Abstract
Highly cross-linked polyethylene has been associated with low in vitro wear, but also has decreased in vitro ultimate yield strength. We therefore asked whether highly cross-linked polyethylene would result in lower outcome scores, wear, or early failure in a young patient population. Seventy THAs in 64 patients were performed using a highly cross-linked (electron beam-irradiated to 9 Mrads) acetabular liner and a cobalt-chrome femoral head. The average age of the patients at surgery was 41 years (range, 19–50 years). The minimum followup was 2.4 years (average, 4 years; range, 2.4–6.5 years). We recorded demographic and clinical data, including Harris hip score. Polyethylene wear measurements were analyzed with a validated, computer-assisted, edge detection method. The average Harris hip score improved from 53 to 92 at last followup. There was no evidence of acetabular or femoral loss of fixation, subsidence, or loosening. Linear wear was undetectable at this followup interval. No patient experienced catastrophic failure or underwent revision surgery. These data show low polyethylene wear rates and no catastrophic failures at early followup in a young patient cohort.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Implant wear and secondary osteolysis are major causes of THA failure and are of particular concern in young patients undergoing THA because of long lifespans [7, 20, 26, 33, 38]. Therefore, alternative bearing surfaces have been introduced to minimize wear particle-induced implant failure. The goal of using highly cross-linked polyethylene (HCLPE) in THA is to reduce wear and thereby minimize secondary osteolysis and enhance implant survivorship.
Previous studies have shown a direct correlation between the amount of polyethylene wear occurring in THAs and the rate of osteolysis [14, 43]. According to one study, wear rates less than 0.1 mm per year were associated with a zero incidence of osteolysis, but rates exceeding 0.3 mm per year were associated with a 100% incidence of osteolysis [14]. Alternative bearing surfaces such as ceramic-on-ceramic, metal-on-metal, and metal-on-HCLPE endeavor to decrease the production of biologically active particulate matter, thereby theoretically decreasing the incidence of osteolysis. In vivo and in vitro hip simulator studies have shown considerably [34] less linear and volumetric wear of HCLPE compared with conventional polyethylene [19, 30]. It is presumed that a decrease in the wear of the implant and reduction in the production of particulate matter will increase the longevity of the implant.
In general, younger patients have been associated with higher activity levels and increased polyethylene wear [20, 33, 38]. In addition, the demands that are placed on the implant in younger patients are produced for longer times compared with those for older patients. Younger patients have consistently had higher polyethylene wear rates than older patients [3, 8]. Potential concerns regarding the use of HCLPE include a reduction in the ultimate yield strength and ultimate tensile strength and a possibility of catastrophic failure owing to the cross-linking process [29]. There have been case reports of early failure [3] in which impingement has been implicated and retrieval studies citing polyethylene cracking and microfracture [2, 41]. The reduction in material properties observed in HCLPE in lower-demand patients implies a theoretical risk of failure in a young high-demand patient population. HCLPE is associated with considerably less wear than conventional polyethylene for short- and intermediate-term results in older (mean ages ranging from 55–60 years) patient populations [12, 22, 28]. It is unclear, however, whether these wear rates apply to a younger population in whom one might anticipate relatively higher wear rates. Conventional polyethylene has had a linear wear rate with time that allows the prediction of long-term polyethylene wear [14]. HCLPE also may have a linear wear rate with time such that short-term wear characteristics may predict long-term performance.
Because long-term implant performance may be predicted from shorter-term results, we determined the clinical outcome scores, polyethylene wear, and any occurrences of catastrophic polyethylene failure associated with a HCLPE acetabular liner in a young patient cohort treated with primary THA.
