Abstract
Forearm nonunion frequently changes the relationship between the radius and ulna and may lead to impairment of forearm function. We propose a new surgical technique for aseptic forearm nonunions combining a fibular cortical autograft strut with a metal plate and a fibular intercalary autograft in cases with a segmental bone defect. We retrospectively reviewed 20 patients with a mean age of 31 years (range, 17–48 years) at the time of surgery. Minimum followup was 12 years (mean, 14 years; range, 12–21 years). There were no intraoperative or postoperative complications. At last followup, all forearm bones had remodeled. The mean visual analog pain scale was 1 (range, 0–3). Forearm function improved; there were no radiographic signs of ankle arthritis at followup. Surgical treatment of aseptic forearm nonunions by combining a massive fibular cortical autograft strut with a plate and associating a fibular intercalary autograft in case of a segmental bone defect led to bone healing, improved forearm function, and a durable outcome with long-term followup.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Aseptic forearm nonunions are an infrequent complication of diaphyseal fractures of the forearm, partly because of the wide use and success of evolved surgical techniques and instruments. Treating forearm nonunions is a challenge for the orthopaedic surgeon. Reviewing the literature of this subject reveals that despite the use of various techniques for treating forearm nonunions, the results are not completely satisfying and there is still debate regarding which type of technique to use [13, 14, 19, 20].
Forearm nonunions usually are associated with a complex injury, inadequate initial reduction of the fracture, unstable fracture fixation, or early limb mobilization. Nonunions may be atrophic or hypertrophic based on the characteristics of the bone ends [13, 14, 17]; an atrophic nonunion always is associated with segmental bone loss (Fig. 1) [13, 14, 17]. Diaphyseal fractures of the forearm differ from other diaphyseal fractures because of the intimate relationship between the radius and ulna and their reciprocal movement [19]. In the sense that anatomic restoration of the radius and ulna is necessary, they could be considered like articular fractures [19]. The shape, length, and distance between them is reflected in movements of the elbow and fine movements of the hand, and these should be restored. Pronation and supination of the forearm occur at the radiohumeral, proximal radioulnar, and distal radioulnar joints. Therefore, when the relationship between the radius and ulna changes, the proximal or distal articulations also may be altered, with impairment of forearm function.
Fig. 1A–B.
An illustration shows the bone ends characteristic of a (A) hypertrophic forearm nonunion and an (B) atrophic one.
The aim of surgical treatment of forearm nonunions is to restore proper bone length and the reciprocal three-dimensional shapes and relationships of the radius and ulna [7, 9, 13]. A surgical technique must provide bone stability and stimulate bone repair to regain normal flexion-extension of the elbow and pronation and supination and grip strength of the wrist. Numerous studies have been published regarding treatment of forearm nonunions with a wide variety of surgical options (Table 1).
Table 1.
Studies of surgical options for treatment of forearm nounions
| Year of study | Authors | Surgical option |
|---|---|---|
| 1954 | Spira [24] | Iliac graft fixed with an intramedullary nail |
| 1956 | Nicoll [18] | Cancellous bone graft and plate fixation |
| 1965 | Scaglietti et al. [21] | Tibial cortical autograft and screws |
| 1971 | Dabezies et al. [5] | Iliac bone graft fixed with plate |
| 1972 | Ilizarov et al. [11] | Ilizarov bone transport technique |
| 1976 | Weber and Cech [28] | Cancellous bone alone or combined with plating |
| 1979 | Muller et al. [16] | Compression plate combined with bone graft |
| 1981 | Shelton and Sage [23] | Iliac autograft and compression plating |
| 1989 | Williamson et al. [29] | Vascularized fibula |
| 1995 | Moroni et al. [13] | Intercalary autograft and opposite allograft fixed with a plate |
| 2004 | Ring et al. [20] | Iliac intercalary autograft and compression plate |
| 2006 | Hong et al. [9] | Iliac bone graft and interlocking intramedullary nail |
| 2009 | Current authors | Plate with opposite and intercalary fibular autograft |
We propose an original surgical technique for aseptic forearm nonunions consisting of combining a fibular cortical autograft strut with a plate and a fibular intercalary autograft in case of a segmental bone defect. We determined (1) whether a fibular autograft combined with a plate reliably achieved union; and (2) whether this surgical technique could restore alignment and improve elbow and wrist motion.
