Abstract
1,25-dihydroxyvitamin D3 (1,25D) induces differentiation of myeloid leukemia cells, but resistant cells are also encountered. We studied the mechanistic basis for the resistance in a model system using enhancers of 1,25D, the antioxidant carnosic acid and a kinase inhibitor SB202190. Knock-down (KD) of JNK2p54 unexpectedly increased the intensity of differentiation induced by the 1,25D, carnosic acid and SB202190 (DCS) combination. This was associated with upregulation of activated JNK1p46, and the transcription factors regulated by the JNK pathway, c-Jun, ATF2 and JunB, as well as C/EBP β. In contrast, KD of JNK1p46 reduced the intensity of DCS-induced differentiation, and partially abrogated activation of c-Jun/AP-1 transcription factors.
Keywords: vitamin D, AML, differentiation, JNK pathway, drug resistance, nuclear localization
1. Introduction
The transition of an undifferentiated progenitor cell into a mature, biologically functional phenotype is a complex and highly regulated process. Multiple intracellular signaling pathways have been implicated in the regulation of differentiation, with varied downstream targets that execute the differentiation program [1-6]. Furthermore, 1,25D is a potent regulator of several of these intracellular signaling pathways, such as the protein kinase C pathway [7], calcium-dependent pathways [8], the PI3/AKT-kinase pathway [9-12], and the mitogen-activated protein kinase (MAPK) pathways [13-17]. In particular, the MAPK family, which includes the extracellular regulated kinase (ERK), p38 MAPK, and the c-Jun N-terminal kinase (JNK) pathways, has been observed to regulate differentiation of a variety of human cell types, e.g. the hematopoietic, adipocyte and neuronal lineages [18-21]. Most recently, microRNAs have been found to participate in cell cycle control of myeloid leukemia cells induced to differentiate by 1,25D [22].
Induction of terminal differentiation of proliferating, immature, cancer cells by 1,25D offers a potentially less toxic alternative for treatment of acute myeloid leukemia (AML). However, in addition to the risk of severe hypercalcemia that can result from such therapy, it is apparent that while some AML cells obtained from patients respond to vitamin D derivatives, other cells are resistant [23]. Thus, it is important to determine the basis for this resistance, and then to design more effective regimens for differentiation therapy [24].
Studies in human models of AML have indicated that the MAPK pathways regulate 1,25D-induced monocytic differentiation [13, 14, 18, 25, 26]. Prior work in our laboratory has demonstrated a regulatory function in 1,25D-induced differentiation of JNK activity and the consequent phosphorylation of the c-Jun protein, a component of AP-1 transcription factor dimer, [13, 27]. However, the relative roles of the principal universally expressed isoforms of JNK, JNK1p46 (JNK1) and JNK2p54 (JNK2), were unclear, and their involvement in the resistance to 1,25D-induced differentiation was not investigated. In the current study we focused on the relative importance of these isoforms of JNK in signaling of differentiation induced by a 1,25D-based “differentiation cocktail” DCS, known to overcome the vitamin D resistance [23, 28], and conclude that JNK2 is a negative regulator of monocytic differentiation, while the role of JNK1 is positive in this regard. Further, an investigation of the participation of nuclear proteins that dimerize to form the AP-1 transcription factor, previously reported to be required for differentiation of HL60 cells induced by 1,25D [29], showed that the presumptive downstream targets of JNK1, c-Jun, JunB and ATF2 may all participate in overcoming the resistance to 1,25D.
2. Materials and methods
2.1. Cell Culture
HL60-G [30], and 40AF cells derived from HL60 ATCC cells by cultivation in presence of 1,25D [31], are differentiation-sensitive and resistant subclones of HL60 cells, respectively. The cells were cultured in RPMI 1640 medium supplemented with 10% bovine calf serum (Hyclone, Logan, UT), and were kept in 5% CO2 at 37°C. For all experiments the cells were suspended in fresh medium containing 1,25D or the equivalent volume of ethanol as a vehicle control (Veh). Each experimental condition was repeated at least three times.
2.2. Isolation of the mononuclear blasts from patients' peripheral blood
The specimens of peripheral blood from patients with myeloid leukemia and acute lymphocytic leukemia (ALL) were obtained following the patient's informed consent according to the Institutional IRB protocol. Cells were separated by Ficoll gradient centrifugation, and cultured with 1,25D, carnosic acid (CA), SB202190 (SB), or DCS as described in individual experiments, for periods of time ranging from 48 to 184 h in RPMI medium plus 10% bovine calf serum. The duration of the exposure depended on the time that the cells maintained their viability.
2.3. Reagents and antibodies
1,25D was a kind gift from Dr. Milan Uskokovic (BioExcell, Nutley, NJ). SB202190 was obtained from Calbiochem (La Jolla, CA), carnosic acid from Alexis Pharmaceuticals (Lausen, Switzerland). Antibodies which detect surface differentiation markers Mo1-FITC (CD11b) and MY4-RD1 (CD14), and isotype controls were from Beckman Coulter (Fullerton, CA).
