Abstract
The Knee Outcome Survey-Activities of Daily Living Scale (KOS-ADLS), originally developed in English, is a valid and reliable self-reported instrument used for patients with various painful knee conditions. We adapted the KOS-ADLS to Turkish and tested its reliability and validity. We enrolled 142 patients with knee pain in the study. The patients were randomized into two groups: Group 1 (n = 75) completed the questionnaire twice a week for assessing test-retest reliability and Group 2 (n = 67) answered the questionnaire and performed additional tests for assessing validity. The intraclass correlation coefficient ranged from 0.98 to 0.99 with high internal consistency (Cronbach’s alpha, 0.89). Validity-related tests included pain measurement with a visual analog scale and functional tests, including time measurements for the get-up-and-go and ascending/descending stairs tests. The visual analog scale score correlated with total score (r = 0.56), function total score (r = 0.53), and symptom total score (r = 0.45). The ascending/descending stairs test correlated with total score (r = 0.47), function total score (r = 0.49), and symptom total score (r = 0.31). The get-up-and-go test weakly correlated with all three scores. The Turkish version of the KOS-ADLS is reliable and valid in evaluating the functional limitations of patients with knee pain.
Introduction
Pain is a common symptom of knee disorders and mostly is associated with functional limitation at various levels. One of the main assessment parameters of clinical outcome is evaluation of pain and functional capacity in daily living activities [19]. Different instruments for evaluating functional capacity and disability have been developed and used for specific knee conditions [2, 8, 11–13].
Although patients mainly report having pain, pain can result in limitations of daily living activities or disability. Physical function can be measured by activities such as the ability to stand, walk, or rise from a chair. However, in knee disorders such as osteoarthritis (OA), symptoms are not always directly related to functional limitation and disability [8]. Therefore, it is important to have self-reported instruments that are reliable, valid, and related to symptoms and functional capacity. Such instruments should be easy to use for patients with various painful knee conditions. The Knee Outcome Survey-Activities of Daily Living Scale (KOS-ADLS) combines questions on symptoms and functional limitations [9]. It originally was developed in the English language. The German version is reportedly reliable and valid for patients with various knee impairments including those with cruciate ligament injury, patellofemoral pain (PFP) syndrome, and TKA [3].
We translated and cross-culturally adapted the English KOS-ADLS to Turkish. We then asked two questions: (1) Is the Turkish version of the KOS-ADLS valid and reliable for patients with knee pain? (2) In the context of validity, can the KOS-ADLS differentiate among different knee abnormalities?
Materials and Methods
The KOS-ADLS is a self-administered questionnaire designed to determine the symptoms and functional limitations in usual daily activities experienced within the last few days [9]. It contains six questions concerning symptoms: pain, stiffness, swelling, giving way, weakness, and limping. The responses are given in Likert-type format and graded on a scale from 0 to 5, with 5 being no symptom and 0 being the highest limitation caused by the symptom. Questions regarding functional limitations include eight items: walking, ascending and descending stairs, standing, kneeling, squatting, sitting, and rising from a chair. The responses are graded on a 0 to 5 scale, where 5 indicates no limitation and 0 indicates a high level of functional limitation. The symptom score and function score together make the total score; lower total scores indicate lower levels of function and/or higher limitation.
The translation and cross-cultural adaptation of the English KOS-ADSL into Turkish followed the recommended standard procedure [1, 23]. Two native Turkish speakers independently produced the forward translation of the KOS-ADLS into the Turkish language. One of them was a medical doctor and the other was an engineer. The results of these two translators were synthesized by a third native Turkish speaker and then the synthesized version was translated back into English by two English first-language teachers who spoke fluent Turkish. To obtain a prefinal Turkish version of the questionnaire, an expert committee including translators, statisticians, and health professionals worked on resolution of discrepancies. This resulted in a Turkish test version that was administered to 30 selected patients of different ages and social, ethnic, and educational backgrounds. These patients were interviewed to see if they understood the questions clearly. After checking these pilot results, the committee began formal assessment study of the Turkish version of the KOS-ADLS in patients with knee pain.