Materials and Methods
We retrospectively reviewed 31 patients with forearm nonunions treated from 1987 to 1996 with a fibular cortical autograft strut with a plate and fibular intercalary autograft in case of a segmental bone defect. We included patients with: (1) a diaphyseal nonunion of one or both forearm bones, (2) absence of other fractures in the same limb at the time of the primary forearm injury, and (3) no infection after the primary fracture. We considered a fracture a nonunion when there were no signs of healing at a minimum of 7 months after the initial treatment. We excluded 11 patients having this same reconstruction: seven patients with an associated fracture of the same limb (four with a fracture of the olecranon, two with a wrist fracture, and one with a distal humerus fracture), two in whom the forearm fracture evolved into a septic nonunion, and two who were lost to followup at 12 and 24 months. This left 20 patients for study. There were four females and 16 males. The initial fracture was a high-energy fracture in all cases and involved the radius alone in two forearms, the ulna alone in five forearms, and the radius and ulna in 13 forearms. Two patients sustained an open fracture. The initial treatment consisted of fixation with a plate and screws in eight patients, an intramedullary rod in six, external fixation in two, and cast immobilization in four. Eighteen of the 20 patients had only one treatment course before the nonunion evolved, whereas in two patients, nonunion occurred after two surgical procedures. Of the initial fractures, nonunion of the radius alone evolved in two, nonunion of the ulna alone evolved in 14, and nonunion of the radius and ulna evolved in four. The nonunion was atrophic in 18 cases and hypertrophic in six (Table 2). The average time between the initial fracture and surgical treatment of the nonunion was 29 months (range, 7–180 months). The mean age of the patients at the time of surgery was 31 years (range, 17–48 years). Minimum followup was 12 years (mean, 14 years; range, 12–21 years).
Table 2.
Nonunion types in study patients
| Patient number | Hypertrophic | Atrophic | ||
|---|---|---|---|---|
| Radius | Ulna | Radius | Ulna | |
| 1 | * | |||
| 2 | * | |||
| 3 | * | * | ||
| 4 | * | |||
| 5 | * | * | ||
| 6 | * | |||
| 7 | * | |||
| 8 | * | |||
| 9 | * | |||
| 10 | * | * | ||
| 11 | * | |||
| 12 | * | |||
| 13 | * | |||
| 14 | * | |||
| 15 | * | |||
| 16 | * | |||
| 17 | * | |||
| 18 | * | |||
| 19 | * | |||
| 20 | * | * | ||
All procedures were performed with the patient under general anesthesia on a routine operating table, in the supine position, an arm tourniquet inflated, the elbow flexed 90°, and the forearm resting on the table. We used the dorsal Thompson approach [26] to expose the radius in all cases. For the ulna, the direct posterior approach [2] was used. We removed loose implants when present and widely exposed the bone ends. The bone ends were freshened, removing dense and dysvascular bone and fibrous tissues until exposing healthy cancellous bone. In all cases, the medullary canal was opened using a drill to ensure bone bleeding. We measured the lengths of segmental bone defects.
Only after measuring the bone defect and considering the length of the massive cortical graft needed were the grafts harvested from the fibula of the ipsilateral lower limb. Using an oscillating saw, we removed a cortical strut 7 to 10 cm long from the fibula, with a thickness equal to half of the fibula’s diameter, leaving its medial aspect intact. We then removed a full-thickness fibular cylinder of the needed length. When nonunion involved the radius and ulna, a longer massive cortical autograft was needed; in these cases, we harvested a circumferential fibular graft and then divided it into two cortical struts.
In case of radius and ulna nonunion, fixation of the ulna was performed first to restore bone length and alignment [3]. Length was restored using the parameters regarding the anatomy of the wrist described by Szabo and Weber [25]: proper length of the radius and ulna was defined by a distance of approximately 12 mm along the axis of the forearm from the tip of the radial styloid to the ulnar head on an anteroposterior (AP) view [25]. We determined proper length using an intraoperative image intensifier.
