Abstract
Osteonecrosis (ON) is commonly caused by high doses of glucocorticoids or ethanol intake. We previously reported suppression of BMP-2 by dexamethasone was more pronounced and enhancement by lovastatin was less pronounced in bone marrow stromal cells (BMSCs) from patients with ON than from patients without ON. We therefore presumed the BMSCs might be dysfunctional in patients with ON and performed this pilot study. We obtained BMSCs from 10 patients with ethanol-induced ON, 10 patients with glucocorticoid-induced ON, and 12 patients without ON as control subjects, checking third passage cells for osteogenic and adipogenic gene expression and differentiation ability. BMSCs from patients with glucocorticoid-induced ON possessed less osteogenic gene expression and less osteogenic differentiation, whereas BMSCs from patients with ethanol-induced ON possessed more adipogenic gene expression and more adipogenic differentiation. Dysfunction of BMSCs may be one of the causes of ON, with differing dysfunction in ethanol-induced ON and glucocorticoid-induced ON. Glucocorticoids may possess more of a suppressive effect on osteogenesis than ethanol, whereas ethanol may possess a more potent adipogenic effect than glucocorticoids on BMSCs.
Introduction
Osteonecrosis is commonly caused by high doses of glucocorticoids or ethanol intake [1]. The pathogenesis of nontraumatic ON is unclear, although numerous risk factors include corticosteroid treatment, alcoholism, smoking, hyperlipidemia, and hyperviscosity [1, 9, 19, 25]. Numerous proposed theories relate to apoptosis of osteoblasts and osteocytes [10], fat emboli [12], intravascular coagulation [13], intraosseous hypertension, or fatty necrosis of osteocytes [14]. However, no clear connection between the differentiation potentials of BMSCs and ON has been established.
Previous animal studies revealed dexamethasone shifts the osteogenic differentiation to adipogenic differentiation in the bone marrow progenitor cells of mice [6], induces ON in femoral heads of chickens [7], increases fat cell size and intraosseous pressure [24], and decreases femoral head blood flow with lipid vesicle accumulation in osteocytes in rabbits [23]. Ethanol, however, induces adipogenesis and also produces intracellular lipid deposits resulting in the death of osteocytes in rabbits [25]. In vitro studies also suggest dexamethasone induces adipogenesis in a murine pluripotent marrow cell linage [5, 8] with lipid vesicle accumulation in the cell, upregulates expression of adipogenic genes (AP2 and peroxisome proliferator-activated receptor γ [PPARγ]), and downregulates expression of osteogenic genes, including Type I collagen, Runx2, and osteocalcin [5]. Ethanol, however, induces adipogenic differentiation and diminishes alkaline phosphatase (ALP) activity and expression of osteocalcin in murine marrow stromal cells [25].
We previously showed BMSCs from patients with ON, especially those with glucocorticoid-induced ON, have decreased mRNA expression of bone morphogenetic protein 2 (BMP-2) and osteocalcin and may be more susceptible to glucocorticoids [2]. Therefore, we reasoned there might be dysfunction in BMSCs from patients with ON. Also, the ability of BMSCs to differentiate might differ between patients with and without ON.
We therefore hypothesized (1) gene expression related to osteogenesis is lower and gene expression related to adipogenesis is higher in BMSCs from patients with ON; (2) gene expression of BMSCs from glucocorticoid-induced ON and ethanol-induced ON differs; [3] BMSCs from patients with ON possess less capability to differentiate into osteoblasts and more potential to differentiate into adipocytes; and (4) the ability of BMSCs to differentiate differs in glucocorticoid-induced ON and ethanol-induced ON.
Materials and Methods
We monitored ability of BMSCs to differentiate from patients with and without ON. To confirm the relationship between the abnormal constitutional gene expression and ON, we analyzed the gene expression related to osteogenesis and adipogenesis by quantitative real-time polymerase chain reaction (qPCR). To further confirm the osteogenic and adipogenic abilities of BMSCs, we analyzed the activity of ALP and mineralization of calcium deposits by alizarin red S stain and lipid droplet formation by oil red stain. The study was approved by the Institutional Review Board of the Kaohsiung Medical University Hospital. Informed consent for participating in the study was obtained from all patients.
