Abstract
Fracture healing is normally assessed through an interpretation of radiographs, clinical evaluation, including pain on weight bearing, and a manual assessment of the mobility of the fracture. These assessments are subjective and their accuracy in determining when a fracture has healed has been questioned. Viewed in mechanical terms, fracture healing represents a steady increase in strength and stiffness of a broken bone and it is only when these values are sufficiently high to support unrestricted weight bearing that a fracture can be said to be healed. Information on the rate of increase of the mechanical properties of a healing bone is therefore valuable in determining both the rate at which a fracture will heal and in helping to define an objective and measurable endpoint of healing. A number of techniques have been developed to quantify bone healing in mechanical terms and these are described and discussed in detail. Clinical studies, in which measurements of fracture stiffness have been used to identify a quantifiable end point of healing, compare different treatment methods, predictably determine whether a fracture will heal, and identify factors which most influence healing, are reviewed and discussed.
Introduction
Flexibly fixed fractures of diaphyseal bone usually heal by secondary fracture healing with the formation of a callus. The callus tissue builds a bridge between the fragments and leads to a reduction of the interfragmentary movement, which occurs in the fracture under loading. After the early phase of inflammation a soft callus proliferates, which greatly increases its mechanical stiffness when the callus calcifies by endochondral and intramembranous ossification. When the peripheral callus has built a bony bridge between the fragments, the interfragmentary movement is reduced substantially, enabling cortical healing to take place [9]. The healing area will finally remodel and the callus will be totally resorbed.
Unfortunately, there is no definition when the fracture is healed enough to allow normal function of the bone. Usually the assessment of fracture healing is performed on the basis of radiographs where the callus healing is visible in terms of callus bridging and disappearance of the fracture line. The assessment of fracture healing, however, is subjective, and neither radiological nor manual examination [45] allows reliable determination of bone healing. Studies have shown that radiographic analyses are subjective and inaccurate [21], that the amount of callus does not correlate to the stiffness of the healed bone [34], and that both the general appearance and cortical bridging poorly predict the bone healing results [29].
A number of studies have attempted to use quantitative radiology to measure the changes in callus mineralization in both experimental [1, 13] and clinical fractures [5], but the relationship between these changes and the mechanical properties of the healing fracture is not always clear, unless there is a consistent fracture gap, which is often not the case in clinical fractures.
Even though a definition of an endpoint for fracture healing might be difficult, it would be very helpful to be able to define a time point at which healing is complete, as this is important in guiding clinical decisions which have to be made during the treatment of the patients.
One such decision is the time point for the safe removal of fixation devices. Early removal of external fixators stabilizing tibia fractures has led to refractures in up to 11% [12, 27, 40, 41] of patients. To avoid too early a removal of the external fixation device, an objective parameter for the mechanical quality of the healing bone would be very helpful. Such a parameter would also be helpful to aid decision making about the time when increased weight bearing on the fracture can be permitted and also whether further operations are necessary to achieve healing. Clinical studies comparing various methods for treating fractures also need a certain healing time point to allow statistical comparison between different treatment groups.
A number of techniques have, therefore, been developed to permit the noninvasive determination of a healing time. The principal parameter measured is the stiffness of the healing fracture as stiffness correlates with the bone strength during callus formation [6, 46]. Most of the techniques have been applied to tibial fractures treated by external fixator or cast. Tibial fractures are more critical in regard to complications and delayed healing and therefore need more careful assessment of the healing process. Tibial fractures also have less soft tissue and muscle cover, which makes carrying out measurements on this bone potentially easier. For patients treated with external fixation or a cast, noninvasive measurements of the callus stiffness have been performed in a number of studies while measurements on fractures treated with intramedullary nails [37] and internal fixation [33] are rare.
We will describe the various techniques used for the measurements of fracture healing, their possibilities and limitations, and we will give an overview of studies that used these techniques.
Principles of Mechanical Measurements of Callus Stiffness
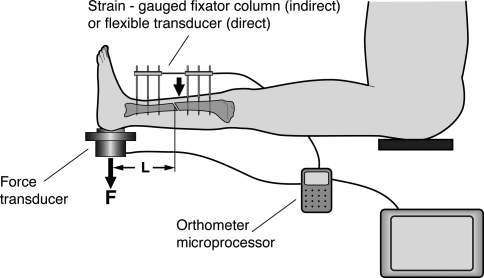
For a direct measurement of callus stiffness, the most accurate method to determine the stiffness of the fracture callus is the measurement of the deflection of the healing bone under loading with all the fixation devices removed [21, 23, 24, 32]. In principle, this is only really possible for patients who are treated by external fixators or by cast. After removal of an external fixator the remaining bone pins can be used to fix a goniometric system for the measurement of the deflection of the fracture under the application of a known load. With the lower leg in a horizontal position a load applied vertically by a weight or manually (and measured by a load cell underneath the heel) produces a bending moment in the sagittal plane (Fig. 1). With the known distances (L) between heel support and load application point the bending moment can be calculated. Together with the measured angular deflection from the goniometer, a bending stiffness in Nm/degree can be calculated. Similar measurements can be performed on patients treated by cast, where a deflection, which occurs under a bending moment, can be detected [21, 39].
