Abstract
Evaluation of patient activity is essential for clinical decision making before THA. To correlate age progression to patient activity after THA, we determined the number of walking cycles of 105 patients in different age groups by decades. Patients on average performed 6144 walking cycles per day (2.24 million cycles per year). Men were more active than women. The highest activity occurred in patients between 50 and 59 years of age, with a constant decrease in activity with advancing age. However, within age groups, we observed up to sixfold differences in the number of walking cycles per day. In addition to declining activity with advancing age, higher body mass index correlated with lower step counts. The high mean measured number of walking cycles, which were even higher than those reported for subjects without an arthroplasty, suggests patients benefit from THA. Female gender, advanced age, and obesity correlated with lower activity. Owing to the high intragroup variability of our results, preoperative evaluation of patient activity levels, individual patient factors, and patient demands, should be considered in clinical practice.
Introduction
After THA, wear of the bearing surfaces and subsequent debris-induced osteolysis are the main causes for aseptic loosening [4, 6, 12]. Although different variables such as gender, weight, diagnosis, and biomechanical reconstruction of the affected joint might influence the wear rate [13], orthopaedic surgeons often select the type of implant and particularly the bearing couple according to patients’ age [13]. This practice is based on the assumption of decreased time of use with shorter life expectancy and lower activity levels of older patients, factors that would lead to decreased wear with advancing age. Furthermore, economic considerations may influence decisions related to more expensive hard-on-hard bearings, although a higher wear rate of traditional non-crosslinked polyethylene liners has been observed [12]. Schmalzried et al. [13] reported wear is dependent predominantly on the number of walking cycles and not time of use. Therefore, the choice of hard-on-hard bearings for younger patients with a longer life expectancy and consequentially more walking cycles during the entire lifetime seems justified, although activity levels undoubtedly can be decreased owing to other variables [8, 14–16, 19]. Choosing implant components using advanced age alone as a surrogate for shorter time of use and lower activity levels is questionable considering activity levels can differ as much as 45-fold in patients approximately 70 years of age [13, 21]. In addition, life expectancy of older people and their demand for leisure activities have been increasing [7, 20]. Although pedometers may underestimate the step count [11, 13, 14, 17], studies using such a device have shown a correlation of declining activity with age [3, 8, 13, 14, 16, 21, 22].
Therefore, we determined (1) the activity levels of patients after THA using a more accurate device [17] with focus on development of activity with advancing age by decade, (2) the variability within age groups, and (3) whether gender, body mass index (BMI), type of prosthesis, and preoperative diagnosis correlated with the measured walking cycles.
Materials and Methods
We studied the activity levels of 105 patients (45 men, 60 women) at least 1 year after THA (mean, 3.4 years; range, 1–6 years). Two hundred fifteen patients were invited to participate in the study, and of them, 105 (49%) agreed to do so. All patients had a well-functioning primary THA without perioperative or postoperative complications. We assessed the influence of different variables (age at the time of surgery, gender, BMI, preoperative diagnosis, and type of prosthesis) on activity. According to the patient characteristics, we assigned the patients to different subgroups of these variables (Table 1). The patients’ ages ranged between 32 and 79 years (mean, 57.6 years; standard deviation [SD], 11.3 years) at the time of surgery. Twenty-two patients were younger than 50 years, 41 were 50 to 59 years old, 23 were 60 to 69 years old, and 19 were 70 to 79 years old. The mean BMI was 27.4 kg/m2 (range, 20–40 kg/m2; SD, 4.1). The preoperative diagnoses were osteoarthritis in 71 patients, developmental dysplasia of the hip in 20, osteonecrosis in seven, and posttraumatic osteoarthritis in four. There was one case of femoral neck fracture and one secondary arthritis after bacterial infection.
Table 1.