Based on a power analysis with a 95% confidence interval and 5% significance level, we recruited 142 patients (122 females/20 males) with knee pain seen during 3 months in our outpatient clinic. Patients with severe knee effusion, previous knee arthroplasty, and who were illiterate were excluded. Patients were randomized into two groups through computer-generated numbers. Patients in Group 1 (n = 75) completed the questionnaire twice in a week to assess test-retest reliability, and patients in Group 2 (n = 67) answered the questionnaire and performed additional tests to assess validity. These tests included pain measurement (visual analog scale [VAS] [18]) and functional tests (time measurements for the get-up-and-go [GUG] [16] and ascending-descending stairs [A/D stairs] tests [3]). All patients fully completed the questionnaires. There were no differences in age, gender, and diagnosis between the reliability and validity assessment groups (Table 1). The diagnosis in the majority of patients was knee OA. Our study was approved by the Ufuk University Human Research Ethics Committee, and all participants were informed of the trial and signed written informed consent.
Table 1.
Demographics of the reliability and validity groups
| Demographic | Reliability group | Validity group |
|---|---|---|
| Age (years)* | 54.32 (11.57) | 57.68 (11.54) |
| Gender (female/male) | 64/11 | 58/9 |
| Diagnosis | ||
| Knee osteoarthritis | 54 | 51 |
| Patellofemoral pain | 21 | 16 |
* Data are presented as means, with standard deviations in parentheses.
We used SPPS® for Windows® Release 15.0 (SPSS Inc, Chicago, IL) for all statistical analysis. We tested all data for normality using the Kolmogorov-Smirnov test to meet the assumptions to perform the subsequent tests on reliability, validity, and further analysis. We used the chi square test to detect potential differences between the reliability and validity test groups for ordinal data (gender) and Student’s t test to detect differences in nominal data (age).
Test-retest reliability gives an indication of the stability of the test instrument with time. The standard error of measurement (SEM), which represents the error between test and retest, was calculated for symptom scores, function scores, and total scores. We used the intraclass correlation coefficient (ICC) to assess test-retest reliability. It ranges from 0 to 1, and values greater than 0.75 were considered adequate for reliability. The minimal detectable change (MDC), which shows the real individual change over and above measurement error, also was computed [12]. Student’s t test for independent samples was used to detect differences between consecutive measurements. A scatterplot and linear regression were used for total score on test and retest to further assess reliability. Internal consistency is an estimation of strength of interrelated items in the test instrument and was assessed by calculating Cronbach’s alpha, which ranges from 0 to 1, with higher values indicating higher internal consistency reliability [20, 21].
Validity evaluates if a questionnaire measures what it is intended to measure. It mostly was assessed by using other related tests and calculating the Pearson correlation coefficient. Generally, we considered a value between 0 and 0.25 as reflecting no or poor correlation, 0.26 and 0.50 moderate correlation, 0.51 and 0.75 good correlation, and greater than 0.75 very good correlation [20]. We assessed validity by correlating the total scores (symptom, function, and total scores) with the VAS scores, GUG test scores, and A/D stairs test scores. In the GUG test, patients stood up from a standard height chair and walked along a corridor, and the time it took for the patients to walk a 15-m distance was measured in seconds [7]. The A/D stairs score was calculated by measuring the time it took patients to ascend and descend 12 steps in the hospital [3]. In the KOS-ADLS, the time for ascending and descending stairs was assessed separately. Additional analyses were performed to find any effects of diagnostic subgroups on the KOS-ADLS questionnaire assessment. Based on clinical examinations, two major subgroups were diagnosed as having tibiofemoral OA (n = 105) and PFP (n = 37). For this evaluation, the KOS-ADLS scores were compared between the two subgroups by using Student’s t test.
