Abstract
Nonsteroidal antiinflammatory drugs (NSAIDs) are used to reduce inflammatory response and pain. These drugs have been reported to impair bone metabolism. Parecoxib, a specific COX-2 inhibitor, exerts an inhibitory effect on the mineralization of fracture callus after a tibial fracture in rats. Decreased bone mineral density (BMD) at a fracture site may indicate impairment of early healing, casting doubt on the safety of using COX-2 inhibitors during the early treatment of diaphyseal fractures. Forty-two female Wistar rats were randomly allocated to three groups. They were given parecoxib, indomethacin, or saline intraperitoneally for 7 days after being subjected to a closed tibial fracture stabilized with an intramedullary nail. Two and 3 weeks after surgery, the bone density at the fracture site was measured using dual energy xray absorptiometry (DEXA). Three weeks after the operation the rats were euthanized and the healing fractures were mechanically tested in three-point cantilever bending. Parecoxib decreased BMD at the fracture site for 3 weeks after fracture, indomethacin for 2 weeks. Both parecoxib and indomethacin reduced the ultimate bending moment and the bending stiffness of the healing fractures after 3 weeks. These results suggest COX inhibitors should be avoided in the early phase after fractures.
Introduction
Since the mid 1970s the effects of conventional nonsteroidal antiinflammatory drugs (NSAIDs), general cyclooxygenase (COX) inhibitors, on bone metabolism and fracture healing have been studied. These drugs inhibit disordered osteoclast activity, retard woven-fibered bone tissue formation, and impair fracture healing [1, 2, 23, 34, 37, 52, 54, 62, 63]. COX inhibitors are, however, very efficient drugs in the treatment of postoperative inflammation and pain in orthopaedic trauma and surgery. Several studies have proven their superior effects compared to other analgesics like acetaminophen, codeine, and opioids [10, 11, 25, 44, 48]. It has been demonstrated that the analgesic and antiinflammatory effects of conventional COX inhibitors are attributed to the inhibition of COX-2, which is the COX isoform involved in the induction of pain and inflammation [18, 57].
In the management of perioperative pain, the newer selective COX-2 inhibitors have lately been introduced with potential advantages. In contrast to the conventional COX inhibitors, selective COX-2 inhibitors do not impair platelet function. It is COX-1 that mediates platelet activation and aggregation by the generation of thromboxane A2 [26]. Due to this, COX-2 inhibitors may be safer concerning perioperative bleeding [7, 31, 40, 41, 68]. COX-2 inhibitors can therefore be given preoperatively, and it has been shown that COX-2 inhibition effectively reduce postoperative pain [14, 27].
The effects of COX-2 inhibitors on bone metabolism and fracture healing have not been adequately elucidated, but it has been demonstrated that COX-2 is required for both intramembranous and endochondral bone formation [69], and COX-2 function is essential for fracture healing [59]. A recent study also demonstrated that the lack of COX-2 caused a detrimental effect on bone graft healing (Unpublished data; Xie C, Schwarts EM, Guldberg RE, O’Keefe RJ, Zhang X. COX-2 deficient microenvironment impairs periosteal progenitor cell activation and differentiation in cortical bone grafting. 53rd Annual Meeting of the Orthopaedic Research Society 2007; Poster no 0416). Several studies indicate that COX-2 inhibitors may delay fracture healing [6, 21, 22, 28, 30, 60], and concerns have been raised using COX inhibitors in orthopaedic fracture treatment due to these possible negative effects [3, 5, 12, 19].
In an earlier study we demonstrated that parecoxib given perioperatively for 1 week in doses analogous to those used in humans reduced the bone mineral density (BMD) at the fracture site in rats [16]. The difference in BMD between the animals given parecoxib and placebo, however, decreased with time and the mechanical strength after 6 weeks was not substantially reduced among the parecoxib animals. Parecoxib is a selective and potent inhibitor of COX-2 [65] for parenteral use. Due to this and the lack of antiplatelet effects, parecoxib has the potential to become the antiinflammatory drug of choice for parenteral treatment of postoperative pain in the future [39, 55, 61]. The conventional COX inhibitor indomethacin has a much higher potency against COX-1 than COX-2 [67], and is proven to delay fracture healing [54, 59, 63].
The present study was designed to investigate if parecoxib delays early fracture healing and to compare its effects with indomethacin. We asked whether (1) parecoxib and indomethacin reduce BMD at the fracture site in healing fractures and (2) parecoxib and indomethacin have negative effects on the mechanical properties of healing fractures in the early fracture healing process.
Materials and Methods
A total of 42 female Wistar rats (Harland, Horst, Netherlands) with mean weight of 226.5 g were randomly allocated into three groups; one parecoxib group, one indomethacin group, and one placebo group of 14 animals each. The animals were kept in pairs in wire-topped plastic cages with free access to tap water and standard laboratory rodent chow (with 1.1% calcium, 0.8% phosphorus, and 1500 IU/kg vitamin D3) in a 12-hour light and 12-hour dark cycle. For surgery and bone density measurements, the animals were anesthetized with a combination of Hypnorm (fluanisone 5 mg/mL, fentanyl citrate 0.1575 mg/mL, Jansen Pharmaceutica BV, Beerse, Belgium) and Dormicum (midazolam 2.5 mg/mL, Hoffmann La Roche, Basel, Switzerland) administered subcutaneously in a dose of 0.2 mL/100 g body weight for surgery and 0.15 mL/100 g body weight for bone density measurements. Temgesic (buprenorphin 0.3 mg/mL, Schering-Plough, Kenilworth, NJ), 0.005 mg/100 g body weight was given subcutaneously immediately after surgery and on the first 2 days after operations. The experiments conformed to the Norwegian Council of Animal Research Code for the Care and Use of Animals for Experimental Purposes.