Fig. 1.
Fracture stiffness measurement after removal of the external fixator. A gomiometer system is used to measure the deflection of the bone under loading (L: Distance between fracture and load cell, F: load at the heel). (Adapted and reproduced with permission and copyright © of the British Editorial Society of Bone and Joint Surgery from Richardson JB, Cunningham JL, Goodship AE, O’Connor BT, Kenwright J. Measuring stiffness can define healing of tibial fractures. J Bone Joint Surg Br. 1994;76:389–394.)
The advantage of this method is that the calculated callus stiffness data allow a good estimation of the load capacity of the healing bone [6, 46]. This method, however, has one major limitation in that the fixator has to be removed for each measurement and, in the early phase of fracture healing, up to 6 weeks postfracture, it is not usually possible to remove the fixation device as the risk of loss of reduction of the fractured bone under loading would be too high. The method is therefore only applicable for the later phases of the fracture healing process.
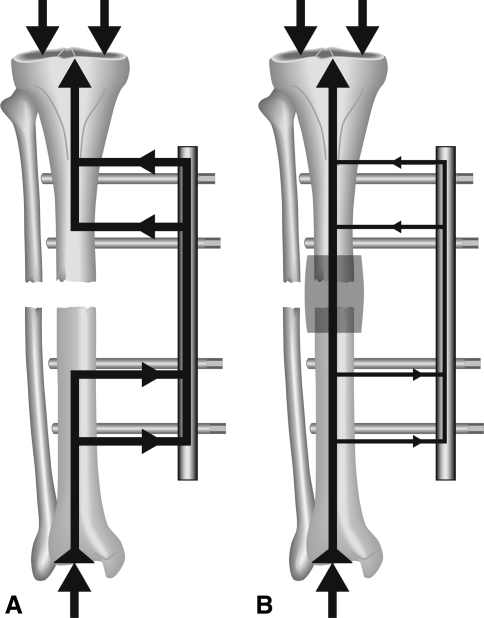
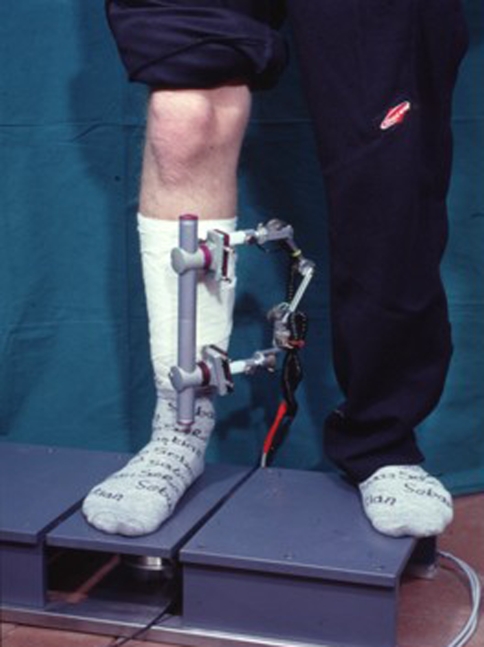
In indirect measurements of callus stiffness the load, which is applied to a fractured and stabilized bone, is shared by the fixation device and the bone. This load sharing depends mainly on the reduction of the fragments and the biomechanical quality of the developing callus formation. If the reduction of the fragments leads to a fracture gap, immediately postoperatively the load is mainly carried by the fixation device. With progressive healing the growing callus will increase in diameter and stiffness (due to calcification) and will be able to carry an increasing part of the applied load (Fig. 2). This will lead to reduced loading of the fixation device with increasing healing time. To detect the changes in load sharing during healing, a standardized load has to be applied to the treated bone. In cases with a fracture gap an external loading can be applied by an axial load (Fig. 2). In cases of a good reduction and contact of the fragments, a bending moment can be applied as previously described in the direct measurement of callus stiffness (Fig. 1). With the exception of very few cases [35, 37], these measurements are performed on patients treated by external fixation because only the external fixator is easily accessible for measuring devices to which it can be attached. Changes in fixator loading lead to changes in fixator deformation. The main deformations are caused by the deflection of the bone pins and the deformation of the fixator body. One of the first surgeons who used strain-gauge-equipped fixators to analyze the increase in bone stiffness was Burny [3]. In the following years several measuring devices were developed, which detected mainly the deflection of the fixation pins or the bending of the fixator body [2, 4, 7, 8, 10, 26]. As the fixator is eccentrically arranged to the load axis of the bone, the deformation of the fixator is a complex deformation, which is usually different in the three translations and rotations. This could be shown in a few investigations using six-degrees-of-freedom goniometric systems [18] (Fig. 3). For clinical applications, however, most often only the deformation in the longitudinal axis of the bone was measured. Another possibility to measure the load sharing between bone and fixator is the integration of a load cell in the fixator body [17, 38] (Fig. 4).