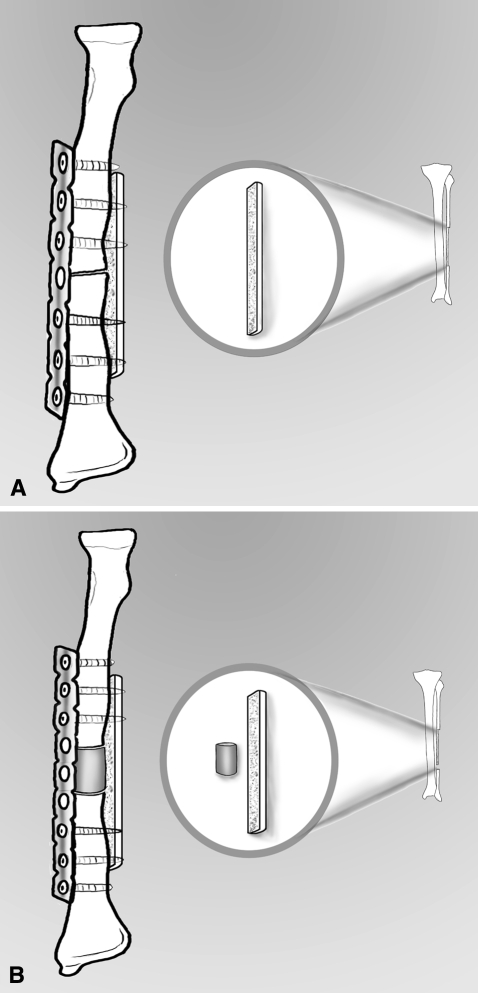
Alignment was restored under visual and c-arm control in three projections: AP, lateral, and oblique. A metal plate of adequate length to find appropriate bone tissue for screw fixation (Sherman plate in 14 cases treated from 1987 to 1992 and DCP 3.5 plate in 10 cases treated from 1993 to 1996) was fixed temporally by bone clamps and the opposite massive cortical fibular autograft was applied. We then used 3.5-mm screws to fix the plate and the graft. In all 18 cases of atrophic nonunion, the cylinder of the fibular autograft was driven into the bone defect as an intercalary graft (Fig. 2). We had no specific positions for the plate and the graft, and rather fitted them according to the situation, but we ensured the cortical graft was covered adequately by muscle to promote graft vascularization and avoid infection. Moreover, we avoided placing the plate or cortical graft too close to the interosseous membrane that might have resulted in an obstacle between the radius and ulna compromising pronation-supination. We inserted two vacuum drains, one in the forearm and one in the donor site. Closure was routine. A cortical fibular autograft strut with a mean length of 8 cm (range, 7–10 cm) was combined with a metal plate in six cases with hypertrophic nonunion. The bone defect in the 18 cases with atrophic nonunions measured an average of 2 cm (range, 1–4 cm); in these cases, in addition to the metal plate and cortical opposite autograft strut, we implanted an intercalary fibular graft of the same length as the defect.
Fig. 2A–B.
(A) For hypertrophic nonunions, a metal plate is associated with an opposite massive cortical autograft harvested from the fibula. (B) For an atrophic nonunion with a bone defect, a metal plate is associated with an opposite massive fibular cortical autograft and a fibular intercalary autograft.
Postoperative radiographs were obtained of the forearm and the leg in two projections. With the elbow flexed 90° and the forearm in intermediate rotation, we applied a comfortable long-arm cast for 4 weeks. To favor upper limb circulation, maintain muscle tone, and prevent disuse osteopenia, patients were instructed before discharge by a physiotherapist to vigorously move the fingers on the surgically treated arm (extension and flexion) daily for 10 minutes every hour until cast removal.
To protect the donor site, we applied a below-knee plaster cast with the ankle at 90° for 4 weeks but without weightbearing the first 3 to 4 days postoperatively (while patients were at bed rest or using a wheelchair); subsequently patients were encouraged to walk with partial weightbearing using one crutch under the untreated arm until the cast on the donor site was removed. Patients were discharged from the hospital 1 to 3 days postoperatively. After both plaster casts were removed, radiographs of the forearm and leg were repeated and rehabilitation was initiated.
All patients were checked monthly until there was radiographic evidence of bone healing, and thereafter, they were checked at 1 year, 2 years, and at last available followup at a minimum of 12 years postoperatively.
