b | H | (R)-CH3 | 27b | H | (R)-CH3 |
| 25c, 26c | H | (S)-CH2OH | 27c | H | (S)-CH2OH |
| 25d, 26d | H | (R)-CH2OH | 27d | H | (R)-CH2OH |
| 25e, 26e | (S)-CH3 | (R)-CH3 | 27e | (S)-CH3 | (R)-CH3 |
| 25f, 26f | (S)-CH3 | (S)-CH2OH | 27f | (S)-CH3 | (S)-CH2OH |
| 25g, 26g | (S)-CH3 | (R)-CH2OH | 27g | (S)-CH3 | (R)-CH2OH |
| 25h, 26h | (R)-CH3 | (S)-CH2OH | 27h | (R)-CH3 | (S)-CH2OH |
| 25i, 26i | (R)-CH3 | (R)-CH2OH | 27i | (R)-CH3 | (R)-CH2OH |
| 26j | H | H | 6a | H | H |
| 26k | (S)-CH3 | (S)-CH3 | 27j | (S)-CH3 | (S)-CH3 |
| 26l | (R)-CH3 | (R)-CH3 | 27k | (R)-CH3 | (R)-CH3 |
Compound 26j upon treatment with primary or secondary amine [12b] and subsequent deprotection with bromotrimethylsilane afforded N2-substituted bisphosphonates 28a–f (Table 3). 2-Methylsulfonyl derivative 26j was hydrolyzed by sodium hydroxide in a mixture of water and THF [13] to give 2-hydroxy derivative 29a; treatment of 26j with sodium methylate in methanol [14] gave 2-methoxy derivative 29b. Treatment of 29a and 29b with bromotrimethylsilane led to the decomposition of pyrimidine derivatives. 2-Methylsulfanyl derivative 21 was reduced with Raney-Nickel [11] to afford 4,6-bis[2-(phosphonomethoxy)ethoxy]pyrimidine, which was subsequently deprotected by bromotrimethylsilane to give free phosphonic acid 30.
Table 3.
Substituents at position 2 of compound 28.
| Compd. 28 | R3 | R4 |
|---|---|---|
| a | H | cyclopropyl |
| b | H | cyclopentyl |
| c | H | methyl |
| d | H | benzyl |
| e | H | 4-methoxybenzyl |
| f | - | morpholino |
2.3. Bisphosphonates derived from 2-amino-4,6-disulfanylpyrimidne
Pyrimidine [15] 10 was converted to the disulfanyl analogue 31 by reaction with thiourea (Scheme 4) [16]. Alkylation of 31 with phosphonate 20a–c (Table 1) gave unequivocally S–alkylated product 32a–c (Table 4). Sulfur derivatives are better nucleophiles than their oxygen and nitrogen analogs, so the alkylation of sulfur at positions 4 and 6 took place smoothly even at room temperature. Alkylation of pyrimidine 31 with one equivalent of alkylating agent 20a or 20b gave monoalkylated product 34a and 34b together with dialkylated product. Further alkylation of 34a–b afforded pyrimidine with two different substituets 35a–c. Diisopropyl esters of bisphosphonates 32a–c and 35a–c were cleaved by standard procedure with bromotrimethylsilane.
Scheme 4.

Alkylation of 2-amino-4,6-(disulfanyl)pyrimidine.
Table 4.
Substitution pattern of compounds 32, 33, 34, 35 and 36.
| Compd. 32, 33, 34 | R1 | Compd. 35, 36 | R1 | R2 |
|---|---|---|---|---|
| a | H | a | H | (S)-CH3 |
| b | (S)-CH3 | b | H | (R)-CH3 |
| c | (R)-CH3 | c | (S)-CH3 | (R)-CH3 |
2.4. Alkoxyalkyl esters of bisphosphonates
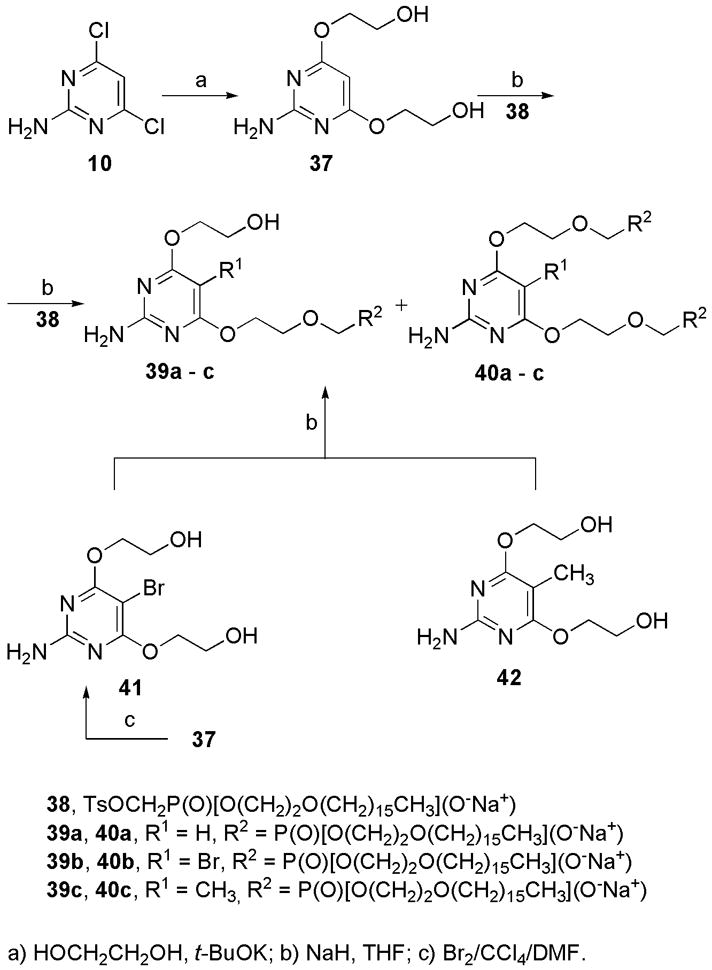
For further biological activity screening lipid esters of compound 6a and its 5-bromo and 5-methyl congener (Scheme 5) were prepared by method described by J. R. Beadle and K. Y. Hostetler [17].
Scheme 5.
Synthesis of alkoxyalkyl esters of bisphosphonates.
Pyrimidine 10 in neat ethyleneglycol in the presence of tBuOK gave hydroxyethoxy derivative 37 in 71% yield (Scheme 5). Pyrimidine 37 in THF was treated with NaH, heated to 50 °C and then hexadecyloxyethyl toluenesulfonyloxymehylphosphonate (38) was added. Monoalkylated derivative 39a was isolated together with bis derivative 40a. Alkylation in DMF or in mixture of triethylamine and THF (1:1) gave lower yields; reaction in triethylamine [17] as solvent did not proceed at all. Bromination of 37 with elemental bromine in DMF/CCl4 [9a] gave smoothly the 5-bromo derivative 41. 5-Substituted derivatives 41 and 42 [Petr Jansa, unpublished results] were similarly converted to esters 39b and 39c and dialkylated products 40b and 40c by above described alkylation. Monoalkylated compounds 39a–c were fully characterized and submitted for biological activity screening, however dialkylated products 40a–c were nearly insoluble in any solvent; therefore their NMR spectra could not be measured. Thus compounds 40a–c were characterized only by mass spectroscopy and elemental analysis and were not tested for biological activity. Our attempts to convert compound 6a to cycloSal, cycloAmb [18] or POM [19] esters failed due to instability of ether bonds at positions 4 and 6 under reaction conditions.
2.5. Capillary electrophoresis
Enantiomerical purity of compounds 27a and 27b were analyzed by capillary electrophoresis. Baseline separation of enantiomers 27a and 27b, with resolution 1.67, was achieved in chiral background electrolyte (BGE) composed of 50 mM borax, adjusted by NaOH to pH 10.0, with chiral selector β-cyclodextrin (20 mg/ml), (Figure 3A). The compound 27a was found enantiomerically pure as demonstrated by single peak of CE analysis of this compound in chiral BGE (Figure 3B) whereas a very small admixture of enantiomer 27a was found in the CE analysis of compound 27b (Figure 3C).
Figure 3.
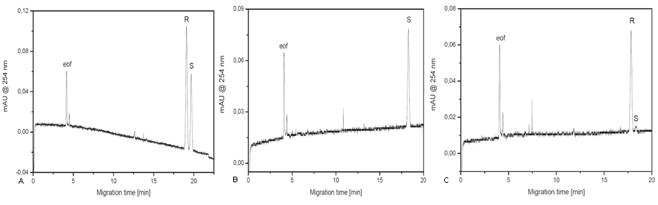
A: CE separation of enantiomers 27a (S), 0.1mM and 27b (R), 0.2 mM in chiral BGE (background electrolyte) composed of 50 mM borax, adjusted by NaOH to pH 10.0, with chiral selector β-cyclodextrin (20 mg/ml). B: CE analysis of enantiomer 27a in the above chiral BGE. C: CE analysis of enantiomer 27b in the above chiral BGE. eof = electroosmotic flow marker.
It was confirmed that optically active phosphonates are stable and do not tend to racemize. No racemization did occur during the whole multistep synthesis from chiral precursors.
3. Conclusion
In conclusion, in the SAR studies of “open-ring“ ANPs a series of bisphosphonates derived from 2-amino-4,6-(dihydroxy)pyrimidine was prepared. Bisphosphonates bearing two identical or different achiral or chiral phosphonoalkoxy chains were prepared either by nucleophilic aromatic substitution of 2-amino-4,6-dichloropyrimidine (10) or by alkylation of 4,6-(dihydroxy)-2-(methylsulfanyl)pyrimidine (19). The second method proved to be the universal method for regioselective preparation of O–alkylated pyrimidines at positions 4 and 6; furthermore 2-methylsulfanyl function is a versatile leaving group for introduction of various substituents at position 2 of the pyrimidine moiety. Disulfanylpyrimidine 31 was alkylated in the same manner to give exclusively S–alkylated product. Alkoxyalkyl esters of selected bisphosphonates were prepared to improve their bioavailability; however introduction of two lipid esters to bisphosphonates dramatically decreased their solubility. Enantiomerical purity of compounds 27a and 27b was successfully determined by capillary electrophoresis.
The whole series of bisphosphonates (6a, 13b, 14b, 18b, 27a–k, 28a–f, 30, 33a–c, 36a–c and 39a–c) was investigated for their inhibitory activity against several DNA and retroviruses. None of the prepared bisphosphonates showed any appreciable antiviral activity. Finally, antiretroviral activity of resynthesized parent compound 6a was not confirmed. The previously reported activity [8a] might probably had been caused by undetectable admixture of several orders more active monoalkylated 2-amino-4-hydroxy-6-[2-(phosphonomethoxy)ethoxy]pyrimidine. Compounds are devoid of any measurable toxicity to cell cultures.
4. Experimental Section
4.1. Materials and general procedures
Solvents were dried by standard procedures. Tetrahydrofuran (THF) was freshly distilled from sodium/benzophenone under argon. NMR spectra were recorded with Bruker Avance 500 (500 MHz for 1H and 125.8 MHz for 13C) and Bruker Avance 400 (1H at 400, 13C at 100.6 MHz) spectrometers in CDCl3, DMSO-d6, or D2O. Chemical shifts (in ppm, δ scale) were referenced to TMS (for 1H NMR spectra in CDCl3) and/or to the solvent signal (CDCl3 δ = 7.26 ppm for 1H NMR and δ = 77.0 ppm for 13C NMR; DMSO-d6 for 1H NMR δ = 2.5 ppm and for 13C δ = 39.7). Chemical shifts in D2O were referenced to 1,4-dioxane for 1H NMR δ = 3.75 and for 13C NMR δ = 67.19. Melting points were determined on a Büchi Melting Point B-545 aparatus and are uncorrected. TLC was performed on plates of Kieselgel 60 F254 (Merck). Mass spectra were measured on a ZAB-EQ (VG Analytical) spectrometer using FAB (ionization by Xe, accelerating voltage 8 kV, glycerol matrix) or on a LCQ classic spectrometer using electrospray ionization (ESI). Preparative HPLC purification was performed on a column packed with 10 μm C18 reversed phase (Luna), 250 × 21 mm; in ca 300 mg portions of mixtures using linear gradient 0.1 M triethylammonium hydrogen carbonate (TEAB) in water and in 50% MeOH (linear gradient of TEAB in 50% MeOH, 0–100%).
All new compounds were fully characterized by mass spectrometry, elemental analysis or high resolution mass spectrometry (for intermediates) and NMR spectroscopy (including complete assignment of all NMR signals using a combination of H,H-COSY, H,C-HSQC, and H,C-HMBC methods). Diastereoisomers gave identical NMR spectra. To prove that we have two different diasostereoisomers we prepared mixed samples of two diastereosisomers; two sets of signals were found for OCH2-1′ protons in 1H NMR spectra.
4.1.1. General procedure 1 (GP1) – Deprotection of diisopropylesters of bisphosphonates
Bisphosphonate (1 mmol) in acetonitrile (20 mL) was treated with bromotrimethylsilane (1.5 mL) at r.t. overnight. Volatiles were removed under reduced pressure, the residue was codistilled with water (3 × 50 mL) and 0.1 M TEAB (2 × 50 mL). Crude products were purified by preparative HPLC using linear gradient 0.1 M triethylammonium hydrogen carbonate in water and in 50% MeOH (linear gradient of TEAB in 50% MeOH, 0–100%) and triethylammonium salts of phosphonates were converted to free phosphonic acids by application onto a column of Dowex 50 × 8 in H+ form and elution with water.
4.1.2. General procedure 2 (GP2) – Dialkylation of 4,6-dihydroxy-2-(methylsulfanyl)pyrimidine (19) in DMSO
Pyrimidine 19 (0.16 g, 1 mmol) in DMSO (5 mL) was treated with NaH (0.084 g, 60% in paraffin oil, 2.1 mmol) and heated at 80 °C for 30 min; appropriate phosphonate 20a–e (2.1 mmol) was added and the resulting mixture was heated at 120 °C for 8–16 hr. The mixture was cooled to r.t., DMSO was evaporated in vacuo at 60 °C. The residue was codistilled with DMF and EtOH, taken to CHCl3 (100 mL) and washed with water (3 × 50 mL). Organic fraction was dried over MgSO4 and taken down in vacuo. The residue was separated by flash chromatography (CHCl3/MeOH).
4.1.3. General procedure 3 (GP3) – Monoalkylation of 4,6-dihydroxy-2-(methylsulfanyl)-pyrimidine (19) in DMSO
Pyrimidine 19 (0.16 g, 1 mmol) and NaH (0.04 g, 60% in paraffin oil, 1 mmol) in DMSO (5 mL) was heated at 60 °C for 30 min., phosphonate 20a–e (1 mmol) in DMSO (1 mL) was added dropwise and the reaction mixture was heated at 120 °C for 8 hr, cooled to r.t. and evaporated in vacuo at 60 °C. The residue was codistilled with DMF and EtOH, diluted with CHCl3, washed with 3 portions of water and dried over MgSO4. Products were separated by flash chromatography (CHCl3/MeOH).
4.1.4. General procedure 4 (GP4) – 2nd Alkylation of monoalkylated product 23
Pyrimidine 23 (1 mmol) and NaH (0.044 g, 60% in paraffin oil, 1.1 mmol) in DMF (5 mL) was heated at 40 °C for 30 min., appropriate phosphonate 20a–e (1.1 mmol) was added and the mixture was stirred at 100 °C for 8–12 hr, evaporated in vacuo, and codistilled with EtOH.
4.1.5. General procedure 5 (GP5) – Oxidation of 2-methylsufanyl group to 2-methylsulfonyl group by m-CPBA
2-Methylsulfanyl derivative (1 mmol) in CH2Cl2 (6 ml) was treated with m-chloroperoxybenzoic acid (3 mmol) at r.t. for 3–12 hr. The reaction mixture was diluted with CH2Cl2, washed with saturated aqueous Na2S2O5, saturated NaHCO3 and water. Organic fraction was dried over MgSO4 and purified by flash chromatography (CHCl3/MeOH).
4.1.6. General procedure 6 (GP6) – Ammonolysis of 2-methylsulfonyl group to amino group
To a cooled (−78 °C) and stirred solution of compound 26 (1 mmol) in dry THF (30 mL), in a pressure tube, 20–30 mL of liquid ammonia was added. The pressure tube was sealed and allowed to warm to r.t. and the reaction mixture was stirred for 5–12 hr. The reaction mixture was concentrated in vacuo and the crude product in CHCl3 was applied onto a pad of silica gel and washed with 10% MeOH in CHCl3 (150 mL).