Materials and Methods
We retrospectively evaluated the prospectively collected clinical and radiographic data of 64 patients (70 hips) undergoing primary THA with a HCLPE acetabular liner between September 1999 and May 2002. The cohort initially was assembled as a subset of 90 patients in a prospective, blinded trial comparing conventional polyethylene with HCLPE in patients of all ages. For this study, we included all 33 patients in the randomized trial who were 50 years or younger and randomized to receive HCLPE liners. After completion of enrollment for the randomized study, 31 additional patients 50 years or younger received HCLPE (in a consecutive fashion) and comprised the remainder of the 64 patients. The average age of the patients was 41 years (range, 19–50 years) at the time of surgery. Thirty-two patients were men and 27 were women, representing 54% and 46% of the cohort, respectively. The average patient weight was 88 kg (range, 38–146 kg) and average height was 173 cm (range, 147–196 cm). Their primary diagnoses that resulted in arthroplasty included osteoarthritis (25 patients [36%]), osteonecrosis (20 patients [29%]), hip dysplasia (14 patients [20%]), rheumatoid arthritis (five patients [8%]), posttraumatic arthritis (two patients [3%]), hip fusion takedown (one patient [2%]), and previous resection for infection (one patient [2%]). Six patients underwent contralateral THA during the study period. Thirty-six hips (51%) had THAs performed on the right side, whereas 34 (49%) had THAs on the left side. None of the patients had a prior hip infection. Twenty patients (29%) had at least one prior surgical procedure on the ipsilateral hip, including core decompression, pelvic osteotomy, proximal femoral osteotomy, open reduction and internal fixation, arthroscopic débridement, trochanteric advancement, or hip fusion. One patient had a greater trochanter osteotomy and fixation during the course of the initial hip fusion takedown and conversion to primary THA. The minimum followup was 2.4 years (average, 4 years; range, 2.4–6.5 years). We had prior Institutional Review Board approval. Patients consented and agreed to participate in the study before their enrollment.
All surgeries were performed by one of the two senior authors (JCC, WJM). All patients were implanted with a HCLPE acetabular liner (Longevity®; Zimmer, Inc, Warsaw, IN) in a cementless socket (Trilogy®; Zimmer). A 22-, 26-, or 28-mm cobalt-chrome head was used in all cases. One patient received a 22-mm head, nine patients received a 26-mm head, and 60 patients received a 28-mm head. Various stems were used and included 45 VerSys™ beaded midcoats (Zimmer), nine fiber metal midcoats (Zimmer), two Heritage™ cemented (Zimmer), eight fiber metal tapers (Zimmer), and six Bantam porocoats (DePuy Orthopaedics, Inc, Warsaw, IN). All patients underwent a posterolateral surgical approach. The HCLPE was electron beam-irradiated to 9 Mrad and gas plasma-sterilized. Twenty-eight patients received a 10° lipped acetabular liner and 42 patients received a 20° lipped acetabular liner. Sixty-four patients (70 THAs) had a minimum followup of 24 months (mean, 47 months; range, 24–77 months).
We recorded preoperative demographic data, including age, gender, height, weight, diagnoses, laterality, and ipsilateral prior surgical procedures. Clinical outcomes were measured, including the Harris hip score and a modified WOMAC score (at final followup only) as disease-specific outcome measures and the SF-12 as a general health measure [32, 40]. WOMAC scores were available for 63 of 70 hips (90%) and SF-12 scores were available for 30 of 70 hips (43%). Harris hip scores were available for all patients preoperatively and at final followup. We recorded activity measures using the University of California–Los Angeles (UCLA) activity score and these were available for all patients preoperatively and at final followup. This measure evaluates the amount of activity as self-reported by the patient from a high score of 10 correlating with “regularly participating in impact sports” to a low score of 1 correlating with “wholly inactive.” This evaluation of patient activity has been correlated with pedometer data [45].
