Abstract
We designed a new pedicled fasciocutaneous flap for large sacral defects that combined a classic superior gluteal artery perforator flap and an acentric axis perforator pedicled propeller flap. We asked whether this technique would be simple and result in few complications. Six patients with large sacral defects had reconstruction using this technique in one stage. The size of the defect and postoperative complications in each patient were assessed. The minimum followup was 6 months (mean, 20.1 months; range, 6–38 months). All wounds healed with no recurrence during followup. Five patients achieved healing primarily, and another with minimal drainage achieved healing by secondary intention after a dressing change. No patients had deep infection, wound dehiscence, necrosis, or partial loss or shrinkage of the flap at final followup. The buttocks were symmetric. We consider this a good alternative for reconstructing large sacral defects because it is a relatively simple procedure and results in few complications.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Débridement of pressure sores and excision of aggressive tumors in the sacral region often result in extensive soft tissue defects that cannot be closed primarily and are further associated with increased risk of flap ischemia, wound dehiscence, and deep infection. Numerous surgical methods have been used to correct these defects, including skin grafting [14], local flaps [15], muscle flaps [5], and free flaps [11]. Local flaps in the gluteal region are the first choice for reconstruction of sacral defects [1].
The flap we have used for large sacral defects combines a classic superior gluteal artery perforator (SGAP) flap [12] and an acentric axis perforator pedicled propeller (PPP) flap [7]. The SGAP flap evolved from a gluteus maximus myocutaneous flap. The PPP flap is a propeller flap elevated as an island flap and rotated as needed. We termed the combined flap the pedicled multi-island fasciocutaneous flap. Hyakusoku et al. [7] reported PPP flaps rotated 180° to cover the recipient sites, but they sometimes required skin grafts to close the donor sites. Rajacic et al. [16] used an inferior gluteus maximus musculocutaneous island flap to repair pressure sores. However, wound dehiscence at the donor sites occurred in eight of 27 patients. Stamate and Budurca [18] reconstructed sacral pressure sores with gluteal flaps, including rotation, transposition, or V-Y advancement flaps. However, two transposition flaps developed marginal necrosis and 10 patients required additional surgery.
The SGAP and PPP flaps often need the donor sites closed with skin grafts. However, the multi-island pedicled fasciocutaneous flap we propose easily closes the recipient and donor sites in one stage without skin grafts. The risk of wound dehiscence is less than with other fasciocutaneous flaps. We described this technique in a previous publication [20].
In the current study, we describe additional uses of the procedure and provide illustrations.
Materials and Methods
We retrospectively reviewed six patients with large sacral defects who underwent the pedicled multi-island fasciocutaneous flap between 2003 and 2006. There were four men and two women ranging in age from 28 to 67 years (mean, 51.3 years). Diagnoses consisted of cervical fracture accompanied by quadriplegia (n = 2), thoracic vertebrae or lumbar vertebrae fracture accompanied by paraplegia (n = 2), giant cell tumor (n = 1), and cerebrovascular accident (n = 1). Five patients were treated with a unilateral multi-island flap and one with bilateral multi-island flaps.
The sacral bed sores in most patients were caused by long-term bed rest and formed large sacral defects and/or were accompanied by bone exposure. The scar contracture and bone exposure in one patient were caused by repeated drainage and infection of the sacral wound for 20 years after excision of a sacroiliac giant cell tumor. Three patients had bed sores with full-thickness skin loss with damage to subcutaneous tissue, not involving underlying fascia (Grade III using the criteria of Keong et al. [9]) and three patients had sores with extensive destruction to muscle, bone, or supporting structure (Grade IV). The sizes of the sacral defects ranged from 10 cm × 12 cm to 20 cm × 25 cm (mean, 16 cm × 20 cm). Most of the patients had severe soft tissue and bone infections with a large area and deep wound, and one patient had accompanying extensive scar contractures around the wound, which was difficult to reconstruct. Minimum followup was 6 months (mean, 20.1 months; range, 6–38 months). In the followup, four patients underwent physical examination, and the other two patients underwent only indirect subjective evaluation (eg, by mail or telephone).