Patient demographics and measured walking cycles according to patient factor
| Demographic | Number of patients (n = 105) | Walking cycles/day | Standard deviation (cycles/day) | Walking cycles/year | Standard deviation (cycles/year) |
|---|---|---|---|---|---|
| Age | |||||
| < 50 years | 22 | 6598 | 1825 | 2,408,170 | 666,294 |
| 50–59 years | 41 | 6722 | 1896 | 2,453,485 | 692,026 |
| 60–69 years | 23 | 5900 | 2447 | 2,153,341 | 893,168 |
| 70–79 years | 19 | 4669 | 1808 | 1,704,300 | 659,783 |
| Gender | |||||
| Male | 45 | 6881 | 2061 | 2,511,719 | 752,263 |
| Female | 60 | 5592 | 1998 | 2,040,898 | 729,297 |
| Prosthesis | |||||
| Resurfacing | 7 | 6599 | 2503 | 2,408,687 | 913,423 |
| Cementless | 62 | 6576 | 1899 | 2,400,140 | 693,174 |
| Hybrid | 20 | 6109 | 2094 | 2,229,730 | 764,230 |
| Cemented | 16 | 4318 | 1966 | 1,576,070 | 717,593 |
| Body mass index | |||||
| < 24 kg/m2 | 26 | 6308 | 2198 | 2,302,420 | 802,270 |
| 25–29 kg/m2 | 52 | 6316 | 2170 | 2,305,340 | 792,050 |
| > 30 kg/m2 | 27 | 5657 | 1919 | 2,064,805 | 700,435 |
Different types of implants and fixation techniques were used. Most patients received a cementless stem with a press-fit acetabular component (n = 62). Hybrid fixation with a cemented stem and a press-fit cup was used in 20 patients and cemented fixation in 16 patients. Seven patients received a total hip resurfacing arthroplasty (Table 1).
All patients were provided with a SAM (StepWatch™ Activity Monitor; Ortho Care Innovations, Washington, DC) to measure the number of walking cycles per day; a walking cycle consists of two steps. SAM reportedly is more accurate than pedometers [17]. This device contains a two-dimensional accelerometer and was worn at the ankle during the entire day (Fig. 1).
Fig. 1.
A photograph shows the StepWatch™ Activity Monitor (SAM) worn at the ankle.
The study period varied between 5 and 14 days (mean, 9 days). Collected data were downloaded from the accelerometer by an infrared docking station and analyzed with the provided software. For the measured loading cycles, the mean, SD, minimum, and maximum were calculated. To decide which statistical tests were appropriate, the data structure was evaluated and tested with the Kolmogorov-Smirnov test for normal distribution. We performed univariate variance analysis to assess the influence of age, gender, BMI, type of prosthesis, and preoperative diagnosis on patient activity. With paired comparisons, the subgroups of these variables were matched to each other. Subsequently the post hoc Bonferroni test was used to analyze differences in activity levels between these subgroups (eg, different age groups). As type of implanted prosthesis (uncemented, hybrid, cemented, surface replacement) could be influenced by the age of the patient [13], we performed a covariance analysis with age as a covariate to adjust for age. Effect sizes (d) also were determined to describe the extent of difference between the subgroups in a standardized manner. Regression analyses were performed to analyze the influence of quantitative variables (age, BMI) on the measured loading cycles. SPSS® for Windows® 15.0 (SPSS Inc, Chicago, IL) was used for the statistical analysis.
Results
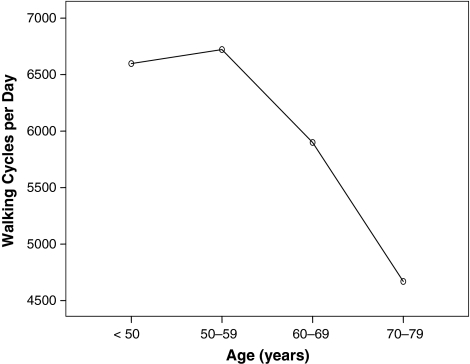
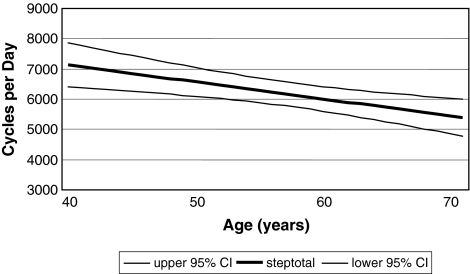
Patients on average walked 6144 cycles/day (range, 1207–16,450 cycles/day), which corresponds to 2.24 million cycles/year. We observed differences (p = 0.003) in counted mean walking cycles/day across the age groups. Patients younger than 50 years (p = 0.016; d = 1.06) and between 50 and 59 years (p = 0.002; d = 1.1) performed more walking cycles than patients 70 years or older. Thus, patients younger than 50 years (6598 cycles/day) and between 50 and 59 years (6722 cycles/day) had similar (p = 1.00) activity levels, whereas the number of measured walking cycles declined with advancing age (patients 60 to 69 years: 5900 cycles/day; patients 70 years and older: 4669 cycles/day) (Fig. 2). This correlation also was confirmed in regression analysis (p = 0.002; R2[corr] = 0.081; walking cycles per year = −56.2 × age + 9382) (Fig. 3).