The medical records and radiographs were reviewed by two of the authors (DSS, MFS) independent of the treating surgeons. Patients were seen at followup 6 weeks after surgery, at 3 months, and annually thereafter. We (DSS, MFS) assessed three radiographic views of each patient; an anteroposterior (AP) view of the pelvis and AP and cross-table lateral views of the affected hip were obtained. We analyzed all followup radiographs. Nondigital radiographs obtained at our institution before 2001 were converted to a digital format using a commercially available radiographic scanner and imaging software. We assessed the acetabular position by determining the vertical and horizontal positions of the component. The vertical position was determined by measuring the distance between the interteardrop line and a parallel line tangential to the most superior aspect of the acetabular component. The horizontal position was determined by measuring the distance between a vertical line drawn through the medial aspect of the teardrop and a parallel line drawn tangential to the most medial aspect of the acetabular component [8]. Subsidence resulting from loosening was defined as any change in acetabular component position greater than 4 mm in either the vertical or horizontal position in relationship to the teardrop [35]. We examined the acetabular component interface radiographically for lucency according to the methods described by DeLee and Charnley [11]. Lysis was defined as any area of radiolucency greater than 3 mm at the implant-bone interface. If there was greater than 2 mm lucency in all three DeLee and Charnley zones or if the implant had subsided, the acetabular component was considered loose. We determined the acetabular component inclination by measuring the theta angle as defined by the acute angle formed from a line drawn horizontally across the inferior border of the ischial tuberosities and a line drawn diagonally across the acetabular component. Acetabular version was assessed using the cross-table lateral radiograph. The acetabular version was classified as retroverted, neutral, or anteverted.
We evaluated femoral loosening using the AP and frog leg lateral radiographs, observing for lucency at the bone-implant interface in Zones 1 through 14 according to the method described by Gruen et al. [21]. Any subsidence of the femoral stem greater than 2 mm also classified the implant as loose [18]. The presence of a subsided stem was determined by measuring the distance from the tip of the trochanter to the top of the femoral stem. We assessed ingrowth by the presence or absence of reactive lines adjacent to the porous-coated portion of the implant and the presence of spot welds of endosteal bone [18]. Heterotopic ossification was observed radiographically and quantified according to the classification system of Brooker et al. [5].
We used a computerized, semiautomated, edge detection method to determine the two-dimensional vector wear by one orthopaedic surgeon (DSS) trained and validated in the use and function of the program (Martell Hip Analysis Suite™, Version 8.0.1.7; University of Chicago, Chicago, IL). As described by Hui et al. and Martell and Berdia [23, 27], calculation of linear and volumetric wear was performed with this validated computer algorithm for all AP radiographs taken 6 weeks postoperatively and at yearly intervals thereafter.
We determined the true linear wear rate by two methods. In the first method, the linear wear was determined using the 1-year postoperative radiograph as a baseline. All wear measurements were based on the 1-year radiograph and all the patients had their wear measurements plotted as total wear versus time. The slope of a least squares trend line through the data set was taken as the true wear rate for the series. In the second method, the 1-year film was compared with the longest followup film available for each patient. The wear observed between these two films was divided by the radiographic interval to calculate a yearly wear rate for each patient. The mean of these values was taken as the population wear rate. The radiographic software reports the difference in pelvic rotation (flexion/extension) between each film pair analyzed. This value is calculated based on changes in the magnification-corrected perpendicular distance from the center of the acetabular component to the tuberosity line. For this study, radiographic pairs with greater than a 25° rotational difference were excluded. One patient was excluded from radiographic wear analysis based on these criteria.
Two calculations were performed regarding wear rates and the bedding-in process. The bedding-in process included values calculated using the 28-day and the 120-day films as a baseline for subsequent wear measurements. This reference value approximates the nonwear-related head migration (bedding-in) such as creep and the liner settling into the metal acetabular component. The bedding-in excluded calculation uses comparison films that are older than 120 days, thereby reducing the influence bedding-in has on true wear measurements.
Results
The mean Harris hip score improved from 53 points preoperatively to an average of 92 points at final followup (Table 1). The mean UCLA activity score also improved from a preoperative score of 3.5 to a postoperative score of 6.3. The mean postoperative modified WOMAC scores were 90 for pain (range, 45–100), 86.7 for stiffness (range, 37–100), and 90.9 for physical function (range, 53–100). Of the original cohort of 70 THAs, eight hips in seven patients were associated with followup Harris hip scores less than 80 points. Two of these seven patients had prior surgeries, including one patient who had a previous hip arthrodesis and one patient who had a resection arthroplasty for native hip infection.