We obtained cells from 10 patients with ethanol-induced ON, 10 patients with glucocorticoid-induced ON, and 12 patients without ON. The non-ON group included four patients who had THA for dysplastic arthritis of the hip and eight patients who had internal fixation for a fresh fracture at the acetabulum, pelvis, or femoral shaft within 2 days after injury. Frequent alcohol consumption greater than 400 mL ethanol per week was reported for all patients with ethanol-induced ON. We excluded patients with impaired renal or liver function, those receiving hormone therapy, and those with malignancy or diabetes mellitus. Age, gender, and body mass index were similar between groups (Table 1). We performed a pilot study based on our hypotheses. The sample size was based on a power calculation. In previous studies, we found dexamethasone decreased mRNA expression of Type I collagen in murine BMSCs by 30% and the standard deviation was less than 15% [7, 22]. Using an effect size of 30% and a standard deviation of 15%, a power calculation indicated at least 10 subjects were required per group (α = 0.05, β = 0.8, n = 10).
Table 1.
Patient demographics
| Demographic | Non-ON (n = 12) | Ethanol-induced ON (n = 10) | Glucocorticoid-induced ON (n = 10) |
|---|---|---|---|
| Gender (male:female) | 6:6 | 7:3 | 5:5 |
| Average age (years) | 48.2 | 47.3 | 50.1 |
| Glucocorticoid (number of patients) | 0 | 0 | 10* |
| Heavy alcohol consumption (number of patients) | 0 | 10 | 0 |
| Smoking (number of patients) | 3 | 4 | 2 |
| Diving (number of patients) | 0 | 0 | 0 |
| Hyperlipidemia (number of patients) | 2 | 3 | 2 |
| Average body mass index (average) | 24.21 | 23.78 | 24.33 |
| Heavy physical working (number of patients) | 4 | 5 | 3 |
* The 10 patients with glucocorticoid-induced ON include three systemic lupus erythematosus, two chronic dermatitis, one idiopathic thrombocytopenia, one transient nephrotic syndrome, and three glucocorticoid abusers; ON = osteonecrosis.
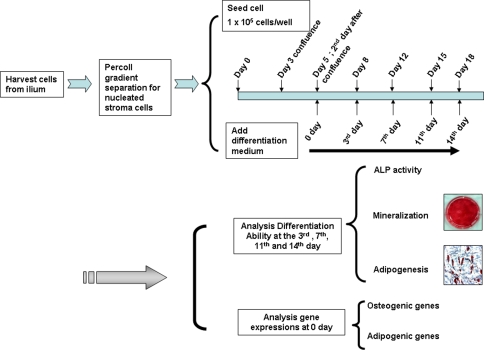
The detailed procedures of BMSC culture were described previously [2]. Briefly, we aspirated 5 mL bone marrow from the iliac crest for BMSC culture during surgery. Cells from 32 patients were cultured and tested separately. The cells were separated by a 70% Percoll™ (Amersham Pharmacia, Piscataway, NJ) gradient; the nucleated stroma cells were collected for primary cell cultures [2, 26]. The third passage of culture was used for all experiments. A flowchart illustrating all experimental procedures is shown (Fig. 1). All independent experiments containing at least three tests were repeated at least three times.
Fig. 1.
A flowchart illustrates the experimental procedures. The cells from the ilium were separated by a 70% Percoll™ (Amersham Pharmacia) gradient and the nucleated stroma cells were collected for primary cell cultures. Cells were seeded at a density of 1 × 105 cells/well. The cells reached first confluence 3 days later and second confluence 5 days after seeding. At the time of second confluence, gene expressions were analyzed. ALP activity and osteogenic and adipogenic differentiation were analyzed at the third, seventh, eleventh, and fourteenth days after the second confluence.
First, we analyzed the gene expression related to osteogenesis, including BMP-2, Runx2, osteocalcin, osteopontin, and osteonectin, and the gene expression related to adipogenesis, including PPARγ and adipsin, by qPCR using an iQ5™ Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA). For each gene, total RNA (1 μg) was treated with 2 U DNase I (Ambion, Carlsbad, CA) and reverse-transcribed with oligo dT as primers with the Clontech RT-for-PCR Kit (BD Biosciences, San Jose, CA). The reagent was the SYBR® Green Supermixes for Real-Time PCR Kit (Bio-Rad Laboratories) instrument. Melting curve analysis was performed for each reaction to ensure a single peak. Amplicons were observed after electrophoresis on a 1.4% agarose gel to ensure presence of a single amplicon. All primer sequences confirmed on the NCBI Web site are listed (Table 2). For each gene, the qPCR experiments were performed with at least three independent batches of cDNAs. Changes (x-fold) in gene expression level were calculated by the 2−ΔΔct method [18].
Table 2.