Fig. 2.
A graph shows the highest number of walking cycles per day in patients between 50 and 59 years and subsequent decline with advancing age.
Fig. 3.
The correlation of declining activity with advancing age in regression analysis (walking cycles per year = −56.2 × age + 9382) is shown. CI = confidence interval.
The SD from the mean number of walking cycles of different age groups was relatively high (Table 1). This variability in activity was especially evident in the older age groups. For 60- to 69-year-old patients, we observed a sixfold deviation from the mean number of measured walking cycles.
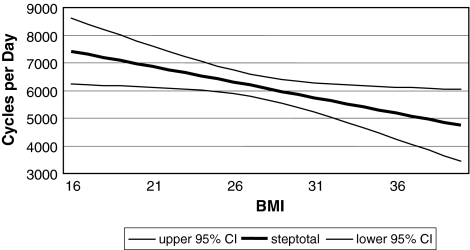
At a similar average age (57.5 and 57.7 years), men were more active (p = 0.002; d = 0.64) than women with 6881 cycles/day compared with 5592 cycles/day. BMI correlated with activity in the regression analysis (p = 0.025; R2[corr] = 0.038; walking cycles per year = −111.85 × BMI + 9212) (Fig. 4). Prosthesis type influenced (p = 0.001) activity level. Patients with cementless implants performed a higher (p = 0.001; d = 1.18) number of mean walking cycles per day compared with patients with cemented prostheses (6576 versus 4318 cycles/day, respectively); with age as a covariate, however, there was no correlation (p = 0.112) between prosthesis type and activity. We observed no correlation between the diagnosis that led to the THA and measured walking cycles (p = 0.649). Probability values for all comparisons are provided (Table 2).
Fig. 4.
A graph shows the correlation of lower activity with higher BMI in regression analysis (walking cycles per year = −111.85 × BMI + 9212). CI = confidence interval.
Table 2.
Probability values of statistical comparisons
| Variable | Subgroup 1 | Subgroup 2 | p Value |
|---|---|---|---|
| Age | < 50 years | 50–59 years | 1.000 |
| < 50 years | 60–69 years | 1.000 | |
| < 50 years | > 70 years | 0.016 | |
| 50–59 years | 60–69 years | 0.707 | |
| 50–59 years | > 70 years | 0.002 | |
| 60–69 years | > 70 years | 0.300 | |
| Gender | Male | Female | 0.002 |
| Body mass index | < 25 kg/m2 | 25–29 kg/m2 | 1.000 |
| < 25 kg/m2 | > 30 kg/m2 | 0.796 | |
| 25–29 kg/m2 | > 30 kg/m2 | 0.577 | |
| Type of prosthesis | Cementless | Cemented | 0.001 |
| Cementless | Hybrid | 1.000 | |
| Cementless | Surface replacement | 1.000 | |
| Surface replacement | Cemented | 0.077 | |
| Surface replacement | Hybrid | 1.000 | |
| Hybrid | Cemented | 0.051 | |
| Diagnosis | Osteoarthritis | Dysplasia | 1.000 |
| Osteoarthritis | Posttraumatic | 1.000 | |
| Osteoarthritis | Osteonecrosis | 1.000 | |
| Osteoarthritis | Secondary arthritis | 1.000 | |
| Osteoarthritis | Fracture | 1.000 | |
| Dysplasia | Posttraumatic | 1.000 | |
| Dysplasia | Osteonecrosis | 1.000 | |
| Dysplasia | Secondary arthritis | 1.000 | |
| Dysplasia | Fracture | 1.000 | |
| Posttraumatic | Osteonecrosis | 1.000 | |
| Posttraumatic | Secondary arthritis | 1.000 | |
| Posttraumatic | Fracture | 1.000 | |
| Osteonecrosis | Secondary arthritis | 1.000 | |
| Osteonecrosis | Fracture | 1.000 | |
| Secondary arthritis | Fracture | 1.000 |
Discussion
Assessment of patient activity after THA as a function of generated walking cycles, which predicts wear [13], is an important aspect in clinical decision making before THA. The types of implant and bearing surface often are chosen in relation to patients’ age as a surrogate for activity [13, 22]. However, other factors influence activity apart from age [8, 14–16, 19]. Some studies have used pedometers, which underestimate activity levels [11, 13, 14, 17]. Therefore, we determined (1) the activity levels of patients after THA using a more accurate device [17] with focus on development of activity with advancing age by decade, (2) the variability within age groups, and (3) whether gender, BMI, type of prosthesis, and preoperative diagnosis correlated with the measured walking cycles.
We identified subjects by contacting patients at least 1 year after surgery. Of the 215 invited, 105 agreed to participate. This might lead to a selection of satisfied and motivated patients. As we excluded THA with perioperative complications, which could influence patient activity levels, such a selection might be amplified. Consequently, the data obtained in this study might overestimate activity levels in a nonselected patient population. However, because this selection affects all our patients, comparisons between subgroups should provide valid results. Lack of compliance in wearing the monitor also could underestimate activity levels. To minimize recording failures, each patient was instructed personally in the use of the device by one of the authors. Patients also reported their daily activities in a diary. Despite these efforts, we still cannot entirely exclude a possible influence of inadequate compliance. We presume patient activity can be assessed with measuring the walking cycles as the UCLA activity score (which considers various activities) correlates with counted steps after THA [22]. To interpret our data, accuracy of the measuring device is another important factor. Comparing activity in 31 patients with a pedometer and SAM, Schmalzried et al. [13] noted approximately 50% more gait cycles according to SAM. Shepherd et al. [17] analyzed the accuracy of these two measuring devices, comparing the reported steps with those determined by an escorting investigator using a handheld counter. With a mean error of 0.54%, they reported higher accuracy with SAM than with the pedometers (2.82%). This higher accuracy occurred across all subjects and all activities, including briskly and slowly walking and ascending and descending stairs [17]. Correspondingly, it is unlikely walking style of the subjects or different daily activities compromise the accuracy of results obtained with SAM. Explanations for the higher accuracy of SAM could be a higher sensitivity of the two-dimensional accelerometer of SAM, whereas the pendulum of the pedometer might be less precise. Also different positioning of the devices could influence their accuracy. The pedometer is fixed at the waist, which was considered problematic especially in obese subjects with soft tissue hindering the detection of pelvic oscillations and a higher error probability compared with SAM [17]. As the latter is worn at the ankle (Fig. 1), a greater amplitude during the stride might facilitate a more accurate recording of walking cycles. Finally, we did not consider social and occupational factors in our analysis although these influence activity. We suspect these sorts of factors, along with comorbidities, explain the high variance of our data; age explained 8.1% of the variability and BMI 3.8%.
One to 6 years after THA, we found high activity levels in our patients. With a mean of 2.24 million cycles/year, we observed even higher activity levels compared with results reported in subjects without an arthroplasty [1, 15, 16]. In these studies, 3000 (range not given) [15], 3350 (range, 1820–4880) [1], and 4830 (range, 600–17,750) [16] mean walking cycles/day have been recorded, which are less than the 6144 (range, 1207–16,450) determined in our study. Differences in study populations and the use of a pedometer, which underestimates the step count compared with the SAM [11, 13, 14, 17], in the cited studies [1, 15, 16] limit direct comparison with our data. This is also true for the comparison of our results with those in studies involving patients undergoing arthroplasty reporting between 1988 and 3398 walking cycles/day [8, 13, 14, 18, 21, 22]. A previous SAM study [13], however, reported 5275 average walking cycles/day (range, 1737–11,805) in patients after THA, which is in the same range as our results and corresponds to 1.9 million walking cycles/year.