Table 1.
Summary of clinical outcomes
| Clinical measure | Preoperative | Postoperative |
|---|---|---|
| Harris hip score | 53 (26–86) | 92 (61–100) |
| SF-12 Physical Component | 26 (19–42) | 50 (28–59) |
| SF-12 Mental Component | 54 (43–62) | 54 (39–65) |
| UCLA activity score | 3.5 (1–7) | 6.3 (2–10) |
Values are expressed as means with ranges in parentheses; UCLA = University of California—Los Angeles.
Radiographic examinations showed no implant loosening or periprosthetic osteolysis. Each of the acetabular components was placed in an anteverted position. The average theta angle was 46.4° (range, 25.1°–68.7°). All of the femoral stems were well fixed without signs of subsidence or osteolysis.
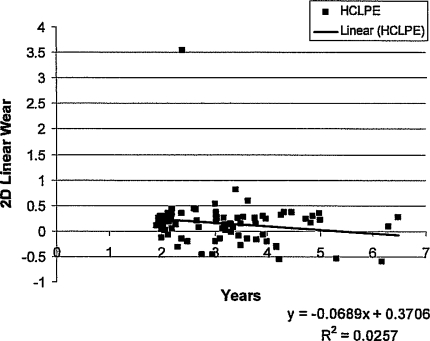
We observed low wear rates with this HCLPE liner. The true linear wear rate, based on the 1-year films and calculated by the slope of the wear versus time regression line, was 0.003 mm per year with bedding-in included in the analysis. With the bedding-in phenomenon excluded, the wear rate was −0.036 mm per year, representing undetectable wear at this followup (Fig. 1).
Fig. 1.
The true linear wear rate for the highly cross-linked polyethylene acetabular liner calculated using the 1-year film compared with the longest followup film available for each patient yielded a mean wear rate of 0.026 mm per year ± 0.135 mm per year with bedding-in included and 0.033 mm per year with bedding-in excluded. 2D = two-dimensional. The one outlier resulted from inadequate radiographic technique. The difference in radiographic projections of the two AP pelvis radiographs was greater than 25° rotation, the maximum rotation allowed by the software. For this reason, the radiographic software inappropriately assigned an abnormally high wear rate. This one outlier was removed from our final analysis of the wear but was included in the graphic analysis for completeness
None of the 70 THAs underwent revision or reoperation during the followup. Our cohort experienced three dislocations after surgery. One patient with rheumatoid arthritis experienced dislocation in the recovery room and returned emergently to the operating room for closed reduction. That patient did not have any additional surgery. One patient with juvenile rheumatoid arthritis experienced dislocation 3 months postoperatively. The patient was treated by closed reduction, wore a brace for 6 weeks, and had no additional episodes of instability. An obese patient with a body mass index greater than 38 kg/m2 experienced dislocation as a result of a fall 4 years after her THA. She underwent closed reduction, wore a brace, and required no additional treatment. Finally, one patient sustained an ipsilateral greater trochanter fracture in a motor vehicle accident 4 years after THA. This was treated nonoperatively and he regained full abductor function. At final followup, 18 hips had Type I heterotopic ossification, two had Type II, six had Type III, and none had Type IV heterotopic ossification. None of these patients underwent surgery for heterotopic ossification.
Discussion
HCLPE has been associated with low in vitro wear properties, but also has shown decreased in vitro material properties that could influence early wear or failure. Early results therefore are important to establish the safety and reliability of this bearing surface in young patients. Accordingly, we asked whether HCLPE would result in lower outcome scores, wear, or early failure in a young patient population.
The lack of a control cohort is an inherent weakness of our study. Nevertheless, we have used common clinical and radiographic outcome measures that can be interpreted relative to reported historical results. The short-term followup of this study could be viewed as a potential limitation to the study. Early followup, however, is important to determine safety of new implants and provide some information regarding possible long-term performance. For example, initial performance of a conventional polyethylene liner has been shown to be predictive of long-term wear [14]. The use of 26- and 28-mm heads in our cohort also may be perceived as a weakness given that larger heads have become more popular to increase hip stability. We believe the performance of this relatively new technology should be defined with traditional head sizes before larger femoral heads are used on a widespread basis.