The sequences of real-time PCR primer used
| Oligo name | Forward sequence (5′–3′) | Reverse sequence (5′–3′) | Length (bp) |
|---|---|---|---|
| 18S | 5′-CCG CAG CTA GGA ATA ATG GAA TAG GAC-3′ | 5′-ACG ACG GTA TCT GAT CGT CTT CG-3′ | 220 |
| Osteocalcin | 5′-GTGCAGAGTCCAGCAAGGT-3′ | 5′-CGATAGGCCTCCTGA AAGC-3′ | 202 |
| BMP-2 | 5′-GGAATGACTGGATTGTGGCT-3′ | 5′-TGAGTTCTGTCGGGACACAG-3′ | 171 |
| Runx2 | 5′-AGA TGG GAC TGT GGT TAC TG-3′ | 5′-GTA GCT ACT TGG GGA GGA TT-3′ | 189 |
| Osteopontin | 5′-AGC CAG GAC TCC ATT GAC TCG AAC-3′ | 5′-GTT TCA GCA CTC TGG TCA TCC AGC-3′ | 250 |
| Osteonectin | 5′-AGA TGC TGG ACT GGA GAT TGG-3′ | 5′-TTC TAC CAA TCC GAT CCA TGG-3′ | 300 |
| PPARγ | 5′-GAG CCC AAG TTT GAG TTT GC-3′ | 5′-CTG TGA GGA CTC AGG GTG GT-3′ | 189 |
| Adipsin | 5′-TGC TAC AGC TGT CGG AGA AG-3′ | 5′-ATG ACT TCA TTG CTC GGG AC-3′ | 200 |
| GAPDH | 5′-TCT CCT CTG ACT TCA ACA GCG AC-3′ | 5′-CCC TGT TGC TGT AGC CAA ATT C-3′ | 126 |
BMP-2 = bone morphogenetic protein-2; PPARγ = peroxisome proliferator-activated receptor γ; GAPDH = glyceraldehyde-3-phosphate dehydrogenase.
We evaluated the ALP activity of BMSCs to reflect osteogenic differentiation. Cells (1 × 105 cells/well) were seeded at 1 × 104 cells/cm2 in a six-well plate in the presence of 10 mmol/L β-glycerol phosphate. Cells were cultured for 7 and 14 days and then harvested and washed twice with Ca2+-, Mg2+-, and NaHCO3-free phosphate-buffered saline (PBS). Cell lysate (100 μL) was assayed for ALP activity by a chemiluminescent method (Applied Biosystems, Branchburg, NY). Total protein was determined using a Bio-Rad protein assay kit (Bio-Rad Laboratories). The specific activity of ALP was expressed as light unit per mg protein [4].
We further analyzed the phenotypes of BMSCs after induction into osteogenesis and adipogenesis. To induce osteogenic differentiation, cells were treated with osteogenic medium for 12 days. The differentiation medium was changed every other day. Osteogenic medium consists of Iscove’s Modified Dulbecco’s Medium supplemented with 0.01 µmol/L dexamethasone (Sigma-Aldrich, St Louis, MO), 50 μmol/L β-glycerol phosphate (Sigma-Aldrich), and 0.2 mmol/L ascorbic-2-phosphate (Sigma-Aldrich) [16]. Human BMSCs were cultured in osteogenic differentiation condition medium and analyzed on the third, seventh, eleventh, and fourteenth days by alizarin red S stain [11]. The cultures were rinsed briefly with PBS followed by fixation (ice-cold 4% formalin) for 15 minutes and stained for 10 minutes with 40 mmol/L alizarin red S. Stained cultures were photographed followed by a quantitative destaining procedure using 10% (w/v) cetylpyridinium chloride in 10 mmol/L sodium phosphate for 15 minutes at room temperature. Aliquots of these alizarin red S extracts were diluted 10-fold in the 10% cetylpyridinium chloride solution and the alizarin red S concentration determined by absorbance measurement at 562 nm by an enzyme-linked immunosorbent assay reader (Bio-Rad Laboratories) using an alizarin red S standard curve in the same solution. After removing all alizarin red S stain and isolating DNA from all wells, DNA content was determined from replicate cultures using the high-salt (2 mol/L NaCl) Tris-NaCl-EDTA fluorometric approach with the Hoechst 33258 dye binding assay in a TKO 100 Minifluorometer (Hoefer Scientific, San Francisco, CA) [17, 20].
To induce adipogenic differentiation, cells were treated with adipogenic medium for 12 days. The medium was changed every other day and adipogenesis was assessed at weekly intervals. Adipogenic medium consists of Dulbecco’s Modified Eagle’s Medium supplemented with 0.5 mmol/L 3-isobutyl-1-methylxanthine (Sigma-Aldrich), 1 µmol/L dexamethasone (Sigma-Aldrich), and 10 µg/mL insulin. Cells were washed twice with PBS and fixed with 10% formalin in PBS for 1 hour; then they were washed three times with water and dried. Cells were stained with oil red O for 15 minutes. Excess stain was removed by washing with 70% ethanol, and then stained cells were washed with water, dissolving the stained oil droplets in the cell monolayer with 4% Nonidet® P-40 in isopropanol for 5 minutes [11].