Results
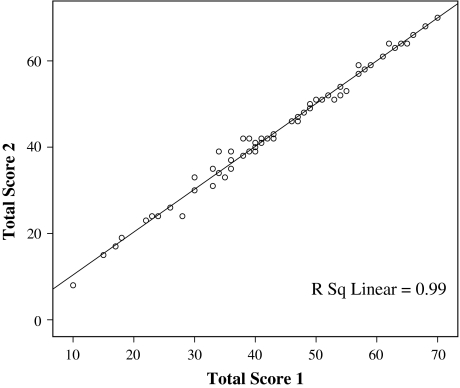
The Turkish version of the KOS-ADLS was reliable (Table 2) for subscores (total symptom and total function) and total score. The ICCs ranged from 0.98 to 0.99. The computed SEM values were low (range, 1.07–1.84), supporting the reliability values obtained, and the MDC values (2.59 for the total score), which reflect the individual change above the measurement error and indicate minimal clinical change. These observations also are supported by the linear regression and the scatterplot (regression coefficient, 0.99) of total scores in two consecutive measurements (Fig. 1). This indicates a strong relationship between the data collected twice a week, showing a strong reliability of data collected on these two occasions. There were no differences between the means of test-retest values with respect to the symptom, function, and total scores. As for internal consistency, the Turkish version of the KOS-ADLS had a Cronbach’s alpha of 0.89 when all 14 items were considered.
Table 2.
The results of test-retest reliability scores
| KOS-ADLS score | Test* | Retest* | ICC† | SEM† | MDC† |
|---|---|---|---|---|---|
| Symptom total | 22.29 (5.27) | 22.37 (5.36) | 0.986 (0.978–0.991) | 1.21 (0.96–1.52) | 1.70 (1.35–2.14) |
| Function total | 22.64 (8.8) | 22.70 (8.79) | 0.996 (0.994– 0.998) | 1.07 (0.76–1.33) | 1.50 (1.07–1.87) |
| Total | 44.93 (13.33) | 45.08 (13.30) | 0.995 (0.992– 0.997) | 1.84 (1.43–2.33) | 2.59 (2.01–3.28) |
* Data are presented as means, with standard deviations in parentheses; †results were calculated, with 95% confidence intervals in parentheses; KOS-ADLS = Knee Outcome Survey-Activities of Daily Living Scale; ICC = intraclass correlation coefficient; SEM = standard error of measurement; MDC = minimal detectable change.
Fig. 1.
A test-retest scatterplot and linear regression for KOS-ADLS total scores indicate a strong relationship (regression coefficient, 0.99) between the total scores collected in two consecutive measurements (twice in a week).
The Turkish version of the KOS-ADLS appeared to be valid (ICC range, 0.98–0.99) as assessed using the VAS score and the GUG and A/D stairs test scores (Table 3). The VAS score correlated with total score (r = 0.56), function total score (r = 0.53), and symptom total score (r = 45). The VAS score had the highest correlation with the pain question of the KOS-ADLS. The A/D stairs test correlated with total score (r = 0.47), function total score (r = 0.49), and symptom total score (r = 0.31). The GUG test, however, weakly correlated with all three scores (Table 4). The VAS score correlated with walking, ascending and descending stairs, kneeling, sitting, squatting, and rising from a chair (Table 5). Also, the A/D stairs test correlated with walking, kneeling, squatting, and ascending and descending stairs (Table 5). The GUG test did not correlate (p > 0.05) with any of the items.
Table 3.
Descriptive information for pain and functional tests
| Test | Number of patients | Score* |
|---|---|---|
| VAS | 67 | 5.28 (2.02) |
| GUG | 67 | 26.07 (14.23) |
| A/D stairs | 67 | 22.45 (13.00) |
* Data are presented as means, with standard deviations in parentheses; VAS = visual analog scale; GUG = get-up-and-go test; A/D stairs = ascending/descending stairs test.
Table 4.
Correlations (r values and p values) between pain and functional tests and KOS-ADLS scores
| KOS-ADLS | VAS | p Value | A/D stairs | p Value | GUG | p Value |
|---|---|---|---|---|---|---|
| Symptom total | −0.45 | 0.000 | −0.31 | 0.010 | −0.22 | 0.070 |
| Function total | −0.53 | 0.000 | −0.49 | 0.000 | −0.22 | 0.070 |
| Total score | −0.56 | 0.000 | −0.47 | 0.000 | −0.25 | 0.031 |
KOS-ADLS = Knee Outcome Survey-Activities of Daily Living Scale; VAS = visual analog scale, A/D stairs = ascending/descending stairs test; GUG = get-up-and-go test.