Various conditions, including osteonecrosis, posttraumatic arthritis, inflammatory arthritis, chondrolysis, and early secondary osteoarthritis, can occur in young patients leading to advanced intraarticular hip deterioration [9]. The surgical options for these patients are limited and THA is commonly used for symptomatic, end-stage disease. Controversy exists regarding the choice of implant design and bearing surfaces. Younger patients tend to be more active, especially patients with osteonecrosis, posttraumatic arthritis, and secondary osteoarthritis, who do not have multiple joint involvement. This increased activity is presumed to result in increased polyethylene wear. Berger et al. [1] reported polyethylene wear rate is related to the patient’s age when using ultrahigh-molecular-weight polyethylene. In the series published by Dunkley et al. [16] for patients 50 years or younger followed for a mean 7 years, six of 55 polyethylene liners were revised for excessive wear and an additional four liners had an eccentric wear pattern that was evident on radiographs. This increased wear rate in young patients also was reported by Dowdy and colleagues who described a 60% (12 of 20) incidence of considerable polyethylene wear and osteolysis in patients younger than 50 years [15].
The material properties of HCLPE, including its reduced ultimate yield and tensile strength compared with conventional polyethylene, could result in early catastrophic failure [29]. This concern is heightened in the young patient population because of higher activity levels [38]. In the short term, there is potential concern for early catastrophic failure when using HCLPE, yet in our young, demanding patient population, we observed no such failures.
Component loosening and osteolysis have been associated with polyethylene particulate disease and high degrees of polyethylene wear [6, 31, 36, 37]. A clinical reduction of 80% in the generation of wear debris can prevent osteolysis in the majority of patients, thereby increasing the survivorship of THAs [28]. Reducing wear in THA is of particular importance in the younger patient population. Conventional polyethylene historically has had varying wear rates from 0.1 mm per year to greater than 0.25 mm per year [24–26, 39]. These wear rates are considerably higher than those in our cohort of patients. Other investigators also have reported improved wear rates with HCLPE.
Martell et al. [28] found a 42% reduction in the linear wear of HCLPE versus conventional polyethylene in older patients. Heisel et al. [22] reported an 81% reduction in clinical wear and a 72% reduction in wear per million cycles in HCLPE compared with conventional polyethylene in early followup. The 5-year wear data for Durasul® (Zimmer, Warsaw, IN) HCLPE in patients with a mean age in their 60s had a linear wear rate of 0.029 mm per year, well below the 0.10 mm per year average threshold for osteolysis [13, 14, 43, 44]. Durasul® is manufactured by electron beam radiation with 9.5 Mrad at 120°C; it then is remelted at 150°C and stored in ethylene oxide. The calculated annual wear for Crossfire™ (Stryker, Kalamazoo, MI) HCLPE in a study with an average followup of 5 years was 0.036 mm per year with a mean patient age of 57 years [10]. In our series, the wear rate was 0.026 mm per year ± 0.135 at a mean of 4.0 years followup. This wear rate is comparable to rates in other series that have reported low wear rates of HCLPE in older patient populations [4, 42]. In hip simulator studies, cross-linked polyethylene has been associated with an 80% to 90% reduction in wear when compared with conventional polyethylene [17, 27]. Differing manufacturing processes lend different material properties to the polyethylene. The dosage of radiation influences the degree of cross-linking and the postradiation processing also affects the material properties of the polyethylene [34]. Longevity® HCLPE was introduced in 1999 and is produced by a process of electron beam irradiation with 9 Mrad and remelt annealing. The differing commercially available highly cross-linked polyethylenes are produced by somewhat differing processes with differing properties. The optimal process for cross-linking continues to be investigated.
