Abstract
Mechanical stimuli are critical to the growth, maintenance, and repair of the skeleton. The adaptation of bone to mechanical forces has primarily been studied in cortical bone. As a result, the mechanisms of bone adaptation to mechanical forces are not well-understood in cancellous bone. Clinically, however, diseases such as osteoporosis primarily affect cancellous tissue and mechanical solutions could counteract cancellous bone loss. We previously developed an in vivo model in the rabbit to study cancellous functional adaptation by applying well-controlled mechanical loads to cancellous sites. In the rabbit, in vivo loading of the lateral aspect of the distal femoral condyle simulated the in vivo bone-implant environment and enhanced bone mass. Using animal-specific computational models and further in vivo experiments we demonstrate here that the number of loading cycles and loading duration modulate the cancellous response by increasing bone volume fraction and thickening trabeculae to reduce the strains experienced in the bone tissue with loading and stiffen the tissue in the loading direction.
Introduction
Describing the influences of mechanical stimuli on bone in patients with skeletal conditions such as osteoporosis and osteoarthritis is important for understanding the etiology of these diseases and for developing effective therapies. The majority of our knowledge regarding the skeletal response to mechanical forces is based on data from predominantly cortical bone sites such as the diaphysis [11, 12, 15, 22, 23]. However, osteoporotic fractures occur at corticocancellous sites such as the hip and spine that contain large volumes of cancellous bone [24]; therefore, understanding adaptive mechanisms in cancellous bone is critical.
Few in vivo models have been developed to study the functional adaptation of cancellous bone to mechanical forces [2, 8, 14]. Models of reduced loading have demonstrated functional adaptation of cancellous tissue but these results do not provide insights into the effects of increased loading [17, 18]. Applying and controlling forces to the epiphysis and metaphysis is more difficult than the cortical diaphysis, due to the smaller volumes of cancellous bone and the surrounding cortical shell. We have developed two in vivo models for the study of cancellous bone adaptation. In the mouse, noninvasive cyclic compression of the tibia allowed the response of the corticocancellous metaphysis to be examined [6]. Loading enhanced cancellous bone volume in healthy mice and inhibited the loss of cancellous bone following hormone deficiency [7]. In the rabbit, we have developed a novel in vivo loading device to apply controlled mechanical loads directly to native cancellous bone in the distal femur [25]. This model simulates the environment around total joint replacement. Loading had an anabolic effect on the tissue beneath the loading device.
Here we focus on the rabbit loading model and our primary findings regarding the response to in vivo loading. This model allows us to relate specific loading parameters to the tissue response, and we examined two specific hypotheses. First, we hypothesized that the increased bone mass and altered architecture observed following loading would enhance the mechanical properties of the cancellous bone. We specifically asked whether the apparent modulus (based on finite element models of the specimen-specific geometry) would increase when subjected to applied loads. Second, we hypothesized that loading duration would affect the cancellous tissue response. To address this hypothesis we examined two questions: first whether bone mass (bone volume fraction) changed as a function of loading duration in loaded limbs relative to contralateral controls; and, second whether bone mass adaptation was evident in altered trabecular architecture of the loaded cancellous tissue.
Materials and Methods
To examine the influence of controlled mechanical loading on cancellous bone adaptation, a novel device and animal model of bone adaptation were developed to apply known compressive loads to cancellous bone in the rabbit distal femur [25]. We characterized the quantity and architecture of cancellous bone subjected to specified cyclic loading parameters including magnitude, duration, and number of cycles.
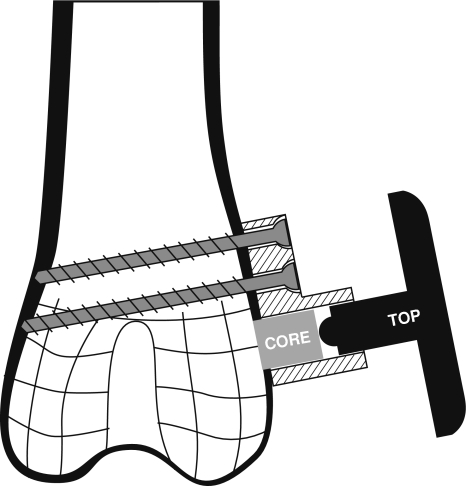
An implantable loading device for rabbits was developed to apply controlled compressive loads to cancellous bone in situ. The device consisted of a stationary base mounted on the lateral femoral condyle with two bicortical screws, a loading core within the base that contacted exposed cancellous bone and a top that articulated with the core (Fig. 1), all fabricated from Ti-6AL-4 V alloy. When the top was manually twisted percutaneously, either manually or with a calibrated torque wrench, the 5-mm diameter core slid within the base and compressed the underlying bone with a known load. Devices were calibrated in the laboratory to confirm load production. For in vivo device validation, the core of the device was instrumented with uniaxial strain gauges for calibration and to examine the in vivo loading magnitude and profile.