Age influences activity levels [1, 8, 10, 14, 15, 19, 21, 22]. We found activity as reflected by walking cycles reached a maximum in our 50- to 59-year-old patients who had THA and subsequently declined steadily with advancing age (Fig. 2). This finding is supported by the results of studies conducted with a pedometer [3, 8, 13, 14, 16, 21, 22]. We suspect the lower activity levels in younger patients is a reflection of their sedentary lifestyle attributable to long-standing preexisting hip disorders, such as hip dysplasia in 36% of patients, which led to THA early in life. Higher activity levels of patients 50 to 59 years of age likely reflects disease of more recent onset because only 27% had longer preexisting hip disorders and the subsequent continuous decline can be explained by different demands in professional life or retirement and decreasing overall health with advancing age.
Although we established differences in activity regarding age, great variability in each group should be considered when interpreting our results. This corresponds to results in the literature with ninefold to 45-fold reported differences [9, 14, 22]. An increased variability of activity with older age is likely the result of higher variability in physical ability attributable to comorbidities such as cardiopulmonary and musculoskeletal disorders of patients with advancing age.
The literature is unclear regarding whether gender predicts activity level. Although Seedhom and Wallbridge [16] observed a higher step count in female patients (4769 mean walking cycles/day for males and 4920 for females), Schmalzried et al. [14] and Zahiri et al. [22] reported higher activity in male patients after THA (2790 and 2927 mean walking cycles/day for males and 2182 for females). We found a greater number of walking cycles in male patients than in female patients at a similar age. Although we cannot provide an explanation for this difference, higher activity of patients undergoing THA may be the reason men are at higher risk for revision surgery [2, 10].
Our data confirm the literature [8, 14] suggesting there is a lower activity with a higher BMI. Whether this finding can be explained by obesity leading to inactivity or vice versa is unclear [5]. However, low activity with higher BMI may explain why wear in obese patients is not necessarily increased [8]. The elevated risk of revision of THA in overweight patients [2, 10] may be the result of factors other than wear.
Higher numbers of walking cycles in patients with hip resurfacing or cementless fixation of conventional THAs are attributable to the younger age of patients with such implants (60 of 63 patients younger than 60 years) compared with older patients having at least partially cemented prostheses (33 of 42 patients older than 60 years). Accordingly, we observed no correlation between type of prosthesis and activity after adjusting for age as a covariate. Younger patients with a better general health status probably will be more active compared with patients with comorbidities. Therefore, not the implant, but selection of the implant by the surgeon in relation to age and assessed activity most likely explains differences between the prosthesis types. The choice of prosthesis type and fixation also is influenced by demands of patients on activities and consequently on the prosthesis.
Contrary to our expectations, we did not observe a relationship between preoperative diagnosis and activity levels after THA. Patients with primary osteoarthritis and developmental hip dysplasia had similar activity levels. Age at the time of surgery and diagnosis-related way of living can overlap in their potentially diverse influence on activity.
Patients benefit from THA as indicated by an average of 2.24 million walking cycles 1 to 6 years after implantation. This high number of load changes exceeds currently accepted tribologic test guidelines by one million cycles per year [12, 13, 16]. We suggest readjusting these guidelines to the results of Schmalzried et al. [13] with 1.9 million walking cycles and with our findings. This is substantiated by the higher accuracy of SAM [17], which was used in these studies. Advanced age, female gender, and higher BMI are associated with lower activity levels. Taking into account the large variability of our results, the choice of implant, bearing couple, and type of fixation should not be made based solely on these factors. Individual patient activity should be considered by the surgeon.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at University of Heidelberg.
References
- 1.Bassey EJ, Dallosso HM, Fentem PH, Irving JM, Patrick JM. Validation of a simple mechanical accelerometer (pedometer) for the estimation of walking activity. Eur J Appl Physiol Occup Physiol. 1987;56:323–330. [DOI] [PubMed]
- 2.Flugsrud GB, Nordsletten L, Espehaug B, Havelin LI, Meyer HE. The effect of middle-age body weight and physical activity on the risk of early revision hip arthroplasty. Acta Orthop. 2007;78:99–107. [DOI] [PubMed]
- 3.Goldsmith AA, Dowson D, Wroblewski BM, Siney BA, Fleming PA, Lane JM, Stone MH, Walker R. Comparative study of the activity of total hip arthroplasty patients and normal subjects. J Arthroplasty. 2001;16:613–619. [DOI] [PubMed]
- 4.Harris WH. Wear and periprosthetic osteolysis. Clin Orthop Relat Res. 2001;393:66–70. [DOI] [PubMed]
- 5.Heisel C, Silva M, dela Rosa MA, Schmalzried TP. The effects of lower-extremity total joint replacement for arthritis on obesity. Orthopedics. 2005;28:157–159. [DOI] [PubMed]
- 6.Kabo MJ, Gebhard JS, Loren G, Amstutz HC. In vivo wear of polyethylene acetabular components. J Bone Joint Surg Br. 1993;75:254–258. [DOI] [PubMed]
- 7.Mason JB. The new demands by patients in the modern era of total joint arthroplasty: a point of view. Clin Orthop Relat Res. 2008;446:146–152. [DOI] [PMC free article] [PubMed]
- 8.McClung CD, Zahiri CA, Higa JK, Amstutz HC, Schmalzried TP. Relationship between body mass index and activity in hip or knee arthroplasty patients. J Orthop Res. 2000;18:35–39. [DOI] [PubMed]
- 9.Morlock M, Schneider E, Bluhm A, Vollmer M, Bergmann G, Müller V, Honl M. Duration and frequency of every day activities in total hip patients. J Biomech. 2001;34:873–881. [DOI] [PubMed]
- 10.Münger P, Röder C, Ackermann-Liebrich U, Busato A. Patient-related risk factors leading to aseptic stem loosening in total hip arthroplasty. Acta Orthop. 2006;77:567–574. [DOI] [PubMed]
- 11.Saris WH, Binkhorst RA. The use of pedometer and actometer in studying daily physical activity in man. Part II: Validity of pedometer and actometer measuring the daily physical activity. Eur J Appl Physiol Occup Physiol. 1977;37:229–235. [DOI] [PubMed]
- 12.Schmalzried TP, Callaghan JJ. Wear in total hip and knee replacements. J Bone Joint Surg Am. 1999;81:115–136. [DOI] [PubMed]
- 13.Schmalzried TP, Shepherd EF, Dorey FJ, Jackson WO, dela Rosa M, Fa’vae F, McKellop HA, McClung CD, Martell J, Moreland JR, Amstutz HC. Wear is a function of use, not time. Clin Orthop Relat Res. 2000;381:36–46. [DOI] [PubMed]
- 14.Schmalzried TP, Szuszczewicz ES, Northfield MR, Akizuki KH, Frankel RE, Belcher G, Amstutz HC. Quantitative assessment of walking activity after total hip or knee replacement. J Bone Joint Surg Am. 1998;80:54–59. [DOI] [PubMed]
- 15.Seedhom BB, Dowson D, Wright V. Wear of solid phase formed high density polyethylene in relation to the life of artificial hips and knees. Wear. 1973;24:35–51. [DOI]
- 16.Seedhom BB, Wallbridge NC. Walking activities and wear of prostheses. Ann Rheum Dis. 1985;44:838–843. [DOI] [PMC free article] [PubMed]
- 17.Shepherd EF, Toloza E, McClung CD, Schmalzried TP. Step activity monitor: increased accuracy in quantifying ambulatory activity. J Orthop Res. 1999;17:703–708. [DOI] [PubMed]
- 18.Silva M, McClung CD, dela Rosa MA, Dorey FJ, Schmalzried TP. Activity sampling in the assessment of patients with total joint arthroplasty. J Arthroplasty. 2005;20:487–491. [DOI] [PubMed]
- 19.Stevens M, Wagenmakers R, Groothoff JW, Bulstra SK, van den Akker-Scheek I, Zijlstra W. Physical activity behavior after total hip arthroplasty (THA): a prediction based on patient characteristics. Patient Educ Couns. 2007;69:196–199. [DOI] [PubMed]
- 20.Suckel A, Best R. [Golf with total joint replacement of the hip and knee] [in German]. Sportverletz Sportschaden. 2006;20:127–131. [DOI] [PubMed]
- 21.Wallbridge N, Dowson D. The walking activity of patients with artificial hip joints. Eng Med. 1982;11:95–96. [DOI] [PubMed]
- 22.Zahiri CA, Schmalzried TP, Szuszczewicz ES, Amstutz HC. Assessing activity in joint replacement patients. J Arthroplasty. 1998;13:890–895. [DOI] [PubMed]