We found the early wear rates of this HCLPE in a young patient population are comparable to published data for older populations [10, 28]. In addition, the polyethylene wear rate that we determined was less than that of previously reported conventional polyethylene historic controls [10, 24–26, 28, 39]. Finally, no patient underwent revision, had evidence of accelerated wear, or had evidence of early failure during the followup period. Although these data are encouraging, additional study is essential to assess the long-term durability and clinical performance of HCLPE in primary THA.
Footnotes
One or more of the authors (JCC) have received funding from the Curing Hip Disease Fund.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Berger RA, Jacobs JJ, Quigley LR, Rosenberg AG, Galante JO. Primary cementless acetabular reconstruction in patients younger than 50 years old. 7- to 11-year results. Clin Orthop Relat Res. 1997;344:216–226. [DOI] [PubMed]
- 2.Bradford L, Baker DA, Graham J, Chawan A, Ries MD, Pruitt LA. Wear and surface cracking in early retrieved highly cross-linked polyethylene acetabular liners. J Bone Joint Surg Am. 2004;86:1271–1282. [DOI] [PubMed]
- 3.Bradford L, Kurland R, Sankaran M, Kim H, Pruitt LA, Ries MD. Early failure due to osteolysis associated with contemporary highly cross-linked ultra-high molecular weight polyethylene: a case report. J Bone Joint Surg Am. 2004;86:1051–1056. [DOI] [PubMed]
- 4.Bragdon CR, Kwon YM, Geller JA, Greene ME, Freiberg AA, Harris WH, Malchau H. Minimum 6-year followup of highly cross-linked polyethylene in THA. Clin Orthop Relat Res. 2007;465:122–127. [DOI] [PubMed]
- 5.Brooker AF, Bowerman JW, Robinson RA, Riley LH Jr. Ectopic ossification following total hip replacement: incidence and a method of classification. J Bone Joint Surg Am. 1973;55:1629–1632. [PubMed]
- 6.Cates HE, Faris PM, Keating EM, Ritter MA. Polyethylene wear in cemented metal-backed acetabular cups. J Bone Joint Surg Br. 1993;75:249–253. [DOI] [PubMed]
- 7.Clohisy JC, Calvert G, Tull F, McDonald D, Maloney WJ. Reasons for revision hip surgery: a retrospective review. Clin Orthop Relat Res. 2004;429:188–192. [DOI] [PubMed]
- 8.Clohisy JC, Harris WH. The Harris-Galante porous-coated acetabular component with screw fixation: an average ten-year follow-up study. J Bone Joint Surg Am. 1999;81:66–73. [DOI] [PubMed]
- 9.Clohisy JC, Keeney JA, Schoenecker PL. Preliminary assessment and treatment guidelines for hip disorders in young adults. Clin Orthop Relat Res. 2005;441:168–179. [DOI] [PubMed]
- 10.D′Antonio JA, Manley MT, Capello WN, Bierbaum BE, Ramakrishnan R, Naughton M, Sutton K. Five-year experience with Crossfire highly cross-linked polyethylene. Clin Orthop Relat Res. 2005;441:143–150. [DOI] [PubMed]
- 11.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed]
- 12.Digas G, Karrholm J, Thanner J, Malchau H, Herberts P. Highly cross-linked polyethylene in cemented THA: randomized study of 61 hips. Clin Orthop Relat Res. 2003;417:126–138. [DOI] [PubMed]
- 13.Dorr LD, Wan Z, Shahrdar C, Sirianni L, Boutary M, Yun A. Clinical performance of a Durasul highly cross-linked polyethylene acetabular liner for total hip arthroplasty at five years. J Bone Joint Surg Am. 2005;87:1816–1821. [DOI] [PubMed]