The patients were mostly cachectic, anemic, and had poor nutrition and a water-electrolyte imbalance. Based on their preoperative conditions, we used whole blood, plasma, and human serum albumin to ensure levels of hemoglobin greater than 90 g/L and plasma protein greater than 50 g/L preoperatively. Any fluid-electrolyte imbalance was corrected. We performed débridements and dressing changes preoperatively and cultured the wounds to ensure sensitive antibiotics. We obtained electrocardiograms, liver and renal functions, and blood coagulation profiles preoperatively to ensure proper assessment.
The patients were placed prone on the operating table under general anesthesia. The wound was washed intraoperatively with hydrogen peroxide and bromogeramine and any necrotic tissue was débrided as completely as possible. Any bone tissue in the wound (such as apophysis of sacrococcyx) was removed in small segments if judged infected or if it would disturb coverage by the flap.
According to the size of the sacral defects, the plus-shaped multi-island flap and the pedicle were drawn on the body surface with methylene blue (see description below). The skin incision was the same as that for a classic gluteal rotation flap. The flap elevation was begun with an incision along the boundaries of the flap. We elevated the flap subfascially from around the borders until obtaining a pedicle approximately 3 cm × 3 cm while preserving a small amount of gluteus maximus muscle about the pedicle; we ensured the blood vessels in the pedicle would not be constrained. We widely undermined the tissue around the defect to facilitate direct closure. The color of the flap was observed during the operation to ensure the blood supply was not injured or constricted. The wound was sutured and a negative pressure drain placed under the flap. The volume of blood loss estimated by the suction container during surgery ranged from 200 to 600 mL (mean, 380 mL). The multi-island flaps ranged in area from 12 cm × 16 cm to 25 cm × 30 cm (mean, 19 cm × 24 cm) (see Case Reports).
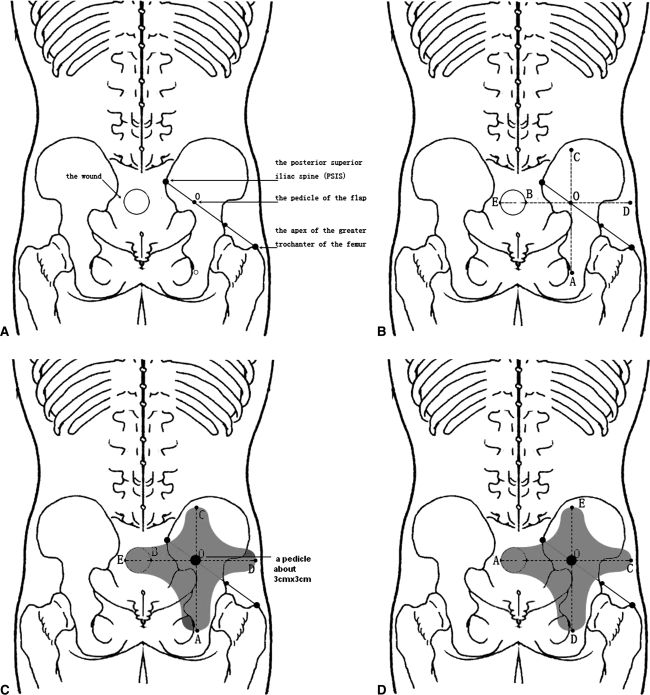
To create the flap, point O was set at the border of the sacrum at the junction of the medial one-third and lateral two-thirds of a line drawn between the posterior-superior iliac spine (PSIS) and the apex of the greater trochanter of the femur preoperatively (Fig. 1A). If necessary, the perforator of the flap was identified by Doppler [16]. Point E was the farthest point from point O to the distal edge of the wounds and O-E was set as the horizontal axis to draw the horizontal line of the flap. Point B was the crossed point of axis O-E and the proximal edge of the wound and axis O-A, which was perpendicular to axis O-B, and was drawn through point O. The distance measured downward from axis O-A was the same as that of O-E and point A could be marked. In the same way, O-C = O-B + 1/3E-B and point C was marked and O-D = O-B + 2/3E-B and point D was marked (Fig. 1B). According to the longitudinal diameter of the wound, the flap width on both sides of axis O-E and axis O-A then were marked (Fig. 1C). After a 90° clockwise or counterclockwise turn of the flap, the recipient and donor sites were closed in a one-stage procedure (Fig. 1D).