All data are presented as mean ± standard error. For our first hypothesis, we determined differences in gene expression of BMSCs including BMP-2, Runx2, osteocalcin, osteopontin, osteonectin, PPARγ, and adipsin between the ON and non-ON groups using one-way analysis of variance (ANOVA). The variables in the test are the ON and non-ON groups. For the second hypothesis, we determined differences in gene expression of BMSCs including BMP-2, Runx2, osteocalcin, osteopontin, osteonectin, PPARγ, and adipsin between the ethanol-induced ON and glucocorticoid-induced ON groups using one-way ANOVA. The variables in the test are the ethanol-induced ON and glucocorticoid-induced ON groups. For the third hypothesis, we determined differences in ALP activity, alizarin red S stain, and oil red O stain in BMSCs between the ON and non-ON groups using one-way ANOVA. The variables in the test are the ON and non-ON groups. For the fourth hypothesis, we determined differences in ALP activity, alizarin red S stain, and oil red O stain in BMSCs between the glucocorticoid-induced ON and ethanol-induced ON groups using one-way ANOVA. The variables in the test are the ethanol-induced ON and glucocorticoid-induced ON groups. ANOVA was performed using Excel® 2003 software (Microsoft Corp, Redmond, WA).
Results
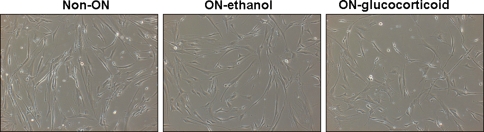
The cells isolated from patients without ON, patients with ethanol-induced ON, and patients with glucocorticoid-induced ON all appeared similar (Fig. 2). The expression of genes related to osteogenesis, including BMP-2, Runx2, osteocalcin, osteopontin, and osteonectin, was higher in the non-ON group than in the ethanol-induced ON and glucocorticoid-induced ON groups, except we found no difference in osteocalcin expression between the ethanol-induced ON and non-ON groups (Table 3). However, expression of adipogenic genes (PPARγ and adipsin) was lower in the non-ON group than in the ethanol-induced ON and glucocorticoid-induced ON groups, except we observed similar expression of adipsin in the glucocorticoid-induced ON and non-ON groups (Table 3).
Fig. 2.
Photomicrographs show similar appearances of the cells isolated from patients without ON, ethanol-induced ON, and glucocorticoid-induced ON in the third passage of culture (Stain, no stain under phase-contrast microscopy; original magnification, ×100).
Table 3.
Osteogenic and adipogenic mRNA expression* by real-time PCR in 32 samples of BMSCs
| Gene | Non-ON (n = 12) | Ethanol-induced ON (n = 10) | Glucocorticoid-induced ON (n = 10) | |||
|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | P1 | Mean ± SE | P1 | P2 | |
| BMP-2 | 100 ± 6 | 61 ± 10 | 0.045 | 12 ± 4 | < 0.001 | < 0.001 |
| Runx2 | 100 ± 12 | 68 ± 11 | 0.060 | 11 ± 10 | < 0.001 | 0.001 |
| Osteocalcin | 100 ± 17 | 71 ± 5 | 0.132 | 9 ± 3 | < 0.001 | 0.002 |
| Osteopontin | 100 ± 9 | 42 ± 4 | 0.008 | 6 ± 1 | < 0.001 | < 0.001 |
| Osteonectin | 100 ± 2 | 37 ± 4 | 0.033 | 4 ± 4 | < 0.001 | < 0.001 |
| PPARγ | 100 ± 12 | 281 ± 17 | 0.002 | 230 ± 6 | 0.003 | 0.32 |
| Adipsin | 100 ± 6 | 222 ± 17 | 0.004 | 126 ± 12 | 0.350 | 0.036 |
* Changes (x-fold) in gene expression level were calculated by the 2−ΔΔct method [18]; PCR = polymerase chain reaction; BMSCs = bone marrow stromal cells; ON = osteonecrosis; SE = standard error; P1 = ON versus non-ON; P2 = ethanol-induced ON versus glucocorticoid-induced ON; BMP-2 = bone morphogenetic protein 2; PPARγ = peroxisome proliferator-activated receptor γ.