4.1.7. General procedure 7 (GP7) – Alkylation of hydroxyethoxy derivatives 37, 41 and 42 with phosphonate 38
2-Hydroxyethoxy derivative (0.25 mmol), NaH (30 mg, 60% in paraffin oil, 0.75 mmol) and 4-dimethylaminopyridine (6 mg) in THF was heated at 50 °C for 30 min. and phosphonate 38 (292 mg, 0.525 mmol) was added in one portion. The resulting mixture was heated at 70 °C for 16 hr and taken down in vacuo. The residue in CHCl3 (50 mL) was washed with brine (2 × 50 mL) and water (1 × 50 mL) and evaporated under reduced pressure. Flash chromatography (EtOAc:EtOH:acetone:H2O, 6:1:1:0.5 and EtOAc:EtOH:acetone:H2O, 4:1:1:1) gave compounds 39 and 40.
4.2. 2-Amino-4,6-(2R,2′R)-bis(1,2-dihydroxypropoxy)pyrimidine (12)
(S)-1,2-Isopropylidene-glycerol (11) (7.53 mL, 61 mmol) in THF (15 mL) was added dropwise to a stirred suspension of NaH (2.5 g, 60% in paraffin oil, 61 mmol) in THF (80 mL) at r. t. After stirring for 1 h, pyrimidine 10 (5 g, 30.5 mmol) was added in one portion and the reaction mixture was refluxed for 6 h. After cooling to r.t., solvent was removed under reduced pressure and the residue was dissolved in hot CHCl3 and filtered through Celite. Chromatography on silica gel (CHCl3/MeOH 0–2%) afforded intermediate 2-amino-4,6-(S,S)-bis(2,2-dimethyl-4-methoxy-1,3-dioxolane)pyrimidine (10.1 g, 93%) as a yellow oil. 1H NMR (DMSO-d6): δ = 6.60 (br s, 2H, NH2), 5.36 (s, 1H, H-5), 4.33 (m, 2H, H-2′), 4.22 (dd, J(1′a,2′) = 4.6, Jgem = 11.1, 2H, H-1′a), 4.16 (dd, J(1′b,2′) = 6.3, Jgem = 11.1, 2H, H-1′b), 4.04 (dd, J(3′a,2′) = 6.4, Jgem = 8.4, 2H, H-3′a), 3.68 (dd, J(3′b,2′) = 6.2, Jgem = 8.4, 2H, H-3′b), 1.33 and 1.28 (2 × s, 2 × 6H, CH3) ppm. 13C NMR (DMSO-d6): δ = 171.19 (2C, C-4 and C-6), 162.73 (C-2), 108.96 (2C, CHMe2), 78.56 (C-5), 73.59 (2C, C-2′), 66.37 and 65.93 (2 × 2C, C-1′, C-3′), 26.81 and 25.52 (2 × 2C, CH3) ppm. MS (FAB): m/z (%) = 356.1 (85) [MH]+. Anal. C16H25N3O6 (C, H, N).
Solution of the intermediate (9 g, 25.3 mmol) in methanol/water mixture (1:4, 100 mL) was acidified with hydrochloric acid to pH 2 and stirred for 4 h at r.t. Reaction mixture was applied onto a column of Dowex 50 × 8, washed with water until neutral reaction of eluate and eluted with 2.5% ammonia. UV absorbing eluate was collected and evaporated. Crystallization from ethanol/ether mixture afforded 12 as a white solid (4.74 g, 66%); m.p. 110 °C. 1H NMR (DMSO-d6): δ = 6.50 (br s, 2H, NH2), 5.32 (s, 1H, H-5), 4.90 and 4.63 (2 × br s, 2 × 2H, OH), 4.17 (dd, J(1′a,2′) = 4.3, Jgem = 10.9, 2H, H-1′a), 4.06 (dd, J(1′b,2′) = 6.6, Jgem = 10.9, 2H, H-1′b), 3.72 (m, 2H, H-2′), 3.38 (d, J(3′,2′) = 4.0, 4H, H-3′) ppm. 13C NMR (DMSO-d6): δ = 171.62 (2C, C-4, C-6), 162.82 (C-2), 78.58 (C-5), 69.89 (2C, C-2′), 67.73 (2C, C-1′), 62.97 (C-3′) ppm. MS (FAB): m/z (%) = 276.1 (100) [MH]+. Anal. C10H17N3O6.1/2 H2O (C, H, N).
4.3. 2-Amino-4,6-(2R,2′R)-bis[2-(diisopropoxyphosphorylmethoxy)-3-hydroxypropoxy]pyrimidine (13a)
Compound 12 (3 g, 11.7 mmol) in pyridine (400 mL) was treated with DMTrCl (11.95 g, 35 mmol) and the mixture was stirred for 3 h. The reaction was quenched by addition of EtOH and the solvent was removed in vacuo. The residue was partitioned between CHCl3 and saturated aqueous NaHCO3, and the separated organic layer was washed with brine, dried (MgSO4) and concentrated in vacuo. The residue in DMF (150 mL) was treated with NaH (1 g, 60% suspension in mineral oil, 25 mmol) at 0 °C and stirred for 30 min; diisopropoxyphosphorylmethyl bromide (6.25 g, 25 mmol) was added and the mixture was stirred at r.t. overnight; the solvent was removed in vacuo and the residue was dissolved in 80% acetic acid (100 mL). After stirring at r.t. for 1 h, acetic acid was evaporated and the residue was codistilled with water. Flash chromatography in CHCl3/MeOH (0–3%) afforded colorless oil (2.6 g, 39%). 1H NMR (DMSO-d6): δ = 6.57 (br s, 2H, NH2), 5.29 (s, 1H, H-5), 4.78 (t, J(OH,3′) = 5.4, 2H, OH), 4.58 (m, 4H, CHipr.), 4.32 (dd, J(1′a,2′) = 3.6, Jgem = 11.5, 2H, H-1′a), 4.19 (dd, J(1′b,2′) = 6.1, Jgem = 11.5, 2H, H-1′b), 3.90 (dd, J(P,CH) = 8.7, Jgem = 13.8, 2H) and 3.86 (dd, J(P,CH) = 8.9, Jgem = 13.8, 2H, PCH2), 3.69 (m, 2H, H-2′), 3.51 (t, J(3′,2′) = J(3′,OH) = 5.4, 4H, H-3′), 1.235 (d, 6H), 1.23 (d, 6H), 1.22 (d, 6H) and 1.21 (d, J(CH3,CH) = 6.2, 6H, CH3) ppm. 13C NMR (DMSO-d6): δ = 171.34 (2C, C-4, C-6), 162.77 (C-2), 80.53 (d, 2C, J(P,C) = 11.7, C-2′), 78.46 (C-5), 70.37 (d, 2C) and 70.32 (d, 2C, J(P,C) = 6.3, CHipr.), 65.25 (2C, C-1′), 63.99 (d, 2C, J(P,C) = 164.6, PC), 60.08 (2C, C-3′), 23.99 (d, 4C, J(P,C) = 3.9) and 23.82 (d, 4C, J(P,C) = 4.4, CH3) ppm. MS (FAB): m/z (%) = 632.6 (56) [MH]+. Anal. C24H47N3O12P2 (C, H, N, P).
4.4. 2-Amino-4,6-(2R,2′R)-bis[2-(phosphonomethoxy)-3-hydroxypropoxy]pyrimidine (13b)
Compound 13a (2 g, 3.17 mmol) was deprotected by GP1 to give 13b (1.05 g, 71%) as colorless foam. 1H NMR (D2O): δ = 4.56 (dd, J(1′a,2′) = 3.8, Jgem = 11.2, 2H, H-1′a), 4.43 (dd, J(1′b,2′) = 5.6, Jgem = 11.2, 2H, H-1′b), 3.94 (m, 2H, H-2′), 3.89 (dd, 2H) and 3.84 (dd, J(P,CH) = 9.3, Jgem = 13.3, 2H, PCH2), 3.82 (dd, J(3′a,2′) = 4.3, Jgem = 12.2, 2H, H-3′a), 3.75 (dd, J(3′b,2′) = 5.7, Jgem = 12.2, 2H, H-3′b) ppm. 13C NMR (D2O): δ = 169.14 (2C, C-4, C-6), 155.56 (C-2), 79.62 (C-5), 79.57 (d, J(P,C) = 11.2, 2C, C-2′), 68.16 (2C, C-1′), 65.74 (d, J(P,C) = 158.7, 2C, PC), 60.11 (2C, C-3′) ppm. MS (FAB): m/z (%) = 464 (49) [MH]+. [α]25D = −1.0 (c 0.502, H2O). Anal. C12H23N3O12P2.H2O (C, H, N, P).’
4.5. 2-Amino-4,6-(2S,2′S)-bis[2-(diisopropoxyphosphorylmethoxy)-3-hydroxypropoxy]pyrimidine (14a)
Prepared from 10 and 16 by the same procedure as compound 13a. 2-Amino-4,6-(2R,2′R)-bis(2,2-dimethyl-4-methoxy-1,3-dioxolane)pyrimidine, yellow oil, yield 9.6 g (89%). 1H NMR (DMSO-d6) and 13C NMR (DMSO-d6) identical with (2S,2′S) enantiomer. MS (FAB): m/z (%) = 356.0 (54) [MH]+. Anal. C16H25N3O6 (C, H, N).
2-Amino-4,6-(2S,2′S)-bis(1,2-dihydroxypropoxy)pyrimidine, white solid, yield 5 g (70%); m.p. 99 °C. 1H NMR (DMSO-d6) and 13C NMR (DMSO-d6) identical with (2R,2′R) enantiomer. MS (FAB): m/z (%) = 276.0 (80) [MH]+. ‘Anal. C10H17N3O6.1/2 H2O (C, H, N).’
2-Amino-4,6-(2S,2′S)-bis[2-(diisopropoxyphosphorylmethoxy)-3-hydroxypropoxy]pyrimidine (14a), colorless oil, yield 2.2 g (33%). 1H NMR (DMSO-d6) and 13C NMR (DMSO-d6) identical with 13a. MS (FAB): m/z (%) = 632.6 (100) [MH]+. Anal. C24H47N3O12P2 (C, H, N, P).
4.6. 2-Amino-4,6-(2S,2′S)-bis[2-(phosphonomethoxy)-3-hydroxypropoxy]pyrimidine (14b)
Prepared from 14a by the same procedure as compound 13b. Colorless foam, yield 0.95 g (64%). 1H NMR (D2O) and 13C NMR (D2O) identical with 13b. MS (FAB): m/z (%) = 464 (15) [MH]+. [α]25D = +1.9 (c 0.267, H2O). Anal. C12H23N3O12P2.H2O (C, H, N, P).
4.7. 2-Amino-4-chloro-6-(2S)-(2,2-dimethyl-4-methoxy-1,3-dioxolane)pyrimidine (15)
Compound 11 (12.34 mL, 100 mmol) was added dropwise to the suspension of NaH (4 g, 60% suspension in mineral oil, 100 mmol) in THF (130 mL); the mixture was stirred for 1 h and pyrimidine 10 (16.4 g, 100 mmol) was added in one portion. Reaction mixture was heated at reflux for 6 h, cooled to r.t. and evaporated in vacuo. The residue was taken to chloroform and washed with brine; the organic extract was dried over magnesium sulfate and evaporated. Chromatography in chloroform/methanol (0–3%) afforded 23.1 g (89%) of compound 15 as a white solid, m.p. 130 °C. 1H NMR (DMSO-d6): δ = 7.10 (br s, 2H, NH2), 6.11 (s, 1H, H-5), 4.35 (m, 1H, H-2′), 4.28 (dd, J(1′a,2′) = 4.4, Jgem = 11.0, 1H, H-1′a), 4.21 (dd, J(1′b,2′) = 6.3, Jgem = 11.0, 1H, H-1′b), 4.04 (dd, J(3′a,2′) = 6.5, Jgem = 8.6, 1H, H-3′a), 3.70 (dd, J(3′b,2′) = 6.0, Jgem = 8.6, 1H, H-3′b), 1.32 and 1.27 (2 × s, 2 × 3H, CH3) ppm. 13C NMR (DMSO-d6): δ = 170.48 (C-6), 162.95 (C-2), 160.21 (C-4), 109.05 (CHMe2), 94.48 (C-5), 73.35 (C-2′), 66.85 and 65.82 (C-1′, C-3′), 26.79 and 25.51 (CH3) ppm. MS (ESI): m/z (%) = 282.43 (100) [MNa]+, 260.0 (37) [MH]+. Anal. C10H14ClN3O3 (C, H, Cl, N).
4.8. 2-Amino-4,6-(2R,2′S)-bis(1,2-dihydroxypropoxy)pyrimidine (17)
Compound 16 (1.43 mL, 11.6 mmol) was added dropwise to the suspension of NaH (0.46 g, 60% suspension in mineral oil, 11.6 mmol) in THF (10 mL); the mixture was stirred for 1 h and compound 15 (3 g, 11.6 mmol) in THF (5 mL) was added. Reaction mixture was heated at reflux for 6 h, filtered through Celite while hot, Celite was washed with chloroform and combined organic extracts were evaporated in vacuo. Flash chromatography in chloroform/methanol (0–2%) gave protected intermediate [2-amino-4,6-(2R,2′S)-bis(2,2-dimethyl-4-methoxy-1,3-dioxolane)pyrimidine, 2.95 g, 72%] as a yellow oil. 1H NMR (DMSO-d6) and 13C NMR (DMSO-d6) identical with 2-amino-4,6-(2S,2′S)-bis(2,2-dimethyl-4-methoxy-1,3-dioxolane)pyrimidine. MS (FAB): m/z (%) = 356.0 (100) [MH]+. Anal. C16H25N3O6 (C, H, N).
The intermediate was deprotected by the same procedure as was described for compound 12. White solid, yield 69%; m.p. 116 °C. 1H NMR (DMSO-d6):δ = 6.51 (br s, 2H, NH2), 5.32 (s, 1H, H-5), 4.90 and 4.65 (2 × br s, 2 × 2H, OH), 4.17 (dd, J(1′a,2′) = 4.3, Jgem = 10.9, 2H, H-1′a), 4.06 (dd, J(1′b,2′) = 6.6, Jgem = 10.9, 2H, H-1′b), 3.72 (m, 2H, H-2′), 3.38 (d, J(3′,2′) = 4.0, 4H, H-3′) ppm. 13C NMR (DMSO-d6): δ = 171.62 (2C, C-4, C-6), 162.82 (C-2), 78.58 (C-5), 69.89 (2C, C-2′), 67.73 (2C, C-1′), 62.97 (C-3′) ppm. MS (FAB): m/z (%) = 276.1 (100) [MH]+. Anal. C10H17N3O6.1/2 H2O (C, H, N).
4.9. 2-Amino-4,6-(2S,2′R)-bis[2-(diisopropoxyphosphorylmethoxy)-3-hydroxypropoxy]pyrimidine (18a)
Prepared from compound 17 by the same procedure as was described for 13a, colorless oil, yield 2.7 g (41%). 1H NMR (DMSO-d6) and 13C NMR (DMSO-d6) identical with 13a. MS (FAB): m/z (%) = 632.1 (100) [MH]+. Anal. C24H47N3O12P2 (C, H, N, P).
4.10. 2-Amino-4,6-(2S,2′R)-bis[2-(phosphonomethoxy)-3-hydroxypropoxy]pyrimidine (18b)
Prepared by the same procedure as compound 13b, colorless foam, yield 63%. 1H NMR (D2O) and 13C NMR (D2O) identical with 13b. MS (FAB): m/z (%) = 464 (35) [MH]+. [α]25D = +0.02 (c 0.383, H2O). Anal. C12H23N3O12P2.H2O (C, H, N, P).
4.11. 4,6-Bis[2-(diisopropoxyphosphorylmethoxy)ethoxy]-2-(methylsulfanyl)pyrimidine (21) and 4-[2-(diisopropoxyphosphorylmethoxy)ethoxy]-1-[2-(diisopropoxyphosphorylmethoxy)ethyl]-2-(methylsulfanyl)pyrimidine-6(1H)-one (22)
GP2, pyrimidine 19 (3 g, 19 mmol), phosphonate 20a (10.6 g, 40 mmol), yield 6.15 g (54%) of 21, colorless syrup. 1H NMR (DMSO-d6): δ = 5.88 (s, 1H, H-5), 4.58 (dh, J(CH,P) = 7.7, J(CH,CH3) = 6.3, 4H, CHipr.), 4.43 (m, 4H, H-1′), 3.82 (m, 4H, H-2′), 3.79 (d, J(C,H,P) = 8.3, 4H, PCH2), 2.48 (s, 3H, SCH3), 1.23 (m, 24H, CH3ipr.) ppm. 13C NMR (DMSO-d6): δ = 170.61 (C-2), 170.26 (2C, C-4,6), 85.55 (C-5), 70.57 (d, J(2′,P) = 11.9, C-2′), 70.36 (d, J(CH,P) = 6.2, CHipr.), 65.67 (C-1′), 64.95 (d, J(C,P) = 164.6, PCH2), 24.00 (d, J(CH3,P) = 3.7) and 23.85 (d, J(CH3,P) = 4.6, CH3ipr.), 13.66 (SCH3) ppm. MS (ESI): m/z (%) = 625.1 (100) [MNa]+, 603 (15) [MH]+. HR MS (FAB) calcd. for C23H45N2O10P2S [MH]+ 603.2192, found 603.2185. Anal. C23H45N2O10P2S (C, H, N, P, S).