To assess the time of healing of the fractures, we (SG, CF, SP, MN) used a combined clinical and radiographic assessment. The clinical parameters evaluated at the last followup were: (1) absence of pain or tenderness on palpation, (2) absence of pain when strength gripping, and (3) full range of motion at the adjacent joint. The three radiographic key parameters for the evaluation were: (1) bridging of the fracture site by bone, callus, or trabeculae; (2) bridging of the fracture seen at three cortices; and (3) obliteration of the fracture line or cortical continuity [15]. Each of the four observers independently evaluated each of the monthly radiographs to determine time of healing. Afterward each evaluation was compared with the evaluation made by the other observers. We considered the graft remodeled when it appeared integrated with the native bones and there no longer was any distinction in the graft and radius or ulna.
At last followup, three of us (CF, SP, MN, SM) evaluated the clinical and functional results. Pain in the upper limb was evaluated using the visual analog scale (VAS) from 0 to 10, in which 0 represents absence of pain and 10 represents maximum pain [22]. We rated forearm functional results using the Anderson system [1], as modified by us, which classifies the results into three main groups: elbow flexion-extension, elbow pronation-supination, and wrist flexion-extension (Tables 3 and 4). Return to activities of daily living (ADLs) was evaluated and the outcome was divided into three groups: no ADL limitation, slight ADL limitation, and severe ADL limitation. We also evaluated the patient’s grip strength and divided the outcome into three groups: normal, slight limitation, and severe limitation (Table 5).
Table 3.
Preoperative functional assessment
| Patient number | Elbow flexion-extension | Elbow pronation-supination | Wrist flexion-extension | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal or movement loss < 10° | Movement loss < 20° | Movement loss > 20° | Normal or movement loss < 25% | Movement loss < 50% | Movement loss > 50° | Normal or movement loss < 10° | Movement loss < 20° | Movement loss < 50% | |
| 1 | * | * | * | ||||||
| 2 | * | * | * | ||||||
| 3 | * | * | * | ||||||
| 4 | * | * | * | ||||||
| 5 | * | * | * | ||||||
| 6 | * | * | * | ||||||
| 7 | * | * | * | ||||||
| 8 | * | * | * | ||||||
| 9 | * | * | * | ||||||
| 10 | * | * | * | ||||||
| 11 | * | * | * | ||||||
| 12 | * | * | * | ||||||
| 13 | * | * | * | ||||||
| 14 | * | * | * | ||||||
| 15 | * | * | * | ||||||
| 16 | * | * | * | ||||||
| 17 | * | * | * | ||||||
| 18 | * | * | * | ||||||
| 19 | * | * | * | ||||||
| 20 | * | * | * | ||||||
Table 4.
Functional assessment at last followup
| Patient number | Pain (VAS) | Elbow flexion-extension | Elbow pronation-supination | Wrist flexion-extension | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal or movement loss < 10° | Movement loss < 20° | Movement loss < 30° | Normal or movement loss < 25% | Movement loss < 50% | Movement loss > 50° | Normal or movement loss < 10° | Movement loss < 20° | Movement loss < 50% | ||
| 1 | 0 | * | * | * | ||||||
| 2 | 2 | * | * | * | ||||||
| 3 | 1 | * | * | * | ||||||
| 4 | 3 | * | * | * | ||||||
| 5 | 3 | * | * | * | ||||||
| 6 | 1 | * | * | * | ||||||
| 7 | 0 | * | * | * | ||||||
| 8 | 1 | * | * | * | ||||||
| 9 | 1 | * | * | * | ||||||
| 10 | 0 | * | * | * | ||||||
| 11 | 1 | * | * | * | ||||||
| 12 | 2 | * | * | * | ||||||
| 13 | 1 | * | * | * | ||||||
| 14 | 0 | * | * | * | ||||||
| 15 | 0 | * | * | * | ||||||
| 16 | 0 | * | * | * | ||||||
| 17 | 0 | * | * | * | ||||||
| 18 | 1 | * | * | * | ||||||
| 19 | 2 | * | * | * | ||||||
| 20 | 2 | * | * | * | ||||||
VAS = visual analog scale.
Table 5.