In the genes related to osteogenesis, the expression of BMP-2, Runx2, osteocalcin, osteopontin, and osteonectin was higher in the ethanol-induced ON group than in the glucocorticoid-induced ON group. In the genes related to adipogenesis, expression of adipsin was higher in the ethanol-induced ON group than in the glucocorticoid-induced ON group. However, expression of PPARγ was nearly the same in the ethanol-induced ON and glucocorticoid-induced ON groups (Table 3).
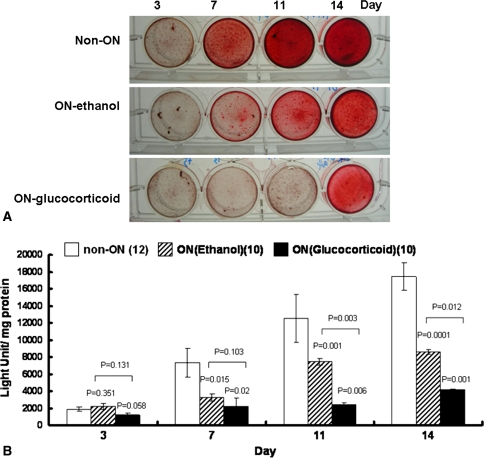
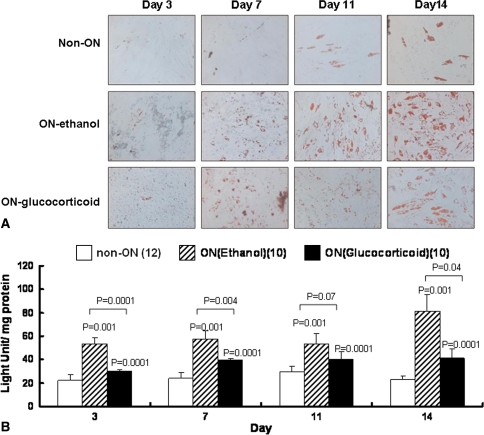
The ALP activity was higher in the non-ON group than in the ethanol-induced ON and glucocorticoid-induced ON groups in the first week (Table 4). However, we observed no difference in the second week between the non-ON and ethanol-induced ON groups, whereas ALP activity was lower in the glucocorticoid-induced ON group than in the other two groups (Table 4). The time of mineralization differed among groups (Table 5). Mineralization appeared on the third day in the non-ON group, on the seventh day in the ethanol-induced ON group, and on the eleventh day in the glucocorticoid-induced ON group. The extent of mineralization increased with time. The difference in mineralization became noteworthy on the eleventh and fourteenth days among all groups. By comparison, mineralization was highest in the non-ON group and lowest in the glucocorticoid-induced ON group (Fig. 3; Table 5). The time lipid droplets also appeared to differ among groups. In the non-ON group, cells expressed triglycerol lipid droplets on the fourteenth day. However, lipid droplets appeared on the third day in the ethanol-induced ON group and on the seventh day in the glucocorticoid-induced ON group. The lipid droplets became more prominent with time. The cells in the ethanol-induced ON group had the highest ability to produce lipid droplets, and conversely, the cells in the non-ON group had the lowest ability to produce lipid droplets. The difference became noteworthy after 7 days of incubation (Fig. 4; Table 5).
Table 4.
ALP activity (expressed as light unit per mg protein) in 32 samples of BMSCs
| Week | Non-ON (n = 12) | Ethanol-induced ON (n = 10) | Glucocorticoid-induced ON (n = 10) | |||
|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | P1 | Mean ± SE | P1 | P2 | |
| First | 9.3 ± 1.2 | 0.47 ± 0.1 | < 0.001 | 0.58 ± 0.18 | < 0.001 | 0.28 |
| Second | 17.26 ± 2.3 | 16.67 ± 2.1 | 0.13 | 13.53 ± 1.15 | 0.012 | 0.003 |
ALP = alkaline phosphatase; ON = osteonecrosis; SE = standard error; P1 = ON versus non-ON; P2 = ethanol-induced ON versus glucocorticoid-induced ON; BMSCs = bone marrow stromal cells.
Table 5.