Table 5.
Correlations (r values and p values) between KOS-ADLS functional items and pain and functional tests
| KOS-ADLS (functional limitation items) | VAS | p Value | A/D stairs | p Value | GUG | p Value |
|---|---|---|---|---|---|---|
| Walking | 0.49 | 0.000 | 0.41 | 0.001 | 0.18 | 0.143 |
| Ascending stairs | 0.37 | 0.002 | 0.51 | 0.000 | 0.21 | 0.080 |
| Descending stairs | 0.48 | 0.000 | 0.57 | 0.000 | 0.28 | 0.020 |
| Standing | 0.15 | 0.202 | 0.20 | 0.096 | 0.21 | 0.077 |
| Kneeling | 0.37 | 0.002 | 0.43 | 0.000 | 0.23 | 0.057 |
| Squatting | 0.41 | 0.000 | 0.38 | 0.001 | 0.13 | 0.295 |
| Sitting | 0.47 | 0.000 | 0.33 | 0.005 | 0.12 | 0.324 |
| Rising from a chair | 0.22 | 0.067 | 0.38 | 0.001 | 0.15 | 0.227 |
KOS-ADLS = Knee Outcome Survey-Activities of Daily Living Scale; VAS = visual analog scale, A/D stairs = ascending/descending stairs test; GUG = get-up-and-go test.
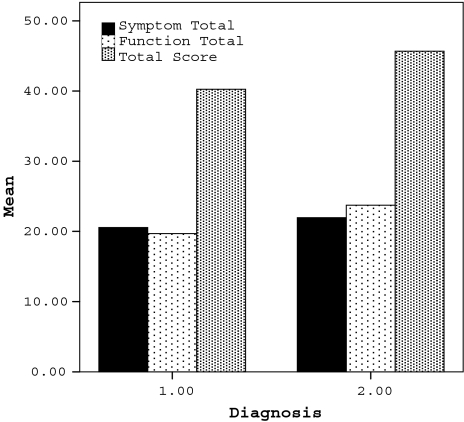
The KOS-ADLS distinguished knee pain in tibiofemoral OA and PFP. The function total scores and total scores were lower in the patients with OA (p = 0.14 and p = 0.33, respectively) than in the patients with PFP, which indicated higher disability in the OA group (Fig. 2). The PFP group had higher disability in descending stairs, standing, kneeling, and squatting (p = 0.025, p = 0.047, p = 0.018, and p = 0.027, respectively).
Fig. 2.
The results of the KOS-ADLS for patients with OA and PFP show the function total scores and total scores were lower in the patients with OA than in the patients with PFP, which indicated higher disability in the OA group. Diagnosis 1 = OA; Diagnosis 2 = PFP.
Discussion
The original KOS-ADLS is a valid and reliable instrument. The objectives of this study were to adapt the KOS-ADLS to Turkish and to provide a reliable and valid basis for use of the KOS-ADLS by the Turkish medical community.
A limitation of this study is the coverage with respect to types of abnormalities and the number of patients. We cannot ensure the reliability or validity for abnormalities such as ligamentous or meniscal injuries, or in postoperative patients.
The KOS-ADLS has been widely used in patients with knee disorders, not only for those with OA [5] or PFP syndrome [3], but also for patients with acute ligament [4] or meniscal injuries [14]. It was first developed for evaluation of patients with anterior cruciate ligament injury and arthrosis. Marx et al. [14] investigated the reliability, validity, and responsiveness of four knee outcome scales, including the KOS-ADLS, in athletic patients. As a result, the KOS-ADLS was considered reliable (ICC, 0.93), valid, and responsive for use in clinical research. Also, it was easily understood and was completed in a short time (although we did not collect data regarding time for completion). Compared with the other scales, it had better validity and responsiveness, which allowed its use with various kinds of knee disorders, including athletic injuries [14].