ADL assessment at last followup
| Patient number | No ADL limitation | Slight ADL limitation | Severe ADL limitation | Grip strength |
|---|---|---|---|---|
| 1 | * | Normal | ||
| 2 | * | Slight limitation | ||
| 3 | * | Slight limitation | ||
| 4 | * | Severe limitation | ||
| 5 | * | Slight limitation | ||
| 6 | * | Slight limitation | ||
| 7 | * | Slight limitation | ||
| 8 | * | Normal | ||
| 9 | * | Normal | ||
| 10 | * | Slight limitation | ||
| 11 | * | Slight limitation | ||
| 12 | * | Normal | ||
| 13 | * | Slight limitation | ||
| 14 | * | Slight limitation | ||
| 15 | * | Slight limitation | ||
| 16 | * | Normal | ||
| 17 | * | Normal | ||
| 18 | * | Slight limitation | ||
| 19 | * | Normal | ||
| 20 | * | Normal |
ADL = activities of daily living.
To quantify the donor-site functional result, three of us (CF, SP, MN, SM) evaluated the following parameters: hindfoot alignment: normal (5°–7° valgus), minimal malalignment (7°–10° valgus), and severe malalignment (valgus greater than 10°); ankle stability: stable or unstable; and limitation of ankle movement: no limitation, slight limitation, and severe limitation (Table 6). We noted the time of return to the original occupation before the initial injury and time of return to usual sports activities for patients involved in sports.
Table 6.
Clinical assessment of donor site at last followup
| Patient number | Hindfoot alignment | Ankle stability | Ankle movement limitation | |||||
|---|---|---|---|---|---|---|---|---|
| Normal (5°–7° valgus) | Minimal malalignment (7°–10° valgus) | Severe malalignment (> 10° valgus) | Stable | Unstable | No movement limitation | Slight movement limitation | Severe movement limitation | |
| 1 | * | * | * | |||||
| 2 | * | * | * | |||||
| 3 | * | * | * | |||||
| 4 | * | * | * | |||||
| 5 | * | * | * | |||||
| 6 | * | * | * | |||||
| 7 | * | * | * | |||||
| 8 | * | * | * | |||||
| 9 | * | * | * | |||||
| 10 | * | * | * | |||||
| 11 | * | * | * | |||||
| 12 | * | * | * | |||||
| 13 | * | * | * | |||||
| 14 | * | * | * | |||||
| 15 | * | * | * | |||||
| 16 | * | * | * | |||||
| 17 | * | * | * | |||||
| 18 | * | * | * | |||||
| 19 | * | * | * | |||||
| 20 | * | * | * | |||||
We obtained radiographs of the donor site (including the entire length of the leg) at the last available followup to identify any ankle arthritis using the criteria published by Van Dijk et al. [27].
Results
There were no intraoperative or postoperative complications and no early or late infections. All forearm bones healed. At last followup, all forearm bones had remodeled (Figs. 3, 4).
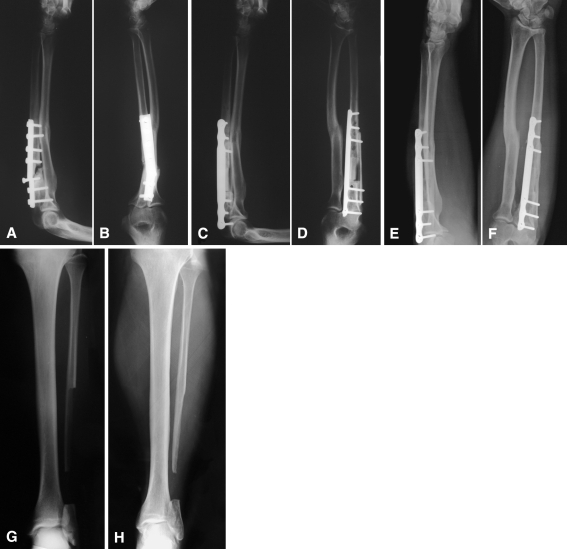
Fig. 3A–H.
(A) Lateral and (B) anteroposterior radiographs show an isolated atrophic nonunion of the ulna in a 27-year-old man. (C) Lateral and (D) anteroposterior radiographs taken 3 months after surgery, signs of bone healing are evident. (E) Lateral and (F) anteroposterior radiographs show complete remodeling of the grafts at the 14-year followup. (G) A postoperative AP radiograph of the fibula donor site is shown. (H) Good alignment of the hindfoot and no signs of ankle arthritis are evident on this radiograph taken at the 14-year followup.