Osteogenic and adipogenic differentiation (expressed as light unit per mg protein) by stain in 32 samples of BMSCs
| Stain | Day | Non-ON (n = 12) | Ethanol-induced ON (n = 10) | Glucocorticoid-induced ON (n = 10) | |||
|---|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | P1 | Mean ± SE | P1 | P2 | ||
| Alizarin red S | 3 | 100 ± 5 | 118 ± 6 | 0.351 | 62 ± 4 | 0.058 | 0.131 |
| 7 | 393 ± 24 | 172 ± 4 | 0.015 | 117 ± 4 | 0.002 | 0.103 | |
| 11 | 673 ± 46 | 401 ± 22 | < 0.001 | 127 ± 5 | < 0.001 | 0.012 | |
| 14 | 941 ± 61 | 463 ± 23 | < 0.001 | 224 ± 23 | < 0.001 | 0.003 | |
| Oil red O | 3 | 100 ± 5 | 239 ± 5 | < 0.001 | 136 ± 12 | 0.09 | 0.02 |
| 7 | 106 ± 5 | 259 ± 6 | < 0.001 | 177 ± 9 | 0.012 | 0.059 | |
| 11 | 133 ± 4 | 240 ± 8 | < 0.001 | 181 ± 6 | 0.014 | 0.07 | |
| 14 | 132 ± 4 | 365 ± 14 | < 0.001 | 185 ± 7 | 0.013 | 0.04 | |
ON = osteonecrosis; SE = standard error; P1 = ON versus non-ON; P2 = ethanol-induced ON versus glucocorticoid-induced ON; BMSCs = bone marrow stromal cells.
Fig. 3A–B.
(A) Photomicrographs (Stain, alizarin red S; original magnification, ×100) and (B) graphs show the osteogenic differentiation ability of isolated BMSCs. Cells were cultured in osteoinduction medium for 14 days and stained with alizarin red S for mineralization ability. Early mineralization appeared on the third day in the non-ON group, whereas mineralization appeared on the seventh day in the ethanol-induced ON group. The least and latest mineralization appeared in the glucocorticoid-induced ON group on the eleventh to fourteenth days.
Fig. 4A–B.
(A) Photomicrographs (Stain, oil red O; original magnification, ×100) and (B) graphs show the adipogenic differentiation ability of isolated BMSCs. Cells were fixed and stained with oil red O stain at the third, seventh, eleventh, and fourteenth days. Adipogenesis appeared early on the third day in the ethanol-induced ON group, whereas it appeared on the seventh day in the glucocorticoid-induced ON group. The least and latest adipogenesis appeared in the non-ON group on the eleventh to fourteenth days.
We observed no difference in ALP activity between the ethanol-induced ON and glucocorticoid-induced ON groups in the first week; however we noted a difference in the second week (Table 4). In mineralization, the stain was greater in the ethanol-induced ON group than in the glucocorticoid-induced ON group on the eleventh and fourteenth days but not on the third and seventh days (Fig. 3; Table 5). BMSCs from the ethanol-induced ON group generated more lipid droplets than BMSCs from the glucocorticoid-induced ON group. We observed a difference on the third and fourteenth days but not on the seventh and eleventh days (Fig. 4; Table 5).
Discussion
A previous study indicated BMSCs from individuals with ON are more susceptible to glucocorticoid by decreasing the mRNA expression of BMP-2 and osteocalcin [2]. Accordingly, we reasoned the BMSCs from patients with and without ON possess different osteogenic and adipogenic differentiation abilities. Consequently, four hypotheses were raised. We therefore hypothesized (1) gene expression related to osteogenesis is lower and gene expression related to adipogenesis is higher in BMSCs from patients with ON; (2) gene expression of BMSCs from glucocorticoid-induced ON and ethanol-induced ON differs; (3) BMSCs from patients with ON possess less capability to differentiate into osteoblasts and more potential to differentiate into adipocytes; and (4) the ability of BMSCs to differentiate differs in glucocorticoid-induced ON and ethanol-induced ON.
There are some limitations of this study. First, the control subjects in this study were not exposed to a substantial amount of glucocorticoid or ethanol; we do not know whether they would have ON develop if they were exposed to excessive glucocorticoid and/or ethanol. Nevertheless, cells from patients without ON exhibited different gene expression from the ON groups. Second, there are some discrepancies in the gene and differentiation expression between groups. For example, osteocalcin gene expression in patients with ethanol-induced ON is not different from that in patients without ON. Adipsin gene expression in patients with glucocorticoid-induced ON is not different from that in patients without ON. ALP in the second week in the patients with ethanol-induced ON is not different from that in patients without ON. Third, we did not test the in vivo osteogenic and adipogenic differentiation of BMSCs from these three populations. We did not know whether the in vivo results are the same as the in vitro results. Fourth, we do not know whether normal BMSCs respond to ethanol or corticosteroids similarly to those from patients with ON who had high intake of each substance. Finally, because ON is a focal disease, we are not certain cells from different areas will behave in the same way. The behavior of BMSCs from the ilium cannot completely represent the behavior of BMSCs from the femoral head.