- 14.Dowd JE, Sychterz CJ, Young AM, Engh CA. Characterization of long-term femoral-head-penetration rates: association with and prediction of osteolysis. J Bone Joint Surg Am. 2000;82:1102–1107. [DOI] [PubMed]
- 15.Dowdy PA, Rorabeck CH, Bourne RB. Uncemented total hip arthroplasty in patients 50 years of age or younger. J Arthroplasty. 1997;12:853–862. [DOI] [PubMed]
- 16.Dunkley AB, Eldridge JD, Lee MB, Smith EJ, Learmonth ID. Cementless acetabular replacement in the young: a 5- to 10-year prospective study. Clin Orthop Relat Res. 2000;376:149–155. [DOI] [PubMed]
- 17.Endo M, Tipper JL, Barton DC, Stone MH, Ingham E, Fisher J. Comparison of wear, wear debris and functional biological activity of moderately crosslinked and non-crosslinked polyethylenes in hip prostheses. Proc Inst Mech Eng [H]. 2002;216:111–122. [DOI] [PubMed]
- 18.Engh CA Jr, Culpepper WJ 2nd, Engh CA. Long-term results of use of the anatomic medullary locking prosthesis in total hip arthroplasty. J Bone Joint Surg Am. 1997;79:177–184. [DOI] [PubMed]
- 19.Engh CA Jr, Stepniewski AS, Ginn SD, Beykirch SE, Sychterz-Terefenko CJ, Hopper RH Jr, Engh CA. A randomized prospective evaluation of outcomes after total hip arthroplasty using cross-linked marathon and non-cross-linked Enduron polyethylene liners. J Arthroplasty. 2006;21(suppl 2):17–25. [DOI] [PubMed]
- 20.Griffith MJ, Seidenstein MK, Williams D, Charnley J. Socket wear in Charnley low friction arthroplasty of the hip. Clin Orthop Relat Res. 1978;137:37–47. [PubMed]
- 21.Gruen TA, McNeice GM, Amstutz HC. ‘Modes of failure’ of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;141:17–27. [PubMed]
- 22.Heisel C, Silva M, dela Rosa MA, Schmalzried TP. Short-term in vivo wear of cross-linked polyethylene. J Bone Joint Surg Am. 2004;86:748–751. [DOI] [PubMed]
- 23.Hui AJ, McCalden RW, Martell JM, MacDonald SJ, Bourne RB, Rorabeck CH. Validation of two and three-dimensional radiographic techniques for measuring polyethylene wear after total hip arthroplasty. J Bone Joint Surg Am. 2003;85:505–511. [DOI] [PubMed]
- 24.Kim YH, Kim JS, Cho SH. Primary total hip arthroplasty with a cementless porous-coated anatomic total hip prosthesis: 10- to 12-year results of prospective and consecutive series. J Arthroplasty. 1999;14:538–548. [DOI] [PubMed]
- 25.Kim YH, Kook HK, Kim JS. Total hip replacement with a cementless acetabular component and a cemented femoral component in patients younger than fifty years of age. J Bone Joint Surg Am. 2002;84:770–774. [DOI] [PubMed]
- 26.Maloney WJ, Galante JO, Anderson M, Goldberg V, Harris WH, Jacobs J, Kraay M, Lachiewicz P, Rubash HE, Schutzer S, Woolson ST. Fixation, polyethylene wear, and pelvic osteolysis in primary total hip replacement. Clin Orthop Relat Res. 1999;369:157–164. [DOI] [PubMed]
- 27.Martell JM, Berdia S. Determination of polyethylene wear in total hip replacements with use of digital radiographs. J Bone Joint Surg Am. 1997;79:1635–1641. [DOI] [PubMed]
- 28.Martell JM, Verner JJ, Incavo SJ. Clinical performance of a highly cross-linked polyethylene at two years in total hip arthroplasty: a randomized prospective trial. J Arthroplasty. 2003;18(suppl 1):55–59. [DOI] [PubMed]
- 29.McKellop H, Shen FW, Lu B, Campbell P, Salovey R. Development of an extremely wear-resistant ultra high molecular weight polyethylene for total hip replacements. J Orthop Res. 1999;17:157–167. [DOI] [PubMed]