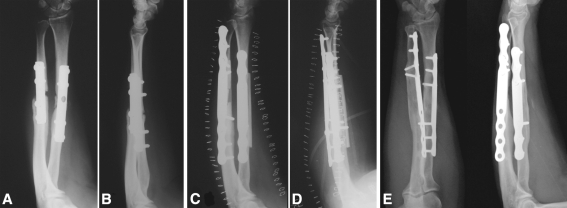
Fig. 4A–E.
(A) Anteroposterior and (B) lateral radiographs show an atrophic nonunion of the radius and ulna in a 34-year-old woman. (C) Anteroposterior and (D) lateral postoperative radiographs show an opposite cortical graft and an intercalary graft were used for both bones. (E) Complete remodeling of the grafts was evident at the 15-year followup. Proximal screws were removed 10 years after the first operation because the patient experienced tenderness adjacent to the site of these screws.
At last followup, the mean VAS for pain was 1 (range, 0-3). Forearm functional results according to the Anderson scale modified by us were: elbow flexion and extension: loss less than 10° in 12 patients, loss less than 20° in seven patients, and loss greater than 30° in one patient; elbow pronation-supination: normal or a loss less than 25% in 13 patients, loss less than 50% in seven patients, and loss greater than 50% in none of the patients; and wrist flexion and extension: 70° flexion and 80° extension or a loss less than 10° in 15 patients, loss less than 20° in four patients, and a loss greater than 50% in one patient (Tables 3 and 4). Patients resumed ADLs 2 months after surgery and their original work activity at a mean of 3 months (range, 2–4 months) after surgery for sedentary work and a mean of 4 months (range, 3–5 months) for strenuous jobs (eg, one patient who had a fruit stand returned to work and lifting heavy weights after 3 months from surgery). Young and active patients began sports activities 4 to 5 months after surgery (eg, one patient used to compete in motorcycle races and returned to racing after 4 months from surgery); grip strength was normal in eight patients, slightly limited in 11 patients, and severely limited in one (Table 5).
Nineteen patients had normal hindfoot alignment (5°–7° valgus), one had minimal malalignment (7°–10° valgus), and none had malalignment greater than 10° valgus. The ankle was stable in all 20 patients. Eighteen patients had no limitation of ankle motion, two patients had slight limitation, and none had severe limitation (Table 6). At last available followup no patients had radiographic signs of ankle arthritis (Fig. 3G–H).
No patients had reoperation at the nonunion site except for one who asked us to remove four of the eight plate screws (Fig. 4).
Discussion
Aseptic forearm nonunions are a challenge for orthopaedic surgeons. Failed healing of the fracture modifies the normal relationship between radius and ulna, with impairment of forearm, elbow, and wrist function. The aims of surgical treatment are to regain proper length and relationship between forearm bones and recover function of the forearm in terms of flexion-extension of the elbow and wrist, pronation and supination, and grip strength. Treatment of forearm nonunions is a debated topic and many surgical techniques have been described [4, 5, 7–9, 11–14, 16, 18, 20, 21, 23, 24, 28, 29]. We propose a new surgical technique for aseptic forearm nonunions combining a fibular cortical autograft strut with a metal plate and a fibular intercalary autograft in cases with a segmental bone defect. We evaluated whether using a fibular autograft combined with a plate resulted in reliable and rapidly achieved union, and if our surgical technique could restore alignment and improve elbow and wrist motion during long-term followup.
The main limitation of our study relates to the selection of patients; all patients were preselected before surgery. We lost two patients to last followup and the remaining group of patients selected might not represent all such patients. However, based on the data, we believe the sample we have included in the study is heterogeneous enough to provide a reliable assessment of the outcome in the general population. Three of the observers were not directly involved in the treatment and we believe those clinical and radiographic assessments are less subject to bias.