Further elution of column gave 22, yield 1.35 g (12%), colorless syrup. 1H NMR (DMSO-d6): δ = 5.40 (s, 1H, H-5), 4.58 (m, 4H, CHipr.), 4.31 (m, 2H, H-1″), 4.10 (t, J(1′,2′) = 5.4, 2H, H-1′), 3.82 (m, 2H, H-2″), 3.79 (d, 2H) and 3.76 (d, J(P,CH) = 8.3, 2H, PCH2), 3.75 (t, J(2′,1′) = 5.4, 2H, H-2′), 2.54 (s, 3H, SCH3), 1.24 (d, 6H), 1.23 (d, 6H), 1.22 (d, 6H) and 1.20 (d, J(CH3,CH) = 6.2, 6H, CH3) ppm. 13C NMR (DMSO-d6): δ = 167.34 (C-2), 162.75 (C-4), 159.48 (C-6), 85.65 (C-5), 70.76 (d, J(C,P) = 11.9, C-2′), 70.44 (d, J(C,P) = 11.89, C-2″), 70.04 (m, CHipr.), 69.53 (d, J(C,P) = 6.61, CHipr.), 65.31 (C-1′), 64.80 (d, J(C,P) = 164.41, PCH2′), 64.75 (d, J(C,P) = 163.72, PCH2″), 43.69 (C-1″), 23.61 (m, CH3ipr.), 14.43 (SCH3) ppm. MS (ESI): m/z (%) = 625.1 (100) [MNa]+. HR MS (FAB) calcd. for C23H45N2O10P2S [MH]+ 603.2192, found 603.2193. Anal. C23H45N2O10P2S (C, H, N, P, S).
4.12. 6-[2-(Diisopropoxyphosphorylmethoxy)ethoxy]-4-hydroxy-2-(methylsulfanyl)pyrimidine (23a)
GP3, pyrimidine 19 (3 g, 19 mmol), phosphonate 20a (4.8 g, 18.1 mmol), yield 2.67 g (37%) of 23a, colorless syrup. 1H NMR (DMSO-d6): δ = 12.32 (br s, 1H, OH), 5.39 (br s, 1H, H-5), 4.59 (dh, J(CH,CH3) = 6.2, J(CH,P) = 7.8, 2H, CHipr.), 4.33 (m, 2H, H-1′), 3.80 (m, 2H, H-2′), 3.79 (d, J(CH2,P) = 8.3, 2H, CH2P), 2.47 (s, 3H, SCH3), 1.24 (d, J(CH3,CH) = 6.0, 6H) and (1.22 d, J(CH3,CH) = 6.0, 6H, CH3ipr.) ppm. 13C NMR (DMSO-d6): δ = 169.19 (C-4), 86.06 (C-5), 70.66 (d, J(2,P) = 11.9, C-2′), 70.43 (d, J(C,O,P) = 6.3, CHipr.), 65.83 (C-1′), 64.97 (d, J(C,P) = 164.5, CH2P), 24.03 (d, J(CH3,P) = 3.6) and 23.89 (d, J(CH3,P) = 4.4, CH3ipr.), 13.12 (SCH3) ppm. MS (ESI): m/z (%) = 403 (100) [MNa]+. HR MS (FAB) calcd. for C14H26N2O6PS [MH]+ 381.1249, found 381.1265. Compound 21 (4.18 g, 36%) was isolated as a side product.
4.13. 6-(2S)-[2-(Diisopropoxyphosphorylmethoxy)propoxy]-4-hydroxy-2-(methylsulfanyl) pyrimidine (23b) and 4,6-(2S,2′S)-bis[2-(diisopropoxyphosphorylmethoxy)propoxy]-2-(methylsulfanyl)pyrimidine (24a)
GP3, from pyrimidine 19 (2 g, 12.6 mmol) and phosphonate 20b (5.68 g, 13.9 mmol). Yield 1.25 g (25%) of 23b, colorless oil. 1H NMR (CDCl3): δ = 12.3 (br s, 1H, OH), 5.40 (br s, 1H, H-5), 4.58 (m, 2H, CHipr.), 4.23 (dd, Jgem = 11.3, J(1′a,2′) = 3.8, 1H, H-1′a), 4.16 (dd, Jgem = 11.3, J(1′b,2′) = 6.1, 1H, H-1′b), 3.87 (m, 2H, H-2′), 3.80 (m, 2H, OCH2P), 2.47 (s, 3H, SCH3), 1.22 (m, 12H, CH3ipr.), 1.15 (d, J(CH3,2′) = 6.4, 3H, H-3′) ppm. 13C NMR (CDCl3): δ = 170.52 (C-2), 169.15 (C-4), 162.50 (C-6), 86.08 (C-5), 75.24 (d, J(2′,P) = 12.8, C-2′), 70.32 (m, 2C, CHipr.), 69.20 (C-1′), 63.01 (d, J(OCH2,P) = 165.2, OCH2P), 23.92 (m, 4C, CH3ipr.), 16.28 (C-3′), 13.10 (SCH3) ppm. MS (FAB): m/z (%) = 395 (100) [MH]+. HR MS (FAB) calcd. for C15H28N2O6PS [MH]+ 395.1405, found 395.1418.
Dialkylated derivative 24a was isolated as a second product. Yield 1.75 g (22%), colorless oil. 1H NMR (CDCl3): δ = 5.69 (s, 1H, H-5), 4.74 (m, 4H, CHipr.), 4.307 (d, J(1′,2′) = 5.17, 4H, H-1′), 3.89 (m, 2H, H-2′), 3.82 (d, J(CH2,P) = 9.11, 4H, OCH2P), 2.49 (s, 3H, SCH3), 1.31 (m, 24H, CH3ipr.), 1.23 (d, J(3′,2′) = 6.39, 6H, H-3′) ppm. 13C NMR (CDCl3): δ = 170.92 (C-2), 170.17 (C-4,6), 86.12 (C-5), 75.85 (d, J(2′,P) = 11.98, C-2′), 71.05 (d, J(C,P) = 6.68) and 70.96 (d, J(C,P) = 6.66, CHipr.), 69.35 (C-1′), 64.04 (d, J(C,P) = 168.56, PCH2), 24.06 (d, J(C,P) = 3.69) and 23.92 (d, J(C,P) = 4.64, CH3ipr.), 16.55 (C-3′), 14.00 (SCH3) ppm. MS (ESI): m/z (%) = 653 (100) [MNa]+, 631 (100) [MH]+. HR MS (FAB) calcd. for C25H49N2O10P2S [MH]+ 631.2583, found 631.2579.
4.14. 6-(2R)-[2-(Diisopropoxyphosphorylmethoxy)propoxy]-4-hydroxy-2-(methylsulfanyl)-pyrimidine (23c) and 4,6-(2R,2′R)-bis[2-(diisopropoxyphosphorylmethoxy)propoxy]-2-(methylsulfanyl)pyrimidine (24b)
GP3, from pyrimidine 19 (4 g, 25.2 mmol) and phosphonate 20c (10.3 g, 25.2 mmol). Yield 2.5 g (25%) of compound 23c, colorless oil. 1H NMR (CDCl3) and 13C NMR (CDCl3) identical with compound 23b. MS (FAB): m/z (%) = 395 (100) [MH]+. HR MS (FAB) calcd. for C15H28N2O6PS [MH]+ 395.1405, found 395.1413. Compound 24b isolated as a colorless oil, yield 1.2 g (7.5%). 1H NMR (CDCl3) identical with compound 24a. MS (FAB): m/z (%) = 631 (100) [MH]+. HR MS (FAB) calcd. for C25H49N2O10P2S [MH]+ 631.2583, found 631.2599.
4.15. 4-[2-(Diisopropoxyphosphorylmethoxy)ethoxy]-6-(2S)-[2-(diisopropoxyphosphorylmethoxy)propoxy]-2-(methylsulfonyl)pyrimidine (26a)
GP4, 23a (800 mg, 2.1 mmol), 20b (0.94 g, 2.3 mmol). The crude product was taken to CHCl3, filtered through a pad of silica gel and washed with 10% MeOH in CHCl3 (150 mL). The filtrate was taken down in vacuo. The residue was without further purification oxidized by m-CPBA by GP5 to give 26a (0.52 g, 38%), colorless oil. 1H NMR (DMSO-d6): δ = 6.52 (s, 1H, H-5), 4.58 (m, 4H, CHipr.), 4.52 (m, 2H, H-1′), 4.43 (dd, Jgem = 11.4, J(1″a,2″) = 3.5, 1H, H-1″a), 4.34 (dd, Jgem = 11.4, J(1″b,2″) = 6.0, 1H, H-1″b), 3.92 (m, 2H, H-2″), 3.86 (m, 2H, H-2′), 3.81 (m, 4H, 2 × PCH2), 3.38 (s, 3H, SO2CH3), 1.19 – 1.24 (m, 24H, CH3ipr.), 1.19 (d, J(CH3, 2″) = 6.5, 3H, H-3″) ppm. 13C NMR (DMSO-d6): δ = 171.51 and 171.45 (C-4, C-6), 164.26 (C-2), 92.66 (C-5), 75.05 (d, J(2″,P) = 12.5, C-2″), 70.12 – 70.40 (m, 6C, CHipr., C-2′ and C-1″), 66.95 (C-1′), 64.95 (d, J(C,P) = 164.5, C-3′), 63.03 (d, J(C,P) = 165.3, C-3″), 38.94 (CH3SO2), 23.82 – 24.01 (m, 8C, CH3ipr.), 16.21 (C-3″) ppm. MS (FAB): m/z (%) = 671 (23) [MNa]+, 649 (32) [MH]+. HR MS (FAB) calcd. for C24H47N2O12P2S [MH]+ 649.2325, found 649.2328.
4.16. 4-[2-(Diisopropoxyphosphorylmethoxy)ethoxy]-6-(2R)-[2-(diisopropoxyphosphorylmethoxy)propoxy]-2-(methylsulfonyl)pyrimidine (26b)
Prepared by the same procedure as described for compound 26a from 23a (800 mg, 2.1 mmol) and phosphonate 20c. Colorless oil, 460 mg (34%). 1H NMR spectra identical with compound 26a. MS (ESI): m/z (%) = 671.1 (100) [MNa]+. HR MS (FAB) calcd. for C24H47N2O12P2S [MH]+ 649.2325, found 649.2318.
4.17. 4-[2-(Diisopropoxyphosphorylmethoxy)ethoxy]-6-(2S)-[2-(diisopropoxyphosphorylmethoxy)-3-hydroxypropoxy]-2-(methylsulfonyl)pyrimidine (26c)
Prepared by the same procedure (GP4 and GP5) as compound 26a from 23a (800 mg, 2.1 mmol) and phosphonate 20d (1.07 g, 2.52 mmol). Colorless oil, 870 mg (62%). 1H NMR (CDCl3): δ = 6.21 (s, 1H, H-5), 4.59 (m, 2H, H-1′), 4.51 (m, 2H, H-1″), 4.05 (dd, Jgem = 14.2, J(H,C,P) = 7.3, 1H, PCH2″a), 3.95 (m, 2H, H-2′), 3.88 (m, 2H, H-2″), 3.83 (dd, Jgem = 14.2, J(H,C,P) = 8.4, 1H, PCH2″b), 3.81 (d, J(H,C,P) = 8.2, 2H, PCH2′), 3.80 (m, 1H, H-3″a), 3.70 (dd, Jgem = 12.3, J(3″b,2″) = 5.9, 1H, H-3″b), 3.30 (s, 3H, CH3SO2), 1.34 (m, 24H, CH3ipr.) ppm. 13C NMR (CDCl3): δ = 171.35 (C-6), 171.21 (C-4), 164.18 (C-2), 93.69 (C-5), 81.49 (d, J(2″,P) = 7.4, C-2″), 71.78 (d, J(CH,P) = 6.6), 71.40 (d, J(CH,P) = 6.9) and 71.15 (d, J(CH,P) = 6.5, CHipr.), 70.73 (d, J(2′,P) = 10.5, C-2′), 66.86 (2C, C-1′,1″), 66.06 (d, J(C,P) = 167.7, PCH2′), 65.35 (d, J(C,P) = 168.8, PCH2″), 61.62 (C-3″), 38.88 (CH3SO2), 23.97 (m, CH3ipr.) ppm. MS (ESI): m/z (%) = 687 (100) [MNa]+, 665 (44) [MH]+. HR MS (FAB) calcd. for C24H47N2O13P2S [MH]+ 645.2274, found 645.2268.
4.18. 4-[2-(Diisopropoxyphosphorylmethoxy)ethoxy]-6-(2R)-[2-(diisopropoxyphosphorylmethoxy)-3-hydroxypropoxy]-2-(methylsulfonyl)pyrimidine (26d)
Prepared by the same procedure (GP4 and GP5) as compound 26a from 23a (800 mg, 2.1 mmol) and phosphonate 20e (1.07 g, 2.52 mmol). Colorless oil, 700 mg (50%). 1H NMR (CDCl3) spectra identical with compound 26c. MS (ESI): m/z (%) = 687 (25) [MNa]+, 665 (12) [MH]+. HR MS (FAB) calcd. for C24H47N2O13P2S [MH]+ 645.2274, found 645.2288.
4.19. 4,6-(2R,2′S)-Bis[2-(diisopropoxyphosphorylmethoxy)propoxy]-2-(methylsulfonyl)pyrimidine (26e)
Prepared by the same procedure (GP4 and GP5) as compound 26a from 23b (500 mg, 1.27 mmol) and phosphonate 20c (0.57 g, 1.4mmol). Colorless oil, 740 mg (88%). 1H NMR (DMSO-d6): δ = 6.35 (s, 1H, H-5), 4.58 (m, 4H, CHipr.), 4.43 (dd, J(1′a,2′) = 3.4, Jgem = 11.4, 2H, H-1′a), 4.34 (dd, J(1′b,2′) = 5.9, Jgem = 11.4, 2H, H-1′b), 3.92 (m, 2H, H-2′), 3.82 (m, 4H, OCH2P), 2.38 (s, 3H, SO2CH3), 1.18–1.24 (m, 30H, CH3ipr., H-3′) ppm. 13C NMR (DMSO-d6): δ = 171.50 (C-4,6), 164.25 (C-2), 92.66 (C-5), 75.05 (d, J(2′,P) = 12.7, C-2′), 70.30 (m, CHipr), 70.13 (C-1′), 63.04 (d, J(C,P) = 165.5, PCH2), 38.95 (CH3SO2), 23.92 (m, CH3ipr.), 16.22 (C-3′) ppm. MS (ESI): m/z (%) = 685 (100) [MNa]+. HR MS (FAB) calcd. for C25H49N2O12P2S [MH]+ 663.2476, found 663.2469.
4.20. 4-(2S)-[2-(Diisopropoxyphosphorylmethoxy)-3-hydroxypropoxy]-6-(2S)-[2-(diisopropoxyphosphorylmethoxy)propoxy]-2-(methylsulfonyl)pyrimidine (26f)
GP4, GP5, 23b (1.25 g, 3.17 mmol), 20d (1.75 g, 4.12 mmol). Colorless oil, 394 mg (18%). 1H NMR (DMSO-d6): δ = 6.21 (s, 1H, H-5), 4.75 (m, 4H, CHipr.), 4.52 (dd, Jgem = 11.4, J(1″a,2″) = 5.9, 1H, H-1″a), 4.49 (dd, Jgem = 11.4, J(1″b,2″) = 4.8, 1H, H-1″b), 4.44 (dd, Jgem = 11.3, J(1′a,2′) = 3.8, 1H, H-1′a), 4.40 (dd, Jgem = 11.3, J(1′b,2′) = 6.2, 1H, H-1′b), 4.06 (dd, Jgem = 14.2, J(C,H,P) = 7.3, 1H, PCH2″a), 3.95 (m, 2H, H-2′), 3.87 (m, 2H, H-2″), 3.83 (m, 4H, PCH2″b, PCH2′, H-3″), 3.70 (m, 2H, H-3b″), 3.30 (s, 3H, SO2CH3), 1.34 (m, 24H, CH3ipr.), 1.28 (d, J(3′ 2′) = 6.4, 3H, H-3′) ppm. 13C NMR (DMSO-d6): δ = 171.39 (C-4), 171.20 (C-6), 164.17 (C-2), 93.65 (C-5), 81.53 (d, J(2″,P) = 7.5, C-2″), 75.54 (d, J(2′,P) = 11.9, C-2′), 71.81 (d, J(C,O,P) = 6.7), 71.42 (d, J(C,O,P) = 6.9) and 71.08 (d, 2C, J(C,O,P) = 6.7, CHipr.), 70.54 (C-1′), 66.83 (C-1″), 65.35 (d, J(C,P) = 168.8, PCH2″), 64.00 (d, J(C,P) = 169.2, PCH2′), 61.62 (C-3″), 38.89 (CH3SO2), 24.00 (m, CH3ipr.), 16.34 (C-3′) ppm. MS (ESI): m/z (%) = 679 (10) [MH]+, 701 (100) [MNa]+. HR MS (FAB) calcd. for C25H49N2O13P2S [MH]+ 679.2430, found 679.2429.
4.21. 4-(2R)-[2-(Diisopropoxyphosphorylmethoxy)-3-hydroxypropoxy]-6-(2S)-[2-(diisopropoxyphosphorylmethoxy)propoxy]-2-(methylsulfonyl)pyrimidine (26g)
GP4, GP5, 23b (1.25 g, 3.17 mmol), 20e (1.75 g, 4.12 mmol). Colorless oil, 360 mg (16%). 1H NMR (DMSO-d6) and 13C NMR (DMSO-d6) identical with compound 26f. MS (ESI): m/z (%) = 679 (6) [MH]+, 701 (100) [MNa]+. HR MS (FAB) calcd. for C25H49N2O13P2S [MH]+ 679.2430, found 679.2438.
4.22. 4-(2S)-[2-(Diisopropoxyphosphorylmethoxy)-3-hydroxypropoxy]-6-(2R)-[2-(diisopropoxyphosphorylmethoxy)propoxy]-2-(methylsulfonyl)pyrimidine (26h)
GP4, GP5, 23c (1.25 g, 3.17 mmol), 20d (1.75 g, 4.12 mmol). Colorless oil, 960 mg (42%). 1H NMR (DMSO-d6): δ = 6.21 (s, 1H, H-5), 4.75 (m, 4H, CHipr.), 4.51 (m, 2H, H-1″), 4.54 (dd, Jgem = 11.4, J(1′a,2′) = 3.9, 1H, H-1′a), 4.41 (dd, Jgem = 11.3, J(1′b,2′) = 6.1, 1H, H-1′b), 4.05 (dd, Jgem = 14.1, J(C,H,P) = 7.4, 1H, PCH2″a), 3.95 (m, 2H, H-2′), 3.78–3.89 (m, 5H, H-2″, PCH2″b, PCH2′, H-3″), 3.70 (dd, Jgem = 12.4, J(3″b,2″) = 5.9, 1H, H-3″b), 3.30 (s, 3H, SO2CH3), 1.33 (m, 24H, CH3ipr.), 1.28 (d, J(3′,2′) = 6.4, 3H, H-3′) ppm. 13C NMR (DMSO-d6): δ = 171.35 (C-4), 171.17 (C-6), 164.14 (C-2), 93.60 (C-5), 81.43 (d, J(2″,P) = 7.7, C-2″), 75.51 (d, J(2′,P) = 11.8, C-2′), 71.75 (d, J(C,O,P) = 6.7), 71.37 (d, J(C,O,P) = 6.9), 71.06 (d, J(C,O,P) = 6.7) and 71.04 (d, J(C,O,P) = 6.5, CHipr.), 70.52 (C-1′), 66.84 (C-1″), 65.28 (d, J(C,P) = 168.8, PCH2″), 63.95 (d, J(C,P) = 169.1, PCH2′), 61.53 (C-3″), 38.89 (CH3SO2), 23.96 (m, CH3ipr.), 16.30 (C-3′) ppm. MS (ESI): m/z (%) = 679 (35) [MH]+, 701 (15) [MNa]+. HR MS (FAB) calcd. for C25H49N2O13P2S [MH]+ 679.2430, found 679.2441.
4.23. 4-(2R)-[2-(Diisopropoxyphosphorylmethoxy)-3-hydroxypropoxy]-6-(2R)-[2-(diisopropoxyphosphorylmethoxy)propoxy]-2-(methylsulfonyl)pyrimidine (26i)
GP3, GP4, 23c (1.25 g, 3.17 mmol), 20e (1.75 g, 4.12 mmol). Colorless oil, 1.05 g (46%). NMR spectra identical with 26h. MS (ESI): m/z (%) = 679 (18) [MH]+, 701 (100) [MNa]+. HR MS (FAB) calcd. for C25H49N2O13P2S [MH]+ 679.2430, found 679.2432.
4.24. 4,6-Bis[2-(diisopropoxyphosphorylmethoxy)ethoxy]-2-(methylsulfonyl)pyrimidine (26j)
GP4, 21 (5.8 g, 9.62 mmol). Colorless oil, yield 4.2 g (69%). 1H NMR (DMSO-d6): δ = 6.20 (s, 1H, H-5), 4.58 (dh, J(CH,P) = 7.6, J(CH,CH3) = 6.4, 4H, CHipr.), 4.58 (m, 4H, H-1′), 3.94 (m, 4H, H-2′), 3.80 (d, J(C,H,P) = 8.17, 4H, PCH2), 3.30 (s, 3H, SO2CH3), 1.33 (d,12H) and 1.32 (d, 12H, J(CH3,CH) = 6.16, CH3ipr.) ppm. MS (FAB): m/z (%) = 635 (100) [MH]+. HR MS (FAB) calcd. for C23H45N2O12P2S [MH]+ 635.2168, found 635.2152.
4.25. 4,6-(2S,2′S)-Bis[2-(diisopropoxyphosphorylmethoxy)propoxy]-2-(methylsulfonyl)pyrimidine (26k)
GP5, 24a (840 mg, 1.33 mmol). Yield 800 mg (72%), colorless oil. 1H NMR (DMSO-d6) identical with spectra of 26e. MS (ESI): m/z (%) = 685 (100) [MNa]+. HR MS (FAB) calcd. for C25H49N2O12P2S [MH]+ 663.2476, found 663.2475.
4.26. 4,6-(2R,2′R)-Bis[2-(diisopropoxyphosphorylmethoxy)propoxy]-2-(methylsulfonyl)pyrimidine (26l)
GP5, 24b (1.2 g, 1.9 mmol). Yield 1.1 g (87%), colorless oil. 1H NMR (DMSO-d6) identical with spectra of 26e. MS (ESI): m/z (%) = 685 (100) [MNa]+. HR MS (FAB) calcd. for C25H49N2O12P2S [MH]+ 663.2476, found 663.2484.
4.27. Preparation of compounds 27 – general method
2-Methylsulfonyl derivatives 26 were converted to 2-amino derivatives by GP6 and diisopropyl esters of 2-amino pyrimidines were without further purification deprotected to free phosphonic acids by GP1.
4.27.1. 2-Amino-4-[2-(phosphonomethoxy)ethoxy]-6-(2S)-[2-(phosphonomethoxy)propoxy] pyrimidine (27a)
26a (500 mg, 0.77 mmol), freeze dried, white hydroscopic foam, yield 210 mg (61%). 1H NMR (DMSO-d6): δ = 5.92 (s, 1H, H-5), 4.52 (m, 5H, H-1′), 4.45 (dd, Jgem = 11.2, J(1″a,2′) = 2.9, 1H, H-1″a), 4.30 (dd, Jgem = 11.3, J(1″b,2″) = 6.3, 1H, H-1″b), 4.05 (dt, J(2″,3″) = J(2″,1″b) = 6.4, J(2″,1″a) = 3.0, 2H, H-2″), 3.98 (m, 2H, H-2′), 3.74–3.84 (m, 4H, PCH2), 1.27 (d, J(3″,2″) = 6.5, 3H, H-3″) ppm. 13C NMR (DMSO-d6): δ = 169.98 (C-4), 169.91 (C-6), 156.14 (C-2), 79.67 (C-5), 76.06 (d, J(2″,P) = 11.0, C-2″), 72.42 (C-1), 70.72 (d, J(2′,P) = 10.3, C-2′), 67.19 (d, J(P,CH2) = 157.2, PCH2), 65.05 (d, J(P,CH2) = 159.1, PCH2), 15.72 (C-3″) ppm. MS (ESI): m/z (%) = 418 (100) [MH]+. [α]25D = +12.1 (c 0.248, H2O). Anal. C11H21N3O10P2.H2O (C, H, N, P).
4.27.2. 2-Amino-4-[2-(phosphonomethoxy)ethoxy]-6-(2R)-[2-(phosphonomethoxy)propoxy] pyrimidine (27b)
26b (400 mg, 0.62 mmol), freeze dried, white hydroscopic foam, yield 190 mg (70%). NMR spectrum identical with compound 27a. MS (ESI): m/z (%) = 418 (100) [MH]+. [α]25D = −10.3 (c 0.347, H2O). Anal. C11H21N3O10P2.H2O (C, H, N, P).
4.27.3. 2-Amino-4-[2-(phosphonomethoxy)ethoxy]-6-(2S)-[2-(phosphonomethoxy)-3-hydroxypropoxy]pyrimidine (27c)
26c (870 mg, 1.3 mmol), freeze dried, white hydroscopic foam, yield 320 mg (54%). 1H NMR (DMSO-d6): δ = 5.36 (s, 1H, H-5), 4.29 (m, 2H, H-1′), 4.25 (dd, Jgem = 11.3, J(1″a,2″) = 4.3, 1H, H-1″a), 4.20 (dd, Jgem = 11.3, J(1″b,2″) = 5.7, 1H, H-1″b), 3.75 (m, 2H, H-2′), 3.71 (dd, Jgem = 13.6, J(H-C-P) = 8.7, 1H, OCH2P″a), 3.67 (dd, Jgem = 13.6, J(H-C-P) = 8.9, 1H, OCH2P″b), 3.67 (m, 2H, H-2″), 3.58 (d, J(H-C-P) = 8.7, 2H, OCH2P′), 3.51 (m, 2H, H-3″) ppm. 13C NMR (DMSO-d6): δ = 171.46 (C-6), 171.36 (C-4), 162.82 (C-2), 80.41 (d, J(2″,P) = 9.9, C-2″), 78.64 (C-5), 70.76 (d, J(2′,P) = 11.4, C-2′), 66.97 (d, J(C,P) = 160.4, OCH2P), 65.93 (d, J(C,P) = 160.4, OCH2P″), 65.39 (C-1″), 64.93 (C-1′), 60.33 (C-3″) ppm. MS (ESI): m/z (%) = 434 (100) [MH]+. [α]25D = +5.8 (c 0.625, H2O). Anal. C11H21N3O11P2.H2O (C, H, N, P).
4.27.4. 2-Amino-4-[2-(phosphonomethoxy)ethoxy]-6-(2R)-[2-(phosphonomethoxy)-3-hydroxypropoxy]pyrimidine (27d)
26d (700 mg, 1.05 mmol), freeze dried, white hydroscopic foam, yield 215 mg (45%). NMR spectrum identical with compound 27c. MS (ESI): m/z (%) = 434 (100) [MH]+. [α]25D = − 6.7 (c 0.341, H2O). Anal. C11H21N3O11P2.H2O (C, H, N, P).
4.27.5. 2-Amino-4,6-(2R,2′S)-bis[2-(phosphonomethoxy)propoxy]pyrimidine (27e)
26e (840 mg, 1.27 mmol), freeze dried, white hydroscopic foam, yield 280 mg (49%). 1H NMR (DMSO-d6): δ = 3.58 (s, 1H, H-5), 4.18 (dd, Jgem = 11.1, J(1′a,2′) = 5.8, 2H, H-1′a), 4.12 (dd, Jgem = 11.1, J(1′b,2′) = 4.4, 2H, H-1′b), 3.82 (m, 2H, H-2′), 3.60 (d, J(CH2,P) = 9.3, 4H, PCH2), 1.14 (d, J(H-3′,H-2′) = 6.4, 6H, H-3′) ppm. 13C NMR (DMSO-d6): δ = 171.40 (C-4, C-6), 162.77 (C-2), 78.54 (C-5), 74.96 (d, J(C-2′,P) = 11.4, C-2′), 68.58 (C-1′), 65.07 (d, J(CH2,P) = 161.6, PCH2), 16.80 (C-3′) ppm. MS (ESI): m/z (%) = 432.1 (100) [MH]+, 454.1 (37) [MNa]+. [α]25D = +0.9 (c 0.521, H2O). Anal. C12H23N3O10P2.H2O (C, H, N, P).
4.27.6. 2-Amino-4-(2S)-[2-(phosphonomethoxy)-3-hydroxypropoxy]-6-(2S)-[2-(phosphonomethoxy)propoxy]pyrimidine (27f)
26f (394 mg, 0.64 mmol), freeze dried, white hydroscopic foam, yield 200 mg (67%). 1H NMR (DMSO-d6): δ = 5.36 (s, 1H, H-5), 4.26 (dd, Jgem = 11.3, J(1″a,2″) = 4.3, 1H, H-1″a), 4.20 (dd, Jgem = 11.3, J(1″b,2″) = 5.8, 1H, H-1″b), 4.16 (dd, Jgem = 11.1, J(1′a,2′) = 5.8, 1H, H-1′a), 4.12 (dd, Jgem = 11.1, J(1′b,2′) = 4.4, 1H, H-1′b), 3.81 (m, 1H, H-2′), 3.71 (dd, Jgem = 13.6, J(C-H-P) = 8.7, 1H, OCH2,P″a), 3.67 (dd, Jgem = 13.6, J(C-H-P) = 8.9, 1H, OCH2,P″b), 3.67 (m, 1H, H-2″), 3.60 (d, 2H, J(C-H-P) = 9.4, OCH2,P′), 3.51 (m, 2H, H-3″), 1.13 (d, J(3′,2′) = 6.4, 3H, H-3′) ppm. 13C NMR (DMSO-d6): δ = 171.48, 171.42 (C-4, C-6), 162.83 (C-2), 80.40 (d, J(2″,P) = 9.7, C-2″), 78.59 (C-5), 75.08 (d, J(2′,P) = 11.7, C-2′), 68.63 (C-1′), 66.00 (d, J(P,C) = 161.0, PCH2″), 65.45 (C-1″), 65.01 (d, J(P,C) = 161.8, PCH2′), 60.35 (C-3″), 16.83 (C-3′) ppm. MS (ESI): m/z (%) = 448.1 (100) [MH]+. [α]25D = +12.2 (c 0.181, H2O). Analysis C12H23N3O11P2.H2O (C, H, N, P).
4.27.7. 2-Amino-4-(2R)-[2-(phosphonomethoxy)-3-hydroxypropoxy]-6-(2S)-[2-(phosphonomethoxy)propoxy]pyrimidine (27g)
From 26g (360 mg, 0.58 mmol), freeze dried, white hydroscopic foam, yield 200 mg (73%). 1H NMR (DMSO-d6): δ = 5.36 (s, 1H, H-5), 4.25 (dd, Jgem = 11.3, J(1″a,2″) = 4.3, 1H, H-1″a), 4.20 (dd, Jgem = 11.3, J(1″b,2″) = 5.8, 1H, H-1″b), 4.17 (dd, Jgem = 11.1, J(1′a,2′) = 5.8, 1H, H-1′a), 4.11 (dd, Jgem = 11.1, J(1′b,2′) = 4.4, 1H, H-1′b), 3.81 (m, 1H, H-2′), 3.71 (dd, Jgem = 13.6, J(C-H-P) = 8.7, 1H, OCH2,P″a), 3.67 (dd, Jgem = 13.6, J(C-H-P) = 8.9, 1H, OCH2,P″b), 3.67 (m, 1H, H-2″), 3.60 (d, J(C-H-P) = 9.4, 2H, OCH2,P′), 3.51 (m, 2H, H-3″), 1.13 (d, J(3′,2′) = 6.4, 3H, H-3′) ppm. 13C NMR (DMSO-d6): δ = 171.48 and 171.42 (C-4, C-6), 162.83 (C-2), 80.40 (d, J(2″,P) = 9.7, C-2″), 78.59 (C-5), 75.08 (d, J(2′,P) = 11.7, C-2′), 68.63 (C-1′), 66.00 (d, J(P,C) = 161.0, PCH2″), 65.45 (C-1″), 65.01 (d, J(P,C) = 161.8, PCH2′), 60.35 (C-3″), 16.83 (C-3′) ppm. MS (ESI): m/z (%) = 448.1 (100) [MH]+, 470.1 (35) [MNa]+. [α]25D = +8.6 (c 0.561, H2O). Anal. C12H23N3O11P2.H2O (C, H, N, P).
4.27.8. 2-Amino-4-(2S)-[2-(phosphonomethoxy)-3-hydroxypropoxy]-6-(2R)-[2-(phosphonomethoxy)propoxy]pyrimidine (27h)
From 26h (960 mg, 1.55 mmol), freeze dried, white hydroscopic foam, yield 605 mg (83%). NMR spectra identical with compound 27g. MS (ESI): m/z (%) = 448.2 (100) [MH]+. [α]25D = −4.4 (c 0.182, H2O). Anal. C12H23N3O11P2.H2O (C, H, N, P).
4.27.9. 2-Amino-4-(2R)-[2-(phosphonomethoxy)-3-hydroxypropoxy]-6-(2R)-[2-(phosphonomethoxy)propoxy]pyrimidine (27i)
From 26i (1 g, 1.62 mmol), freeze dried, white hydroscopic foam, yield 616 mg (81%). NMR spectra identical with compound 39f. MS (ESI): m/z (%) = 448.0 (100) [MH]+. [α]25D = −5.8 (c 0.312, H2O). Anal. C12H23N3O11P2.H2O (C, H, N, P).
4.27.10. 2-Amino-4,6-(2S,2′S)-bis[2-(phosphonomethoxy)propoxy]pyrimidine (27j)
26k (350 mg, 0.58 mmol), yield 135 mg (52%), colorless foam. 1H NMR (DMSO-d6): δ = 3.57 (s, 1H, H-5), 4.17 (dd, Jgem = 11.1, J(1′a,2′) = 5.8, 2H, H-1′a), 4.11 (dd, Jgem = 11.1, J(1′b,2′) = 4.4, 2H, H-1′b), 3.81 (m, 2H, H-2′), 3.60 (d, J(CH2,P) = 9.3, 4H, PCH2), 1.14 (d, J(H-3′,H-2′) = 6.3, 6H, H-3′) ppm. 13C NMR (DMSO-d6): δ = 171.41 (C-4, C-6), 162.80 (C-2), 78.54 (C-5), 75.02 (d, J(C-2′,P) = 11.6, C-2′), 68.59 (C-1′), 65.02 (d, J(CH2,P) = 161.7, PCH2), 16.82 (C-3′) ppm. MS (ESI): m/z (%) = 432.1 (100) [MH]+; 454.1 (26) [MNa]+. [α]25D = +20.9 (c 0.291, H2O). Anal. C12H23N3O10P2.H2O (C, H, N, P).
4.27.11. 2-Amino-4,6-(2R,2′R)-bis[2-(phosphonomethoxy)propoxy]pyrimidine (27k)
From 26l (1.2 g, 1.8 mmol), white hydroscopic foam, yield 450 mg (55%). NMR spectra identical with compound 27j. MS (ESI): m/z (%) = 432.0 (100) [MH]+; 454.1 (14) [MNa]+. [α]25D = −20.5 (c 0.254, H2O). Anal. C12H23N3O10P2.H2O (C, H, N, P).
4.28. 2-Substituted bisphosphonates
4.28.1. 2-Cyclopropylamino-4,6-bis[2-(phosphonomethoxy)ethoxy]pyrimidine (28a)
Compound 26j (1 g, 1.57 mmol) in dry THF (45 mL) was treated with cyclopropylamine (5.5 mL) and the mixture was refluxed in a sealed flask for 2 hr. Volatiles were removed in vacuo and the residue was purified by flash chromatography (EtOAc/EtOH 0–10%) to give 330 mg (34%) of 2-cyclopropylamino-4,6-bis[2-(diisopropoxyphosphorylmethoxy)ethoxy]pyrimidine as a thick oil. 1H NMR (DMSO-d6): δ = 7.25 (br d, J(NH,CH) = 3.6, 1H, NH), 5.32 (s, 1H, H-5), 4.58 (m, 4H, P-OCH), 4.34 (m, 4H, C-1′), 3.79 (m, 4H, C-2′), 3.78 (d, J(OCH2,P) = 8.3, 4H, PCH2), 2.67 (m, 1H, CH cycloprop.), 1.23 (d, 12H) and 1.22 (d, J(CH3,CH) = 6.3, 12H, CH3), 0.62 (m, 2H) and 0.45 (m, 2H, CH2 cycloprop.) ppm. 13C NMR (DMSO-d6): δ = 170.99 2C (C-4, C-6), 162.51 (C-2), 78.66 (C-5), 70.80 (C-2′), 70.34 (d, 4C, J(CH,P) = 6.4, P-OCH), 64.96 (d, J(C-3′,P) = 164.5, P-OCH2), 64.48 (C-1′), 23.87 (CH cycloprop.), 23.92 (CH3), 6.34 (CH2 cycloprop.) ppm. MS (FAB): m/z (%) = 612 (100) [MH]+. HR MS (FAB) calcd. for C25H48N3O10P2 [MH]+ 612.2814, found 612.2813. Diisopropyl esters were deprotected by GP1 to give 28a (140 mg, 62%) as a white foam. 1H NMR (D2O): δ = 4.53 (m, 4H, H-1′), 3.98 (m, 4H, H-2′), 3.76 (d, J(P,CH2) = 9.2, 4H, PCH2), 2.72 (m, 1H, CH cycloprop.), 0.93 and 0.71 (2 × m, 2 × 2H, CH2 cycloprop.) ppm. 13C NMR (D2O): δ = 171.05 (C-4, C-6), 159.38 (C-2), 78.79 (C-5), 70.85 (d, J(C-2′,P) = 10.4, C-2′), 68.23 (C-1′), 67.60 (d, J(CH2,P) = 156.4, PCH2), 23.41 (CH cycloprop.), 7.19 (CH2 cycloprop.) ppm. MS (ESI): m/z (%) = 444.1 (100) [MH]+, 466.0 (26) [MNa]+. Anal. C13H23N3O10P2.H2O (C, H, N, P).
4.28.2. 2-Cyclopentylamino-4,6-bis[2-(phosphonomethoxy)ethoxy]pyrimidine (28b)
Compound 26j (1 g, 1.57 mmol) in dry THF (30 mL) was treated with cyclopentylamine (1.6 mL) and the mixture was refluxed in a sealed flask for 3 hr. THF was removed under reduced pressure and the residue was purified by flash chromatography (CHCl3/MeOH 0–2%) to give 2-cyclopentylamino-4,6-bis[2-(diisopropoxyphosphorylmethoxy)ethoxy]pyrimidine as an oil, yield 540 mg (54%). 1H NMR (CDCl3): δ = 5.37 (s, 1H, H-5), 4.88 (d, J(NH-1′) = 7.0, 1H, NH), 4.76 (dh, J(CH,CH3) = 6.2, J(CH,P) = 7.7, 4H, CH ipr.), 4.39 (m, 4H, H-1′), 4.18 (m, 1H, H-1″), 3.89 (m, 4H, H-2′), 3.82 (d, J(P,CH2) = 8.2, 4H, PCH2), 2.01 (m, 2H, H-2″a), 1.71 and 1.61 (2 × m, 2 × 2H, H-3″), 1.45 (m, 2H, H-2″b), 1.33 (m, 24H, CH3ipr.) ppm. 13C NMR (CDCl3): δ = 171.20 (C-4, C-6), 161.10 (C-2), 79.48 (C-5), 71.33 (d, J(2′-P) = 11.0, C-2′), 71.08 (d, J(CH-P) = 6.6, CH ipr.), 65.97 (d, J(3′-P) = 167.1, PCH2), 64.76 (C-1′), 52.91 (C-1″), 33.29 (C-2″), 24.07 (d, J(CH3-P) = 3.7) and 23.93 (d, J(CH3-P) = 4.6, CH3ipr.), 23.72 (C-3″) ppm. MS (FAB): m/z (%) = 640.5 (60) [MH]+. HR MS (FAB) calcd. for C27H52N3O10P2 [MH]+ 640.3128, found 640.3140.
Diisopropyl esters were cleaved by GP1 to afford 28b (220 mg, 53%) as a white hydroscopic foam. 1H NMR (DMSO-d6): δ = 5.34 (s, 1H, H-5), 4.31 (m, 4H, H-1′), 4.09 (m, 1H, H-1″), 3.76 (m, 4H, H-2′), 3.58 (d, J(CH2P) = 8.7, 4H, PCH2), 1.88 (m, 2H, H-2″a), 1.65 (m, 2H, H-3″a), 1.49 (m, 4H, H-2″b and H-3″b) ppm. 13C NMR (DMSO-d6): δ = 171.04 (C-4, C-6), 161.23 (C-2), 78.17 (C-5), 70.73 (d, J(2,P) = 11.2, C-2′), 66.95 (d, J(3,P) = 160.3, PCH2), 64.83 (C-1′), 52.60 (C-1″), 32.44 (C-2″), 23.71 (C-3″) ppm. MS (FAB): m/z (%) = 472.1 (100) [MH]+. Anal. C15H27N3O10P2.H2O (C, H, N, P).
4.28.3. 2-Methylamino-4,6-bis[2-(phosphonomethoxy)ethoxy]pyrimidine (28c)
2-Methylsulfonyl derivative 26j (1 g, 1.57 mmol) in EtOH (33.75 mL) was treated with methylamine (8 M solution in EtOH, 11.25 mL) and the reaction mixture was heated at 50 °C in a sealed tube for 6 h. Volatiles were removed under reduced pressure and the residue was purified by flash chromatography (EtOAc/EtOH 0–5%) to give colorless oil of 2-methylamino-4,6-bis[2-(diisopropoxyphosphorylmethoxy)ethoxy]pyrimidine, yield 440 mg (48%). 1H NMR (DMSO-d6): δ = 6.97 (br q, J(NH,CH3) = 4.7, 1H, NH), 5.28 (s, 1H, H-5), 4.59 (dh, J(CH,CH3) = 6.2, J(CH,P) = 7.8, 4H, CHipr.), 4.33 (m, 4H, H-1′), 3.78 (m, 8H, H-2′, H-3′), 2.75 (d, J(CH3,NH) = 4.7, 3H, CH3NH) ppm. 13C NMR (DMSO-d6): δ = 170.82 (C-2), 161.74 (C-4, C-6), 77.83 (C-5), 70.36 (m, CHipr.), 64.97 (d, J(CH2,P) = 164.2, CH2P), 64.49 (C-1′), 27.86 (CH3NH), 23.93 m (CH3ipr.) ppm. MS (FAB): m/z (%) = 586.2 (75) [MH]+. HR MS (FAB) calcd. for C23H46N3O10P2 [MH]+ 586.2658, found 586.2659.
Diisopropyl esters were deprotected by GP1 to give 28c (210 mg, 67%) as a white hydroscopic foam. 1H NMR (D2O): δ = 4.54 (m, 4H, H-1′), 3.98 (m, 4H, H-2′), 3.77 (d, J(CH2,P) = 9.2, 4H, PCH2), 3.00 (s, 3H, CH3) ppm. 13C NMR (D2O): δ = 173.02 (C-4, C-6), 162.95 (C-2), 70.72 d (C-2′), 69.21 (C-1′), 67.20 (d, PCH2), 28.21 (CH3) ppm. MS (FAB): m/z (%) = 418 (100) [MH]+. Anal. C11H21N3O10P2.H2O (C, H, N, P).
4.28.4. 2-Benzylamino-4,6-bis[2-(phosphonomethoxy)ethoxy]pyrimidine (28d)
Pyrimidine 26j (1 g, 1.57 mmol) in dry THF was treated with benzylamine (6 mL) and the reaction mixture was heated at 50 °C in a sealed tube for 4 hr. The solvent was removed under reduced pressure and the residue was purified by flash chromatography (EtOAc/EtOH 0–5%) to afford 2-benzylamino-4,6-bis[2-(diisopropoxyphosphorylmethoxy)ethoxy]pyrimidine (390 mg, 37%) as a colorless oil. 1H NMR (DMSO-d6): δ = 7.63 (br t, J(NH,H-1″) = 6.4, 1H, NH), 7.15–7.34 (m, 5H, arom.), 5.29 (s, 1H, H-5), 4.58 (m, 4H, CH ipr.), 4.41 (d, J(H-1″,NH) = 6.3, 2H, H-1″), 4.29 (m, 4H, H-1′), 3.75 (m, 8H, H-2′, PCH2), 1.23 (d, J(CH3,CH) = 6.2, 12H) and 1.21 (d, J(CH3,CH) = 6.2, 12H, CH3 ipr.) ppm. 13C NMR (DMSO-d6): δ = 170.94 (C-4, C-6), 161.46 (C-2), 140.71, 128.28, 127.42, 126.67 (arom.), 78.59 (C-5), 70.74 (C-2′), 70.35 (d, J(CH,P) = 6.3, 4C, CH ipr.), 64.95 (d, J(CH2,P) = 164.3, 2C, PCH2), 64.58 (2C, C-1′), 44.39 (C-1″), 24.00 (d, J(CH3,P) = 3.6, 4C) and 23.84 (d, J(CH3,P) = 4.3, 4C, CH3ipr.) ppm. MS (FAB): m/z (%) = 662 (25) [MH]+. HR MS (FAB) calcd. for C29H50N3O10P2 [MH]+ 662.2971, found 662.2981.
The intermediate was treated with bromotrimethylsilane (GP1) to give 28d, white hydroscopic foam, yield 120 mg (42%). 1H NMR (DMSO-d6): δ = 7.66 (br t, J(NH, H-1″) = 6.0, 1H, NH), 7.16–7.34 (m, 5H, arom.), 5.37 (s, 1H, H-5), 4.42 (d, J(H-1″, NH) = 5.6, 2H, H-1″), 4.27 (m, 4H, H-1′), 3.71 (m, 4H, H-2′), 3.53 (m, 4H, PCH2) ppm. 13C NMR (DMSO-d6): δ = 171.15 (C-4, C-6), 161.52 (C-2), 140.75, 128.35, 127.46, 126.70 (arom.), 78.68 (C-5), 70.60 (d, J(C-2′,P) = 10.9, C-2′), 67.18 (d, J(CH2,P) = 160.6, PCH2), 64.97 (C-1′), 44.41 (C-1″) ppm. MS (FAB): m/z (%) = 494 (100) [MH]+, 516 (35) [MNa]+. Anal. C17H25N3O10P2.H2O (C, H, N, P).
4.28.5. 2-(4-Methoxybenzyl)amino-4,6-bis[2-(phosphonomethoxy)ethoxy]pyrimidine (28e)
2-Methylsulfonyl derivative 26j (1 g, 1.57 mmol) in dry THF (30 mL) was treated with 4-methoxybenzylamine (0.61 mL, 4.1 mmol) and the reaction mixture was refluxed in a sealed tube for 2 h. The solvent was removed under reduced pressure and the residue was purified by flash chromatography to give 2-(4-methoxybenzyl)amino-4,6-bis[2-(diisopropoxy-phosphorylmethoxy)ethoxy]pyrimidine (550 mg, 50%) as colorless oil. 1H NMR (CDCl3): δ = 7.25 (m, 2H, Ph-2), 6.86 (m, 2H, Ph-3), 5.40 (s, 1H, H-5), 5.20 (t, J(NH,CH2) = 5.9, 1H, NH), 4.75 (dh, J(CH,CH3) = 6.2, J(CH,P) = 7.7, 4H, CHipr.), 4.49 (d, J(CH2,NH) = 5.9, 2H, PhCH2NH), 4.33 (m, 4H, H-1), 3.86 (m, 4H, H-2), 3.81 (d, J(CH2,P) = 8.3, 4H, PCH2), 3.80 (s, 3H, OCH3), 1.33 (m, 24H, CH3ipr.) ppm. 13C NMR (CDCl3): δ = 171.29 (C-4, C-6), 161.20 (C-2), 158.73 (Ph-4), 131.41 (Ph-1), 128.74 (Ph-2), 113.85 (Ph-3), 80.01 (C-5), 71.29 (d, J(C-2′,P) = 11.1, C-2′), 71.08 (d, J(CH,P) = 6.6, CHipr.), 65.96 (d, J(CH2,P) = 167.1, PCH2), 64.86 (C-1′), 55.24 (OCH3), 44.90 (PhCH2NH), 24.06 (d, J(CH3,CH) = 3.7) and 23.93 (d, J(CH3,CH) = 4.6, CH3ipr.) ppm. MS (FAB): m/z (%) = 692.2 (15) [MH]+. HR MS (FAB) calcd. for C30H52N3O11P2 [MH]+ 692.3077, found 692.3074.
Diisopropyl esters were deprotected by GP1. The final product was applied onto a column of Dowex 50 × 8 in Na+ form and eluted with water to give 28e (160 mg, 23%) as a tetrasodium salt; white hydroscopic foam. 1H NMR (DMSO-d6): δ = 7.60 (br t, J(NH,CH2) = 6.4, NH), 7.23 (m, 2H, H-2″), 6.85 (m, 2H, H-3″), 5.36 (d, 1H, H-5), 4.33 (d, J(CH2,NH) = 6.0, 2H, CH2N), 4.25 (m, 4H, H-1′), 3.70 (s, 3H, OCH3), 3.70 (m, 4H, H-2′), 3.50 (d, 4H, J(H,P) = 8.6, PCH2). 13C NMR (DMSO-d6): δ = 171.14 (C-4,6), 161.46 (C-2), 158.22 (C-4″), 132.68 (C-1″), 128.83 (C-2″), 113.76 (C-3″), 78.60 (C-5), 70.54 (d, J(2′,P) = 10.6, C-2′), 67.57 (d, J(C,P) = 159.5, PCH2), 64.97 (C-1′), 55.21 (OCH3), 43.80 (CH2N). MS (ESI): m/z (%) = 524.1 (100) [MH]+, 546.1 (69) [MNa]+. Anal. C18H23N3Na4O11P2 (C, H, N, P).