Fig. 2A–B.
The principle of load sharing is demonstrated when the fracture is reduced with a remaining fracture gap. In the early phase of healing all loads are carried by the fixator leading there to a measurable deformation (A). With increasing callus stiffness more load is carried by the bone leading to a reduced load and deformation at the fixator (B).
Fig. 3.
Partial load bearing is measured on a six-degrees-of-freedom force plate. The deformation of the screws and fixator can be measured by a six-degrees-of-freedom goniometer system.
Fig. 4.

Strain-gauged transducer device is attached to the fixator column to measure the loads acting on the fixation.
In contrast to the direct measurement of callus stiffness discussed previously, the measured values in this load-sharing method depend on the stiffness of the fixator; the stiffer the fixator the lower the measured deflection for a given applied load. For external fixators with a relatively low stiffness (ie, ring fixator with wire fixation), it is possible to use commercially available measuring systems to detect the deformation in the fracture healing zone [16]. For more rigid fixators, special transducers need to be developed [17, 18].
For the normal healing process a hyperbolic decrease in fixator deformation with time is observed. When the signals are close to zero and show an asymptotic behavior, it is assumed that the callus is stiff enough to carry nearly all loads and the bridging of the fracture by a calcified and strong and stiff callus has taken place. Theoretical calculations have shown that the callus healing led to a bone that has approximately 20% to 50% of the strength of the normal bone [2].
For very rigid fixator systems, the measured signal is small. This leads to the disadvantage of a limited accuracy in the late phase of healing when the fixator carries usually only small loads and the consequent deflection of the fixator is small. The advantage of this method, however, is that the fixator does not have to be removed during the early phase of healing and it is possible to measure the load sharing, and indirectly the callus stiffness, from the first day postoperatively.
General Limitations of Stiffness Measurements
The calculation of the callus stiffness requires the measurement of the deformation under a defined load. The external loads applied (eg, at the foot of the patient) are influenced by internal loads, which occur when the muscles are contracted. Because there is no control of the muscle activity of the patient during the measurement, the forces acting at the level of the fracture are not exactly known and consequently are a source of inaccuracy of the measurement. Another important source of measurement inaccuracy is loosening of fixator pins, which are either used to measure the deformation of the bone (direct measurement) or to transfer load to the fixator body (indirect measurement). Therefore, if pin loosening is detected, any measurements made should either be interpreted with caution (in the case of direct measurements of stiffness) or excluded (in the case of indirect measurements).
Other Methods to Measure the Changes in Callus Stiffness
A substantial amount of research has been carried out in vibrational measurements of fracture healing, and some promising results have been obtained. However, this technique has never been widely accepted or used, and it remains very much a research tool. Good quantitative results have been obtained using both wave propagation [11, 30, 31, 42] and resonant frequency analysis. Of these two methodologies, resonant frequency analysis is the more widely researched and appears to be the more repeatable. When using vibrational techniques, fracture healing appears to be best quantified in terms of the nonfractured contralateral limb (the vibrational properties of this representing the “healed” state) and there is some limited agreement between different studies as to what constitutes a “healed” fracture in vibrational terms. With all vibrational techniques however, the experimental protocol used can significantly influence the results and therefore much care needs to be taken to standardize measurements.
The use of acoustic emission to determine the mechanical status of a healing bone was investigated by Watanabe et al. [44]. They found that under increasing loading, the acoustic emission signal from the fracture site could be detected prior to the failure and that the load required to initiate the acoustic emission increased proportionately with the increasing mechanical properties of the healing fracture. This technique was applied clinically in 35 patients with long bone fractures treated with external fixation and a reliable criteria for healing was found to be no initiation of the acoustic emission signal of full weight bearing [22].
Changes in both ultrasound velocity and attenuation across a healing fracture have been used to quantify fracture healing [11, 19, 20, 28, 36]. Of these two modalities of measurement (velocity and attenuation), it has been demonstrated that attenuation is by far the more sensitive to the presence of a fracture which results in a large loss in signal amplitude [14]. Bridging of the fracture with an external callus substantially reduces the loss in signal amplitude [15], suggesting that measuring the change in signal amplitude across a healing fracture could be an effective method of quantifying fracture healing. However, the in vivo measurement of ultrasound velocity and attenuation is problematic due to the surrounding soft tissues and to date there is no evidence connecting ultrasound measurements to bone stiffness and strength.
Clinical Studies Using Bone Stiffness Measurements
Unfortunately, there are only a few studies [4, 8, 21, 23, 25, 32, 41] which monitored the stiffness of healing fractures in a sufficiently large number of patients to be able to draw conclusions from the results.
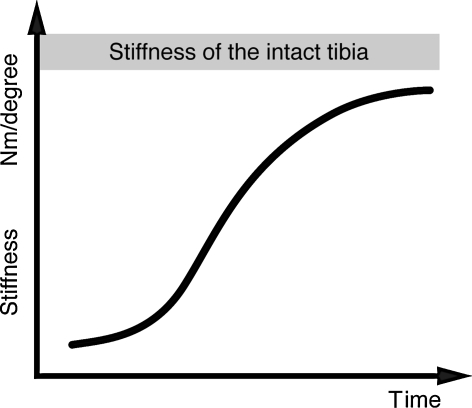
The largest study of direct measurement of bone stiffness was presented by Richardson et al. [32] on 212 patients with tibial fractures treated by external fixation. The measurements were performed after removal of the fixator as described before. The typical course of a normal healing tibia was characterized by an exponential increase in stiffness versus healing time (Fig. 5). The measurements started 6 weeks postfracture due to the necessity of removing the fixator to carry these out. The patients were split into two groups. In Group 1 the clinical healing time and time of fixator removal was based on clinical assessments, whereas in Group 2 the biomechanical healing time was that taken for the fracture stiffness to reach 15 Nm/degree. In Group 1 (clinical definition of healing) eight of 117 patients showed refractures after fixator removal. In Group 2, with the biomechanical definition of healing time, no patient showed a refracture on fixator removal. The authors concluded that the stiffness measurements provided an objective method to determine the healing time, and this was also associated with a reduction in the refracture rate and in the time taken to achieve independent weight bearing.
Fig. 5.
Characteristic changes in fracture stiffness of healing tibial fractures are shown. (Adapted and reproduced with permission and copyright © of the British Editorial Society of Bone and Joint Surgery from Richardson JB, Cunningham JL, Goodship AE, O’Connor BT, Kenwright J. Measuring stiffness can define healing of tibial fractures. J Bone Joint Surg Br. 1994;76:389–394.)
A study on 157 patients with conservatively treated tibial shaft fractures was reported by Hammer et al. [21]. A bending moment in the frontal plane was applied to the tibia and the angular deflection of the fracture was determined by two radiographs, one in the unloaded and one in the loaded situation. It was found that for all fractures with a deflection ratio below a certain value, the strength of the union was sufficient for the plaster to be removed and for full unprotected loading of the leg during walking to be permitted. In 68 patients the cast could be removed on average 4 weeks earlier based on the measurement in comparison to conventional clinical assessments. In 22 patients additional surgery was avoided because repeated stability measurements indicated progressive union.
Jernberger [23] investigated 40 patients with tibial fractures and clinically assessed delayed healing. After cast removal he implanted two screws onto which he attached a stiffness measurement system. He started the measurements relatively late, on average 19 weeks postfracture, and determined the bending deflection and bending stiffness. He found the time course of healing detected by the stiffness measurements was helpful in deciding whether a patient with a delayed union should undergo surgery.
Wade et al. [43] measured the progression of healing in 103 patients with unstable tibial fractures using fracture stiffness measurements. In 73 of these patients, the decision to remove the fixator was based on achieving a fracture stiffness of 15 Nm/degree in the sagittal plane only. After fixator removal, angulation at the fracture site occurred in four patients. In 23 of the patients, fracture stiffness was measured in several planes (sagittal plane and in planes perpendicular and parallel to the fixator pins) and fixator removal only occurred when a stiffness of 15 Nm/degree was reached in each plane. There were no refractures or angulation on fixator removal. It was found that fracture stiffness in two orthogonal planes can differ by a large amount and it was suggested that only when the fracture stiffness reaches 15 Nm/degree in both planes should the fixator be removed.
Indirect Measurement
Studies which assessed healing by the indirect method of measurement included those by Burny et al. [4] using indirect measurements of bone healing with external fixators equipped with strain gauges over decades and have reported on around 500 patients. A complete followup was possible for 231 tibia fractures. They described seven different types of time courses of healing when they monitored the fixator deformation against the healing time and divided them into four classes: fast healing (healing achieved in fewer than 100 days), normal healing (healing in 101 to 150 days), slow healing (healing after 150 days), and nonunion requiring new surgery. For each class he calculated the linear regression of the measured fixator deformation (normalized to 100% to the first postoperative value) versus the healing time. He found that the linear regression coefficient, describing approximately the slope of the curve, was largest in the fast-healing group (r = −0.7) and decreased with increasing time required for healing (normal healing: −0.68, slow healing: −0.61; nonunion: −0.11). The fact that the curves are normally nonlinear and that no information about the standard deviations for the various regression curves are given, makes these results difficult to apply as a general tool for the analysis of the fracture healing processes. The general outcome seems to be that a lack of progressive increase of bone stiffness up to 200 days postfracture, as indicated by a decrease in fixator deformation, is a good indicator of a nonunion.
Kenwright et al. [25] used indirect measurement of fracture stiffness as a method of comparing two different treatment modalities in 80 patients treated with external fixation for a tibial diaphyseal fracture. In one group the fixator was modified to allow for 1 mm of axial movement on the application of a low load (12 N) while in the other group the fixator remained rigid. A fracture stiffness of 15 Nm/degree was used to represent clinical union and a substantially shorter healing time (13 compared to 18 weeks) was found in the group with axial micromovent compared to the rigid fixation group.
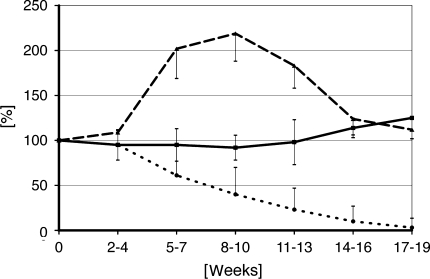
Claes et al. [8] performed another study measuring the deformation of the bone screws of external fixators under axial or bending loads. This clinical multicenter study was carried out on 100 tibial fractures stabilized by various types of external fixators and monitored for healing up to 28 weeks postoperatively. The deformation of the bone screws was different depending on the application technique and the fixator used. To compare the measurements for the different patients, the first postoperative signal was defined as 100% and the following signals measured during the healing process were expressed as a percentage of the postoperative value. Fractures were defined as “healed” when the deformation of the bone screws under loading was below 10% of the initial postoperative level or was below the accuracy of the measuring device [7]. There were three different types of time course in the reduction of fixator deformation (Fig. 6). Ninety-two patients showed a steady decrease in deformation indicating a steady increase in stiffness of the healing bone. Eighty-two of them “healed” in a maximum of 19 weeks (on average after 12.1 ± 4.3 weeks) and 10 patients needed on average 24 ± 4.3 weeks for healing to be completed. All 92 patients were defined as normal healing patients. The course of their measured signals could be characterized graphically. Eight patients showed no steady decrease in the measured signal; five of them nearly constant values of deformation and three even exhibited an increased signal (perhaps indicating ongoing bone resorption with consequent reduction in fracture stability). All eight patients were reoperated and an intramedullary nail was implanted. The indication for this was based on the stiffness measurements and the radiographic assessment. A prediction as to whether a fracture will heal or is likely to develop a nonunion appeared to be possible by about weeks 5 to 7 (p < 0.05). From this time onward the values on the three patients with increasing signals (Fig. 6) were always above the 95% confidence level (2 SD) of all the patients that healed. The five “nonresponding” patients with nearly constant measurement values showed a different course of the signals than the “normal healing” group. Differences between these two groups, however, occurred only from weeks 8 to 10 onwards at a confidence level of 66% (1 SD). The method of indirect measurement of fracture stiffness revealed the healing time on average about 2.5 weeks earlier than the radiological assessment. The use of the stiffness measurement technique would therefore appear to allow shortening of the treatment time. Factors that affect the fracture healing process could additionally be analyzed based on this objective data, eg, the healing time of closed fractures was on average 11.3 ± 3.2 weeks and for open fractures was on average 14 ± 4.9 weeks. It could be demonstrated that the healing time increases with the complexity of the fracture: 11.3 weeks for Type A, 13.1 weeks for Type B, and 15.1 weeks for Type C (AO classification). It could also be demonstrated that large fracture gaps tended to delay the healing process.
Fig. 6.
Changes in fixator deformation as indirect indicator of increasing fractures stiffness. Normal healing shows steady decrease of the signal (…) whereas delayed healing shows no significant changes (___) and nonunions are showing an increase of fixator deformation due to bone resorption (_ _ _).
Discussion and Conclusion
The monitoring of the progress of fracture healing by measuring the stiffness of the healing bone is possible. It can be performed using either a direct or an indirect technique. The direct measurement of stiffness requires removing fixation devices like casts or fixators and is limited to healing periods from 6 weeks onward due to the early instability of the fracture. The indirect measurement of bone stiffness can be performed from the first day after fracture but is limited to fracture fixation by external fixators. The main outcome information of these measurements is the fracture stiffness as a function of the healing time. A steady increase in the stiffness of the healing bone indicates progress of the bone healing process. Even though the measured data are objective, a definition of an endpoint for fracture healing is critical. After bridging of the fracture by a calcified callus takes place, the callus stiffness can increase rapidly and consequently the accuracy of the techniques used to measure the fracture stiffness decrease making subsequent changes in stiffness difficult to be detected. Therefore it might be useful to define the time when the calcified callus bridges the fracture as “healing” time. In the study of Claes et al. [8] it was demonstrated that such a definition indicated healing to occur about 2.5 weeks earlier than suggested by radiograph assessment, but that both the stiffness measurements and radiograph assessments were in agreement as to which fractures healed and which did not. The main aim of such studies is to determine when the external fixation device can be removed without risk of a refracture or angulation and when an increased loading of the fracture can be recommended to the patient. Most of the studies [8, 21, 25, 32, 43] provided a good and objective database to inform this decision. Decisions about reoperations or additional operations, which might be necessary, could be supported by the data achieved from these measurements.
The majority of the operatively treated fractures are treated by intramedullary nails and plates. Therefore it would be of great interest to use these kinds of internal fixation devices with instruments which allow the indirect measurement of fracture stiffness. Unfortunately there are no known clinical studies which use such a technology. The reason is that internal fixation devices require a telemetry system for data transfer and a wireless power supply by an inductive coil system or a long-term battery system. These requirements make the measuring system much more complex and more expensive than for systems used for external fixators. In addition the approval of such an implant from the appropriate legislative body (eg, the FDA) is difficult. The standards required for an implant, which include electronic devices that remain for a long time or even forever in patients, are very high. This might be the reason that no instrumented plate or nail is available on the market and no clinical studies have been performed using these devices even though several groups have tried to develop such systems. Further progress in technology may make the use of such devices possible in the future.
A general limitation of all techniques of monitoring fracture healing is that it requires special measuring techniques, experience in its application, and a knowledge and appreciation of possible errors, which can occur with these measurements. It is a method, which requires more effort from medical personnel and takes much more time than the normal clinical assessment. As the past decades have demonstrated, these are limitations which did not allow the use of this method in the routine clinical assessment of fracture healing. These methods therefore can only be a tool for clinical research when objective data on the fracture healing process needs to be determined or when data for statistical comparison between various fracture healing treatment modalities are necessary.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Augat P, Merk J, Genant HK, Claes L. Quantitative assessment of experimental fracture repair by peripheral computed tomography. Calcif Tissue Int. 1997;60:194–199. [DOI] [PubMed]
- 2.Bourgois R, Burny F. Measurement of the stiffness of fracture callus in vivo. A theoretical study. J Biomech. 1972;5:85–91. [DOI] [PubMed]
- 3.Burny F. Study of consolidation of fractures by deformation gauges in clinical medicine. Acta Orthop Belg. 1968;34:917–927. [PubMed]
- 4.Burny F, Donkerwolcke M, Bourgois R, Domb M, Saric O. Twenty years experience in fracture healing measurement with strain gauges. Orthopedics. 1984;7:1823–1826. [DOI] [PubMed]
- 5.Cattermole HC, Cook JE, Fordham JN, Muckle DS, Cunningham JL. Bone mineral changes during tibial fracture healing. Clin Orthop Relat Res. 1997;339:190–196. [DOI] [PubMed]
- 6.Chehade MJ, Pohl AP, Pearcy MJ, Nawana N. Clinical implications of stiffness and strength changes in fracture healing. J Bone Joint Surg Br. 1997;79:9–12. [DOI] [PubMed]
- 7.Claes L. Measuring bone healing in osteosynthesis with external fixator using the Fraktometer FM 100 [in German]. Chirurg. 1991;62:354–355. [PubMed]
- 8.Claes L, Grass R, Schmickal T, Kisse B, Eggers C, Gerngross H, Mutschler W, Arand M, Wintermeyer T, Wentzensen A. Monitoring and healing analysis of 100 tibial shaft fractures. Langenbecks Arch Surg. 2002;387:146–152. [DOI] [PubMed]
- 9.Claes LE, Heigele CA. Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing. J Biomech. 1999;32:255–266. [DOI] [PubMed]
- 10.Cunningham JL, Evans M, Harris JD, Kenwright J. The measurement of stiffness of fractures treated with external fixation. Eng Med. 1987;16:229–232. [DOI] [PubMed]
- 11.Cunningham JL, Kenwright J, Kershaw CJ. Biomechanical measurement of fracture healing. J Med Eng Technol. 1990;14:92–101. [DOI] [PubMed]
- 12.De Bastiani G, Aldegheri R, Renzi Brivio L. The treatment of fractures with a dynamic axial fixator. J Bone Joint Surg Br. 1984;66:538–545. [DOI] [PubMed]
- 13.den Boer FC, Bramer JA, Patka P, Bakker FC, Barentsen RH, Feilzer AJ, de Lange ES, Haarman HJ. Quantification of fracture healing with three-dimensional computed tomography. Arch Orthop Trauma Surg. 1998;117:345–350. [DOI] [PubMed]
- 14.Dodd SP, Cunningham JL, Miles AW, Gheduzzi S, Humphrey VF. Ultrasound transmission loss across transverse and oblique bone fractures: an in vitro study. Ultrasound Med Biol. 2008;34:454–462. [DOI] [PubMed]
- 15.Dodd SP, Miles AW, Gheduzzi S, Humphrey VF, Cunningham JL. Modelling the effects of different fracture geometries and healing stages on ultrasound signal loss across a long bone fracture. Comput Methods Biomech Biomed Engin. 2007;10:371–375. [DOI] [PubMed]
- 16.Duda GN, Sporrer S, Sollmann M, Hoffmann JE, Kassi JP, Khodadadyan C, Raschke M. Interfragmentary movements in the early phase of healing in distraction and correction osteotomies stabilized with ring fixators. Langenbecks Arch Surg. 2003;387:433–440. [DOI] [PubMed]
- 17.Evans M, Kenwright J, Cunningham JL. Design and performance of a fracture monitoring transducer. J Biomed Eng. 1988;10:64–69. [DOI] [PubMed]
- 18.Gardner TN, Evans M, Kyberd PJ. An instrumented spacial linkage for monitoring relative three-dimensional motion between fracture fragments. J Biomech Eng. 1996;118:586–594. [DOI] [PubMed]
- 19.Gerlanc M, Haddad D, Hyatt GW, Langloh JT, St Hilaire P. Ultrasonic study of normal and fractured bone. Clin Orthop Relat Res. 1975;111:175–180. [DOI] [PubMed]
- 20.Glinkowski W, Gorecki A. Clinical experiences with ultrasonometric measurement of fracture healing. Technol Health Care. 2006;14:321–333. [PubMed]
- 21.Hammer RR, Hammerby S, Lindholm B. Accuracy of radiologic assessment of tibial shaft fracture union in humans. Clin Orthop Relat Res. 1985;199:233–238. [PubMed]
- 22.Hirasawa Y, Takai S, Kim WC, Takenaka N, Yoshino N, Watanabe Y. Biomechanical monitoring of healing bone based on acoustic emission technology. Clin Orthop Relat Res. 2002;402:236–244. [DOI] [PubMed]
- 23.Jernberger A. Measurement of stability of tibial fractures. A mechanical method. Acta Orthop Scand Suppl. 1970;135:1–88. [DOI] [PubMed]
- 24.Jorgensen TE. Measurements of stability of crural fractures treated with Hoffmann osteotaxis 4 The complicated, terminal phase of healing of crural fractures. Acta Orthop Scand. 1972;43:280–291. [DOI] [PubMed]
- 25.Kenwright J, Richardson JB, Cunningham JL, White SH, Goodship AE, Adams MA, Magnussen PA, Newman JH. Axial movement and tibial fractures. A controlled randomised trial of treatment. J Bone Joint Surg Br. 1991;73:654–659. [DOI] [PubMed]
- 26.Kershaw CJ, Cunningham JL, Kenwright J. Tibial external fixation, weight bearing, and fracture movement. Clin Orthop Relat Res. 1993;293:28–36. [PubMed]
- 27.Krettek C, Haas N, Tscherne H. The role of supplemental lag-screw fixation for open fractures of the tibial shaft treated with external fixation. J Bone Joint Surg Am. 1991;73:893–897. [PubMed]
- 28.Malizos KN, Papachristos AA, Protopappas VC, Fotiadis DI. Transosseous application of low-intensity ultrasound for the enhancement and monitoring of fracture healing process in a sheep osteotomy model. Bone. 2006;38:530–539. [DOI] [PubMed]
- 29.McClelland D, Thomas PB, Bancroft G, Moorcraft CI. Fracture healing assessment comparing stiffness measurements using radiographs. Clin Orthop Relat Res. 2007;457:214–219. [DOI] [PubMed]
- 30.Nakatsuchi Y, Tsuchikane A, Nomura A. Assessment of fracture healing in the tibia using the impulse response method. J Orthop Trauma. 1996;10:50–62. [DOI] [PubMed]
- 31.Nokes L, Mintowt-Czyz WJ, Fairclough JA, Mackie I, Williams J. Vibration analysis in the assessment of conservatively managed tibial fractures. J Biomed Eng. 1985;7:40–44. [DOI] [PubMed]
- 32.Richardson JB, Cunningham JL, Goodship AE, O’Connor BT, Kenwright J. Measuring stiffness can define healing of tibial fractures. J Bone Joint Surg Br. 1994;76:389–394. [PubMed]
- 33.Rohlmann A, Graichen F, Weber U, Bergmann G. 2000 Volvo Award winner in biomechanical studies: Monitoring in vivo implant loads with a telemeterized internal spinal fixation device. Spine. 2000;25:2981–2986. [DOI] [PubMed]
- 34.Sano H, Uhthoff HK, Backman DS, Yeadon A. Correlation of radiographic measurements with biomechanical test results. Clin Orthop Relat Res. 1999;368:271–278. [DOI] [PubMed]
- 35.Sarmiento A, McKellop HA, Llinas A, Park SH, Lu B, Stetson W, Rao R. Effect of loading and fracture motions on diaphyseal tibial fractures. J Orthop Res. 1996;14:80–84. [DOI] [PubMed]
- 36.Saulgozis J, Pontaga I, Lowet G, Van der Perre G. The effect of fracture and fracture fixation on ultrasonic velocity and attenuation. Physiol Meas. 1996;17:201–211. [DOI] [PubMed]
- 37.Schneider E, Michel MC, Genge M, Perren SM. Loads acting on an intramedullary femoral nail. In: Bergmann G, Rohlmann A, Graichen F, eds. Implantable Telemetry in Orthopedics. Berlin, Germany: Forschungsmitteilung der FU; 1990:221–227.
- 38.Seide K, Weinrich N, Wenzl ME, Wolter D, Jurgens C. Three-dimensional load measurements in an external fixator. J Biomech. 2004;37:1361–1369. [DOI] [PubMed]
- 39.Shah KM, Nicol AC, Hamblen DL. Fracture stiffness measurement in tibial shaft fractures: a non-invasive method. Clin Biomech. 1995;10:395–400. [DOI] [PubMed]
- 40.Steinfield PH, Cobelli NJ, Sadler AH, Szporn MN. Open tibial fractures treated by anterior half-pin frame fixation. Clin Orthop Relat Res. 1988;228:208–214. [PubMed]
- 41.Thakur AJ, Patankar J. Open tibial fractures. Treatment by uniplanar external fixation and early bone grafting. J Bone Joint Surg Br. 1991;73:448–451. [DOI] [PubMed]
- 42.Tower SS, Beals RK, Duwelius PJ. Resonant frequency analysis of the tibia as a measure of fracture healing. J Orthop Trauma. 1993;7:552–557. [DOI] [PubMed]
- 43.Wade RH, Moorcroft CI, Thomas PB. Fracture stiffness as a guide to the management of tibial fractures. J Bone Joint Surg Br. 2001;83:533–535. [DOI] [PubMed]
- 44.Watanabe Y, Takai S, Arai Y, Yoshino N, Hirasawa Y. Prediction of mechanical properties of healing fractures using acoustic emission. J Orthop Res. 2001;19:548–553. [DOI] [PubMed]
- 45.Webb J, Herling G, Gardner T, Kenwright J, Simpson AH. Manual assessment of fracture stiffness. Injury. 1996;27:319–320. [DOI] [PubMed]
- 46.Windhagen H, Kolbeck S, Bail H, Schmeling A, Raschke M. Quantitative assessment of in vivo bone regeneration consolidation in distraction osteogenesis. J Orthop Res. 2000;18:912–919. [DOI] [PubMed]