Various surgical techniques have been proposed for treatment of forearm nonunions [4, 5, 7–9, 11–14, 16, 18, 20, 21, 23, 24, 28, 29] (Table 1). Some are similar to the one we describe in this study. Spira [24] used an iliac graft fixed with an intramedullary nail and reported a success rate of 73%. Grace and Eversmann [7] used iliac bone fixed with plates and reported a success rate of 67%. Cristensen [4] proposed using the Küntscher technique and reported a 75% success rate. Shelton and Sage [23] achieved a union rate of 87% using an iliac graft and compression plate. Hong et al. [9] reported interlocking intramedullary nailing should not be considered a surgical option for treating forearm nonunions.
However, although a success rate of 100% was reported in some studies [8, 11, 12], these treatment techniques have substantial disadvantages. The bone transport technique reported by Ilizarov et al. [11] leads to bone healing of the nonunion but requires more surgical time and cooperative patients because of difficult postoperative management. We recommend the Ilizarov technique only in case of septic forearm nonunion. Han et al. [8] and Jupiter [12] reported high union rates using vascularized bone grafts, which is a valid technique but requires a longer surgical time and specialized equipment.
We preferred the fibula as a massive cortical graft because its curvature ray is similar to the one in the forearm bones. The fibula also is chosen for an intercalary graft, because its shape and diameter approach the anatomic characteristics of the radius and ulna. We do not recommend the iliac autograft because it is difficult to obtain an adequate opposite cortical strut from the iliac crest. In addition, high donor site morbidity was reported when using the iliac crest [6, 10]. When a cortical graft is used opposite a metal plate, the bone is provided with more stability compared with a metal plate alone. If good stability is obtained, bone healing occurs earlier and more easily. In addition, stable fixation permits early, active motion and early recovery of activities. Using this technique, we advise caution regarding the length of the opposite cortical graft, because if it is very short, it may not provide stability in the entire implant. Also, the screws should not be placed close to the nonunion or the intercalary graft when present so that healing would not be compromised.
We found all patients treated for diaphyseal forearm aseptic nonunion achieved bone healing using the described approach. There was a high success rate regarding forearm alignment and functional results; all patients recovered daily and working activities quickly, and none reported pain or discomfort at the fibula donor site.
All patients regained normal upper limb function, even those who had an objective functional limitation of some level. None of the patients had any limitations in their daily activities attributable to the donor site, a point that encourages us to use this donor site rather than the iliac crest when an autograft is needed.
The ability to follow this group of patients over a long period gave us the possibility to evaluate the durability of the outcome from clinical and radiographic points of view. We observed in all cases, the postoperative outcome was maintained during that entire period. This variable also promotes the proposed technique as being reliable for treatment of aseptic nonunions.
Acknowledgments
We thank Olivia Faldini for help in clinical evaluation of the patients during followup.
Footnotes
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
Each author certifies that his or her institution either has waived or does not require approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Anderson LD. Compression plate fixation and the effect of different types of internal fixation on fracture healing. J Bone Joint Surg Am. 1965;47:191–208. [PubMed]
- 2.Boyd HB. Surgical exposure of the ulna and proximal third of the radius through one incision. Surg Gynecol Obstet. 1940;71:86.
- 3.Bronstein AJ, Trumble TE, Tencer AF. The effects of distal radius fracture malalignment on forearm rotation: a cadaveric study. J Hand Surg Am. 1997;22:258–262. [DOI] [PubMed]