Previous in vivo studies revealed glucocorticoid-induced ON was associated with increased fat cell size and accumulated lipid in the femoral head, resulting in fat emboli and ischemia of the femoral head [6, 21, 24]. The size of the mature fat cells in human bone marrow increased after high doses of glucocorticoid treatment, especially during the early stage of glucocorticoid-induced ON [15]. Although we did not measure fat size in BMSCs after adipogenic induction, we found BMSCs from the ethanol-induced ON and glucocorticoid-induced ON groups more easily differentiated into adipocytes than BMSCs from the non-ON group in the phenotype analysis. In addition, adipogenesis of the BMSCs was more obvious in the ethanol-induced ON group than in the glucocorticoid-induced ON group. The relatively high expression of PPARγ in BMSCs from patients with ON, which eventually results in more adipogenesis of BMSCs, may be one of the causes of ON, especially ethanol-induced ON.
A previous study suggested patients with ON are more susceptible to suppression by glucocorticoids on BMP-2 and osteocalcin expression and less susceptible to enhancement by lovastatin on BMP-2 and osteocalcin expression [2]. The relationship between osteogenesis and ON is unclear. We found substantially decreased expression of genes related to osteogenesis, including BMP-2, Runx2, osteocalcin, osteopontin, and osteonectin, in BMSCs from patients with ON, which eventually led to decreased ALP activity and mineralization, especially in glucocorticoid-induced ON.
Although the phenotype of ethanol-induced ON and glucocorticoid-induced ON is similar, the BMSCs from patients with these diseases behave differently to some extent. The BMSCs from patients with ethanol-induced ON possess higher adipogenic expression and higher ability of adipogenic differentiation, even higher than those of BMSCs from patients with glucocorticoid-induced ON. In contrast, the BMSCs from patients with glucocorticoid-induced ON possess less osteogenic expression and less ability of osteogenic differentiation, even much less than those of BMSCs from patients with ethanol-induced ON. We suggest dysfunction of BMSCs with corticosteroid or alcohol may be one of the reasons for ON, and there are differing dysfunctions between ethanol-induced ON and glucocorticoid-induced ON. Glucocorticoids may possess a more suppressive effect on osteogenesis than ethanol, whereas ethanol may possess a more potent adipogenic effect on the BMSCs than glucocorticoids.
Acknowledgments
We thank Yi-Jen Chen for helping in the experiment process.
Footnotes
One or more of the authors (JKC, MLH, CHC, GJW) have received funding from the National Health Research Institute of Taiwan (NHRI-EX94-9316EP and NHRI-EX96-9615EP), Kaohsiung Medical University Hospital (93-KMUH-019), and the Technology Development Program for Academia in Taiwan (96-EC-17-A-17-S1-041).
Each author certifies that his or her institution has approved for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Kaohsiung Medical University Hospital.
Contributor Information
Chung-Hwan Chen, Email: hwan@kmu.edu.tw.
Gwo-Jaw Wang, Email: gwojaw@cc.kmu.edu.tw.
References
- 1.Arlet J. Nontraumatic avascular necrosis of the femoral head: past, present, and future. Clin Orthop Relat Res. 1992;277:12–21. [PubMed]
- 2.Chang JK, Ho ML, Yeh CH, Chen CH, Wang GJ. Osteogenic gene expression decreases in stromal cells of patients with osteonecrosis. Clin Orthop Relat Res. 2006;453:286–292. [DOI] [PubMed]
- 3.Chang JK, Li CJ, Wu SC, Yeh CH, Chen CH, Fu YC, Wang GJ, Ho ML. Effects of anti-inflammatory drugs on proliferation, cytotoxicity and osteogenesis in bone marrow mesenchymal stem cells. Biochem Pharmacol. 2007;74:1371–1382. [DOI] [PubMed]
- 4.Chen CH, Ho ML, Chang JK, Hung SH, Wang GJ. Green tea catechin enhances osteogenesis in a bone marrow mesenchymal stem cell line. Osteoporos Int. 2005;16:2039–2045. [DOI] [PubMed]