We found no differences in the means of test-retest groups when symptoms, function, and total scores were considered. We also observed a high correlation between the total scores of two nonconsecutive measurements. In this study, the SEM for total score (1.12) and the range (1.07–1.84) were lower than the results of the German version. Irrgang et al. [9] reported an ICC of 0.97 for the total score in their original study. This was similar to that found with the German version of the KOS-ADLS (ICC range, 0.94–0.97) [3]. Our ICC was 0.99, indicating high test-retest reliability for the Turkish version.
Beateon et al. and Irrgang et al. [1, 9] reported internal consistency of the KOS-ADLS was very good, with a Cronbach’s alpha of 0.92, when compared with the Lysholm knee scale (Cronbach’s alpha, 0.60). The Cronbach’s alpha calculated in our study was 0.89, the same as that reported by Bizzini and Gorelick [3]. This indicates the Turkish version of the KOS-ADLS has high internal consistency reliability for all items of the questionnaire.
In the original study, validity was based on evaluating the relationship between the individual’s global rating of function on a scale of 0 to 100 and the scores of the KOS-ADLS and Lysholm knee scale [9]. Irrgang et al. reported the global rating of function had a higher correlation with the KOS-ADLS than with the Lysholm knee scale [9]. In our study, the validity was measured using the VAS, GUG test, and A/D stairs test scores, similar to the German version [3]. We observed the VAS score was highly correlated and the A/D stairs test was moderately correlated with the symptom total, function total, and total scores. There was no correlation between the GUG test and the symptom total, function total, and total scores. The German version of the KOS-ADLS correlated with the VAS score and with the functional tests (GUG test, A/D stairs test). A relationship was expected between the GUG test and some of the items, including rising from a chair and walking in the functional limitation group of the KOS-ADLS. However, the lack of correlation may be because some patients exaggerate whereas others minimize their complaints. In addition, the GUG test does not have enough sensitivity and therefore is not recommended for use alone in assessing functional capacity [15]. As mentioned in various studies, there is no gold standard test for measuring validity [10, 15, 20]. We might have used another self-administered instrument in the current study to gain additional insight for assessment of validity, which could be considered a limitation of our study. The WOMAC index, which has been adapted to Turkish, could have been used for this purpose [22]. The adapted version of this test is reportedly reliable and valid for patients with OA, but it takes longer to complete than the KOS-ADLS, and mostly is preferred for patients with OA and not for patients with other disorders, such as PFP.
When we analyzed the OA and PFP subgroups, we observed a difference in function total and total scores. Lower scores indicated higher disability in the OA group. The patients with PFP were more disabled in descending stairs, standing, kneeling, and squatting. This was in accordance with the clinical symptoms of PFP, which were characterized by peripatellar pain provoked by ascending or descending stairs, squatting, or sitting with flexed knees [6, 17].
The Turkish version of the KOS-ADLS met the criteria of reliability and validity in measuring symptoms and functional limitations in patients with knee pain. This will lead to better assessment and followup of the functional capacities in daily living activities of patients and will enrich the instrument set through the addition of a reliable and valid test for the Turkish medical community in this area.
Acknowledgments
We thank Dr. Tuncay Cakir for assistance with the collection of data.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This study was performed at Ufuk University and Kocatepe University.