4.28.6. 2-Morpholino-4,6-bis[2-(phosphonomethoxy)ethoxy]pyrimidine (28f)
Compound 26j (1 g, 1.57 mmol) in dry THF (30 mL) was treated with morpholine (1.4 mL) and the mixture was refluxed in a sealed flask for 2 h and the solvent was removed under reduced pressure. Flash chromatography (CHCl3/MeOH 0–1%) gave thick oil of 2-morpholino-4,6-bis[2-(diisopropoxyphosphorylmethoxy)ethoxy]pyrimidine (720 mg, 71%). 1H NMR (CDCl3): δ = 5.39 (s, 1H, C-5), 4.76 (dh, J(CH,CH3) = 6.2, J(CH,P) = 7.7, 4H, CHipr.), 4.41 (m, 4H, H-1′), 3.90 (m, 4H, H-2′), 3.82 (d, J(CH2,P) = 8.2, 4H, PCH2), 3.72 (s, 8H, H-2″, H-3″), 1.34 (d, J(CH3,CH) = 6.3, 12H) and 1.33 (d, J(CH3,CH) = 6.3, 12H, CH3ipr.) ppm. 13C NMR (CDCl3): δ = 171.13 (C-4, C-6), 160.62 (C-2), 79.50 (C-5), 71.27 (d, J(2′,P) = 10.8, C-2′), 71.07 (d, J(CH,P) = 6.61, CH ipr.), 66.74 (C-3″), 66.02 (d, J(CH,P) = 167.3, PCH2), 64.80 (C-1′), 44.24 (C-2″), 24.07 (d, J(CH3,P) = 3.5) and 23.94 (d, J(CH3,P) = 4.5, CH3ipr.) ppm. MS (FAB): m/z (%) = 642.5 (22) [MH]+. HR MS (FAB) calcd. for C26H50N3O11P2 [MH]+ 642.2921, found 642.2911.
Deprotection of diisopropyl esters by GP1 gave 28f (360 mg, 65%) as a white hydroscopic foam. 1H NMR (DMSO-d6): δ = 5.45 (s, 1H, H-5), 4.33 (m, 4H, H-1′), 3.77 (m, 4H, H-2′), 3.64 (m, 8H, CH2 – morpholine), 3.57 (d, J(P,CH) = 8.7, 4H, PCH2) ppm. 13C NMR (DMSO-d6): δ = 171.13 (2C, C-4, C-6), 160.51 (C-2), 78.84 (C-5), 70.62 (d, J(C-2,P) = 11.2, C-2′), 66.93 (d, J(C-3,P) = 160.4, PCH2), 66.13 (C-3″), 65.10 (C-1′), 44.13 (C-2″) ppm. MS (ESI): m/z (%) = 474 (86) [MH]+. Anal. C14H25N3O11P2.H2O (C, H, N, P).
4.28.7. 4,6-Bis[2-(diisopropoxyphosphorylmethoxy)ethoxy]-2-hydroxypyrimidine (29a)
Solution of NaOH (0.072 g) in water (10 mL) was added in one portion to the 2-methylsulfonyl derivative 26j (1 g, 1.57 mmol) in THF (5 mL) and the resulting mixture was heated at 60 °C for 1hr. Reaction mixture was cooled to r.t., neutralized with acetic acid and volatiles were removed in vacuo. The residue was partioned between CHCl3 and water. Organic fraction was washed with water (3 × 50 mL) and dried with MgSO4. Flash chromatography (CHCl3/MeOH 0–5%) gave colorless oil, 450 mg (50%). 1H NMR (CDCl3): δ = 5.29 (s, 1H, H-5), 4.76 (dh, J(CH,P) = 7.7, J(CH,CH3) = 6.2, CHipr.), 4.38 (m, 4H, H-1′), 3.92 (m, 4H, H-2′), 3.81 (d, J(CH2,P) = 8.2, PCH2), 1.34 and 1.33 (2 × d, J(CH3,CH) = 6.2, 2 × 12H, CH3ipr.) ppm. 13C NMR (CDCl3): δ = 162.57 (C-4,6),158.72 (C-2), 75.35 (C-5), 71.18 (d, J(C,P) = 6.6, CHipr.), 70.59 (d, J(C,P) = 10.6, C-2′), 67.29 (C-1′), 66.08 (d, J(C,P) = 167.6, PCH2), 24.05 (d, J(C,P) = 4.0, CH3ipr.), 23.95 (d, J(C,P) = 4.6, CH3ipr.) ppm. MS (FAB): m/z (%) = 573 (100) [MH]+. HR MS (FAB) calcd. for C22H43N2O11P2 [MH]+ 573.2342, found 573.2347.
4.28.8. 2-Methoxy-4,6-bis[2-(diisopropoxyphosphorylmethoxy)ethoxy]pyrimidine (29b)
26j (2 g, 3.15 mmol) in MeOH (40 mL) was treated with MeONa (1M in MeOH, 3.5 mL) and the resulting mixture was heated at 80 °C for 4 hr. Reaction mixture was cooled to r.t. and solvent was removed in vacuo. Flash chromatography afforded 29b (600 mg, 32%) as an oil. 1H NMR (CDCl3): δ = 5.71 (s, 1H, H-5), 4.76 (dh, J(CH,CH3) = 6.2, J(H,C,P) = 7.7, 4H, CHipr), 4.48 (m, 4H, H-1′), 3.94 (s, 3H, OCH3), 3.92 (m, 4H, H-2′), 3.82 (d, J(H,C,P) = 8.2, 4H, OCH2P), 1.33 (m, 24H, CH3ipr.) ppm. 13 C NMR (CDCl3): δ = 171.99 (C-4, C-6), 164.52 (C-2), 84.20 (C-5), 71.08 (m, CHipr., H-2′), 65.97 (d, J(C,P) = 167.4, OCH2P), 65.51 (C-1′), 54.65 (OCH3), 24.05 (d, J(C,C,O,P) = 3.7) and 23.91 (d, J(C,C,O,P) = 4.6, CH3ipr.) ppm. MS (FAB): m/z (%) = 587 (62) [MH]+. HR MS (FAB) calcd. for C23H45N2O11P2 [MH]+ 587.2498, found 587.2516.
4.28.9. 4,6-Bis[2-(phosphonomethoxy)ethoxy]pyrimidine (30)
Compound 21 (2 g, 3.22 mmol) in MeOH (200 mL) was treated with suspension of Raney-Nickel (ca 15 g) in MeOH (30 mL). The reaction mixture was refluxed for 6 hr, filtered while hot through Celite and the precipitate was washed with MeOH (500 mL). The filtrate was evaporated in vacuo, and the residue was purified by flash chromatography (CHCl3/MeOH 0–5%) to give 4,6-bis[2-(diisopropoxyphosphorylmethoxy)ethoxy]pyrimidine as a thick oil (1.5 g, 81%). 1H NMR (DMSO-d6): δ = 8.38 (d, J(H-2,H-5) = 0.9, 1H, H-2), 6.05 (d, J(H-5,H-2) = 0.9, 1H, H-5), 4.76 (dh, J(CH,CH3) = 6.2, J(CH,P) = 7.7, 4H, CHipr.), 4.50 (m, 4H, H-1′), 3.93 (m, 4H, H-2′), 3.83 (d, J(H,C,P) = 8.2, 4H, POCH2), 1.33 (m, 24H, CH3ipr.) ppm. 13C NMR (DMSO-d6): δ = 170.51 (C-4, C-6), 157.21 (C-2), 91.15 (C-5), 71.12 (d, J(2′,P) = 10.8, C-2′), 71.09 (d, J(CH,P) = 6.7, CHipr.), 65.99 (d, J(C,P) = 167.3, POCH2), 65.52 (C-1′), 24.05 (d, J(CH3,P) = 3.7) and 23.93 (d, J(CH3,P) = 4.6, CH3ipr.) ppm. MS (ESI): m/z (%) = 579.2 (100) [MNa]+. Anal. C22H42N2O10P2 (C, H, N, P).
The intermediate was deprotected by bromotrimethylsilane (GP1) to give 30 (350 mg, 48%), freeze dried, white hydroscopic foam. 1H NMR (DMSO-d6): δ = 8.44 (d, J(H-2,H-5) = 0.9, 1H, H-2), 6.28 (d, J(H-5,H-2) = 0.9, 1H, H-5), 4.39 (m, 4H, H-1′), 3.80 (m, 4H, H-2′), 3.57 (d, J(H,C,P) = 8.7, 4H, OCH2P) ppm. 13C NMR (DMSO-d6): δ = 170.70 (C-4, C-6), 157.79 (C-2), 90.36 (C-5), 70.46 (d, J(2′,P) = 11.0, C-2′), 67.10 (d, J(C,P) = 160.2, PCH2), 66.01 (C-1′) ppm. MS (ESI): m/z (%) = 389 (76) [MH]+. Anal. C10H18N2O10P2.H2O (C, H, N, P).
4.29. 4,6-Disulfanylpyrimidine derivatives
4.29.1. 2-Amino-4,6-disulfanylpyrimidine (31)
Thiourea (9.5 g, 90 mmol) was added to the solution of dichloropyrimidine 10 (5 g, 30 mmol) in EtOH (250 mL) and the reaction mixture was refluxed for 2 hr. Solvent was removed in vacuo and the residue in aq. NaOH (0.5 M, 250 mL) was heated at 80 °C for 16 hr. The reaction mixture was cooled to r.t., acidified with acetic acid to pH 4 and evaporated to half of its volume. The precipitate was filtered off, washed with water and dried to give yellow solid (4.78 g, 98%), m.p. 259 °C dec. 1H NMR (DMSO-d6): δ = 11.2–11.8 (br s, 2H, SH), 7.17 (br s, 2H, NH2), 6.24 (s, 1H, H-5) ppm. 13C NMR (DMSO-d6): δ = 173.91 (2C, C-4, C-6), 149.10 (C-2), 116.36 (C-5) ppm. MS (EI): m/z (%) = 159.2 (100) [M]+. Anal. C4H5N3S2 (C, H, N, S).
4.29.2. 2-Amino-4,6-bis{[2-(diisopropoxyphosphorylmethoxy)ethyl]sulfanyl}pyrimidine (32a) and 2-amino-4,6-bis{[2-(phosphonomethoxy)ethyl]sulfanyl}pyrimidine (33a)
Phosphonate 20a (6.9 g, 26.4 mmol) was added to a stirred mixture of disulfanyl pyrimidine 31 (2 g, 12.56 mmol) and NaH (1.25 g, 60% in paraffin oil, 31 mmol) in DMF (50 mL) and the resulting mixture was stirred at r.t. for 24 hr and evaporated in vacuo. The residue was adsorbed onto silica gel from methanol and separated by flash chromatography (CHCl3/MeOH 0–5%) to give 32a (5.6 g, 74%), pale yellow oil. 1H NMR (DMSO-d6): δ = 6.74 (br s, 2H, NH2), 6.41 (s, 1H, H-5), 4.58 (m, 4H, CHipr.), 3.79 (d, J(P,CH) = 8.3, 4H, PCH2), 3.69 (t, J(1′,2′) = 6.4, 4H, H-1′), 3.26 (t, J(2′,1′) = 6.4, 4H, H-2′), 1.24 (d, 12H) and 1.23 (d, J(CH3,CH) = 6.2, 12H, CH3) ppm. 13C NMR (DMSO-d6): δ = 167.60 (2C, C-4, C-6), 161.79 (C-2), 103.07 (C-5), 71.18 (d, J(P,C) = 12.2, 2C, C-2′), 70.35 (d, J(P,C) = 6.3, 4C, CHipr.), 64.81 (d, J(P,C) = 164.6, 2C, PCH2), 27.60 (2C, C-1′), 24.03 (d, J(P,C) = 3.9, 4C) and 23.92 (d, J(P,C) = 4.4, 4C, CH3) ppm. MS (ESI): m/z (%) = 604.2 (100) [MH]+. Anal. C22H43N3O8P2S2 (C, H, N, P, S).
Subsequent deprotection of 32a (2.6 g, 4.3 mmol) by GP1 gave free phosphonic acid 33a (1.2 g, 65%) as a white foam. 1H NMR (D2O): δ = 6.75 (s, 1H, H-5), 3.97 (t, J(2′,1′) = 6.4, 4H, H-2′), 3.80 (d, J(P,CH) = 8.7, 4H, PCH2), 3.45 (t, J(1′,2′) = 6.4, 4H, H-1′) ppm. 13C NMR (D2O): δ = 169.49 (2C, C-4, C-6), 161.20 (C-2), 104.15 (C-5), 70.59 (d, J(P,C) = 10.7, 2C, C-2′), 66.82 (d, J(P,C) = 156.2, 2C, PCH2), 28.38 (2C, C-1′) ppm. MS (ESI): m/z (%) = 436 (35) [MH]+. Anal. C10H19N3O8P2S2 (C, H, N, P, S).
4.29.3. 2-Amino-4,6-(2S,2′S)-bis{[2-(diisopropoxyphosphorylmethoxy)propyl]sulfanyl}pyrimidine (32b) and 2-amino-4,6-(2S,2′S)-bis{[2-(phosphonomethoxy)propyl]sulfanyl}pyrimidine (33b)
Prepared by the same procedure as compounds 32a and 33a. From pyrimidine 31 (200 mg, 1.25 mmol) and phosphonate 20b (1.06 g, 2.6 mmol). Thick oil, 534 mg (68%). 1H NMR (DMSO-d6): δ = 6.69 (s, 2H, NH2), 6.14 (s, 1H, H-5), 4.59 (m, 4H, CHipr.), 3.78 (d, J(P,CH) = 9.2, 4H, PCH2), 3.74 (m, 2H, H-2′), 3.26 (dd, J(1′a,2′) = 5.5, Jgem = 13.6, 2H, H-1′a), 3.20 (dd, J(1′b,2′) = 5.8, Jgem = 13.6, 2H, H-1′b), 1.24 (d, 12H), 1.23 (d, 6H) and 1.16 (d, J(CH3,CH) = 6.2, 6H, CH3ipr.) ppm. 13C NMR (DMSO-d6): δ = 167.63 (2C, C-4,6), 161.64 (C-2), 103.30 (C-5), 76.19 (d, J(P,C) = 12.7, 2C, C-2′), 70.30 (d, J(P,C) = 6.3, 4C, CHipr.), 62.84 (d, J(P,C) = 165.0, 2C, PCH2), 33.21 (2C, C-1′), 24.02 (d, J(P,C) = 3.6, 4C) and 23.88 (d, J(P,C) = 4.6, 4C, CH3ipr.), 18.73 (2C, C-3′) ppm. MS (ESI): m/z (%) = 654.2 (100) [MNa]+.
Phosphonic acid 33b, yield (280 mg, 73%), white foam. 1H NMR (D2O): δ = 6.81 (s, 1H, H-5), 3.94 (m, 2H, H-2′), 3.77 (dd, 2H) and 3.65 (dd, J(P,CH) = 9.3, Jgem = 13.2, 2H, PCH2), 3.38 (dd, 2H) and 3.35 (dd, J(1′,2′) = 5.5, Jgem = 13.6, 2H, H-1′), 1.29 (d, J(3′,2′) = 6.2, 6H, H-3′) ppm. MS (ESI): m/z (%) = 464.0 (100) [MH]+. [α]25D = +48.3 (c 0.357, H2O). Anal. C12H23N3O8P2S2.H2O (C, H, N, P, S).
4.29.4. 2-Amino-4,6-(2R,2′R)-bis{[2-(diisopropoxyphosphorylmethoxy)propyl]sulfanyl}pyrimidine (32c) and 2-amino-4,6-(2R,2′R)-bis{[2-(phosphonomethoxy)propyl]sulfanyl}pyrimidine (33c)
Prepared by the same procedure as compounds 32a and 33a. From pyrimidine 31 (500 mg, 3.1 mmol) and phosphonate 20c (2.65 g, 6.5 mmol). Thick oil, 1.48 g (75%). NMR spectra identical with compound 32b. MS (ESI): m/z (%) = 654.0 (100) [MNa]+.
Phosphonic acid 33c, yield 702 mg (71%), white foam. NMR spectra identical with compound 33b. MS (ESI): m/z (%) = 464.0 (100) [MH]+. [α]25D = −40.2 (c 0.589, H2O). Anal. C12H23N3O8P2S2.H2O (C, H, N, P, S).