- 5.Cui Q, Wang GJ, Balian G. Steroid-induced adipogenesis in a pluripotential cell line from bone marrow. J Bone Joint Surg Am. 1997;79:1054–1063. [DOI] [PubMed]
- 6.Cui Q, Wang GJ, Balian G. Pluripotential marrow cells produce adipocytes when transplanted into steroid-treated mice. Connect Tissue Res. 2000;41:45–56. [DOI] [PubMed]
- 7.Cui Q, Wang GJ, Su CC, Balian G. The Otto Aufranc Award. Lovastatin prevents steroid induced adipogenesis and osteonecrosis. Clin Orthop Relat Res. 1997;344:8–19. [DOI] [PubMed]
- 8.Diduch DR, Coe MR, Joyner C, Owen ME, Balian G. Two cell lines from bone marrow that differ in terms of collagen synthesis, osteogenic characteristics, and matrix mineralization. J Bone Joint Surg Am. 1993;75:92–105. [DOI] [PubMed]
- 9.Felson DT, Anderson JJ. A cross-study evaluation of association between steroid dose and bolus steroids and avascular necrosis of bone. Lancet. 1987;1:902–906. [DOI] [PubMed]
- 10.Gohel A, McCarthy MB, Gronowicz G. Estrogen prevents glucocorticoid-induced apoptosis in osteoblasts in vivo and in vitro. Endocrinology. 1999;140:5339–5347. [DOI] [PubMed]
- 11.Hung SH, Yeh CH, Huang HT, Wu P, Ho ML, Chen CH, Wang C, Chao D, Wang GJ. Pioglitazone and dexamethasone induce adipogenesis in D1 bone marrow stromal cell line, but not through the peroxisome proliferator-activated receptor-gamma pathway. Life Sci. 2008;82:561–569. [DOI] [PubMed]
- 12.Jones JP Jr, Engleman EP, Najarian JS. Systemic fat embolism after renal homotransplantation and treatment with corticosteroids. N Engl J Med. 1965;273:1453–1458. [DOI] [PubMed]
- 13.Jones JP Jr, Ramirez S, Doty SB. The pathophysiologic role of fat in dysbaric osteonecrosis. Clin Orthop Relat Res. 1993;296:256–264. [PubMed]
- 14.Kawai K, Tamaki A, Hirohata K. Steroid-induced accumulation of lipid in the osteocytes of the rabbit femoral head: a histochemical and electron microscopic study. J Bone Joint Surg Am. 1985;67:755–763. [PubMed]
- 15.Kitajima M, Shigematsu M, Ogawa K, Sugihara H, Hotokebuchi T. Effects of glucocorticoid on adipocyte size in human bone marrow. Med Mol Morphol. 2007;40:150–156. [DOI] [PubMed]
- 16.Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. [DOI] [PubMed]
- 17.Lin RW, Chen CH, Wang YH, Ho ML, Hung SH, Chen IS, Wang GJ. (-)-Epigallocatechin gallate inhibition of osteoclastic differentiation via NF-kappaB. Biochem Biophys Res Commun. 2009;379:1033–1037. [DOI] [PubMed]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. [DOI] [PubMed]
- 19.Mankin HJ. Nontraumatic necrosis of bone (osteonecrosis). N Engl J Med. 1992;326:1473–1479. [DOI] [PubMed]
- 20.Puchtler H, Meloan SN, Terry MS. On the history and mechanism of alizarin and alizarin red S stains for calcium. J Histochem Cytochem. 1969;17:110–124. [DOI] [PubMed]
- 21.Sheng HH, Zhang GG, Cheung WH, Chan CW, Wang YX, Lee KM, Wang HF, Leung KS, Qin LL. Elevated adipogenesis of marrow mesenchymal stem cells during early steroid-associated osteonecrosis development. J Orthop Surg. 2007;2:15. [DOI] [PMC free article] [PubMed]
- 22.Wang GJ, Cui Q, Balian G. The Nicolas Andry Award. The pathogenesis and prevention of steroid-induced osteonecrosis. Clin Orthop Relat Res. 2000;370:295–310. [DOI] [PubMed]
- 23.Wang GJ, Hubbard SL, Reger SI, Miller ED, Stamp WG. Femoral head blood flow in long-term steroid therapy: study of rabbit model. South Med J. 1983;76:1530–1532. [DOI] [PubMed]
- 24.Wang GJ, Sweet DE, Reger SI, Thompson RC. Fat-cell changes as a mechanism of avascular necrosis of the femoral head in cortisone-treated rabbits. J Bone Joint Surg Am. 1977;59:729–735. [PubMed]
- 25.Wang Y, Li Y, Mao K, Li J, Cui Q, Wang GJ. Alcohol-induced adipogenesis in bone and marrow: a possible mechanism for osteonecrosis. Clin Orthop Relat Res. 2003;410:213–224. [DOI] [PubMed]
- 26.Yeh CH, Chang JK, Wang YH, Ho ML, Wang GJ. Ethanol may suppress Wnt/beta-catenin signaling on human bone marrow stroma cells: a preliminary study. Clin Orthop Relat Res. 2008;466:1047–1053. [DOI] [PMC free article] [PubMed]