Fig. 1A–D.
(A) Point O was set at the junction of the medial one-third and lateral two-thirds of a line drawn between the PSIS and the apex of the greater trochanter of the femur preoperatively. (B) The distance measured downward, axis O-A, was the same as that of O-E, and point A could be marked. In the same way, O-C = O-B + 1/3E-B and point C was marked and O-D = O-B + 2/3E-B and point D was marked. (C) According to the longitudinal diameter of the wound, the flap width on both sides of axis O-E and axis O-A could be marked, respectively. (D) After a 90° clockwise or counterclockwise turn of the flap, the recipient and donor sites were closed in a one-stage procedure.
Postoperatively, we used antibiotics, anticoagulation, and fluid replacement after the operation. Postoperatively, all patients received an intravenous injection of cefuroxime sodium (0.75 g) in 10 mL saline solution three times a day for 5 days. All patients received an intravenous drip of 10% dextran-40 in saline solution at a dose of 500 mL per day for the first 7 days postoperatively. We monitored the blood supply of the flap by observing the skin color. The negative pressure drainage was removed 24 to 48 hours after the operation and the sutures were removed 14 days postoperatively. The patient was kept prone or on his side until the flap healed.
After discharge, we followed patients from 6 to 38 months. We made followup visits to the patients every 2 weeks. We recorded the size of the flap and any postoperative complications.
Results
Primary closure of the recipient and donor sites was performed in all patients without skin grafting. The largest defects that were closed with a unilateral multi-island flap and bilateral multi-island flaps were 18 cm and 25 cm in diameter, respectively. All the multi-island flaps survived and five patients achieved healing primarily. One patient with a sacroiliac tumor had minimal drainage and healing by the second intention 32 days after daily dressing change. No patients had deep infection, wound dehiscence, necrosis, recurrence, or partial loss or shrinkage of the flap during the followup period.
The patient with a giant cell tumor retained weightbearing function of both lower extremities. Two patients were able to walk with the assistance of one crutch because of cervical cord fracture accompanied by partial quadriplegia or cerebrovascular accident. The other three patients were unable to walk because of original diseases such as cervical cord fracture accompanied by quadriplegia or thoracic vertebrae or lumbar vertebrae fracture accompanied by paraplegia.
All patients had symmetric buttocks (see Case Reports). However, all six patients reported slight numbness in the donor sites. Various degrees of numbness and discomfort of the flap area remained in all patients at final followup, although it did not interfere with the patients’ daily lives.
Case Reports
Case 1
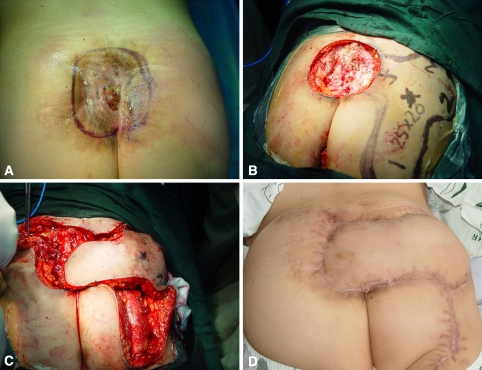
A 28-year-old woman had a sacroiliac giant cell tumor 20 years previously. The chronic sacroiliac osteomyelitis and scar contracture were caused by repeated deep infection after resection of the tumor (Fig. 2A). A wide excision created a 15-cm × 18-cm defect. A 20-cm × 25-cm pedicled multi-island flap was designed, which included the location of the perforator of the superior gluteal artery (Fig. 2B). After a 90° clockwise turn of the flap, the recipient sites and donor sites were closed in a one-stage procedure (Fig. 2C). No skin grafting was used. The flap survived but had minimal drainage after the operation. The patient’s wound healed 32 days after modifying antibiotics based on the sensitivities based on culture and daily dressing changes. During the 7-month followup, the patient retained the weightbearing walking function of both lower extremities, function for stair climbing, and single-limb support, and the appearance of the buttocks was symmetric (Fig. 2D). The patient had persistent numbness of the flap at 7 months. This slight numbness did not impact on the patient’s health-related quality of life in terms of alterations in sleep patterns, concentration, or mood.
Fig. 2A–D.
(A) Preoperatively, the chronic osteomyelitis and scar contracture were caused by repeated deep infections after resection of a sacroiliac tumor. (B) A pedicled fasciocutaneous flap of multi-island design was designed for the large (15-cm × 18-cm) sacral defect. The area of the flap was 20 cm × 25 cm. (C) After a 90° clockwise turn of the flap, the recipient sites and donor sites were closed in a one-stage procedure. (D) The flap survived completely 7 months later, and the appearance of the buttocks was symmetric. (Reprinted with permission from Xu Y, Liang Z, Feng S. [An effect of multi-island flap with shallow branch of gluteus upper artery on repair of sacrum soft tissue defect] [in Chinese]. Zhongguo Xiu FuChong Jian Wai Ke Za Zhi. 2007;21:850–853.)
Case 2
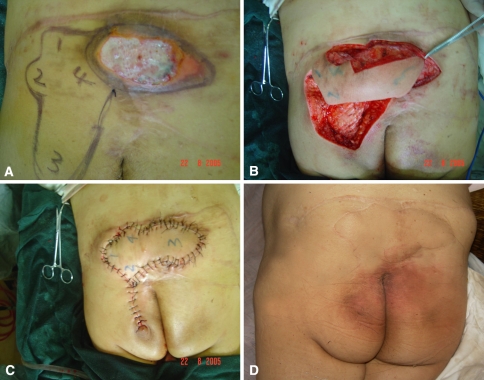
A sacral pressure sore developed in a 40-year-old woman who had a L1–L2 spinal cord injury accompanied by paraplegia after being crushed by a crane 20 years previously Two years after the injury, the sacral pressure sore recurred after reconstruction with bilateral SGAP flaps (Fig. 3A). After débridement of necrotic tissue in the sacral region, the skin and soft tissue defects (10 cm × 12 cm) were reconstructed with the pedicled fasciocutaneous flap of multi-island design (Fig. 3B). The fasciocutaneous pedicle was located on an acentric portion of the flap. The longitude of the multi-island flap was adjusted according to the sacral scar. The area of the flap was 12 cm × 16 cm. The recipient and donor sites of the wound were closed by the pedicled fasciocutaneous flap of multi-island design in a one-stage procedure (Fig. 3C). At followup of 32 months, the flap survived, the pressure sores did not recur, and the appearance of the buttocks was symmetric (Fig. 3D).
Fig. 3A–D.
(A) Preoperative appearance of a sacral pressure sore recurred after reconstruction of a bilateral SGAP flap. The area of the defect was 10 cm × 12 cm. (B) A photograph shows an intraoperative view of the pedicled fasciocutaneous flap of multi-island design. The area of the flap was 12 cm × 16 cm. (C) The recipient and donor sites of the wound were closed by the pedicled fasciocutaneous flap of multi-island design in a one-stage procedure. (D) The flap survived completely 32 months later without recurrence, and the appearance of the buttocks was symmetric.
Discussion
We designed a new pedicled fasciocutaneous flap for large sacral defects, which combined the SGAP and PPP flaps. We asked whether this technique would be relatively simple and result in few complications.
Our preliminary data for six patients suggest the procedure could be performed easily with few complications. However, the case series was limited and for some patients the procedure might not be so straightforward. In addition, the outcomes might not be generalizable to larger groups of patients with more diverse defects, and complication rates cannot be ascertained from a series of so few patients.
Although various degrees of numbness at the donor sites were encountered, all the defects were reconstructed successfully using this technique. The numbness of the donor sites resulted from the superior clunial nerves or the inferior clunial nerves being partly damaged when elevating the flap. However, the numbness did not impact on the patient’s health-related quality of life in terms of alterations in sleep patterns, concentration, or mood.
Perforator flaps usually are used as free flaps but also may be used as pedicled flaps for reconstructing contiguous recipient sites [4, 22]. The pedicled fasciocutaneous flap of multi-island design in the gluteal region is based on the perforators of the superior gluteal artery. The anatomic landmark of the superior gluteal artery is fixed with little variation. It emerges at the border of the sacrum at the junction of the medial one-third and lateral two-thirds of a line drawn between the PSIS and the apex of the greater trochanter of the femur [19]. Doppler flowmetry is useful for identifying perforators preoperatively [3, 8]. Koshima et al. [10] reported the reliability of the blood supply through perforator vessels of flaps that could reach the sacral area without problems.
The multi-island flap rotates 90° and covers the recipient and donor sites simultaneously without skin grafting. However, Hyakusoku et al. [7] suggested the PPP flaps should rotate 180° to cover the recipient site and sometimes required skin grafts to close the donor sites. Traditionally, the V-Y advancement flap has been widely used for reconstruction of cutaneous defects for decades; however, the movement of a V-Y flap sometimes is limited [13]. The multi-island flap not only makes use of the advantage of a rich blood supply but also reduces damage of the gluteus maximus and reserves gluteus function to a large degree.
Pressure sores have high recurrence rates [21]. Rajacic et al. [16] reported the recurrence rate was 11% (three of 27) using the inferior gluteus maximus flap. Chen [2] reported the recurrence rate of pressure sores reconstructed by a V-Y advancement musculocutaneous flap reached 6% (four of 63) postoperatively. Relander and Palmer [17] reported 48% of surgically treated sores recurred, and 56% occurred in patients with spinal cord lesions. This is a major problem after surgery. Recurrence relates to the characteristic of the flap, surgical technique, preoperative and postoperative care, or rehabilitation. We observed no recurrences, contrasting with observations of others [16, 20]. Although the inferior gluteus maximus musculocutaneous island flap closed the recipient and donor sites primarily, a high rate (30%) of wound dehiscence was reported in one series [16].
The pedicled fasciocutaneous flap of multi-island design was used in patients with large sacral defects caused by pressure sores or tumors, especially nonparaplegic patients who needed the weightbearing walking function of both lower extremities. If a unilateral multi-island flap cannot fill the large defect, bilateral multi-island flap repair can be considered [20].
The flap should be dissected gradually from the circumference to the pedicle to avoid interfering with the perforator. An area approximately 3 cm × 3 cm for the nutrition pedicle should be retained and interference of the perforator of the superior gluteal artery minimized. Any necrotic tissue should be cleared thoroughly, especially when infected [6]. At the same time, wound cultures should be done to ensure proper antibiotic selection. Postoperatively, negative pressure drainage should be placed under the flaps for drainage. The patient should be kept prone or on his or her side until the flap is healed to prevent wound dehiscence [12].
We believe the pedicled, multi-island fasciocutaneous flap has advantages, including enhanced blood supply, few complications, and symmetric buttocks. It also is useful in nonparaplegic patients because mobilization can be started early. The new modified flap is easy to handle and eliminates the need for microsurgery.
Acknowledgments
We thank Zhimin Ying (from Taizhou Hospital of Linhai City, Zhejiang province, China) for help in translating the text.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent was obtained.