Fig. 1.
Schematic of in vivo loading device attached to lateral condyle of rabbit distal femur. The device consists of a core and top positioned within a base that is fixed with two screws. Rotation of the top produces translation of the core to compress the underlying cancellous bone.
For all experiments, loading devices were inserted bilaterally into the distal femurs of skeletally mature male New Zealand white rabbits (6–9 months old). Each animal served as its own control with the right side subjected to cyclic compressive loads, while the left side underwent a sham operation with the device implanted and the cortical shell removed, but no load applied to the cancellous bone. We previously reported no left-right differences were present in the rabbit femur [25]. During the entire experiment, animals were permitted unrestricted ambulation in their cages. While control limbs were subjected to forces associated with ambulation, the cancellous bone in the control limbs was not subjected to the applied compressive loads. Daily loading was performed with no anesthesia and was well tolerated by the animals. The procedure was approved by the IACUC at HSS.
Pilot experiments were performed to optimize the surgical protocol, determine load repeatability, and understand the effect of individual cyclic load parameters. When animals were loaded daily to force per nominal area values of 1, 2, or 4 MPa for 10 cycles/day for 4 weeks (n = 6/group), histological sections demonstrated a significant increase in bone volume fraction in the 1 MPa maximum load group (+ 30% BV/TV), and the 4 MPa group did not show increased bone volume fraction. Based on the load magnitude pilot results, 1 MPa peak load magnitude was used first to examine the influence of number of load cycles and then the influence of load duration using the manual device.
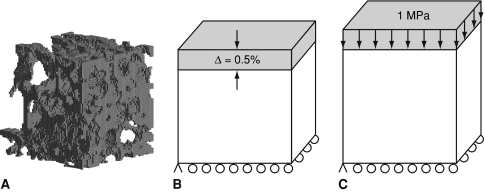
In the first experiment the effect of number of load cycles was examined using three groups of rabbits (n = 6/group) loaded 10, 25, or 50 cycles/day at 0.5 Hz for 4 weeks [8]. Four animals did not complete the 4-week experiment due to infections. Microcomputed tomography (microCT) scans were obtained of both condyles to document bone volume fraction (BV/TV) and trabecular architecture using an in vitro scanner as previously reported [25]. To determine the effect of loading on the apparent mechanical properties of the cancellous bone, finite element (FE) models were created from the scans for a cubic subvolume (3.6 mm × 3.6 mm × 3.6 mm) centered below the loading core within the VOI (Fig. 2). To reduce the total number of degrees of freedom and facilitate solution of the FE models, the microCT scans were first coarsened by combining 9 voxels (3 × 3 × 3) with 19 μm edge lengths into a single cubic voxel with edge lengths of 57 μm. Bone and soft tissue voxels were segmented with a single global threshold for all specimens. Voxels that did not connect with the primary structure were removed prior to coarsening. The coarsened voxels were converted to eight-noded linear brick elements [13, 26]. The tissue was modeled a homogeneous linear elastic material with a Young’s modulus of 10 GPa and Poisson’s ratio of 0.3 [13, 26, 27]. The lateral surface was displaced −0.5% to simulate a uniaxial compression test, and the opposite surface was constrained from displacing out of plane and had one corner fixed to prevent rigid body motions (Fig. 2B). The apparent modulus was calculated as the total reaction force divided by the apparent cross-sectional area. Strains in the tissue were also examined following the application of a 1 MPa compressive load to the model (Fig. 2C). A range of strain outcomes were examined and Von Mises and minimum principal strains are reported here.
Fig. 2A–C.
(A) Representative finite element mesh for 3.6 × 3.6 × 3.6 mm VOI of experimental cancellous bone specimen containing 59,168 elements and 97,981 nodes. Schematic of boundary conditions for finite element analyses for (B) uniaxial compression to 0.5% strain for apparent modulus and (C) compressive loading of 1 MPa for tissue level strains.