Surgery was performed in an operation theatre inside the animal laboratory after shaving and aseptic wash. An incision was made aligned with the patellar tendon of the right tibia of anesthetized animals. An 18-G cannula (BD venflon Pro 18 GA 1.26 IN [1.3 × 32 mm]) was inserted into the medullary canal through the anterior tibial plateau just medial to the patellar tendon in front of the cruciate ligaments. A 19-G cannula (Braun Sterican 19 G/1.5 IN [1.20 × 40 mm]) and the stylet of a 22-G spinal cannula (Braun Spinocan 22 G × 3 ½ IN [0.73 × 88 mm]) were then inserted into the largest cannula and the stylet advanced down through the canal towards the ankle joint. The outer two cannulae were advanced as far distally as possible by combined axial pressure and rotation, and then withdrawn to the proximal part of the tibia. With the stylet remaining inside, the tibia was subjected to a standardized closed midshaft fracture using a specially designed fracture forceps [20]. The two cannulae were readvanced distally over the stylet past the fracture into the distal tibial metaphysis. Comparing the alignment of the foot and the thigh confirmed correct rotation. The nail was cut flush with the tibial plateau and the skin closed with one suture. All fractures were stable at the end of the operation (Fig. 1).
Fig. 1.
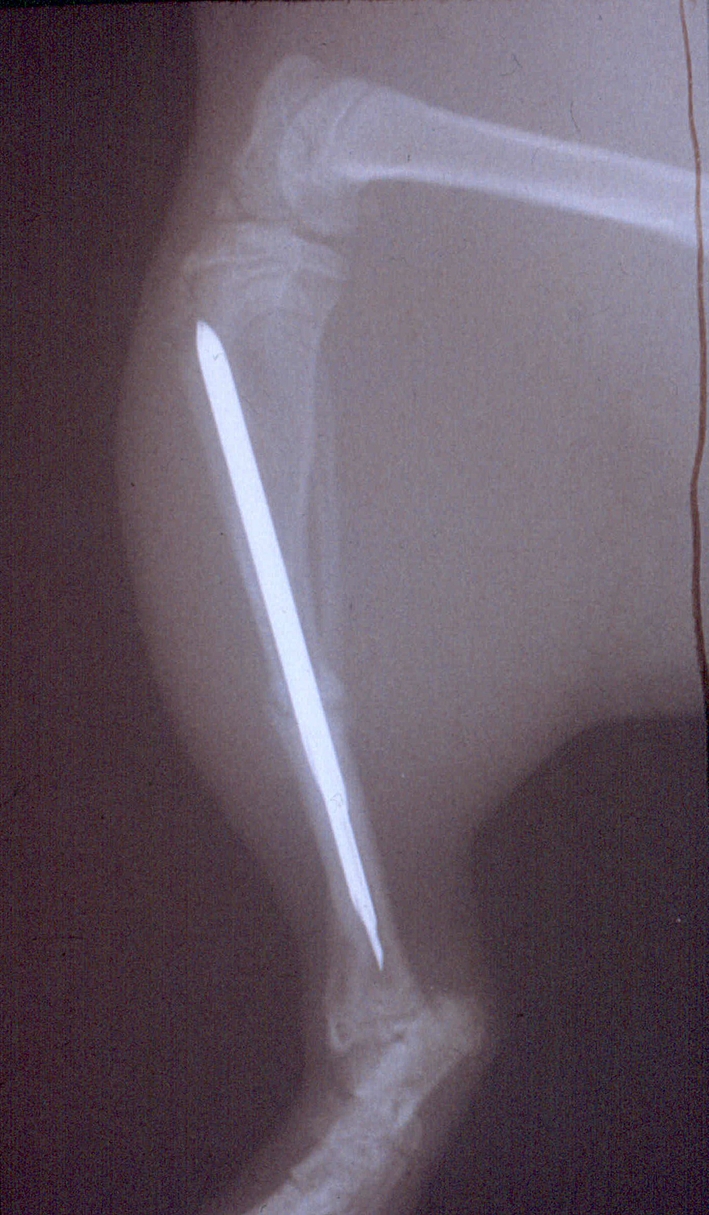
The tibial fracture was stabilized with an intramedullary nail consisting of two cannulae and one stylet.
All animals in the parecoxib group were given Dynastat (parecoxib, Pfizer, Pharmacia Europe EEIG, Kent, Great Britain) 0.05 mg/100 g body weight intraperitoneally in the morning and the evening for 7 days, the first injection immediately before surgery. The animals in the indomethacin group were given Confortid (indomethacin, Dumex-Alpharma A/S, Copenhagen, Denmark) 0.0625 mg/100 g body weight and the animals in the placebo group were given a corresponding volume of saline intraperitoneally at the same time points. The doses for parecoxib and indomethacin were equivalent with doses recommended for human use and proved to be adequate in earlier studies [16, 17].
Two and 3 weeks after surgery the bone density at the fracture site was measured in anesthetized animals using dual energy xray absorptiometry (DEXA) with a bone densitometer designed for measurements on small animals, Lunar PIXIMUS (Lunar, Madison, WI). The smallest possible quadratic area of measurement with the PIXIMUS software, 11 × 11 pixels, was placed aligned with the tibia over the fracture including the cortical bone on the medial side, the callus and the nail. The procedure was performed twice, and the mean BMD value was calculated. The nail was measured alone, and its constant value was subtracted from the calculated BMD values to find the real BMD at the fracture site.
All 42 animals were euthanized by a pentobarbital (pentobarbitalnatrium vet, 100 mg/mL, Norsk Medicinaldepot) overdose. Both tibias were resected, with care taken to leave the periosteum over the fracture. No animals or tibias were excluded. The bones were frozen wet in Ringer-acetate solution (Fresenius Kabi, Oslo, Norway) at −20°C until additional processing [43].
For mechanical testing the tibias were thawed in Ringer-acetate solution. Each fractured tibia was then loaded until fracture in an MTS machine (Model 858 Minin Bionix with Test Star II controller, MTS Systems, Corporation Eden Prairie, MN) in a three-point ventral cantilever bending at a rate of 7.2°/s with the fulcrum placed over the fracture callus. The corresponding left tibia was fractured at the same level in the same manner. The ultimate bending moment, ultimate energy absorption, bending stiffness, and deflection were registered in TestStar II software (MTS Systems) and then calculated in Microsoft Excel software (Microsoft Corporation, Redmond, WA). Nonunions were given nil values for moment, energy, and stiffness, and 86.4° for deflection (maximal bending when the test was terminated after 12 seconds). The ultimate bending moment and BMD at the fracture site were chosen as the primary outcome variables.
Statistical analyses were made in SPSS 16 for Mac (SPSS Inc., Chicago, IL). Results are given as mean values, and dispersion by one standard deviation (SD) and 95% confidence intervals. The groups were compared using analysis of variance (ANOVA) and Scheffe’s post hoc test (p < 0.05).
Results
We found an overall difference in bone density at the fracture sites between the groups (ANOVA) after both 2 weeks (p = 0.002) and 3 weeks (p = 0.043). BMD at the fracture site was 30% lower in the parecoxib group compared to the placebo group after 2 weeks (p = 0.004) and 24% lower after 3 weeks (p = 0.043), both significant differences using Scheffe’s post hoc test. BMD in the indomethacin group was 22% lower after 2 weeks (p = 0.021) and 14% lower after 3 weeks (p = 0.46) compared to the placebo group, a significant difference after 2 weeks. The differences were larger after 2 weeks compared to 3 weeks (Table 1).
Table 1.
Bone mineral density (BMD) at the fracture site after 2 and 3 weeks
| Time elapsed | Parecoxib (mean) n = 14 | Indomethacin (mean) n = 14 | Placebo (mean) n = 14 | Mean difference placebo versus parecoxib (post hoc) | Mean difference placebo versus indomethacin (post hoc) |
|---|---|---|---|---|---|
| 2 weeks (g/cm2) | 0.19 (SD 0.05) | 0.21 (SD 0.05) | 0.27 (SD 0.08) | 0.08 (CI 0.022–0.134) p = 0.004 |
0.06 (CI 0.084–0.120) p = 0.021 |
| 3 weeks (g/cm2) | 0.22 (SD 0.08) | 0.25 (SD 0.07) | 0.29 (SD 0.08) | 0.07 (CI 0.002–0.147) p = 0.043 |
0.04 (CI 0.033–0.112) p = 0.46 |
Groups compared using ANOVA and Scheffe’s post hoc test. Level of significance set at p < 0.05. Results are given as mean values, one standard deviation (SD) and 95% confidence interval of mean difference (CI).
Ultimate bending moment (p = 0.007) and stiffness (p = 0.003) were substantially reduced between the groups after 3 weeks (ANOVA). There were no differences in energy absorption (p = 0.36) and deflection (p = 0.055). Calculations of the bending moment needed for fracturing the tibias in this model, given by a mean ratio between the fractured right tibia and the intact left tibia showed over 60% reduction between the placebo group and the parecoxib group (p = 0.02) and the indomethacin group (p = 0.02) respectively (Scheffe’s post hoc test). Correspondingly, the stiffness was reduced over 80% for the parecoxib group (p = 0.01) and just below 80% for the indomethacin group (p = 0.01). The total energy in both intervention groups was reduced and the deflection was increased (Table 2). There was one nonunion in both the parecoxib group and the indomethacin group, and none in the placebo group.
Table 2.
Ratios for the mechanical properties between the fractured and intact tibia at 3 weeks after fracture and intramedullary nailing
| Property | Parecoxib (mean) n = 14 | Indomethacin (mean) n = 14 | Placebo (mean) n = 14 | Mean difference placebo versus parecoxib (post hoc) | Mean difference placebo versus indomethacin (post hoc) |
|---|---|---|---|---|---|
| Moment (ratio) | 0.11 (SD 0.16) | 0.10 (SD 0.09) | 0.29 (SD 0.22) | 0.18 (CI 0.019–0.334) | 0.18 (CI 0.026–0.340) |
| p = 0.02 | p = 0.02 | ||||
| Total energy (ratio) | 0.12 (SD 0.18) | 0.10 (SD 0.09) | 0.18 (SD 0.16) | 0.05 (CI −0.089–0.195) | 0.08 (CI −0.063–0.221) |
| p = 0.64 | p = 0.37 | ||||
| Stiffness (ratio) | 0.12 (SD 0.22) | 0.14 (SD 0.16) | 0.62 (SD 0.66) | 0.51 (CI 0.108–0.904) | 0.49 (CI 0.088–0.884) |
| p = 0.01 | p = 0.01 | ||||
| Deflection (ratio) | 1.61 (SD 0.74) | 1.68 (SD 0.91) | 1.04 (SD 0.53) | −0.57 (CI −1.286–0.147) | −0.64 (CI −1.360–0.073) |
| p = 0.14 | p = 0.09 |
Groups compared using ANOVA and Scheffe’s post hoc test. Level of significance set at p < 0.05. Results are given as mean values, one standard deviation (SD) and 95% confidence interval of mean difference (CI).
Discussion
Using a rat tibial fracture model, we have demonstrated that parecoxib given perioperatively for 1 week reduced the mechanical strength and BMD after 3 weeks. Accordingly, the mechanical strength in animals given indomethacin was reduced after 3 weeks, and BMD was reduced for 2 weeks.
A limitation of the present study might be the use of three-point cantilever bending test due to concerns related to possible deformation of the soft callus from the fulcrum. However, when doing the testing, the distal tibia is fixed, and the proximal fragment is bent in an anterior direction, the fulcrum is not moving or sagging into the callus. Three-point cantilever bending test gives standardized fractures and reliable results. Another limitation with our method concerning the BMD measurements was that we had to include the intramedullary nail. Due to this, the registered BMD values were artificially high. However, by subtracting the constant nail value, the real differences in fracture callus BMD between the groups could be reliably calculated.
We previously demonstrated that parecoxib exerted an inhibitory effect on the mineralization of fracture callus [16]. These findings were confirmed in the present study; parecoxib had an inhibitory effect on the mineralization in the fracture callus. We also found that indomethacin reduced BMD at the fracture site, but to a lesser degree.
Our findings correspond with Beck’s rat study (2003) where BMD in the healing area of a tibial osteotomy after 3 weeks was lower in animals given diclofenac, a conventional COX inhibitor, compared with animals given tramadol and placebo. In contrast to their study, where BMD was calculated from computer tomography performed after euthanization and nail removal, we were able to measure BMD on anesthetized animals during the study using a bone densitometer specifically designed for measurements on small animals. With this method, we were able to record the development of BMD and differences between the groups during the healing process.
After 3 weeks we found a reduction in the ultimate bending moment and the bending stiffness for both parecoxib- and indomethacin-treated animals compared to placebo. Also, we found reduction of BMD in parecoxib-treated animals after 3 weeks and in indomethacin-treated animals after 2 weeks. According to this, it seems likely that the bone mineralization is involved in the mechanical strength of the healing fractures. Rø et al. [54] concluded in their study in 1978 that the impaired mechanical strength after indomethacin treatment was not caused by an inhibition of collagen synthesis, but by production of lower-quality collagen. The results from our present study also suggest that the conventional COX inhibitors and COX-2 inhibitors interfere with the transformation of immature connective tissue to mineralized callus and bone. Since the osteoblasts produce alkaline phosphatase that initiate mineralization [53], our results may suggest inhibition of the osteoblasts as one of the mechanisms for delaying union.
However, it seems likely that the reason these drugs inhibit bone metabolism and fracture healing is more complex. It has been postulated that COX inhibitors’ detrimental effects on bone metabolism and fracture healing are due to inhibition of prostaglandin (PG) synthesis [54]. Vane [66] showed that the therapeutic effects of sodium salicylate and aspirin-like drugs, the precursors of COX inhibitors, were due to inhibition of the synthesis of PGs. Later, several studies elucidated the role of PGs in the bone healing process. A direct link between PGs and all the characteristic signs of inflammation was demonstrated [33], as well as increased release of PGs locally after a fracture [13]. The dead cells in the area cause an aseptic inflammatory response, and without this inflammation the bone resorption and formation necessary for healing cannot occur adequately [58]. Furthermore, PGs have a direct effect on bone resorption through increased osteoclastic activity [15, 38, 42, 46, 56] and also increase the replication and differentiation of the osteoblasts resulting in enhanced bone formation [42 , 46]. Thus, PGs are involved in regulation of the balance between bone resorption and bone formation [18, 36, 47].
The ability of COX inhibitors to suppress inflammation depends mostly on inhibition of the COX enzymes [66], and decreased production of prostaglandins elicited by COX inhibitors could potentially impair the bone healing process [33].
Concerning the bone metabolism and fracture healing, it is questioned whether COX-1 or COX-2 is the enzyme responsible for the impairing effects. It is demonstrated that COX-2 plays a critical role in bone resorption [49], and induction of COX-2 in osteoblasts is reported to be essential to the acute stress response in a bone remodeling system [51]. Additionally, cytokines associated with inflammation have been found to induce COX-2 when added to bone marrow cells in culture [64]. Furthermore, it is demonstrated that COX-2 is critically involved in bone repair and required for both intramembranous and endochondral bone formation in a study using mice genetically deficient for COX-1 and COX-2 enzyme (COX-1−/− and COX-2−/−) [69]. In another study involving COX-1−/− and COX-2−/− mice, the authors also conclude that COX-2 function is essential for bone healing [59]. Inflammation is present in the early phase after fracture, but does not occur during the later healing process. It was demonstrated that COX-2 mRNA levels showed peak expression during the first 2 weeks of fracture healing and then returned to basal levels by 3 weeks [29]. In a review article on heterotopic ossification (HO), which may represent a mechanism comparable to bone formation in the fracture healing process, it was concluded that indomethacin given for 7 to 11 days prevented HO and was the drug of choice at present [24]. This also supports that the initial inflammation is the critical phase of bone formation and gives further reason to believe that COX-2 inhibitors administered perioperatively even for only a few days will affect bone metabolism and be detrimental in the early phase of fracture healing.
In the present study, relatively large differences between the intervention groups and the placebo animals were found, whereas in a previous study [16] we found no differences in mechanical properties concerning parecoxib animals compared to placebo after 6 weeks, only a tendency with weaker fractures in animals treated with parecoxib. This indicates that the effects of COX-2 inhibition administered in relation to surgery delays fracture healing, but that the effects on the healing fractures decreases, or even disappears, with time.
This is in accordance with the findings of Gerstenfields et al. [28]. Even so, no definitive conclusions regarding clinical short term administration of COX inhibitors could be made on the basis of these experimental studies. In general, however, all fractures showing delayed union, have an increased risk to eventually end up in a non union. In a clinical setting this should be a concern, as short term administration of COX inhibitors might result in increased incidence of non unions, especially in long bone fractures [9].
During the last few years, several studies have demonstrated that COX-2 inhibitors impair fracture healing and suppress bone formation [6, 21, 30, 59, 60, 69]. Controversies still exist, however, regarding the potentially negative effects of the COX-2 inhibitors compared to the conventional COX inhibitors on bone healing and bone repair. The impairing effect on bone healing is less significant with COX-2-specific inhibitors. Gerstenfeldt et al. studied the effects of ketorolac and parecoxib [29]. Both had negative effects on fracture healing, but parecoxib only to a lesser degree. As pointed out by Aspenberg in a comment on this paper [4], this was probably caused by the fact that the animals were given parecoxib by oral gavage only once daily. Due to this, the blood concentration was probably too low much of the time to induce proper inhibition of the COX-2 enzyme. Additionally, male rats, in which COX-2 inhibitors have shown to have a short half-life and are quickly eliminated [32, 50], were used. In Brown et al. [8], there were similar findings; indomethacin delayed fracture healing at 4 weeks, but celecoxib, a specific COX-2 inhibitor, did not. Their use of male rats may be a likely explanation for not finding delayed fracture healing in the celecoxib animals. Recently Gerstenfeld et al. [28] interpreted their previous findings so that inhibition of COX-2 was associated with impaired fracture healing when the medication was prolonged for 21 or 35 days, but not when given for 7 days. Once again, their use of oral gavage once daily commencing 24 hours after surgery and their use of male rats could have resulted in less notable findings compared to female rats or humans. However, they showed that the impairing effects were reversible when the drugs were administered only for 7 days, and this corresponds with our findings.
Another recent study elucidated the effects of celecoxib on fracture healing [60]. Like our present study, they demonstrated that short-term treatment with a COX-2 inhibitor following fracture, delays healing. They also demonstrated that higher doses or longer duration of the celecoxib treatment affected bone healing even more.
The calculation of relevant parecoxib doses for rats is associated with some uncertainty. Due to the quick metabolism of the COX-2 inhibitors celecoxib and rofecoxib in rats [32, 50], Meunier and Aspenberg [45] administered parecoxib in as high doses as 6.4 mg/kg daily using subcutaneous mini pumps with continuous release to compensate for the fast metabolism. However, to our knowledge, the half-life of parecoxib in rats is unknown. Furthermore, no direct correlation is observed between the plasma concentration of COX inhibitors and the magnitude of the pharmacological effects in chronic inflammatory conditions, and different COX inhibitors may also have various potency [35]. Thus, the inhibitory effects on inflammation after fracture could differ between various COX inhibitors. We chose to administer 1 mg/kg daily to our rats, which is equal to the recommended human dosage, as this dosage has previously demonstrated a negative effect on bone mineralization.
The present study was designed to investigate the effects on bone mineralization and fracture healing of short-term perioperative treatment of parecoxib and indomethacin [16]. We used female rats to eliminate the problem with a short half-life for drugs involved, and to ensure the best absorption and sufficient blood concentration, the drug was administered intraperitoneally twice a day. The first injection was given immediately before surgery and prolonged for a week to mimic the use of COX inhibitors in humans. No differences between parecoxib and indomethacin were demonstrated concerning mechanical properties. Our findings, where parecoxib had a higher potential for reducing BMD, might support the presumption that inhibition of the COX-2 enzyme is responsible for impaired fracture healing.
Acknowledgments
We thank the Department of Comparative Medicine, Rikshospitalet-Radiumhospitalet Medical Centre, University of Oslo, Norway, for providing excellent animal facilities and enthusiastic personnel at our disposal.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Allen HL, Wase A, Bear WT. Indomethacin and aspirin: effect of nonsteroidal anti-inflammatory agents on the rate of fracture repair in the rat. Acta Orthop Scand. 1980;51:595–600. [DOI] [PubMed]
- 2.Altman RD, Latta LL, Keer R, Renfree K, Hornicek FJ, Banovac K. Effect of nonsteroidal antiinflammatory drugs on fracture healing: a laboratory study in rats. J Orthop Trauma. 1995;9:392–400. [DOI] [PubMed]
- 3.Aspenberg P. Avoid COX inhibitors after skeletal surgery! Acta Orthop Scand. 2002;73:489–490. [DOI] [PubMed]
- 4.Aspenberg P. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal antiinflammatory drugs. J Orthop Res. 2004;22:684; author reply 685. [DOI] [PubMed]
- 5.Beck A, Salem K, Krischak G, Kinzl L, Bischoff M, Schmelz A. Nonsteroidal anti-inflammatory drugs (NSAIDs) in the perioperative phase in traumatology and orthopedics effects on bone healing. Oper Orthop Traumatol. 2005;17:569–578. [DOI] [PubMed]
- 6.Bergenstock M, Min W, Simon AM, Sabatino C, O’Connor JP. A comparison between the effects of acetaminophen and celeCOXib on bone fracture healing in rats. J Orthop Trauma. 2005;19:717–723. [DOI] [PubMed]
- 7.Blaicher AM, Landsteiner HT, Zwerina J, Leitgeb U, Volf I, Hoerauf K. Effect of non-selective, non-steroidal anti-inflammatory drugs and cyclo-oxygenase-2 selective inhibitors on the PFA-100 closure time. Anaesthesia. 2004;59:1100–1103. [DOI] [PubMed]
- 8.Brown KM, Saunders MM, Kirsch T, Donahue HJ, Reid JS. Effect of COX-2-specific inhibition on fracture-healing in the rat femur. J Bone Joint Surg Am. 2004;86-A:116–123. [DOI] [PubMed]
- 9.Burd TA, Hughes MS, Anglen JO. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J Bone Joint Surg Br. 2003;85:700–705. [PubMed]
- 10.Dahl V, Dybvik T, Steen T, Aune AK, Rosenlund EK, Raeder JC. Ibuprofen vs. acetaminophen vs. ibuprofen and acetaminophen after arthroscopically assisted anterior cruciate ligament reconstruction. Eur J Anaesthesiol. 2004;21:471–475. [DOI] [PubMed]
- 11.Dahl V, Raeder JC, Drosdal S, Wathne O, Brynildsrud J. Prophylactic oral ibuprofen or ibuprofen-codeine versus placebo for postoperative pain after primary hip arthroplasty. Acta Anaesthesiol Scand. 1995;39:323–326. [DOI] [PubMed]
- 12.Dahners LE, Mullis BH. Effects of nonsteroidal anti-inflammatory drugs on bone formation and soft-tissue healing. J Am Acad Orthop Surg. 2004;12:139–143. [DOI] [PubMed]
- 13.Dekel S, Lenthall G, Francis MJ. Release of prostaglandins from bone and muscle after tibial fracture. An experimental study in rabbits. J Bone Joint Surg Br. 1981;63:185–189. [DOI] [PubMed]
- 14.Desjardins PJ, Grossman EH, Kuss ME, Talwalker S, Dhadda S, Baum D, Hubbard RC. The injectable cyclooxygenase-2-specific inhibitor pareCOXib sodium has analgesic efficacy when administered preoperatively. Anesth Analg. 2001;93:721–727. [DOI] [PubMed]
- 15.Dietrich JW, Goodson JM, Raisz LG. Stimulation of bone resorption by various prostaglandins in organ culture. Prostaglandins. 1975;10:231–240. [DOI] [PubMed]
- 16.Dimmen S, Nordsletten L, Engebretsen L, Steen H, Madsen JE. Negative effect of parecoxib on bone mineral during fracture healing in rats. Acta Orthop. 2008;79:438–444. [DOI] [PubMed]
- 17.Dimmen S, Nordsletten L, Engebretsen L, Steen H, Madsen JE. The effect of parecoxib and indometacin on tendon-to-bone healing in a bone tunnel: an experimental study in rats. J Bone Joint Surg Br. 2009;91:259–263. [DOI] [PubMed]
- 18.Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. Faseb J. 1998;12:1063–1073. [PubMed]
- 19.Einhorn TA. Do inhibitors of cyclooxygenase-2 impair bone healing? J Bone Miner Res. 2002;17:977–978. [DOI] [PubMed]
- 20.Ekeland A, Engesaeter LB, Langeland N. Mechanical properties of fractured and intact rat femora evaluated by bending, torsional and tensile tests. Acta Orthop Scand. 1981;52:605–613. [DOI] [PubMed]
- 21.Endo K, Sairyo K, Komatsubara S, Sasa T, Egawa H, Ogawa T, Yonekura D, Murakami R, Yasui N. Cyclooxygenase-2 inhibitor delays fracture healing in rats. Acta Orthop. 2005;76:470–474. [DOI] [PubMed]
- 22.Endo K, Sairyo K, Komatsubara S, Sasa T, Egawa H, Yonekura D, Adachi K, Ogawa T, Murakami R, Yasui N. Cyclooxygenase-2 inhibitor inhibits the fracture healing. J Physiol Anthropol Appl Human Sci. 2002;21:235–238. [DOI] [PubMed]
- 23.Engesaeter LB, Sudmann B, Sudmann E. Fracture healing in rats inhibited by locally administered indomethacin. Acta Orthop Scand. 1992;63:330–333. [DOI] [PubMed]
- 24.Fijn R, Koorevaar RT, Brouwers JR. Prevention of heterotopic ossification after total hip replacement with NSAIDs. Pharm World Sci. 2003;25:138–145. [DOI] [PubMed]
- 25.Fogarty DJ, O’Hanlon JJ, Milligan KR. Intramuscular ketorolac following total hip replacement with spinal anaesthesia and intrathecal morphine. Acta Anaesthesiol Scand. 1995;39:191–194. [DOI] [PubMed]
- 26.Funk CD, Funk LB, Kennedy ME, Pong AS, Fitzgerald GA. Human platelet/erythroleukemia cell prostaglandin G/H synthase: cDNA cloning, expression, and gene chromosomal assignment. Faseb J. 1991;5:2304–2312. [PubMed]
- 27.Gan TJ, Joshi GP, Viscusi E, Cheung RY, Dodge W, Fort JG, Chen C. Preoperative parenteral parecoxib and follow-up oral valdecoxib reduce length of stay and improve quality of patient recovery after laparoscopic cholecystectomy surgery. Anesth Analg. 2004;98:1665–1673. [DOI] [PubMed]
- 28.Gerstenfeld LC, Al-Ghawas M, Alkhiary YM, Cullinane DM, Krall EA, Fitch JL, Webb EG, Thiede MA, Einhorn TA. Selective and nonselective cyclooxygenase-2 inhibitors and experimental fracture-healing. Reversibility of effects after short-term treatment. J Bone Joint Surg Am. 2007;89:114–125. [DOI] [PubMed]
- 29.Gerstenfeld LC, Thiede M, Seibert K, Mielke C, Phippard D, Svagr B, Cullinane D, Einhorn TA. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2003;21:670–675. [DOI] [PubMed]
- 30.Goodman S, Ma T, Trindade M, Ikenoue T, Matsuura I, Wong N, Fox N, Genovese M, Regula D, Smith RL. COX-2 selective NSAID decreases bone ingrowth in vivo. J Orthop Res. 2002;20:1164–1169. [DOI] [PubMed]
- 31.Greenberg HE, Gottesdiener K, Huntington M, Wong P, Larson P, Wildonger L, Gillen L, Dorval E, Waldman SA. A new cyclooxygenase-2 inhibitor, rofecoxib (VIOXX), did not alter the antiplatelet effects of low-dose aspirin in healthy volunteers. J Clin Pharmacol. 2000;40:1509–1515. [PubMed]
- 32.Halpin RA, Geer LA, Zhang KE, Marks TM, Dean DC, Jones AN, Melillo D, Doss G, Vyas KP. The absorption, distribution, metabolism and excretion of rofecoxib, a potent and selective cyclooxygenase-2 inhibitor, in rats and dogs. Drug Metab Dispos. 2000;28:1244–1254. [PubMed]
- 33.Harder AT, An YH. The mechanisms of the inhibitory effects of nonsteroidal anti-inflammatory drugs on bone healing: a concise review. J Clin Pharmacol. 2003;43:807–815. [DOI] [PubMed]
- 34.Hogevold HE, Grogaard B, Reikeras O. Effects of short-term treatment with corticosteroids and indomethacin on bone healing. A mechanical study of osteotomies in rats. Acta Orthop Scand. 1992;63:607–611. [DOI] [PubMed]
- 35.Huntjens DR, Danhof M, Della Pasqua OE. Pharmacokinetic-pharmacodynamic correlations and biomarkers in the development of COX-2 inhibitors. Rheumatology (Oxford). 2005;44:846–859. [DOI] [PubMed]
- 36.Kawaguchi H, Pilbeam CC, Harrison JR, Raisz LG. The role of prostaglandins in the regulation of bone metabolism. Clin Orthop Relat Res. 1995;313:36–46. [PubMed]
- 37.Keller J, Bunger C, Andreassen TT, Bak B, Lucht U. Bone repair inhibited by indomethacin. Effects on bone metabolism and strength of rabbit osteotomies. Acta Orthop Scand. 1987;58:379–383. [DOI] [PubMed]
- 38.Klein DC, Raisz LG. Prostaglandins: stimulation of bone resorption in tissue culture. Endocrinology. 1970;86:1436–1440. [DOI] [PubMed]
- 39.Kranke P, Morin AM, Roewer N, Eberhart LH. Patients’ global evaluation of analgesia and safety of injected parecoxib for postoperative pain: a quantitative systematic review. Anesth Analg. 2004;99:797–806. [DOI] [PubMed]
- 40.Leese PT, Hubbard RC, Karim A, Isakson PC, Yu SS, Geis GS. Effects of celecoxib, a novel cyclooxygenase-2 inhibitor, on platelet function in healthy adults: a randomized, controlled trial. J Clin Pharmacol. 2000;40:124–132. [DOI] [PubMed]
- 41.Leese PT, Talwalker S, Kent JD, Recker DP. Valdecoxib does not impair platelet function. Am J Emerg Med. 2002;20:275–281. [DOI] [PubMed]
- 42.Lin CH, Jee WS, Ma YF, Setterberg RB. Early effects of prostaglandin E2 on bone formation and resorption in different bone sites of rats. Bone. 1995;17:255S–259S. [DOI] [PubMed]
- 43.Madsen JE, Hukkanen M, Aune AK, Basran I, Moller JF, Polak JM, Nordsletten L. Fracture healing and callus innervation after peripheral nerve resection in rats. Clin Orthop Relat Res. 1998;351:230–240. [DOI] [PubMed]
- 44.McLoughlin C, McKinney MS, Fee JP, Boules Z. Diclofenac for day-care arthroscopy surgery: comparison with a standard opioid therapy. Br J Anaesth. 1990;65:620–623. [DOI] [PubMed]
- 45.Meunier A, Aspenberg P. Parecoxib impairs early metaphyseal bone healing in rats. Arch Orthop Trauma Surg. 2006;126:433–436. [DOI] [PubMed]
- 46.Nefussi JR, Baron R. PGE2 stimulates both resorption and formation of bone in vitro: differential responses of the periosteum and the endosteum in fetal rat long bone cultures. Anat Rec. 1985;211:9–16. [DOI] [PubMed]
- 47.Norrdin RW, Jee WS, High WB. The role of prostaglandins in bone in vivo. Prostaglandins Leukot Essent Fatty Acids. 1990;41:139–149. [DOI] [PubMed]
- 48.O’Hara DA, Fragen RJ, Kinzer M, Pemberton D. Ketorolac tromethamine as compared with morphine sulfate for treatment of postoperative pain. Clin Pharmacol Ther. 1987;41:556–561. [DOI] [PubMed]
- 49.Okada Y, Lorenzo JA, Freeman AM, Tomita M, Morham SG, Raisz LG, Pilbeam CC. Prostaglandin G/H synthase-2 is required for maximal formation of osteoclast-like cells in culture. J Clin Invest. 2000;105:823–832. [DOI] [PMC free article] [PubMed]
- 50.Paulson SK, Zhang JY, Breau AP, Hribar JD, Liu NW, Jessen SM, Lawal YM, Cogburn JN, Gresk CJ, Markos CS, Maziasz TJ, Schoenhard GL, Burton EG. Pharmacokinetics, tissue distribution, metabolism, and excretion of celecoxib in rats. Drug Metab Dispos. 2000;28:514–521. [PubMed]
- 51.Pilbeam CC, Fall PM, Alander CB, Raisz LG. Differential effects of nonsteroidal anti-inflammatory drugs on constitutive and inducible prostaglandin G/H synthase in cultured bone cells. J Bone Miner Res. 1997;12:1198–1203. [DOI] [PubMed]
- 52.Reikeraas O, Engebretsen L. Effects of ketoralac tromethamine and indomethacin on primary and secondary bone healing. An experimental study in rats. Arch Orthop Trauma Surg. 1998;118:50–52. [DOI] [PubMed]
- 53.Remedios A. Bone and bone healing. Vet Clin North Am Small Anim Pract. 1999;29:1029–1044, v. [DOI] [PubMed]
- 54.Rø J, Sudmann E, Marton PF. Effect of indomethacin on fracture healing in rats. Acta Orthop Scand. 1976;47:588–599. [DOI] [PubMed]
- 55.Romsing J, Moiniche S. A systematic review of COX-2 inhibitors compared with traditional NSAIDs, or different COX-2 inhibitors for post-operative pain. Acta Anaesthesiol Scand. 2004;48:525–546. [DOI] [PubMed]
- 56.Schelling SH, Wolfe HJ, Tashjian AH, Jr. Role of the osteoclast in prostaglandin E2-stimulated bone resorption: a correlative morphometric and biochemical analysis. Lab Invest. 1980;42:290–295. [PubMed]
- 57.Seibert K, Zhang Y, Leahy K, Hauser S, Masferrer J, Perkins W, Lee L, Isakson P. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci USA. 1994;91:12013–12017. [DOI] [PMC free article] [PubMed]
- 58.Simmons DJ. Fracture healing perspectives. Clin Orthop Relat Res. 1985:100–113. [PubMed]
- 59.Simon AM, Manigrasso MB, O’Connor JP. Cyclo-oxygenase 2 function is essential for bone fracture healing. J Bone Miner Res. 2002;17:963–976. [DOI] [PubMed]
- 60.Simon AM, O’Connor JP. Dose and time-dependent effects of cyclooxygenase-2 inhibition on fracture-healing. J Bone Joint Surg Am. 2007;89:500–511. [DOI] [PubMed]
- 61.Stichtenoth DO, Frolich JC. The second generation of COX-2 inhibitors: what advantages do the newest offer? Drugs. 2003;63:33–45. [DOI] [PubMed]
- 62.Sudmann E. Effect of indomethacin on bone remodelling in rabbit ear chambers. Acta Orthop Scand Suppl. 1975;160:91–115. [PubMed]
- 63.Sudmann E, Dregelid E, Bessesen A, Morland J. Inhibition of fracture healing by indomethacin in rats. Eur J Clin Invest. 1979;9:333–339. [DOI] [PubMed]
- 64.Tai H, Miyaura C, Pilbeam CC, Tamura T, Ohsugi Y, Koishihara Y, Kubodera N, Kawaguchi H, Raisz LG, Suda T. Transcriptional induction of cyclooxygenase-2 in osteoblasts is involved in interleukin-6-induced osteoclast formation. Endocrinology. 1997;138:2372–2379. [DOI] [PubMed]
- 65.Talley JJ, Bertenshaw SR, Brown DL, Carter JS, Graneto MJ, Kellogg MS, Koboldt CM, Yuan J, Zhang YY, Seibert K. N-[[(5-methyl-3-phenylisoxazol-4-yl)-phenyl]sulfonyl]propanamide, sodium salt, parecoxib sodium: A potent and selective inhibitor of COX-2 for parenteral administration. J Med Chem. 2000;43:1661–1663. [DOI] [PubMed]
- 66.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature New Biology. 1971;231:232–235. [DOI] [PubMed]
- 67.Vane JR, Botting RM. New insights into the mode of action of anti-inflammatory drugs. Inflamm Res. 1995;44:1–10. [DOI] [PubMed]
- 68.Weber EW, Slappendel R, Durieux ME, Dirksen R, van der Heide H, Spruit M. COX 2 selectivity of non-steroidal anti-inflammatory drugs and perioperative blood loss in hip surgery A randomized comparison of indomethacin and meloxicam. Eur J Anaesthesiol. 2003;20:963–966. [DOI] [PubMed]
- 69.Zhang X, Schwarz EM, Young DA, Puzas JE, Rosier RN, O’Keefe RJ. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest. 2002;109:1405–1415. [DOI] [PMC free article] [PubMed]