References
- 1.Borman H, Maral T. The gluteal fasciocutaneous rotation-advancement flap with V-Y closure in the management of sacral pressure sores. Plast Reconstr Surg. 2002;109:2325–2329. [DOI] [PubMed]
- 2.Chen TH. Bilateral gluteus maximus V-Y advancement musculocutaneous flaps for the coverage of large sacral pressure sores: revisit and refinement. Ann Plast Surg. 1995;35:492–497. [DOI] [PubMed]
- 3.Colwell AS, Borud LJ. Autologous gluteal augmentation after massive weight loss: aesthetic analysis and role of the superior gluteal artery perforator flap. Plast Reconstr Surg. 2007;119:345–356. [DOI] [PubMed]
- 4.Eo S, Kim D, Jones NF. Microdissection thinning of a pedicled deep inferior epigastric perforator flap for burn scar contracture of the groin: case report. J Reconstr Microsurg. 2005;21:477–450. [DOI] [PubMed]
- 5.Grolleau JL, Collin JF, Chavoin JP, Costagliola M. [Iliac transosseous transposition of rectus abdominis muscle flap to cover a sacral pressure sore] [in French]. Ann Chir Plast Esthet. 1994;39:128–131. [PubMed]
- 6.Hai H, Dai H, Xu Y. [Combined treatment of refractory decubitus ulcers] [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20:909–911. [PubMed]
- 7.Hyakusoku H, Ogawa R, Oki K, Ishii N. The perforator pedicled propeller (PPP) flap method: report of two cases. J Nippon Med Sch. 2007;74:367–371. [DOI] [PubMed]
- 8.Kankaya Y, Ulusoy MG, Oruç M, Yildiz K, Koçer U, Tüccar E. Perforating arteries of the gluteal region: anatomic study. Ann Plast Surg. 2006;56:409–412. [DOI] [PubMed]
- 9.Keong N, Ricketts D, Alakeson N, Rust P. Pressure sore following elective total hip arthroplasty: pitfalls of misinterpretation. Ann R Coll Surg Engl. 2004;86:174–176. [DOI] [PMC free article] [PubMed]
- 10.Koshima I, Moriguchi T, Soeda S, Kawata S, Ohta S, Ikeda A. The gluteal perforator-based flap for repair of sacral pressure sores. Plast Reconstr Surg. 1993;91:678–683. [DOI] [PubMed]
- 11.Lemaire V, Boulanger K, Heymans O. Free flaps for pressure sore coverage. Ann Plast Surg. 2008;60:631–634. [DOI] [PubMed]
- 12.Leow M, Lim J, Lim TC. The superior gluteal artery perforator flap for the closure of sacral sores. Singapore Med J. 2004;45:37–39. [PubMed]
- 13.Li JH, Xing X, Li P, Xu J. Transposition movement of V-Y flaps for facial reconstruction. J Plast Reconstr Aesthet Surg. 2007;60:1244–1247. [DOI] [PubMed]
- 14.Liu Y, Zhang X, Zhang C. [Clinical typing and surgical principle of pressure sore] [in Chinese]. Zhongguo Xiu FuChong Jian Wai Ke Za Zhi. 2007;21:932–936. [PubMed]
- 15.Menke H. [Infected decubitus ulcer] [in German]. Langenbecks Arch Chir Suppl Kongressbd. 1997;114:517–520. [PubMed]
- 16.Rajacic N, Gang RK, Dashti H, Behbehani A. Treatment of ischial pressure sores with an inferior gluteus maximus musculocutaneous island flap: an analysis of 31 flaps. Br J Plast Surg. 1994;47:431–434. [DOI] [PubMed]
- 17.Relander M, Palmer B. Recurrence of surgically treated pressure sores. Scand J Plast Reconstr Surg Hand Surg. 1988;22:89–92. [DOI] [PubMed]
- 18.Stamate T, Budurca AR. The treatment of the sacral pressure sores in patients with spinal lesions. Acta Neurochir Suppl. 2005;93:183–187. [DOI] [PubMed]
- 19.Song WC, Bae SM, Han SH, Koh KS. Anatomical and radiological study of the superior and inferior gluteal arteries in the gluteus maximus muscle for musculocutaneous flap in Koreans. J Plast Reconstr Aesthet Surg. 2006;59:935–941. [DOI] [PubMed]
- 20.Xu Y, Liang Z, Feng S. [An effect of multi-island flap with shallow branch of gluteus upper artery on repair of sacrum soft tissue defect] [in Chinese]. Zhongguo Xiu FuChong Jian Wai Ke Za Zhi. 2007;21:850–853. [PubMed]
- 21.Yamamoto Y, Tsutsumida A, Murazumi M, Sugihara T. Long-term outcome of pressure sores treated with flap coverage. Plast Reconstr Surg. 1997;100:1212–1217. [DOI] [PubMed]
- 22.Zeng A, Xu J, Yan X, You L, Yang H. Pedicled deep inferior epigastric perforator flap: an alternative method to repair groin and scrotal defects. Ann Plast Surg. 2006;57:285–288. [DOI] [PubMed]