- 30.McKellop H, Shen FW, Lu B, Campbell P, Salovey R. Effect of sterilization method and other modifications on the wear resistance of acetabular cups made of ultra-high molecular weight polyethylene: a hip-simulator study. J Bone Joint Surg Am. 2000;82:1708–1725. [DOI] [PubMed]
- 31.Nashed RS, Becker DA, Gustilo RB. Are cementless acetabular components the cause of excess wear and osteolysis in total hip arthroplasty? Clin Orthop Relat Res. 1995;317:19–28. [PubMed]
- 32.Ostendorf M, van Stel HF, Buskens E, Schrijvers AJ, Marting LN, Verbout AJ, Dhert WJ. Patient-reported outcome in total hip replacement: a comparison of five instruments of health status. J Bone Joint Surg Br. 2004;86:801–808. [DOI] [PubMed]
- 33.Perez RE, Rodriguez JA, Deshmukh RG, Ranawat CS. Polyethylene wear and periprosthetic osteolysis in metal-backed acetabular components with cylindrical liners. J Arthroplasty. 1998;13:1–7. [DOI] [PubMed]
- 34.Rimnac C, Pruitt L. How do material properties influence wear and fracture mechanisms? J Am Acad Orthop Surg. 2008;16(suppl 1):S94–S100. [DOI] [PubMed]
- 35.Russotti GM, Harris WH. Proximal placement of the acetabular component in total hip arthroplasty: a long-term follow-up study. J Bone Joint Surg Am. 1991;73:587–592. [PubMed]
- 36.Schmalzried TP, Guttmann D, Grecula M, Amstutz HC. The relationship between the design, position, and articular wear of acetabular components inserted without cement and the development of pelvic osteolysis. J Bone Joint Surg Am. 1994;76:677–688. [DOI] [PubMed]
- 37.Schmalzried TP, Kwong LM, Jasty M, Sedlacek RC, Haire TC, O′Connor DO, Bragdon CR, Kabo JM, Malcolm AJ, Harris WH. The mechanism of loosening of cemented acetabular components in total hip arthroplasty: analysis of specimens retrieved at autopsy. Clin Orthop Relat Res. 1992;274:60–78. [PubMed]
- 38.Schmalzried TP, Szuszczewicz ES, Northfield MR, Akizuki KH, Frankel RE, Belcher G, Amstutz HC. Quantitative assessment of walking activity after total hip or knee replacement. J Bone Joint Surg Am. 1998;80:54–59. [DOI] [PubMed]
- 39.Shih CH, Lee PC, Chen JH, Tai CL, Chen LF, Wu JS, Chang WH. Measurement of polyethylene wear in cementless total hip arthroplasty. J Bone Joint Surg Br. 1997;79:361–365. [DOI] [PubMed]
- 40.Soderman P, Malchau H. Is the Harris hip score system useful to study the outcome of total hip replacement? Clin Orthop Relat Res. 2001;384:189–197. [DOI] [PubMed]
- 41.Tower SS, Currier JH, Currier BH, Lyford KA, Van Citters DW, Mayor MB. Rim cracking of the cross-linked longevity polyethylene acetabular liner after total hip arthroplasty. J Bone Joint Surg Am. 2007;89:2212–2217. [DOI] [PubMed]
- 42.Triclot P, Grosjean G, El Masri F, Courpied JP, Hamadouche M. A comparison of the penetration rate of two polyethylene acetabular liners of different levels of cross-linking: a prospective randomised trial. J Bone Joint Surg Br. 2007;89:1439–1445. [DOI] [PubMed]
- 43.Wan Z, Dorr LD. Natural history of femoral focal osteolysis with proximal ingrowth smooth stem implant. J Arthroplasty. 1996;11:718–725. [DOI] [PubMed]
- 44.Willert HG, Bertram H, Buchhorn GH. Osteolysis in alloarthroplasty of the hip: the role of ultra-high molecular weight polyethylene wear particles. Clin Orthop Relat Res. 1990;258:95–107. [PubMed]
- 45.Zahiri CA, Schmalzried TP, Szuszczewicz ES, Amstutz HC. Assessing activity in joint replacement patients. J Arthroplasty. 1998;13:890–895. [DOI] [PubMed]