References
- 1.Beateon DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross cultural adaptation of self report measures. Spine. 2000;25:3186–3191. [DOI] [PubMed]
- 2.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically-important patient-relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed]
- 3.Bizzini M, Gorelick M. Development of a German version of the knee outcome survey for daily activities. Arch Orthop Trauma Surg. 2007;127:781–789. [DOI] [PubMed]
- 4.Borsa PA, Lephart SM, Irrgang JJ. Comparison of performance-based and patient-reported measures of function in anterior-cruciate-ligament-deficient individuals. J Orthop Sports Phys Ther. 1998;28:392–399. [DOI] [PubMed]
- 5.Briem K, Ramsey DK, Newcomb W, Rudolph KS, Snyder-Mackler L. Effects of the amount of valgus correction for medial compartment knee osteoarthritis on clinical outcome, knee kinetics and muscle co-contraction after opening wedge high tibial osteotomy. J Orthop Res. 2007;25:311–318. [DOI] [PMC free article] [PubMed]
- 6.Heintjes E, Berger MY, Bierma-Zeinstra SM, Bernsen RM, Verhaar JA, Koes BW. Exercise therapy for patellofemoral pain syndrome. Cochrane Database Syst Rev. 2003;4:CD003472. [DOI] [PubMed]
- 7.Hurley MV, Scott DL, Rees J, Newham DJ. Sensorimotor changes and functional performance in patients with knee osteoarthritis. Ann Rheum Dis. 1997;56:641–648. [DOI] [PMC free article] [PubMed]
- 8.Irrgang JJ, Anderson AF. Development and validation of health-related quality of life measures for the knee. Clin Orthop Relat Res. 2002;402:95–109. [DOI] [PubMed]
- 9.Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am. 1998;80:1132–1145. [DOI] [PubMed]
- 10.Koran LM. The reliability of clinical methods, data and judgements. N Engl J Med 1975;293:642–646. [DOI] [PubMed]
- 11.Kücükdeveci AA, McKenna SP, Kutlay S, Gürsel Y, Whalley D, Arasıl T. The development and psychometric assessment of the Turkish version of the Nottingham Health Profile. Int J Rehab Res. 2000;23:31–38. [DOI] [PubMed]
- 12.Lequesne MG, Mery C, Samson M, Gerard P. Indexes of severity for osteoarthritis of the hip and knee: validation-value in comparison with other assessment tests. Scand J Rheumatol Suppl. 1987;65:85–89. [DOI] [PubMed]
- 13.Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with specific emphasis on use of a scoring scale. Am J Sports Med. 1982;10:150–154. [DOI] [PubMed]
- 14.Marx RG, Jones EC, Allen AA, Altchek DW, O’Brien SJ, Rodeo SA, Williams RJ, Warren RF, Wickiewicz TL. Reliability, validity, and responsiveness of four knee outcome scales for athletic patients. J Bone Joint Surg Am. 2001;83:1459–1469. [DOI] [PubMed]
- 15.Nunnally JC, Bernstein IH. Psychometric Theory. New York, NY: McGraw-Hill; 1994.
- 16.Piva SR, Fitzgerald GK, Irrgang JJ, Bouzubar F, Starz TW. Get up and go test in patients with osteoarthritis. Arch Phys Med Rehabil. 2004;85:284–289. [DOI] [PubMed]
- 17.Powers CM, Ward SR, Chen YJ, Chan LD, Terk MR. Effect of bracing on patellofemoral joint stress while ascending and descending stairs. Clin J Sport Med. 2004;14:206–214. [DOI] [PubMed]
- 18.Rejeski WJ, Ettinger WH, Shumaker S, Heuser MD, James P, Monu J, Burns R. The evaluation of pain in patients with knee osteoarthritis: the knee pain scale. J Rheumatol 1995;22:1124–1129. [PubMed]
- 19.Rosemann T, Laux G, Szecsenyi J. Osteoarthritis: quality of life, comorbidities, medication and health service utilization assessed in a large sample of primary care patients. J Orthop Surg. 2007;2:12. [DOI] [PMC free article] [PubMed]
- 20.Sezgin M, Incel NA, Sevim S, Camdeviren H, As I, Erdogan C. Assessment of symptom severity and functional status in patients with carpal tunnel syndrome: reliability and validity of the Turkish version of the Boston Questionnaire. Disabil Rehabil. 2006;28:1281–1285. [DOI] [PubMed]
- 21.Stratford PW, Binkley J, Solomon P, Finch E, Gill C, Moreland J. Defining the minimum level of detectable change for the Roland-Morris Questionnaire. Phys Ther. 1996;76:359–365. [DOI] [PubMed]
- 22.Tüzün EH, Eker L, Aytar A, Daskapan A, Bayramoglu M. Acceptability, reliability, validity and responsiveness of the Turkish version of WOMAC osteoarthritis index. Osteoarthritis Cartilage. 2005;13:28–33. [DOI] [PubMed]
- 23.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey [SF-36]. I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed]