Abstract
Objective
The purpose of this study was to evaluate the relationship of left ventricular mass and geometry measured with cardiac MRI to incident cardiovascular events in the Multi-Ethnic Study of Atherosclerosis (MESA) study.
Background
MRI is highly accurate for evaluation of heart size and structure and has not previously been used in a large epidemiologic study to predict cardiovascular events.
Methods
5098 participants in the MESA study underwent cardiac MRI at the baseline examination and were followed for a median of 4 years. Cox proportional hazard models were constructed to predict the endpoints of coronary heart disease (CHD), stroke and heart failure (HF) after adjustment for cardiovascular risk factors.
Results
216 incident events were observed during the follow-up period. In adjusted models, the endpoints of incident CHD and stroke were positively associated with increased left ventricular mass to volume ratio (coronary heart disease, hazard ratio 2.1 per g/ml, p = 0.02; stroke, hazard ratio 4.2 per g/ml, p =0.005). In contrast, left ventricular mass showed the strongest association with incident HF events (hazard ratio 1.4 per 10% increment, p < 0.0001). HF events occurred primarily in participants with left ventricular hypertrophy, i.e.,≥ 95th percentile of left ventricular mass (hazard ratio 8.6, confidence interval, 3.7 – 19.9, reference group <50th percentile of LV mass).
Conclusions
Left ventricular size was related to incident HF, stroke and CHD in this multi-ethnic cohort. While body-size adjusted left ventricular mass alone predicted incident HF, concentric ventricular remodeling predicted incident stroke and CHD.
Keywords: Heart failure, stroke, coronary heart disease, epidemiology, magnetic resonance imaging, left ventricular hypertrophy
Introduction
The Framingham Study (1-3) as well as other population based studies(4-7) have shown that increased left ventricular (LV) mass, known as left ventricular hypertrophy, is an independent predictor of cardiovascular events in population-based studies using electrocardiograms or echocardiography to define LVH. The value of LV hypertrophy to predict cardiovascular disease events holds for individuals without (1-3,7) as well as with prior known coronary heart disease (5,8) and heart failure (HF) (9,10). Reduction of LV mass as a result of therapeutic intervention reduces cardiovascular events(11-14) indicating LV mass is an important subclinical marker of cardiovascular disease (15).
LV hypertrophy is associated with multiple factors such as increased age, blood pressure or diabetes (16-19), resulting in increased stiffness of the left ventricle. Geometric changes of the ventricle, termed remodeling, have been investigated primarily by echocardiography in relationship to cardiovascular events (20-24). Echocardiographic estimates of LV hypertrophy (defined by LV diameters and wall thickness normalized by body surface area (25) > 125 g/m2) and the ratio of posterior wall thickness to LV radius ≥ 0.45 (22) have been used to define concentric remodeling of the LV. The presence and pattern of ventricular remodeling has been noted to confer cardiovascular risk beyond LV hypertrophy in some studies (22,24,26,27), but not in others (23,28).
Magnetic resonance imaging (MRI) is highly accurate and reproducible for assessing 3-dimensional (3D) ventricular size and shape (29-34) and thus may allow additional insight into the pathophysiology of myocardial remodeling. In this study, we report the relationship between left ventricular mass and volume as determined by MRI to incident cardiovascular disease in a multi-ethnic cohort free from clinical cardiovascular disease at baseline.
Methods
Subjects
The Multi-Ethnic-Study of Atherosclerosis (MESA) has been previously described.(35) In brief, between July 2000 and August 2002, 6,814 men and women who identified themselves as white, African-American, Hispanic, or Chinese and were 45 to 84 years old and free of clinically apparent cardiovascular disease were recruited from six US communities: Baltimore City and Baltimore County, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; Northern Manhattan and the Bronx, New York; and St. Paul, Minnesota. Consenting participants underwent a cardiac MRI scan a median of 16 days after the baseline evaluation; 95 percent were completed by 11 weeks after the baseline examination. The institutional review boards at all participating centers approved the study, and all participants gave informed consent.
Risk factor measures
Standardized questionnaires were used to obtain information about smoking history and medication usage for high blood pressure, high cholesterol, or diabetes. Smoking was defined as current, former, or never. Subjects had measurement of height and weight. Resting blood pressure was measured 3 times with participants in the seated position with a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon). The average of the last 2 measurements was used in analysis. Total and HDL cholesterol, and glucose levels were measured from blood samples obtained after a 12-hour fast. LDL cholesterol was calculated with the Friedewald equation.(36)
Diabetes was defined as fasting glucose ≥126 mg/dL or use of hypoglycemic medication. Impaired fasting glucose was defined as fasting glucose 100 to 125 mg/dL. Hypertension status was classified according to the Seventh Report of the Joint National Committee on the Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7) (37). Body mass index (BMI; kg/m2) was calculated from weight measured to the nearest 0.5 kg and height to the nearest 0.1 cm.
Cardiac MRI
Cardiac MRI was performed with 1.5-T magnets with determination of LV mass and volumes as previously described.(38) Briefly a stack of short axis images covering the entire left ventricle was acquired with TR/ TE 8-10 msec/ 3-5 msec, flip angle 20 degrees, 6 mm slice thickness, 4 mm gap, flow compensation, in plane resolution 1.4-1.6 mm (frequency) x 2.2-2.5 mm. The endocardial and epicardial myocardial borders were contoured using a semi-automated method (MASS 2.4, Medis, The Netherlands). The difference between the epicardial and endocardial areas for all slices was multiplied by the slice thickness and section gap, and then multiplied by the specific gravity of myocardium (1.04 g/ml) to determine the ventricular mass. Papillary muscle mass was included in the left ventricular cavity and excluded from the left ventricular mass. This approach showed better reproducibility than contouring of individual papillary muscles in preliminary data analyses. A study of repeat measurements of LV mass on 79 MESA subjects performed between 3 and 6 months after the initial measurement showed the technical error of measurement percent of the mean (TEM%) was 6% and 4% for LV mass and end diastolic volume respectively, and the intraclass correlation coefficients were 0.98 and 0.98, respectively.(38)
Preliminary evaluation showed MRI measured LV mass and volume indexed by body surface area, height2.7 or height1.9 did not fully remove the correlation of these measures with weight and/or height. Using an allometric approach (39), regression models for body size were derived from a sample of 1746 MESA participants without obesity, hypertension, antihypertensive medication use, diabetes, impaired fasting glucose or hypoglycemic medication use using a multiplicative model estimated by regressing log(LV mass) on log(height), log(weight) and gender. LV mass was adjusted for body size by dividing 100*LV mass by the predicted LV mass based on height, weight and gender, as 100*LV mass / (a * Height0.54 * Weight0.61) where a = 6.82 for women and 8.25 = men with mass in grams, height in meters, weight in kilograms. Similarly, the body size adjusted LV end diastolic volume was computed as 100*LV*volume/(b*Height1.25*Weight0.43) where b = 10.0 for women and 10.5 for males and LV end diastolic volume is in milliliters.
Adjudication of events
Participants were followed for incident cardiovascular events up to 5.2 years from their baseline examinations. In addition to three follow-up MESA examinations, a telephone interviewer contacted each participant every 9 to 12 months to inquire about all interim hospital admissions, cardiovascular outpatient diagnoses, and deaths. In order to verify self-reported diagnoses, copies were requested of all death certificates and medical records for all hospitalizations and outpatient cardiovascular diagnoses. Next of kin interviews for out of hospital cardiovascular deaths were obtained. We were successful in getting medical records on an estimated 98% of hospitalized cardiovascular events and information on 95% of outpatient cardiovascular diagnostic encounters. Follow-up telephone interviews were completed in 92% of living participants.
Trained personnel abstracted any medical records suggesting possible cardiovascular events. Two physicians from the MESA events committee independently reviewed all medical records for endpoint classification and assignment of incidence dates. The reviewers were blinded to the MESA MRI results and used pre-specified criteria. (See Appendix material for detailed criteria for all events.) If the reviewing physicians disagreed on the event classification, they adjudicated differences. If disagreements persisted, the full events committee made the final classification.
Reviewers classified myocardial infarction as definite, probable or absent, based primarily on combinations of symptoms (e.g., chest pain), ECG abnormalities, and cardiac biomarker levels (SEE JOURNAL WEBSITE). CHD death was classified as present or absent based on hospital records and interviews with families. Definite fatal CHD required an MI within 28 days of death, chest pain within the 72 hours before death, or a history of CHD and the absence of a known non-atherosclerotic or non-cardiac cause of death. Adjudicators graded angina based on their clinical judgment as definite, probable or absent. Definite and probable angina required clear documentation of chest pain or anginal equivalent. Definite angina also required objective evidence of reversible myocardial ischemia or obstructive coronary artery disease (e.g., ≥70% coronary artery obstruction or positive stress test). Stroke required documented focal neurologic deficit lasting 24 hours or until death, or if < 24 hours, there was a clinically relevant lesion on brain imaging. Patients with focal neurologic deficits secondary to brain trauma, tumor, infection, or other non-vascular cause were excluded. Definite and probable CHF required clinical symptoms (e.g., shortness of breath) or signs (e.g., edema), as asymptomatic disease was not an endpoint. Probable CHF further required a physician diagnosis of CHF and medical treatment for CHF. Definite CHF also required (a) pulmonary edema/congestion by chest X-ray and/or (b) dilated ventricle or poor LV function by echocardiography or ventriculography, or evidence of left ventricular diastolic dysfunction.
Statistical methods
Unadjusted Cox proportional hazards models were first calculated for each endpoint (CHD events, stroke, HF) for LV mass and end diastolic volume separately as continuous variables (per 10 percent increments) and then for LV mass and end diastolic volume jointly in the same model in order to assess the role of LV geometry. Probable and definite HF and CHD events were considered in the analysis. All stroke events were definite. In additional models, the ratio of LV mass/volume was included both with and without adjustment for body-size. In all instances, there were only minor differences in the fit between these models and for simplicity we only show the results for the ratio of unadjusted LV mass / volume. Then age, gender, ethnicity, diabetes (diabetic, impaired fasting glucose, normal), cigarette smoking (present, former, never), total cholesterol, high density lipoprotein (HDL) cholesterol, use of anti-hypertensive or lipid lowering medication, and systolic and diastolic blood pressure were added to the models.
These analyses were repeated for incident CHD and stroke using quartiles of LV mass / volume. For incident HF, where body-size adjusted LV mass was the best predictor of risk, instead of quartiles, the intervals were constructed to display the non-linearity in risk that was evident from non-linear modeling (results not shown) of the risk in the Cox models. Kaplan-Meier cumulative event rate plots were calculated for the above discrete intervals of the LV measures. Rates in 100 person years are displayed for descriptive purposes for the quartiles of each LV measure.
All analyses were performed using Stata 10.0 for Windows (StataCorp, College Station, Texas). P-values <0.05 are considered statistically significant and presented for descriptive purposes. Confidence intervals are expressed as 95% confidence intervals (CI).
Results
Subject characteristics
Of the 6814 MESA participants, 5098 underwent cardiac MRI (75%) and 5004 (73%) had technically adequate data. Thirty-six participants had no follow-up information leaving 4968 participants in the analysis. Compared to those not included in the analysis (n=1846), those included were slightly younger (2.3 yrs), had lower systolic blood pressure (4.3 mmHg) and body mass index (2.2 units), were less likely to be African American (7.7% less), were more likely to be Asian (4.8% more), and were less likely to have treated hypertension (7.0% less) or diabetes (3.0% less). The mean age of the participants was 62 years (range 45-85): 52% of participants were female, 13% were Chinese-American, 26% were African-American, and 22% were Hispanic and 39% were White.
Cardiovascular events
There were 216 total events through 5.2 years of follow-up (median 4 years). Angina was most frequent (71 events), followed by HF (48 events), myocardial infarction (45 events), stroke (39 events) and CHD death (13 events). Baseline characteristics of participants with and without cardiovascular events are shown in Table 1. Of CHD events, 100 were definite and 15 were probable. Of HF events 33 were definite and 15 were probable. The participants who had cardiovascular events versus no events were more likely to be older at baseline (by 8 years), men (59% versus 47%), diabetic (24% versus 12%), current smokers (except for stroke events), use lipid lowering medication (28% versus 15%), and use hypertension medication (57% versus 35%), respectively. Participants who developed CHF events versus no events were additionally more likely to be black (35% versus 26%), while stroke events versus no events more likely in Hispanics (31% versus 22%) and those with systolic hypertension (29% versus 21%), respectively.
Table 1.
Baseline characteristics of the MESA cohort for participants with and without selected cardiovascular events.
| Characteristic | No Events N=4801 | CHD N=115* | Stroke N=39 | HF N=48 |
|---|---|---|---|---|
| Age, years | 61 (10) | 66 (9) | 71 (8) | 68 (9) |
| Gender, n (%) | ||||
| Women | 2549 (53) | 30 (26) | 22 (56) | 15 (31) |
| Men | 2252 (47) | 85 (74) | 17 (44) | 33 (69) |
| Ethnicity, n (%) | ||||
| White | 1878 (39) | 51 (44) | 17 (44) | 16 (33) |
| Chinese | 633 (13) | 14 (12) | 5 (13) | 4 (8) |
| Black | 1235 (26) | 26 (22) | 5 (13) | 17 (35) |
| Hispanic | 1055 (22) | 24 (21) | 12 (31) | 11 (23) |
| Cigarette smoking, n (%) | ||||
| Never | 2491 (52) | 39 (34) | 21 (54) | 13 (28) |
| Former | 1702 (36) | 52 (45) | 12 (31) | 21 (45) |
| Current | 595 (12) | 24 (21) | 6 (15) | 13 (28) |
| Body mass index, kg/m2 | 28 (5) | 28 (5) | 29 (4) | 30 (6) |
| Diabetes classification, n (%) | ||||
| Normal | 2921 (61) | 49 (43) | 15 (39) | 16 (33) |
| IFG | 1274 (27) | 35 (30) | 11 (29) | 15 (31) |
| Diabetes | 594 (12) | 31 (27) | 12 (32) | 17 (35) |
| Hypertension medication, n (%) | ||||
| No | 3140 (65) | 56 (49) | 14 (36) | 16 (33) |
| Yes | 1658 (35) | 59 (51) | 25 (64) | 32 (67) |
| Systolic blood pressure, mm Hg | 125 (21) | 135 (23) | 149 (29) | 137 (20) |
| Diastolic blood pressure, mm Hg | 72 (10) | 74 (11) | 77 (14) | 74 (11) |
| Lipid lowering medication, n (%) | ||||
| No | 4058 (85) | 77 (67) | 31 (79) | 36 (75) |
| Yes | 740 (15) | 38 (33) | 8 (21) | 12 (25) |
| Total Cholesterol, mg/ dl | 194 (35) | 197 (38) | 200 (38) | 195 (37) |
| HDL Cholesterol, mg/ dl | 51 (15) | 47 (15) | 50 (12) | 50 (15) |
IFG = impaired fasting glucose, CHD=myocardial infarction or angina, HF = heart failure
For continuous variables, mean values ± standard deviations are shown.
14 participants had two CHD events.
Relationship of LV mass and geometry to incident coronary heart disease
The results of the unadjusted and adjusted Cox proportional hazard models are shown in Table 2 for incident CHD events. After adjustment for risk factors, body-size adjusted LV mass and end diastolic volume considered separately were not significant predictors of CHD events. In combination, greater LV mass/ volume ratio was positively associated with incident CHD (hazard ratio (HR) for incident CHD, 2.1 per g/ml, p = 0.02). The LV mass / volume ratio model had a similar fit to the model that included both body-size adjusted LV mass and end diastolic volume (not shown). A similar conclusion was reached for a model based on quartiles of LV mass /volume (HR, 2.3 per for the upper quartile compared to the first quartile, p = 0.01, Figure 1).
Table 2.
The relationship of left ventricle end diastolic volume and mass to coronary heart disease events.
| Cox Models for Incident CHD | ||||
|---|---|---|---|---|
| Unadjusted | Adjusted** | |||
| Model | HR (95% CI) | p-value | HR (95% CI) | p-value |
| LV Mass* (per 10%) | 1.1 (1.0, 1.2) | 0.05 | 1.0 (0.9, 1.1) | 0.39 |
| LV Volume* (per 10%) | 0.9 (0.8, 0.9) | 0.002 | 0.9 (0.8, 1.0) | 0.09 |
| LV Mass /LV Volume (g/ml) | 5.5 (3.3, 9.1) | <0.0001 | 2.1 (1.1, 4.1) | 0.02 |
| LV Mass / LV Volume in Quartiles | ||||
| 1st quartile (0.51-1.0) | 1.0 (Reference) | 1.0 (Reference) | ||
| 2nd quartile (1.0-1.13) | 2.0 (1.0, 4.0) | 0.05 | 1.5 (0.7, 3.0) | 0.30 |
| 3rd quartile (1.13-1.29) | 2.0 (1.0, 4.1) | 0.05 | 1.3 (0.6, 2.6) | 0.63 |
| 4th quartile (1.29-2.89) | 5.3 (2.9, 10.0) | <0.0001 | 2.3 (1.2, 4.4) | 0.01 |
HR = hazard ratio
Adjusted for body size.
Adjusted for the following risk factors: age, gender, race, cigarette smoking, total cholesterol, HDL cholesterol, use of lipid lowering medication, systolic blood pressure, diastolic blood pressure, use of anti-hypertensive drugs and diabetes.
Figure 1.
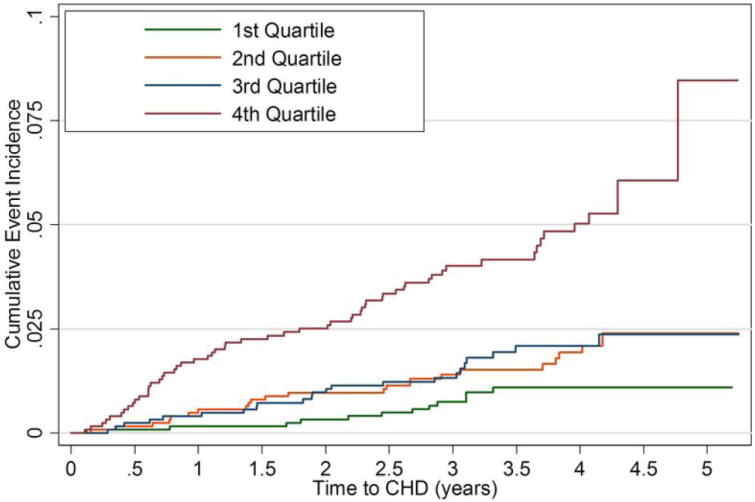
Cumulative event rates for coronary heart disease events by quartiles of LV mass/ volume.
Relationship of LV mass and geometry size to incident stroke
After adjustment for risk factors and in separate models, body-size adjusted LV mass but not LV end diastolic volume was positively associated with incident stroke (LV mass, HR 1.2 per 10% increment, p = 0.01) (Table 3). In the adjusted model, greater LV mass/ volume ratio was positively associated with stroke events (HR 4.2 per g/ml, p =0.005). The LV mass/ volume ratio model had a similar fit to a model that included both body-size adjusted LV mass and end diastolic volume (not shown). With increasing LV mass/volume ratio, the number of stroke events increased in the adjusted model (highest quartile versus lowest quartile, HR 11.1, p = 0.02, Figure 2).
Table 3.
The relationship of left ventricle end diastolic volume and mass to stroke events.
| Cox Models for Incident Stroke | ||||
|---|---|---|---|---|
| Unadjusted | Adjusted** | |||
| Model | HR (95% CI) | p-value | HR (95% CI) | p-value |
| LV Mass* (per 10%) | 1.2 (1.1, 1.4) | <0.0001 | 1.2 (1.0, 1.4) | 0.01 |
| LV Volume* (per 10%) | 0.9 (0.7, 1.1) | 0.16 | 0.9 (0.8, 1.1) | 0.51 |
| LV Mass /LV Volume (g/ml) | 7.8 (3.6, 17.3) | <0.0001 | 4.2 (1.5, 11.2) | 0.005 |
| LV Mass /LV Volume in Quartiles | ||||
| 1st quartile (0.51-1.0) | 1.0 (Reference) | 1.0 (Reference) | ||
| 2nd quartile (1.0-1.13) | 6.0 (0.7, 50.2) | 0.10 | 4.1 (0.5, 50.2) | 0.20 |
| 3rd quartile (1.13-1.29) | 10.2 (1.3, 80.1) | 0.03 | 6.8 (0.9, 54.0) | 0.07 |
| 4th quartile (1.29-2.89) | 23.0 (3.1, 170.5) | 0.003 | 11.1 (1.4, 84.8) | 0.02 |
HR = hazard ratio
Adjusted for body size.
Adjusted for the following risk factors: age, gender, race, cigarette smoking, total cholesterol, HDL cholesterol, use of lipid lowering medication, systolic blood pressure, diastolic blood pressure, use of anti-hypertensive drugs and diabetes.
Figure 2.

Cumulative event rates for stroke events by quartiles of LV mass/ volume.
Relationship of LV mass and geometry to incident heart failure
As shown in Table 4, in separate models both body-size adjusted LV mass and end diastolic volume were positively associated with incident HF before and after adjustment for risk factors (after adjustment, LV mass, HR 1.4 per 10% increment, p < 0.0001; LV volume, HR 1.3 per 10% increment, p < 0.0001). However, unlike incident CHD or stoke, incident HF in the fully adjusted models was not significantly associated with LV mass/ volume ratio (Table 6), (p=0.11). Thus body-size adjusted LV mass alone was the best measure of heart size to predict incident HF. Inclusion of left ventricular ejection fraction in a model with LV mass showed little change in the adjusted HR’s or model fit.
Table 4.
The relationship of left ventricle end diastolic volume and mass to heart failure events.
| Cox Models for Incident HF | ||||
|---|---|---|---|---|
| Unadjusted | Adjusted** | |||
| Model | HR (95% CI) | p-value | HR (95% CI) | p-value |
| LV Mass* (per 10%) | 1.4 (1.3, 1.5) | <0.0001 | 1.4 (1.2, 1.5) | <0.0001 |
| LV Volume* (per 10%) | 1.3 (1.2, 1.5) | <0.0001 | 1.3 (1.2, 1.5) | <0.0001 |
| LV Mass/LV Volume (g/ml) | 7.4 (3.6,15.4) | <0.0001 | 2.3 (0.8, 6.1) | 0.11 |
| LV Mass* In Intervals | ||||
| <50th percentile | 1.0 (Reference) | 1.0 (Reference) | ||
| 50th-90th percentile | 1.7 (0.8, 3.7) | 0.21 | 1.6 (0.7, 3.6) | 0.23 |
| 90-95th percentile | 2.7 (0.6, 12.3) | 0.20 | 2.4 (0.5, 11.1) | 0.27 |
| ≥95th percentile | 13.0 (6.1, 27.7) | <0.0001 | 8.6 (3.7, 19.9) | <0.0001 |
HR = hazard ratio
Adjusted for body size
Adjusted for the following risk factors: age, gender, race, cigarette smoking, total cholesterol, HDL cholesterol, use of lipid lowering medication, systolic blood pressure, diastolic blood pressure, use of anti-hypertensive drugs and diabetes.
Because only 1 HF event occurred in the reference group (1st quartile of LV mass), the HR ratio estimates with this reference group were unstable. Most events occurred in participants with body-size adjusted LV mass greater than or equal to 90% of predicted based on height and weight. In order to examine the gradient of relative risk, 4 categories of LV mass index were compared: below the median (50th) percentile of LV mass index (reference category), the 50-89 percentile, the 90-94th percentile and greater than or equal to the 95th percentile of LV mass index (as previously taken to be the definition of LV hypertrophy (3,4,10,24)) The HR for participants with LV hypertrophy (95th percentile) versus those below the median for LV mass was 8.6 [3.7, 19.9] (p < 0.0001) (Figure 3).
Figure 3.
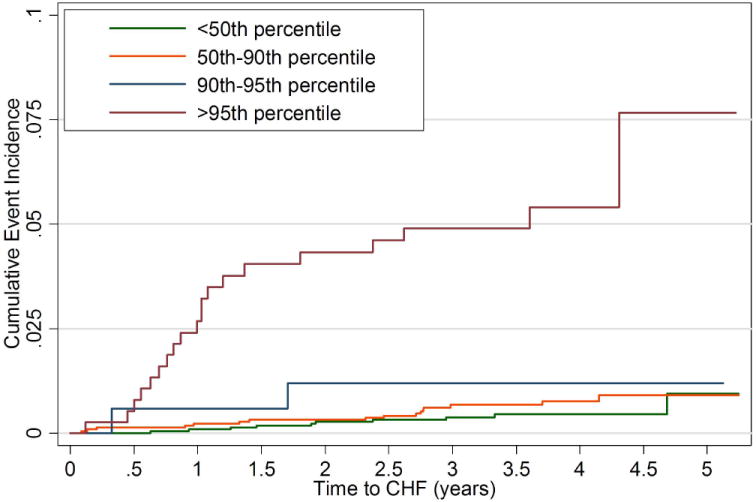
Cumulative event rates for heart failure events by intervals of LV mass (body size adjusted).
Discussion
The pathophysiologic changes in the size and function of the heart in response to cardiovascular risk factors are complex, and increasingly accurate tools are now available to explore these relationships. The MESA study is the first epidemiologic study that has used cardiac MRI in a large cohort to evaluate incident cardiovascular events. There are several conclusions from this study: 1) In a diverse, multi-ethnic cohort, left ventricular hypertrophy confers a substantially elevated risk for incident heart failure, consistent with prior reports from predominantly white or African-American cohorts. 2) Elevated left ventricular mass in most individuals was accommodated over the 4 year period of follow-up, with only the top 5% of the cohort showing increased risk for incident heart failure in adjusted models. 3) Concentric remodeling (defined by elevated LV mass to volume ratio), rather than elevated ventricular mass, was predictive of incident non-heart failure cardiovascular events, specifically stroke and coronary heart disease.
Data from the Framingham study has previously linked LV hypertrophy detected by the electrocardiogram (ECG) to coronary heart disease (myocardial infarction, angina, sudden death) (1). ECG defined LV hypertrophy had a three fold risk of developing clinically apparent coronary heart disease (including HF) compared to the group without LV hypertrophy. In other observational studies, the relative risk of ECG defined LV hypertrophy for incident HF only was 1.4 to 2.9 (3,5,6). ECG is a relatively low cost method for detecting LV hypertrophy (3,40,41) but the sensitivity of ECG for LVH is only 6-20% (3,41). Using echocardiography, the reported relative risk of LV hypertrophy for incident HF in previous observational studies was 1.6 to 3.4 (3,4,7).
For left ventricular mass greater than or equal to the 95th percentile compared to the reference group of <50% percentile, the adjusted hazard ratio for HF in the MESA population was 8.6 (confidence interval, 3.9 – 19.9) using MRI to measure heart size. The greater risk conferred by LV hypertrophy in this study compared to other cohorts is notable. This greater risk may be explained by demographic differences between the cohorts, different approaches to statistical assessment and/or by different methods of heart size assessment (MRI versus echocardiography or ECG). The high accuracy and reproducibility of cardiac MRI (standard errors of about 5% (32,42,43) compared to 20% for echocardiography (44) in single center studies) should facilitate risk estimates for short term studies that by nature will entail fewer events. It is notable that left ventricular mass less than the 95th percentile did not predict incident heart failure events over the 4 year period of follow-up in a cohort that was asymptomatic at baseline.
The relative role of LV hypertrophy versus concentric remodeling associated with cardiovascular events has been unclear. Koren et al (22) originally reported a cardiovascular event rate of 4.2 per 100 patient-years when concentric remodeling was present, versus 1.8 per 100 patient years when there was normal LV geometry. Similar results were identified in other studies (21,24,26,27) but no additional predictive value for concentric hypertrophy beyond LV mass was found in Framingham (23) or in hypertensive subjects studied by Verdecchia et al (28). In general, prior studies have combined types cardiovascular events to examine the relationship to LV mass or LV geometry. The results of this study show that stroke and CHD events were better predicted by elevated LV geometry, whereas HF events were driven primarily by LV mass alone. While our results do not indicate causality, potential mechanisms relating left ventricular remodeling to abnormal arterial structure and function (45) and to stroke and CHD (46) have been previously described.
Reliable evaluation of the relationship of ethnicity in relationship to LV mass and cardiovascular events will require additional follow-up and/or larger sample sizes. The general applicability of our results may be limited by selection and survivor biases. Since MESA participants had no known CVD at baseline, the older individuals undergoing MRI in this cohort represent a particularly healthy sample of the population at large. The mechanisms by which cardiovascular events result from changes in heart size are not elucidated by these observational data. At the time of data collection, only the fast gradient echo MRI pulse sequence was available at all the field centers; the steady state free precession sequence has since been developed for cardiac MRI and this sequence shows better reproducibility for cardiac mass and volume measurement. As indicated in the methods, we did not include the papillary muscle mass as part of the LV mass. The papillary muscle mass is directly related to LV mass over a wide range of values. LV mass methods that include papillary muscles would thus be somewhat larger and mass/volume ratios smaller than we have reported. The diagnosis of HF is not as definitive as other cardiovascular events such as stroke of myocardial infarction. Therefore, we required that participants be symptomatic with physician diagnosed HF documented in medical records that were adjudicated by two physician reviewers.
In conclusion, in an ethnically diverse population free of symptomatic cardiovascular disease at baseline, the end diastolic volume and mass of the left ventricle determined by MRI were strongly associated with cardiovascular events. The association between stroke and coronary heart disease may be mediated through concentric ventricular remodeling, while incident HF was most closely associated with very high levels of left ventricular mass.
Supplementary Material
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. Dr. Kronmal had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
This research was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute.
Abbreviations
- HF
heart failure
- MRI
magnetic resonance imaging
- MESA
Multi Ethnic Study of Atherosclerosis
- CHD
coronary heart disease
- LV
left ventricle
- LVH
left ventricular hypertrophy
- HR
hazard ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kannel WB, Gordon T, Castelli WP, Margolis JR. Electrocardiographic left ventricular hypertrophy and risk of coronary heart disease. The Framingham study. Ann Intern Med. 1970;72:813–22. doi: 10.7326/0003-4819-72-6-813. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Left ventricular mass and incidence of coronary heart disease in an elderly cohort. The Framingham Heart Study. Ann Intern Med. 1989;110:101–7. doi: 10.7326/0003-4819-110-2-101. [DOI] [PubMed] [Google Scholar]
- 3.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 4.Gardin JM, McClelland R, Kitzman D, et al. M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study) Am J Cardiol. 2001;87:1051–7. doi: 10.1016/s0002-9149(01)01460-6. [DOI] [PubMed] [Google Scholar]
- 5.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–37. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 6.Mazza A, Tikhonoff V, Casiglia E, Pessina AC. Predictors of congestive heart failure mortality in elderly people from the general population. Int Heart J. 2005;46:419–31. doi: 10.1536/ihj.46.419. [DOI] [PubMed] [Google Scholar]
- 7.Tsang TS, Barnes ME, Gersh BJ, et al. Prediction of risk for first age-related cardiovascular events in an elderly population: the incremental value of echocardiography. J Am Coll Cardiol. 2003;42:1199–205. doi: 10.1016/s0735-1097(03)00943-4. [DOI] [PubMed] [Google Scholar]
- 8.Ghali JK, Liao Y, Simmons B, Castaner A, Cao G, Cooper RS. The prognostic role of left ventricular hypertrophy in patients with or without coronary artery disease. Ann Intern Med. 1992;117:831–6. doi: 10.7326/0003-4819-117-10-831. [DOI] [PubMed] [Google Scholar]
- 9.Aronow WS, Ahn C, Kronzon I, Koenigsberg M. Congestive heart failure, coronary events and atherothrombotic brain infarction in elderly blacks and whites with systemic hypertension and with and without echocardiographic and electrocardiographic evidence of left ventricular hypertrophy. Am J Cardiol. 1991;67:295–9. doi: 10.1016/0002-9149(91)90562-y. [DOI] [PubMed] [Google Scholar]
- 10.Kupari M, Lindroos M, Iivanainen AM, Heikkila J, Tilvis R. Congestive heart failure in old age: prevalence, mechanisms and 4-year prognosis in the Helsinki Ageing Study. J Intern Med. 1997;241:387–94. doi: 10.1046/j.1365-2796.1997.129150000.x. [DOI] [PubMed] [Google Scholar]
- 11.Devereux RB, Dahlof B, Gerdts E, et al. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. Circulation. 2004;110:1456–62. doi: 10.1161/01.CIR.0000141573.44737.5A. [DOI] [PubMed] [Google Scholar]
- 12.Devereux RB, Wachtell K, Gerdts E, et al. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292:2350–6. doi: 10.1001/jama.292.19.2350. [DOI] [PubMed] [Google Scholar]
- 13.Kjeldsen SE, Dahlof B, Devereux RB, et al. Effects of losartan on cardiovascular morbidity and mortality in patients with isolated systolic hypertension and left ventricular hypertrophy: a Losartan Intervention for Endpoint Reduction (LIFE) substudy. JAMA. 2002;288:1491–8. doi: 10.1001/jama.288.12.1491. [DOI] [PubMed] [Google Scholar]
- 14.Okin PM, Devereux RB, Jern S, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292:2343–9. doi: 10.1001/jama.292.19.2343. [DOI] [PubMed] [Google Scholar]
- 15.Gardin JM, Lauer MS. Left ventricular hypertrophy: the next treatable, silent killer? JAMA. 2004;292:2396–8. doi: 10.1001/jama.292.19.2396. [DOI] [PubMed] [Google Scholar]
- 16.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–62. [PubMed] [Google Scholar]
- 17.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56:56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz AM. Cardiomyopathy of overload. A major determinant of prognosis in congestive heart failure. N Engl J Med. 1990;322:100–10. doi: 10.1056/NEJM199001113220206. [DOI] [PubMed] [Google Scholar]
- 19.Lauer MS, Anderson KM, Levy D. Influence of contemporary versus 30-year blood pressure levels on left ventricular mass and geometry: the Framingham Heart Study. J Am Coll Cardiol. 1991;18:1287–94. doi: 10.1016/0735-1097(91)90549-o. [DOI] [PubMed] [Google Scholar]
- 20.Di Tullio MR, Zwas DR, Sacco RL, Sciacca RR, Homma S. Left ventricular mass and geometry and the risk of ischemic stroke. Stroke. 2003;34:2380–4. doi: 10.1161/01.STR.0000089680.77236.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghali JK, Liao Y, Cooper RS. Influence of left ventricular geometric patterns on prognosis in patients with or without coronary artery disease. J Am Coll Cardiol. 1998;31:1635–40. doi: 10.1016/s0735-1097(98)00131-4. [DOI] [PubMed] [Google Scholar]
- 22.Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–52. doi: 10.7326/0003-4819-114-5-345. [DOI] [PubMed] [Google Scholar]
- 23.Krumholz HM, Larson M, Levy D. Prognosis of left ventricular geometric patterns in the Framingham Heart Study. J Am Coll Cardiol. 1995;25:879–84. doi: 10.1016/0735-1097(94)00473-4. [DOI] [PubMed] [Google Scholar]
- 24.Verdecchia P, Schillaci G, Borgioni C, et al. Adverse prognostic significance of concentric remodeling of the left ventricle in hypertensive patients with normal left ventricular mass. J Am Coll Cardiol. 1995;25:871–8. doi: 10.1016/0735-1097(94)00424-O. [DOI] [PubMed] [Google Scholar]
- 25.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 26.Muiesan ML, Salvetti M, Monteduro C, et al. Left ventricular concentric geometry during treatment adversely affects cardiovascular prognosis in hypertensive patients. Hypertension. 2004;43:731–8. doi: 10.1161/01.HYP.0000121223.44837.de. [DOI] [PubMed] [Google Scholar]
- 27.Pierdomenico SD, Lapenna D, Bucci A, Manente BM, Cuccurullo F, Mezzetti A. Prognostic value of left ventricular concentric remodeling in uncomplicated mild hypertension. Am J Hypertens. 2004;17:1035–9. doi: 10.1016/j.amjhyper.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Verdecchia P, Schillaci G, Borgioni C, et al. Prognostic value of left ventricular mass and geometry in systemic hypertension with left ventricular hypertrophy. Am J Cardiol. 1996;78:197–202. doi: 10.1016/s0002-9149(96)90395-1. [DOI] [PubMed] [Google Scholar]
- 29.Alfakih K, Bloomer T, Bainbridge S, et al. A comparison of left ventricular mass between two-dimensional echocardiography, using fundamental and tissue harmonic imaging, and cardiac MRI in patients with hypertension. Eur J Radiol. 2004;52:103–9. doi: 10.1016/j.ejrad.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Bellenger NG, Burgess MI, Ray SG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J. 2000;21:1387–96. doi: 10.1053/euhj.2000.2011. [DOI] [PubMed] [Google Scholar]
- 31.Bellenger NG, Davies LC, Francis JM, Coats AJ, Pennell DJ. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2000;2:271–8. doi: 10.3109/10976640009148691. [DOI] [PubMed] [Google Scholar]
- 32.Grothues F, Smith GC, Moon JC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 33.Myerson SG, Bellenger NG, Pennell DJ. Assessment of left ventricular mass by cardiovascular magnetic resonance. Hypertension. 2002;39:750–5. doi: 10.1161/hy0302.104674. [DOI] [PubMed] [Google Scholar]
- 34.Tse HF, Cheung BM, Ng W, Chan JK, Devereux RB, Lau CP. Regression of left ventricular hypertrophy after treatment of hypertension: comparison of directed M-echocardiography with magnetic resonance imaging in quantification of serial mass changes. J Card Fail. 2003;9:122–7. doi: 10.1054/jcaf.2003.12. [DOI] [PubMed] [Google Scholar]
- 35.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 36.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 37.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 38.Natori S, Lai S, Finn JP, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186:S357–65. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 39.Dewey FE, Rosenthal D, Murphy DJ, Jr, Froelicher VF, Ashley EA. Does size matter?: clinical applications of scaling cardiac size and function for body size. Circulation. 2008;117:2279–87. doi: 10.1161/CIRCULATIONAHA.107.736785. [DOI] [PubMed] [Google Scholar]
- 40.Rautaharju PM, Park LP, Gottdiener JS, et al. Race- and sex-specific ECG models for left ventricular mass in older populations. Factors influencing overestimation of left ventricular hypertrophy prevalence by ECG criteria in African-Americans. J Electrocardiol. 2000;33:205–18. doi: 10.1054/jelc.2000.7667. [DOI] [PubMed] [Google Scholar]
- 41.Levy D, Labib SB, Anderson KM, Christiansen JC, Kannel WB, Castelli WP. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation. 1990;81:815–20. doi: 10.1161/01.cir.81.3.815. [DOI] [PubMed] [Google Scholar]
- 42.Butler SP, McKay E, Paszkowski AL, Quinn RJ, Shnier RC, Donovan JT. Reproducibility study of left ventricular measurements with breath-hold cine MRI using a semiautomated volumetric image analysis program. J Magn Reson Imaging. 1998;8:467–72. doi: 10.1002/jmri.1880080230. [DOI] [PubMed] [Google Scholar]
- 43.Bottini PB, Carr AA, Prisant LM, Flickinger FW, Allison JD, Gottdiener JS. Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens. 1995;8:221–8. doi: 10.1016/0895-7061(94)00178-E. [DOI] [PubMed] [Google Scholar]
- 44.Myerson SG, Montgomery HE, World MJ, Pennell DJ. Left ventricular mass: reliability of M-mode and 2-dimensional echocardiographic formulas. Hypertension. 2002;40:673–8. doi: 10.1161/01.hyp.0000036401.99908.db. [DOI] [PubMed] [Google Scholar]
- 45.Fernandes VR, Polak JF, Cheng S, et al. Arterial Stiffness Is Associated With Regional Ventricular Systolic and Diastolic Dysfunction. The Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2007 doi: 10.1161/ATVBAHA.107.156950. [DOI] [PubMed] [Google Scholar]
- 46.Roman MJ, Pickering TG, Schwartz JE, Pini R, Devereux RB. Relation of arterial structure and function to left ventricular geometric patterns in hypertensive adults. J Am Coll Cardiol. 1996;28:751–6. doi: 10.1016/0735-1097(96)00225-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