In the second in vivo experiment we examined the effect of experimental duration on bone formation by applying a 1 MPa peak load per unit area to three groups of rabbits (n = 11/group) for 2, 4, or 8 weeks at 0.5 Hz for 50 cycles/day. Two animals did not complete the 8-week experiment. As in the first experiment, microCT scans were obtained of the left and right lateral femoral condyles at 20-μm resolution using an in vitro scanner (MS-8 Small Specimen Scanner, GE Healthcare, London, Ontario, Canada). A global threshold was used to segment bone voxels from soft tissue and background based on statistical segregation of the water and bone peaks. The relative effects of loading were not sensitive to threshold, but absolute magnitudes did change with different threshold choices. BV/TV and trabecular thickness (Tb.Th) and separation (Tb.Sp) were measured for a 4-mm diameter spherical VOI centered directly below the loading device. This same spherical volume was used in our prior experiments [25].
Data were analyzed for the effect of in vivo loading within subjects and either the number of loading cycles (first experiment) or experimental duration (second experiment) between subjects on bone volume and structure by an analysis of variance. Alpha was set at 0.05 for significance.
Results
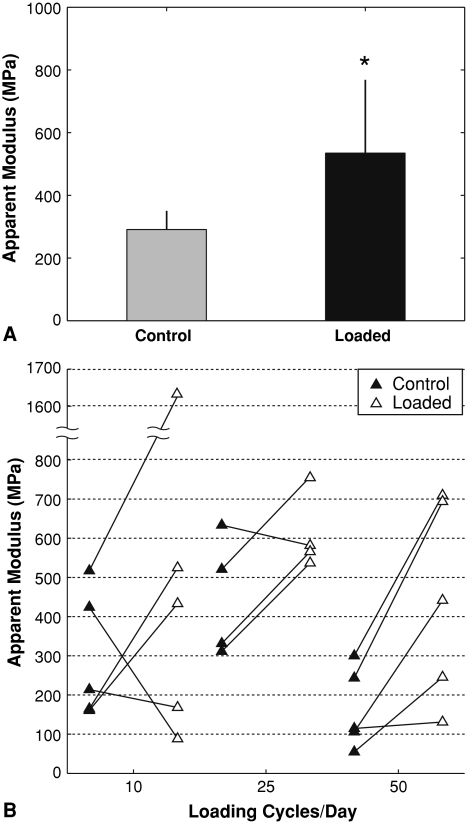
In rabbits loaded daily for 4 weeks at 10, 25 or 50 cycles per day, the apparent modulus increased with loading relative to paired contralateral controls (Fig. 3A), paralleling the increases in cancellous bone volume fraction, trabecular thickness, mean intercept length, and mineral apposition rate that were present with loading. The apparent modulus increased in the loaded limbs of 11 of 14 rabbits compared to the control limbs (Fig. 3B), similar to the increased bone volume fraction in 12 of 14 loaded limbs. The increase in apparent modulus did not depend on the number of loading cycles per day, despite the effect of load on the trabecular thickness and mean intercept length being affected by the number of loading cycles, with 50 cycles per day demonstrating a greater effect than 10 or 25 cycles. Under loading, the mean strains in the cancellous tissue were lower in the animals that had been subjected to 4 weeks of loading than in the contralateral control tissue values. The reduction in mean strains was the result of decreased volume of tissue experiencing high strains accompanied by an increase in the tissue volume experiencing low strains.
Fig. 3A–B.
Apparent modulus from finite element analysis following 4 weeks of loading at 10, 25, or 50 cycles. Data shown for (A) means (SD) of all loaded and control specimens (*p < 0.05), and (B) individual paired specimens separated by number of cycles.
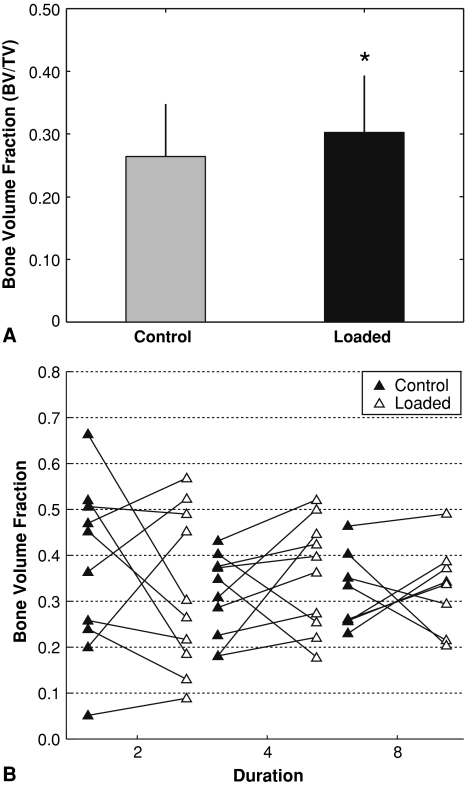
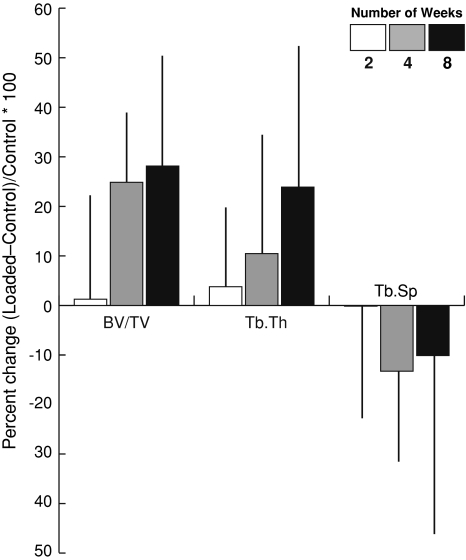
When rabbits were loaded daily for 2, 4 or 8 weeks for 50 cycles per day, bone volume fraction was markedly greater in loaded limbs than control limbs (p < 0.001) (Fig. 4A, Table 1). Compared to the control limb, the loaded limbs in 23 of 28 rabbits had higher BV/TV with loading (Fig. 4B). In particular, bone volume fraction was increased in the loaded limbs of six of 10 rabbits after 2 weeks, 10 of 10 rabbits after 4 weeks, and seven of eight rabbits after 8 weeks. Bone volume fraction depended on (p = 0.039) experimental duration. However, the difference in bone volume fraction with loading did not depend on duration (Table 1). The loaded limb had substantially greater bone volume fraction at 4 weeks (+ 23%, p = 0.002) and 8 weeks (+25%, p = 0.004) (Table 1, Fig. 4). The change in bone volume fraction after 8 weeks of loading was notably greater (p = 0.05) than the difference after 2 weeks of loading (Fig. 5). On the loaded side, the bone volume fraction did not change at any time point examined, and on the control side decreased from 2 to 4 weeks and did not change from 4 to 8 weeks. Compared to the 2-week value, bone volume fraction was lower at 4 weeks (p = 0.005) and 8 weeks (p = 0.012).
Fig. 4A–B.
Bone volume fraction data from microCT analyses following 2, 4, or 8 weeks of loading for 50 cycles per day at 1 MPa. Data shown for (A) means (SD) of all loaded and control specimens (*p < 0.05), and (B) individual paired specimens separated by experimental duration.
Table 1.
The effect of applied loading and duration on cancellous bone morphology in the distal rabbit femur
| Group | Sample size (n) | Bone volume fraction (BV/TV) | Direct trabecular thickness (mm) | Direct trabecular separation (mm) | |||
|---|---|---|---|---|---|---|---|
| Control (left) | Loaded (right) | Control (left) | Loaded (right) | Control (left) | Loaded (right) | ||
| 2 week | 10 | 0.329 (0.05) | 0.333 (0.04) | 0.109 (0.02) | 0.110 (0.02) | 0.225 (0.04) | 0.221 (0.03) |
| 4 week | 10 | 0.260 (0.03) | 0.320 (0.04) | 0.101 (0.02) | 0.111 (0.02) | 0.287 (0.05) | 0.241 (0.05) |
| 8 week | 8 | 0.263 (0.04) | 0.327 (0.06) | 0.097 (0.01) | 0.111 (0.02) | 0.283 (0.08) | 0.240 (0.09) |
All values are mean (SD). Loads were applied to the right femur for 2, 4, or 8 weeks at 1 MPa for 50 cycles per day.
Fig. 5.
Mean percent difference (SD) between loaded and control values for bone volume fraction (BV/TV), trabecular thickness (Tb.Th) and trabecular separation (Tb.Sp) analyses following 2, 4, or 8 weeks of loading for 50 cycles per day at 1 MPa.
The changes in trabecular thickness were similar to those observed in bone volume fraction (Fig. 5): 13% for the loaded side at 4 weeks and 15% greater at 8 weeks compared to the control limb (Fig. 5). Within the same volume, the trabecular separation was lower (p = 0.028) on the loaded side. However, neither the trabecular separation nor the difference in the trabecular separation with loading depended on experimental duration. After 4 weeks, the loaded side had lower (p = 0.046) trabecular separation (Table 1).
Discussion
Mechanical stimuli are critical to the growth, maintenance, and repair of the skeleton. The adaptation of bone to mechanical forces has primarily been studied in cortical bone. As a result, the mechanisms of bone adaptation to mechanical forces are not well-understood in cancellous bone. Using an in vivo rabbit model, we asked whether (1) the apparent modulus (based on finite element models of the specimen-specific geometry) would increase when subjected to applied loads, (2) bone mass (bone volume fraction) changed as a function of loading duration in loaded limbs relative to contralateral controls, and (3) whether bone mass adaptation was evident in altered trabecular architecture of the loaded cancellous tissue.
We note several limitations of our study. First, our paired experimental design reduces animal use but may limit our ability to detect potential systemic effects. Second, the design is also limited by the use of microCT to assess bone formation, a technique that measures the presence of well-mineralized tissue. At the 2-week time point in the duration study dynamic histomorphometry might demonstrate bone formation prior to achieving complete mineralization, while at the later time points, bone formation rates would not be expected to remain elevated [4]. Histological evidence of bone formation would also confirm whether loading stimulated new bone formation or maintained bone volume. This question is particularly critical given that our bone volume fraction values suggest the latter phenomenon: cancellous mass and architecture values were constant in the loaded limbs and declined in the control limbs. We previously reported increased mineral apposition rates from the presence of double calcein labels following loading [25] and would expect to observe the same result here although histological analyses were not performed. In the future, a longitudinal analysis by microCT would help to address this question, particularly for loading duration [28].
The contribution of different characteristics of the applied loading has been examined previously in cortical bone. A relatively low number of cycles was demonstrated to induce cortical bone formation in the turkey ulna [20], consistent with our findings in cancellous bone. In the rat tibial diaphysis, the number of cycles required to induce bone formation was greater at lower load magnitudes [3]. We have not examined the interaction of load magnitude and cycle numbers. For our data, the transient increase in bone mass from 2 to 4 weeks and constant response from 4 to 8 weeks is also consistent with data examining the response of cortical bone mass following loading [4, 20]. In the rat tibia, cortical bone formation rates were elevated at early time points and then decreased with continued loading while bone mass was maintained at later time points [4]. Whether other loading parameters that are known to enhance the cortical anabolic response, such as pauses between cycles and discrete bouts [19, 21], also modulate the response of cancellous bone tissue to mechanical stimuli remains to be determined.
Direct compressive loading of cancellous tissue has been performed in canine and rat models with variable effects on bone mass. In a canine model loading cancellous bone of the distal femur, considerable surgical injury was involved following the amputation of the distal limb [8]. Following surgery a regional acceleratory phenomenon is expected to be present and dominate the early tissue response. The canine model showed clear evidence of a wound healing response [14], whereas our data show bone formation following a less severe surgical procedure. A similar loading approach to ours has recently been used as a mechanism to create microdamage in the distal femur of the rat [29]. Increased bone volume fraction was observed in mature rats 35 days after a single bout of damage-inducing loading, but bone volume was decreased in old animals. Microdamage was present immediately following loading in the loaded limbs of both groups compared to contralateral controls but was no longer present in the mature rats 5 weeks later. Whether damage is created and repaired following loading in our mature rabbit model has not been examined. The apparent pressures applied in both models appear similar or higher in our study, reflecting greater strength and volume fraction of the rabbit tissue.
The focus on loading cancellous bone tissue in situ distinguishes our approach from other studies utilizing either intact bones loaded through surrounding cortical bone [2, 5, 6] or tissue differentiation chambers in which new tissue is formed under varying loading conditions [1, 9, 16]. The increase in cancellous BV/TV seen here exceeds the 15% increase seen with in vivo loading of the tibia [6]. This difference may reflect different load magnitudes as the mechanical stimulus applied to the tibial cancellous bone is difficult to quantify and has shown evidence of a dose response [7]. Greater increases in bone volume fraction are present in the tissue chamber models, but those increases are generally compared to negative controls in which little or no bone forms, a different phenomenon than was examined here.
Finite element models have been used as a surrogate for physical testing to characterize tissue stiffness in prior loading studies. The 2.7-fold increase in apparent stiffness of mechanically loaded specimens found in this study is less than the nearly order of magnitude stiffness increase found in a study using a hydraulic bone chamber [9]. However, the bone chamber study examined loading of newly formed trabecular tissue, not adaptation of preexisting bone tissue that already exhibits substantial stiffness. Our tissue strains were larger than those found in a rat tail loading model [10]. Loads in the rat tail, however, are applied to the cancellous bone through the cortical shell of the vertebrae which dominates the mechanical performance of the rat vertebra, whereas loading from our device is applied directly to cancellous bone tissue after removal of the cortical shell.
Methods to increase cancellous bone mass following disease, disuse or surgical insult are highly desirable clinically. Therefore, in vivo models to understand cancellous bone functional adaptation are critical to advancing our knowledge yet challenging to develop and implement, more so than models loading the cortical diaphysis. Our loading approach is novel and allowed us to isolate the response of cancellous tissue. Strengths of our approach include the isolation of cancellous tissue, well-described loading stimulus and robust adaptive response across experiments. Using this approach we have shown that cancellous bone adapts to mechanical loading by increasing bone mass and thickening trabeculae, thereby reducing tissue strains and increasing apparent stiffness in the direction of applied loading. Much information remains to be learned about cancellous bone adaptation from this and other in vivo loading models. In particular the pathways and mechanisms responsible for the anabolic response need to be elucidated. Incorporating computational bone remodeling algorithms in the analyses will also aid our understanding of cancellous bone functional adaptation.
Acknowledgments
We thank Drs. Timothy Wright and Elizabeth Meyers for their substantial contributions to the model development, Dr. Harrie Weinans for the microCT scanning in the “number of cycles” study, and Dr. Bettina Willie for helpful discussions of the data.
Footnotes
This project was funded by the Oxnard Foundation, the National Science Foundation (BES9753164, BES9875383), the National Institutes of Health (P30-AR46121), the Frese Foundation, the Clark Foundation, and the Kirby Foundation.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Cornell University, Ithaca, NY, and Hospital for Special Surgery, New York, NY.
References
- 1.Aspenberg P, Goodman S, Toksvig-Larsen S, Ryd L, Albrektsson T. Intermittent micromotion inhibits bone ingrowth. Titanium implants in rabbits.Acta Orthop Scand. 1992;63:141–145. [DOI] [PubMed]
- 2.Chambers TJ, Evans M, Gardner TN, Turner-Smith A, Chow JWM. Induction of bone formation in rat tail vertebrae by mechanical loading.Bone Miner. 1993;20:167–178. [DOI] [PubMed]
- 3.Cullen DM, Smith RT, Akhter MP. Bone-loading response varies with strain magnitude and cycle number.J Appl Physiol. 2001;91:1971–1976. [DOI] [PubMed]
- 4.Cullen DM, Smith RT, Akhter MP. Time course for bone formation with long-term external mechanical loading.J Appl Physiol. 2000;88:1943–1948. [DOI] [PubMed]
- 5.De Souza RL, Matsuura M, Eckstein F, Rawlinson SC, Lanyon LE, Pitsillides AA. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element.Bone. 2005;37:810–818. [DOI] [PubMed]
- 6.Fritton JC, Myers ER, Wright TM, van der Meulen MC. Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia.Bone. 2005;36:1030–1038. [DOI] [PubMed]
- 7.Fritton JC, Myers ER, Wright TM, van der Meulen MC. Bone mass is preserved and cancellous architecture altered due to cyclic loading of the mouse tibia after orchidectomy.J Bone Miner Res. 2008;23:663–671. [DOI] [PMC free article] [PubMed]
- 8.Goldstein SA, Matthews LS, Kuhn JL, Hollister SJ. Trabecular bone remodeling: an experimental model.J. Biomech. 1991;24 Suppl 1:135–150. [DOI] [PubMed]
- 9.Guldberg RE, Caldwell NJ, Guo XE, Goulet RW, Hollister SJ, Goldstein SA. Mechanical stimulation of tissue repair in the hydraulic bone chamber.J Bone Miner Res. 1997;12:1295–1302. [DOI] [PubMed]
- 10.Guo XE, Eichler MJ, Takai E, Kim CH. Quantification of a rat tail vertebra model for trabecular bone adaptation studies.J Biomech. 2002;35:363–368. [DOI] [PubMed]
- 11.Haapasalo H, Kontulainen S, Sievanen H, Kannus P, Jarvinen M, Vuori I. Exercise-induced bone gain is due to enlargement in bone size without a change in volumetric bone density: a peripheral quantitative computed tomography study of the upper arms of male tennis players.Bone. 2000;27:351–357. [DOI] [PubMed]
- 12.Hert J, Liskova M, Landa J. Reaction of bone to mechanical stimuli Part 1 Continuous and intermittent loading of tibia in rabbit.Folia Morph, Prague. 1971;19:290–300. [PubMed]
- 13.Hollister SJ, Brennan JM, Kikuchi N. A homogenization sampling procedure for calculating trabecular bone effective stiffness and tissue level stress.J Biomech. 1994;27:433–444. [DOI] [PubMed]
- 14.Hollister SJ, Guldberg RE, Kuelske CL, Caldwell NJ, Richards M, Goldstein SA. Relative effects of wound healing and mechanical stimulus on early bone response to porous-coated implants.J Orthop Res. 1996;14:654–662. [DOI] [PubMed]
- 15.Lanyon LE, Rubin CT. Static vs dynamic loads as an influence on bone remodelling.J. Biomech. 1984;17:897–905. [DOI] [PubMed]
- 16.Moalli MR, Caldwell NJ, Patil PV, Goldstein SA. An in vivo model for investigations of mechanical signal transduction in trabecular bone.J Bone Miner Res. 2000;15:1346–1353. [DOI] [PubMed]
- 17.Morey ER. Spaceflight and bone turnover: correlation with a new rat model of weightlessness.BioSci. 1979;29:168–172. [DOI]
- 18.Morey ER, Baylink DJ. Inhibition of bone formation during space flight.Science. 1978;201:1138–1141. [DOI] [PubMed]
- 19.Robling AG, Burr DB, Turner CH. Partitioning a daily mechanical stimulus into discrete loading bouts improves the osteogenic response to loading.J Bone Miner Res. 2000;15:1596–1602. [DOI] [PubMed]
- 20.Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads.J Bone Joint Surg Am. 1984;66:397–402. [PubMed]
- 21.Srinivasan S, Weimer DA, Agans SC, Bain SD, Gross TS. Low-magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle.J Bone Miner Res. 2002;17:1613–1620. [DOI] [PMC free article] [PubMed]
- 22.Torrance AG, Mosley JR, Suswillo RF, Lanyon LE. Noninvasive loading of the rat ulna in vivo induces a strain-related modeling response uncomplicated by trauma or periostal pressure.Calcif. Tissue Int. 1994;54:241–247. [DOI] [PubMed]
- 23.Turner CH, Akhter MP, Raab DM, Kimmel DB, Recker RR. A noninvasive, in vivo model for studying strain adaptive bone modeling.Bone. 1991;12:73–79. [DOI] [PubMed]
- 24.U.S. Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Office of the Surgeon General; 2004.
- 25.van der Meulen MCH, Morgan TG, Yang X, Baldini TH, Myers ER, Wright TM, Bostrom MPG. Cancellous bone adaptation to in vivo loading in a rabbit model.Bone. 2006;38:871–877. [DOI] [PMC free article] [PubMed]
- 26.van Rietbergen B, Weinans H, Huiskes R, Odgaard A. A new method to determine trabecular bone elastic properties and loading using micromechanical finite-element models.J Biomech. 1995;28:69–81. [DOI] [PubMed]
- 27.Verhulp E, van Rietbergen B, Huiskes R. Comparison of micro-level and continuum-level voxel models of the proximal femur. J Biomech. 2006;39:2951–2957. [DOI] [PubMed]
- 28.Waarsing JH, Day JS, van der Linden JC, Ederveen AG, Spanjers C, De Clerck N, Sasov A, Verhaar JA, Weinans H. Detecting and tracking local changes in the tibiae of individual rats: a novel method to analyse longitudinal in vivo micro-CT data. Bone. 2004;34:163–169. [DOI] [PubMed]
- 29.Waldorff EI, Goldstein SA, McCreadie BR. Age-dependent microdamage removal following mechanically induced microdamage in trabecular bone in vivo.Bone. 2007;40:425–432. [DOI] [PubMed]