- 4.Christensen NO. Kuntscher intramedullary reaming and nail fixation for nonunion of the forearm. Clin Orthop Relat Res. 1976;116:215–221. [PubMed]
- 5.Dabezies EJ, Stewart WE, Goodman FG, Deffer PA. Management of segmental defects of the radius and ulna. JTrauma. 1971;11:778–788. [DOI] [PubMed]
- 6.Fernyhough JC, Schimandle JJ, Weigel MC, Edwards CC, Levine AM. Chronic donor site pain complicating bone graft harvesting from the posterior iliac crest for spinal fusion Spine. 1992;17:1474–1480. [DOI] [PubMed]
- 7.Grace TG, Eversmann WW. The management of segmental bone loss associated with forearm fractures. J Bone Joint Surg Am. 1980;62:1150–1155. [PubMed]
- 8.Han C, Wood MB, Bishop AT, Cooney WP. Vascularized bone transfer. J Bone Joint Surg Am. 1992;74:1441–1449. [PubMed]
- 9.Hong G, Cong-Feng L, Hui-Peng S, Cun-Yi F, Bing-Fang Z. Treatment of diaphyseal forearm nonunions with interlocking intramedullary nails. Clin Orthop Relat Res. 2006;450:186–192. [DOI] [PubMed]
- 10.Hu R, Hearn T, Yang J. Bone graft harvest site as a determinant of iliac crest strength. Clin Orthop Relat Res. 1995;310:252–256. [PubMed]
- 11.Ilizarov GA, Kaplunov AG, Degtiarev VE, Lediaev VI. [Treatment of pseudoarthroses and ununited fractures, complicated by purulent infection, by the method of compression-distraction osteosynthesis] [in Russian]. Ortop Travmatol Protez. 1972;33:10–14. [PubMed]
- 12.Jupiter JB. Complex non-union of the humeral diaphysis. J Bone Joint Surg Am. 1990;72:701–707. [PubMed]
- 13.Moroni A, Caja VL, Sabato C, Rollo G, Zinghi G. Composite bone grafting and plate fixation for the treatment of nonunions of the forearm with segmental bone loss: a report of eight cases. J Orthop Trauma. 1995;9:419–426. [DOI] [PubMed]
- 14.Moroni A, Rollo G, Guzzardella M, Zinghi G. Surgical treatment of isolated forearm non-union with segmental bone loss. Injury. 1997;28:497–504. [DOI] [PubMed]
- 15.Morshed S, Corrales L, Genant H, Miclau T 3rd. Outcome assessment in clinical trials of fracture-healing. J Bone Joint Surg Am. 2008;90:62–67. [DOI] [PubMed]
- 16.Muller ME, Allgower M, Schneider R, Willenegger H. Manual of Internal Fixation. Techniques Recommended by the AO Group. 2nd Ed. New York, NY: Springer; 1979.
- 17.Naimark A, Miller K, Segal D, Kossoff J. Nonunion. Skeletal Radiol. 1981;6:21–25. [DOI] [PubMed]
- 18.Nicoll EA. The treatment of gaps in long bones by cancellous insert grafts. J Bone Joint Surg Br. 1956;38:70–82. [DOI] [PubMed]
- 19.Richard MJ, Ruch DS, Aldridge JM 3rd. Malunions and nonunions of the forearm. Hand Clin. 2007;23:235–243, vii. [DOI] [PubMed]
- 20.Ring D, Allende C, Jafarnia K, Allende BT, Jupiter JB. Ununited diaphyseal forearm fractures with segmental defects: plate fixation and autogenous cancellous bone-grafting. J Bone Joint Surg Am. 2004;86:2440–2445. [PubMed]
- 21.Scaglietti O, Stringa G, Mizzau M. Bone grafting in nonunion of the forearm. Clin Orthop Relat Res. 1965;43:65–76. [DOI] [PubMed]
- 22.Scott J, Huskinsson EC. Graphic representation of pain. Pain. 1976;2:175–184. [DOI] [PubMed]
- 23.Shelton WS, Sage FP. Modified Nicoll-graft treatment of gap nonunions in the upper extremity. J Bone Joint Surg Am. 1981;63:226–231. [PubMed]
- 24.Spira E. Bridging of bone defects in the forearm with iliac graft combined with intramedullary nailing. J Bone Joint Surg Br. 1954;36:642–646. [DOI] [PubMed]
- 25.Szabo RM, Weber SC. Comminuted intraarticular fractures of the distal radius. Clin Orthop Relat Res. 1988;230:39–48. [PubMed]
- 26.Thompson JE. Anatomical methods of approach in operations on the long bones of the extremities. Ann Surg. 1918;68:309–329. [DOI] [PMC free article] [PubMed]
- 27.Van Dijk CN, Tol JL, Verheyen CC. A prospective study of prognostic factors concerning the outcome of arthroscopic surgery for anterior ankle impingement. Am J Sports Med. 1997;25:737–745. [DOI] [PubMed]
- 28.Weber BG, Cech O. Pseudoarthrosis. Vienna, Austria: Hans Huber; 1976.
- 29.Williamson DM, Copeland SA, Landi A. Pseudoarthrosis of the radius treated by free vascularized bone graft. J Hand Surg Br. 1989;14:221–225. [DOI] [PubMed]