4.29.5. 2-Amino-6-{[2-(diisopropoxyphosphorylmethoxy)ethyl]sulfanyl}-4-sulfanylpyrimidine (34a)
To the solution of pyrimidine 31 (3 g, 18.84 mmol) and NaH (0.76 g, 60% in paraffin oil, 19 mmol) in DMF (70 mL) was added dropwise phosphonate 20a (5 g, 19 mmol). The resulting mixture was stirred at r.t. for 3 days and taken down in vacuo. The residue in CHCl3 (200 mL) was washed with water (3 × 100 mL), dried over MgSO4 and taken down under reduced pressure. The residue was separated by flash chromatography (CHCl3/MeOH 0–5%) to give 32a (3.18 g, 28%) and 34a (2.92 g, 40%) as a pale yellow oil. 1H NMR (DMSO-d6): δ = 11.90 (br s, 1H, SH), 7.00 (br s, 2H, NH2), 6.33 (s, 1H, H-5), 4.59 (m, 2H, CHipr.), 3.78 (d, J(P,CH) = 8.3, 2H, PCH2), 3.71 and 3.22 (2 × t, J(1′,2′) = 6.3, 2 × 2H, H-1′, H-2′), 1.24 (d, 6H) and 1.23 (d, J(CH3,CH) = 6.2, 6H, CH3) ppm. 13C NMR (DMSO-d6): δ = 177.97 (C-4), 167.60 (C-2), 154.04 (C-6), 109.86 (C-5), 70.24 (d, J(P,C) = 12.2, C-2′), 70.36 (d, 2C, J(P,C) = 6.3, CHipr.), 64.83 (d, J(P,C) = 164.1, PCH2), 28.33 (C-1′), 23.98 (d, 2C, J(P,C) = 3.9) and 23.91 (d, 2C, J(P,C) = 4.4, CH3) ppm. MS (FAB): m/z (%) = 382 (100) [MH]+. Anal. C13H24N3O4PS2 (C, H, N, P, S).
4.29.6. 2-Amino-6-(2S)-{[2-(diisopropoxyphosphorylmethoxy)propyl]sulfanyl}-4-sulfanylpyrimidine (34b)
To the solution of pyrimidine 31 (1 g, 6.3 mmol) and NaH (0.252 g, 60% in paraffin oil, 6.3 mmol) in DMF (25 mL) wad added dropwise phosphonate 20b (2.57 g, 6.3 mmol) at 0 °C. The resulting mixture was stirred at 60 °C for 8 hr and taken down in vacuo. The residue was purified by flash chromatography to give 32b (750 mg, 19%) and 34b (780 mg, 31%) as an thick oil. 1H NMR (DMSO-d6): δ = 11.88 (br s, 1H, SH), 7.00 (br s, 2H, NH2), 6.33 (s, 1H, H-5), 4.59 (m, 2H, CHipr.), 3.79 (dd, 1H) and 3.75 (dd, Jgem = 13.8, J(P,CH) = 9.2, 1H, PCH2), 3.74 (m, 1H, H-2′), 3.22 (dd, J(1′a,2′) = 5.4, Jgem = 13.6, 1H, H-1′a), 3.17 (dd, J(1′b,2′) = 5.8, Jgem = 13.6, 1H, H-1′b), 1.24 (d, 6H), 1.235 (d, 6H) and 1.18 (d, 3H, J(CH3,CH) = 6.2, CH3) ppm. 13C NMR (DMSO-d6): δ = 178.56 (C-4), 167.86 (C-2), 153.95 (C-6), 109.94 (C-5), 75.97 (d, J(P,C) = 12.8, C-2′), 70.37 and 70.34 (d, J(P,C) = 6.3, CHipr.), 62.78 (d, J(P,C) = 165.6, PCH2), 34.35 (d, J(P,C) = 3.9, C-1′), 24.04 (d, 2C, J(P,C) = 3.0) and 23.90 (2C, J(P,C) = 4.6, CH3), 18.17 (C-3′) ppm. MS (ESI): m/z (%) = 418 (100) [MNa]+. HR MS (FAB) calcd. for C14H27N3O4PS2 [MH]+ 396.1180, found 396.1176.
4.29.7. 2-Amino-4-{[2-(diisopropoxyphosphorylmethoxy)ethyl]sulfanyl}-6-(2S)-{[2-(diisopropoxyphosphorylmethoxy)propyl]sulfanyl}pyrimidine (35a) and 2-amino-4-{[2-(phosphonomethoxy)ethyl]sulfanyl}-6-(2S)-{[2-(phosphonomethoxy)propyl]sulfanyl}pyrimidine (36a)
Monoderivative 34a (300 mg, 0.79 mmol), NaH (0.035 g, 60% in paraffin oil, 0.87 mmol) and phosphonate 20b (0.35 g, 0.86 mmol) in DMF (10 mL) was stirred at 60 °C for 4hr and taken down in vacuo. The residue was treated with hot chloroform and filtered, and the filtrate was evaporated in vacuo. Flash chromatography afforded 35a (400 mg, 82%) as an oil. 1H NMR (DMSO-d6): δ = 6.70 (br s, 2H, NH2), 6.41 (s, 1H, H-5), 4.59 (m, 4H, CHipr.), 3.775 (d, 2H) and 3.77 (d, J(P,CH) = 8.4, 2H, PCH2), 3.76 (m, 1H, H-2″), 3.71 (t, J(2′,1′) = 5.4, 2H, H-2′), 3.28 (dd, J(1″a,2″) = 6.1, Jgem = 13.6, 1H, H-1″a), 3.26 (t, J(1′,2′) = 5.4, 2H, H-1′), 3.20 (dd, J(1″b,2″) = 5.8, Jgem = 13.6, 1H, H-1″b), 1.245 (d, 12H) and 1.23 (d, J(CH3,CH) = 6.2, 12H, CH3ipr.), 1.18 (d, J(CH3,CH) = 6.2, 3H, H-3″) ppm. 13C NMR (DMSO-d6): δ = 167.71 and 167.47 (C-4,6), 161.70 (C-2), 103.83 (C-5), 75.70 (d, J(P,C) = 11.7, C-2″), 71.08 (d, 4C) and 70.64 (d, 4C, J(P,C) = 6.3, CHipr.), 70.03 (d, J(P,C) = 10.7, C-2′), 64.91 (d) and 62,05 (d, J(P,C) = 164.5, PCH2), 32.10 (C-1″), 27.60 (C-1′), 24.66 (d, 4C) and 24.18 (d, 4C, J(P,C) = 3.9), 23.59 (d, 4C) and 23.32 (d, 4C, J(P,C) = 4.4, CH3), 17.20 (C-3″) ppm. MS (FAB): m/z (%) = 618.2 (100) [MH]+. HR MS (FAB) calcd. for C23H46N3O8P2S2 [MH]+ 618.2203, found 618.2205.
Diisopropylester 35a was deprotected by GP1 to give 36a (100 mg, 53%) as a white foam. 1H NMR (D2O): δ = 6.79 (s, 1H, H-5), 3.94 (m, 1H, H-2″), 3.87 (t, J(2′,1′) = 6.1, 2H, H-2′), 3.74 (dd, J(P,CH) = 9.2, Jgem = 13.3, 1H) and 3.70 (d, J(P,CH) = 8.9, 2H) and 3.66 (dd, J(P,CH) = 9.4, Jgem = 13.3, 1H, PCH2), 3.38 (t, J(1′,2′) = 6.1, 2H, H-1′), 3.36 (dd, J(1″a,2″) = 5.4, Jgem = 14.4, 1H, H-1″a), 3.32 (dd, J(1″b,2″) = 6.1, Jgem = 14.4, 1H, H-1″b), 1.29 (d, J(3″,2″) = 6.3, 3H, H-3″). MS (ESI): m/z (%) = 450 (100) [MH]+. [α]25D = +32.5 (c 0.142, H2O). Anal. C11H21N3O8P2S2.H2O (C, H, N, P, S).
4.29.8. 2-Amino-4-{[2-(diisopropoxyphosphorylmethoxy)ethyl]sulfanyl}-6-(2R)-{[2-(diiso-propoxyphosphorylmethoxy)propyl]sulfanyl}pyrimidine (35b) and 2-amino-4-{[2-(phosphonomethoxy)ethyl]sulfanyl}-6-(2R)-{[2-(phosphonomethoxy)propyl]sulfanyl}-pyrimidine (36b)
Prepared by the same procedure as compounds 35a and 36a from pyrimidine 34a and phosphonate 20c.
35b: thick oil, yield 390 mg (80%). NMR spectra identical with compound 35a. MS (FAB): m/z (%) = 618.0 (100) [MH]+. HR MS (FAB) calcd. for C23H46N3O8P2S2 [MH]+ 618.2203, found 618.2206.
36b: white foam, yield 95 mg (50%). NMR spectra identical with compound 36a. MS (ESI): m/z (%) = 450 (100) [MH]+; 472 (50) [MNa]+. [α]25D = −19.3 (c 0.216, H2O). Anal. C11H21N3O8P2S2.H2O (C, H, N, P, S).
4.29.9. 2-Amino-4,6-(2R,2′S)-bis{[2-(diisopropoxyphosphorylmethoxy)propyl]sulfanyl}pyrimidine (35c) and 2-amino-4-(2R,2′S)-bis{[2-(phosphonomethoxy)propyl]sulfanyl}pyrimidine (36c)
Prepared by the same procedure as compounds 35a and 36a from pyrimidine 34b (400 mg, 1.01 mmol) and phosphonate 20c (0.45 g, 1.1 mmol).
35c: thick oil, yield 440 mg (71%). NMR spectra identical with compound 32b. MS (ESI): m/z (%) = 654.0 (100) [MNa]+.
36c: white foam, yield 180 mg (61%). 1H NMR (D2O): δ = 6.86 (s, 1H, H-5), 3.95 (m, 2H, H-2′), 3.79 (dd, Jgem = 13.2, J(CH2,P) = 9.3, 2H) and 3.68 (dd, Jgem = 13.2, J(CH2,P) = 9.5, 2H, PCH2), 3.43 (dd, Jgem = 14.3, J(1,2) = 4.4, 2H) and 3.33 (dd, Jgem = 14.3, J(1,2) = 6.2, 2H, H-1′), 1.30 (d, J(CH3,2′) = 6.7, 6H, H-3′) ppm. MS (ESI): m/z (%) = 464.0 (100) [MH]+. [α]25D = +0.3 (c 0.358, H2O). Anal. C12H23N3O8P2S2.H2O (C, H, N, P, S).
4.30. Alkoxyalkyl esters of bisphosphonates
4.30.1. 2-Amino-4,6-bis(2-hydroxyethoxy)pyrimidine (37)
A solution of t-BuOK (13.5 g, 120 mmol) in ethyleneglycol (50 mL) was heated at 80 °C for 30 min. and dichloropyrimidine 10 (5 g, 30 mmol) was added. The resulting mixture was stirred at 100 °C for 1 hr, cooled to r.t. and neutralized by addition of Dowex 50 × 8; the resulting mixture was diluted with water (100 mL), applied onto a column of Dowex 50 × 8 and washed with water (1L). Column was then eluted with 2.5% aq. ammonia, UV absorbing fraction was collected and evaporated under reduced pressure. The crude product was recrystallized from water to give a white solid (4.65 g, 71%), m.p. 158 °C. 1H NMR (DMSO-d6): δ = 6.48 (br s, 2H, NH2), 5.32 (s, 1H, H-5), 4.82 (t, J(OH,2′) = 5.4, 2H, OH), 4.17 (t, J(1′,2′) = 5.1, 4H, H-1′), 3.63 (q, J(2′,1′) = J(2′,OH) = 5.2, 4H, H-2′) ppm. 13C NMR (DMSO-d6): δ = 171.57 (C-4, C-6), 162.87 (C-2), 76.69 (C-5), 67.53 (C-1′), 59.61 (C-2′) ppm. MS (ESI): m/z (%) = 216 (100) [MH]+. Anal. C8H13N3O4 (C, H, N).
4.30.2. Hexadecyloxyethyl toluenesulfonyloxymehylphosphonate, sodium salt (38)
Prepared by previously described procedure [24]; yield 47%. 1H NMR (CDCl3): δ = 7.77 (br d, J(CH,CH) = 5.81, 2H, CHarom.), 7.30 (br d, J(CH,CH) = 6.0, 2H, CHarom.), 4.07 (br, 2H, PCH2), 3.97 (br, 2H, OCH2), 3.45 (br, 2H, OCH2), 3.34 (br, 2H, OCH2), 2.38 (s, 3H, CH3arom.), 1.48 (br, 2H, OCH2CH2CH2), 1.25 (br s, 26H, 13 × CH2), 0.87 (t, J(CH3,CH2) = 6.83, CH3CH2) ppm. MS (ESI): m/z (%) = 579 (100) [MNa]+. Anal. C26H46NaO7PS (C, H, N, P, S).
4.30.3. 2-(Hexadecyloxy)ethyl 2-amino-4-(2-hydroxyethoxy)-6-[2-(phosphonomethoxy)ethoxy]pyri-midine, sodium salt (39a)
GP7, white solid (47 mg, 31%), m.p. 129 °C. 1H NMR (DMSO-d6): δ = 6.51 (br s, 2H, NH2), 5.31 (s, 1H, H-5), 4.24 (m, 2H, H-1′), 4.15 (t, J(1″,2″) = 5.2, 2H, H-1″), 3.75 (m, 2H, H-4′), 3.68 (m, 2H, H-2′), 3.62 (m, 2H, H-2″), 3.39 (m, 6H, H-3′, 5′, 6′), 1.45 (m, 2H, H-7′), 1.22 (m, 26H, alif.), 0.84 (t, J(CH3,CH2) = 6.9, 3H, CH3) ppm. 13C NMR (DMSO-d6): δ= 171.52 and 171.37 (C-4, C-6), 162.83 (C-2), 78.62 (C-5), 70.79 (d, J(5′,P) = 5.6, C-5′), 70.46 (C-6′), 69.99 (d, J(2′,P) = 8.9, C-2′), 67.47 (C-1″), 64.98 (C-1′), 62.88 (d, J(4′,P) = 5.1, C-4′), 59.55 (C-2″), 31.51 (CH3CH2CH2), 29.52, 29.25, 29.15, 28.92 and 25.90 (alif.), 22.31 (CH3CH2), 14.18 (CH3) ppm. MS (ESI): m/z (%) = 598.4 (80) [M-H]−. Anal. C27H51N3NaO8P (C, H, N, P).
4.30.4. Bis[2-(Hexadecyloxy)ethyl] 2-amino-4,6-bis[2-(phosphonomethoxy)ethoxy]pyrimidine, sodium salt (40a)
GP7, white solid (59 mg, 24%), m.p. 184 °C. MS (ESI): m/z (%) = 962.5 (88) [M-Na+H]+. Anal. C46H89N3Na2O12P2 (C, H, N, P).
4.30.5. 2-(Hexadecyloxy)ethyl 2-amino-5-bromo-4-(2-hydroxyethoxy)-6-[2-(phosphonomethoxy)-ethoxy]pyrimidine, sodium salt (39b)
GP7, white solid (44 mg, 26%), m.p. 83 °C. 1H NMR (DMSO-d6): δ = 6.72 (br s, 2H, NH2), 4.86 (br s, 1H, OH), 4.34 (m, 2H, H-1′), 4.25 (m, 2H, H-1″), 3.61–3.80 (m, 6H, H-2′, 4′, 2″), 3.38 (m, 6H, 2 × OCH2, PCH2), 1.44 (m, 2H, H-7′), 1.22 (m, 26H, alif.), 0.84 (t, J(CH3,CH2) = 6.8, 3H, CH3) ppm. 13C NMR (DMSO-d6): δ = 166.40 and 166.28 (C-4, C-6), 160.87 (C-2), 73.57 (C-5), 70.78 (d, J(5′,P) = 5.7, C-5′), 70.48 (C-6′), 69.88 (C-2′), 68.41 (C-1″), 66.20 (C-1′), 63.02 (C-4′), 59.42 (C-2″), 31.52 (CH3CH2CH2), 29.54, 29.22, 29.18, 28.94, 25.91 (alif.), 22.33 (CH3CH2), 14.19 (CH3) ppm. MS (ESI): m/z (%) = 654 (100) [M-Na]−. Anal. C27H50BrN3NaO8P (C, H, N, P).
4.30.6. Bis[2-(Hexadecyloxy)ethyl] 2-amino-5-bromo-4,6-bis[2-(phosphonomethoxy)ethoxy]pyrimidine, sodium salt (40b)
GP7, white solid (61 mg, 23%), m.p. 160 °C dec. MS (ESI): m/z (%) = 1038 (26) [M-Na]−, 1016 (63) [M-(2 × Na)+H]−. Anal. C46H88BrN3Na2O12P2 (C, H, N, P).
4.30.7. 2-(Hexadecyloxy)ethyl 2-amino-4-(2-hydroxyethoxy)-5-methyl-6-[2-(phosphonomethoxy)-ethoxy]pyrimidine, sodium salt (39c)
GP7, white solid (122 mg, 40%), m.p. 95 °C. 1H NMR (DMSO-d6): δ = 6.20 (br s, 2H, NH2), 4.85 (br, 1H, OH), 4.28 (m, 2H, H-1′), 4.19 (m, 2H, H-1″), 3.62–3.81 (m, 6H, H-2′, 4′, 2″), 3.39 (m, 6H, H-3′, 5′, 6′), 1.77 (s, 3H, 5-CH3), 1.43 (m, 2H, H-7′), 1.22 (m, 26H, alif.), 0.84 (t, J(CH3,CH2) = 7.0, 3H, CH3) ppm. 13C NMR (DMSO-d6): δ = 168.48 and 168.32 (C-4, C-6), 160.37 (C-2), 86.86 (C-5), 70.73 (d, J(5′,P) = 5.5, C-5′), 70.52 (C-6′), 70.41 (C-2′), 67.49 (C-1″), 65.15 (C-1′), 63.11 (br, C-4′), 59.77 (C-2″), 31.57 (CH3CH2CH2), 29.55, 29.31, 29.21, 28.98, 25.94 (alif.), 22.37 (CH3CH2), 14.24 (CH3), 7.09 (5-CH3) ppm. MS (ESI): m/z (%) = 590.3 (100) [M-Na]−. Anal. C28H53N3NaO8P (C, H, N, P).
4.30.8. Bis[2-(Hexadecyloxy)ethyl] 2-amino-5-methyl-4,6-bis[2-(phosphonomethoxy)ethoxy]pyrimidine, sodium salt (40c)
GP7, white solid (106 mg, 21%), m.p. 142 °C. MS (ESI): m/z (%) = 952.5 (48) [M-(2 × Na)+H]−. Anal. C47H91N3Na2O12P2 (C, H, N, P).
4.30.9. 2-Amino-5-bromo-4,6-bis(2-hydroxyethoxy)pyrimidine (41)
Pyrimidine 37 (1 g, 4.65 mmol) in DMF (15 mL) was treated with bromine (0.7 M solution in CCl4, 10 mL) and the mixture was stirred at r.t. overnight. The mixture was taken down in vacuo and codistilled with EtOH (3 × 100 mL). The crude product was recrystallized from EtOH to give pale yellow needles (530 mg, 39%), m.p. 178 °C. 1H NMR (DMSO-d6): δ = 6.71 (br s, 2H, NH2), 4.26 (t, J(1′,2′) = 5.3, 4H, H-1′); 3.67 (t, J(2′,1′) = 5.3, 4H, H-2′) ppm. 13C NMR (DMSO-d6): δ = 166.42 (C-4, C-6), 160.88 (C-2), 73.60 (C-5), 68.46 (C-1′), 59.46 (C-2′) ppm. MS (ESI): m/z (%) = 294 (18) [MH+], 276 (100) [M – H2O+H]+. Anal. C8H12BrN3O4 (C, H, Br, N).
4.31. Capillary electrophoresis
Analyses were performed in a commercial P/ACE MDQ capillary electrophoresis (CE) apparatus (Beckman Coulter, Fullerton, CA, USA), equipped with an internally non-coated fused silica capillary with outer polyimide coating, total length 390 mm, effective length (from injection end to the detector) 288 mm, I.D./O.D. 50/375 μm (Polymicro Technologies, Phoenix, AR, USA). The analytes were monitored by UV-Vis absorption spectrophotometric photodiode array detector (190–600 nm) at two wavelengths, 206 and 254 nm, respectively. The temperature of capillary liquid coolant was set at 20 °C.
The samples were injected hydrodynamically, by pressure 13.8 mbar for 10 s. The analytes were dissolved in deionized water, the concentration of enantiomers in their individual CZE analyses was 0.1 mM, whereas in enantiomeric mixtures the concentration of R-isomer was 0.2 mM and of S-isomer 0.1 mM, in order to distinguish their migration order. Separation voltage was 15 kV.
The analyses were performed both in non-chiral and chiral background electrolytes (BGEs) of the following composition:
Non-chiral BGEs: 25–50 mM borax, adjusted by NaOH to pH 10.0–10.5.
Chiral BGEs: 25–50 mM borax, adjusted by NaOH to pH 10.0–10.5 + chiral selector β-cyclodextrin (5–20 mg/ml).
4.32. Biological activity assays
In vitro cytostatic activity tests (cell growth inhibition) were performed with cultures of murine leukemia L1210 cells (ATCC CCL 219), human promyelocytic leukemia HL60 cells (ATCC CCL 240), human cervix carcinoma HeLa S3 cells (ATCC CCL 2.2) and the human T lymphoblastoid CCRF-CEM cell line (ATCC CCL 119) [20].
The methodology of the antiviral activity assays followed previously described procedures [21], [22].
Analyses indicated by the symbols of the elements or functions were within ± 0.4% of the theoretical values.
Supplementary Material
Acknowledgments
As a part of research project of the Institute of Organic Chemistry and Biochemistry, Czech Academy of Science, v.v.i. OZ40550506. This study was supported by Centre of new antivirals and antineoplastics 1M0508 by the Ministry of Education, Youth and Sports of the Czech Republic, by Gilead Sciences and IOCB Research Centre and by the NIH grant 1UC1AI062540-01. The autors’ thanks are due to Prof. E. De Clercq and his group in the Rega Institute, Katholic University Leuven (Belgium) for the evaluation of antiviral activity; and to Dr I. Votruba of the Institute of Organic Chemistry and Biochemitry for the evaluation of cytostatic activity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.a) De Clercq E, Holý A. Nature Rev Drug Discov. 2005;4:928–940. doi: 10.1038/nrd1877. [DOI] [PubMed] [Google Scholar]; b) Holý A. Curr Pharm Des. 2003;9:2567–2592. doi: 10.2174/1381612033453668. [DOI] [PubMed] [Google Scholar]; c) Khandazhinskaya A, Yasko M, Shirokova E. Curr Med Chem. 2006;13:2953–2980. doi: 10.2174/092986706778521896. [DOI] [PubMed] [Google Scholar]; d) De Clerq E. Biochem Pharmacol. 2007;73:911–922. doi: 10.1016/j.bcp.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 2.a) Naesens L, Snoeck R, Andrei G, Balzarini J, Neyts J, De Clercq E. Antivir Chem Chemother. 1997;8:1–23. [Google Scholar]; b) Ying C, De Clercq E, Neyts J. J Viral Hepat. 2000;7:79–83. doi: 10.1046/j.1365-2893.2000.00192.x. [DOI] [PubMed] [Google Scholar]
- 3.Starrett JE, Jr, Tortolani DR, Hitchcock MJ, Martin JC, Mansuri MM. Antiviral Res. 1992;19:267–273. doi: 10.1016/0166-3542(92)90084-i. [DOI] [PubMed] [Google Scholar]
- 4.a) Perrillo R, Schiff E, Yoshida E, Statler A, Hirsch K, Wright T, Gutfreund K, Lamy P, Murray A. Hepatology. 2000;32:129–134. doi: 10.1053/jhep.2000.8626. [DOI] [PubMed] [Google Scholar]; b) Dando TM, Plosker GL. Drugs. 2003;63:2215–2234. doi: 10.2165/00003495-200363200-00007. [DOI] [PubMed] [Google Scholar]
- 5.a) Tsai CC, Follis KE, Beck TW, Sabo A, Bischofberger N, Dailey PJ. AIDS Res Hum Retroviruses. 1997;13:707–712. doi: 10.1089/aid.1997.13.707. [DOI] [PubMed] [Google Scholar]; b) Srinivas RV, Fridland A. Antimicrob Agents Chemother. 1998;42:1484–1487. doi: 10.1128/aac.42.6.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Suruga Y, Makino M, Okada Y, Tanaka H, De Clercq E, Baba M. J Acquired Immune Defic Syndr Hum Retrovirol. 1998;18:316–322. doi: 10.1097/00042560-199808010-00002. [DOI] [PubMed] [Google Scholar]; d) Van Rompay KK, Miller MD, Marthas ML, Margot NA, Dailey PJ, Canfield DR, Tarara PR, Cherrington JM, Aguirre NL, Bischofberger N, Pedersen NC. J Virol. 2000;74:1767–1774. doi: 10.1128/jvi.74.4.1767-1774.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Grim SA, Romanelli F. Annals of Pharmacother. 2003;37:849–859. doi: 10.1345/aph.1C388. [DOI] [PubMed] [Google Scholar]
- 6.a) De Clercq E. Collect Czech Chem Commun. 1998;63:480–506. [Google Scholar]; b) Safrin SJ, Jaffe HS. Adv Exp Med Biol. 1999;458:111–120. [PubMed] [Google Scholar]
- 7.a) Naesens L, Neyts J, Balzarini J, Holý A, Rosenberg I, De Clercq E. J Med Virol. 1993;39:167–172. doi: 10.1002/jmv.1890390215. [DOI] [PubMed] [Google Scholar]; b) Otová B, Zídek Z, Holý A, Votruba I, Sladká M, Marinov I, Lesková V. In Vivo. 1997;11:163–167. [PubMed] [Google Scholar]; c) Otová B, Francová K, Franěk F, Koutník P, Votruba I, Holý A, Sladká M, Schramlová J. Anticancer Res. 1999;19:3173–3182. [PubMed] [Google Scholar]; d) Rose WC, Crosswell AR, Bronson JJ, Martin JC. J Natl Cancer Inst. 1990;82:510–512. doi: 10.1093/jnci/82.6.510. [DOI] [PubMed] [Google Scholar]; d) Balzarini J, Holý A, Jindřich J, Naesens L, Snoeck R, Schols D, De Clercq E. Antimicrob Agents Chemother. 1993;37:332–338. doi: 10.1128/aac.37.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Balzarini J, Aquaro S, Perno CF, Witvrouw M, Holý A, De Clercq E. Biochem Biophys Res Commun. 1996;219:337–341. doi: 10.1006/bbrc.1996.0234. [DOI] [PubMed] [Google Scholar]
- 8.a) Holý A, Votruba I, Masojídková M, Andrei G, Snoeck R, Naesens L, De Clercq E, Balzarini J. J Med Chem. 2002;45:1918–1929. doi: 10.1021/jm011095y. [DOI] [PubMed] [Google Scholar]; b) Balzarini J, Pannecouque C, De Clercq E, Aquaro S, Perno CF, Egberink H, Holý A. Antimicrob Agents Chemother. 2002;46:2185–2193. doi: 10.1128/AAC.46.7.2185-2193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Ying C, Holý A, Hocková D, Havlas Z, De Clercq E, Neyts J. Antimicrob Agents Chemother. 2005;49:1177–1180. doi: 10.1128/AAC.49.3.1177-1180.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Hocková D, Masojídková M, Holý A. Collect Czech Chem Commun. 2005;70:247–258. [Google Scholar]
- 9.a) Hocková D, Holý A, Masojídková M, Andrei G, Snoeck R, De Clercq E, Balzarini J. J Med Chem. 2003;46:5064–5073. doi: 10.1021/jm030932o. [DOI] [PubMed] [Google Scholar]; b) Hocková D, Holý A, Masojídková M, Andrei G, Snoeck R, De Clercq E, Balzarini J. Bioorg Med Chem. 2004;12:3197–3202. doi: 10.1016/j.bmc.2004.04.002. [DOI] [PubMed] [Google Scholar]; c) Balzarini J, Schols D, Van Laethem K, De Clercq E, Hocková D, Masojídková M, Holý A. J Antimicrob Chemother. 2007;59:80–86. doi: 10.1093/jac/dkl454. [DOI] [PubMed] [Google Scholar]
- 10.a) Vrbovská S, Holý A, Pohl R, Masojídková M. Collect Czech Chem Commun. 2006;71:543–566. [Google Scholar]; b) Vrbková S, Dračínský M, Holý A. Collect Czech Chem Commun. 2007;72:965–983. [Google Scholar]; c) Vrbková S, Dračínský M, Holý A. Tetrahedron. 2007;63:11391–11398. [Google Scholar]; d) Vrbková S, Dračínský M, Holý A. Tetrahedron Asym. 2007;18:2233–2247. [Google Scholar]
- 11.a) Holý A, Günter J, Dvořáková H, Masojídková M, Andrei G, Snoeck R, Balzarini J, De Clercq E. J Med Chem. 1999;42:2064–2086. doi: 10.1021/jm9811256. [DOI] [PubMed] [Google Scholar]; b) Holý A, Dvořáková H, Masojídková M. Collect Czech Chem Commun. 1995;60:1390–1409. [Google Scholar]; c) Holý A, Masojídková M. Collect Czech Chem Commun. 1995;60:1196–1212. [Google Scholar]; d) Hocek M, Masojídková M, Holý A, Andrei G, Snoeck R, Balzarini J, De Clercq E. Collect Czech Chem Commun. 1996;61:1525–1537. [Google Scholar]
- 12.a) Adlington RM, Baldwin JE, Catterick D, Pritchard GJ. J Chem Soc Perkin Trans. 1999;1:855–866. [Google Scholar]; b) Liu Ch, Wrobleski ST, Lin J, Ahmed G, Metzger A, Wityak J, Gillooly KM, Schuster DJ, McIntyre KW, Pitt S, Shen DR, Zhang RF, Zhang H, Doweyko AM, Diller D, Henderson I, Barrish JC, Dodd JH, Schieven GL, Leftheris K. J Med Chem. 2005;48:6261–6270. doi: 10.1021/jm0503594. [DOI] [PubMed] [Google Scholar]; c) Klutchko SR, Hamby JM, Boschelli DH, Wu Z, Kraker AJ, Amar AM, Hartl BG, Shen C, Klohs WD, Steinkampf RW, Driscoll DL, Nelson JM, Elliott WL, Roberts BJ, Stoner ChL, Vincent PW, Dykes DJ, Panek RL, Lu GH, Major TC, Dahring TK, Hallak H, Bradford LA, Hollis Showalter HD, Doherty AM. J Med Chem. 1998;41:3276–3292. doi: 10.1021/jm9802259. [DOI] [PubMed] [Google Scholar]
- 13.Palanki MSS, Erdman PE, Goldman ME, Suto C, Suto MJ. Med Chem Res. 2000;10:19–29. [Google Scholar]
- 14.Herrera A, Martinez-Alvarez R, Ramiro P, Almy J, Molero D, Sanchez A. Eur J Org Chem. 2006:3332–3337. doi: 10.1021/jo052619v. [DOI] [PubMed] [Google Scholar]
- 15.Koppel HC, Springer RH, Robins RK, Cheng CC. J Org Chem. 1961;23:792–803. [Google Scholar]
- 16.Biagi G, Giorgi I, Livi O, Pacchini F, Scartoni V. J Het Chem. 2002;39:885–888. [Google Scholar]
- 17.a) Beadle JR, Wan WB, Ciesla SL, Keith KA, Hartline C, Kern ER, Hostetler KY. J Med Chem. 2006;49:2010–2015. doi: 10.1021/jm050473m. [DOI] [PubMed] [Google Scholar]; b) Kini GD, Beadle JR, Xie H, Aldern KA, Richman DD, Hostetler KY. Antiviral Res. 1997;36:43–53. doi: 10.1016/s0166-3542(97)00039-9. [DOI] [PubMed] [Google Scholar]
- 18.Meier Ch, Görbig U, Müller Ch, Balzarini J. J Med Chem. 2005;48:8079–8086. doi: 10.1021/jm050641a. [DOI] [PubMed] [Google Scholar]
- 19.Starrett JE, Tortolani DR, Hitchcock MJM, Martin JC, Mansuri MM. Antiviral Res. 1992;19:267–273. doi: 10.1016/0166-3542(92)90084-i. [DOI] [PubMed] [Google Scholar]
- 20.Kuchař M, Hocek M, Pohl R, Votruba I, Shih I, Mabery E, Mackman R. Bioorg Med Chem. 2008;16:1400–1424. doi: 10.1016/j.bmc.2007.10.063. [DOI] [PubMed] [Google Scholar]
- 21.a) De Clerq E, Descamps J, Verhelst G, Walker RT, Jones AS, Torrence PF, Shugar D. J Infect Dis. 1980;141:563–574. doi: 10.1093/infdis/141.5.563. [DOI] [PubMed] [Google Scholar]; b) De Clercq E, Sakuma T, Baba M, Pauwels R, Balzarini J, Rosenberg I, Holý A. Antiviral Res. 1987;8:261–272. doi: 10.1016/s0166-3542(87)80004-9. [DOI] [PubMed] [Google Scholar]
- 22.Balzarini J, Naesens L, Slachmuylders J, Niphuis H, Rosenberg I, Holý A, Schellekens H, De Clercq E. AIDS. 1991;5:21–28. doi: 10.1097/00002030-199101000-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.