For Western blotting studies the following antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), Calregulin, Crk-L, cFos, c-Jun, JNK1, JunB, C/EBP β, VDR and RXRα. The following antibodies were obtained from Cell Signaling Technologies (Danvers, MA), JNK, phospho-JNK (Thr183/Tyr185), JNK2, phospho-c-Jun (Ser73), phospho-ATF2 (Thr71), and ATF2. GAPDH and histone H3 antibodies were obtained from Sigma Aldrich (St. Louis, MO).
2.4. Nucleofection of siRNA
40AF cells were transfected with 2.5 μM of JNK1 siRNA (siJNK1), JNK2 siRNA (siJNK2) or with scrambled siRNA (sc siRNA) as control (Dharmacon, CO or Ambion, CA) using Amaxa nucleofector according to the manufacturer's protocol. siRNAs from Dharmacon were pooled siRNAs, each pool containing 4 sequences. The product number of siJNK1 from Ambion is 143169. Following transfection the cells were allowed to recover in RPMI 1640 medium with 10% BCS for 24 h, then were exposed to the indicated compounds for 48 h. Transfected cells were checked by western blotting for the reduction of protein expression. Because of the difficulty of transfection of myeloid cells, only partial KD of the target proteins was achieved, although several different silencing oligonucleotides were tested. The results reported here were performed with the most effective siRNAs and compared with the effects of scrambled siRNA used as controls.
2.5. Determination of differentiation parameters
Aliquots of 1× 106 cells were incubated with 0.3 μl MY4-RD1 and 0.3 μl MO1-FITC in a total volume of 100 μl PBS to analyze the expression of CD14 and CD11b respectively using EPICS XL Flow Cytometer (Beckman Coulter, Fullerton, CA). The acquisition parameters were set for an isotype control. Data analysis was performed using EPICS XL software.
For assessment of non-specific esterase (NSE), also known as monocyte specific esterase (MSE), cell smears were made on slides and fixed in a formaldehyde-acetone mixture buffer, then stained for 45 min at room temperature with the following solution: 8.9 ml of 67 mM phosphate buffer (pH 7.6), 0.6 ml of hexazotized pararosaniline, 1 mg/ml alpha-naphtyl acetate, and 0.5 ml ethylene glycol monomethyl ether. The percentage of NSE-positive cells was determined by counting 100 total cells in triplicate using a light microscope.
2.6. Western blotting and cell fractionation
Western blotting was performed using whole cell extracts or cell fractions as described before [29]. The optical density of each western blot band was quantified using ImageQuant 5.0 software (Molecular Dynamics, Sunnyvale, CA).
Cytosolic/membrane (C/M) and nuclear (N) fractions were prepared by the procedure of Andrews and Fuller [32]. Briefly, 2 × 107 cells were resuspended in 0.2 ml hypotonic cell extract buffer (10 mM HEPES-KOH, pH 7.9, 1.5 mM MgCl2, 10 mM KCL, 0.5 mM dithiothreitol (DTT), 0.2 mM phenylmethylsulfonyl (PMSF) and Complete Protease Inhibitor (CPI) cocktail (Hoffmann La Roche, Nutley, NJ). The cells were kept on ice for 10 min, vortexed, and centrifuged at 4°C at 16,000 g for 30 s. The supernatant was saved as the “C/M” extract. The pellet was then resuspended in 30 μl of nuclear extraction buffer (20 mM HEPES-KOH, pH 7.9, 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 0.2 mM PMSF and CPI cocktail), placed on ice for 20 min, and centrifuged at 4°C at 16,000 g for 2 min. The supernatant was saved as the “N” extract.
2.7. Statistical methods
Significance of the differences between mean values was assessed by student's t-test. The effects of 1,25D alone or in combination with SB202190 and carnosic acid were assessed on the following measures: 1) CD11b and CD14 expression, 2) levels of proteins of interest. The p values are reported in the figures and in the figure legends. All computations were performed with an IBM-compatible personal computer and used Microsoft EXCEL.
3. Results
3.1. Vitamin D resistance can be abrogated by the DCS “cocktail” in primary AML blasts and in HL60 40AF cells, a model cell line
This laboratory has previously reported that the addition of carnosic acid, an antioxidant, and SB202190, a p38 MAPK inhibitor, to low concentrations of 1,25D enhances differentiation of human myeloid leukemic cells freshly obtained from patients with AML [23]. In that study only cells of the more mature subtypes of AML, M2 and M4, were responsive to this combination, and the small amount of cells available for analysis precluded studies of the underlying mechanisms. We therefore undertook additional studies aimed at elucidating the role of intracellular signaling in the induction of differentiation of cells resistant to 1,25D, and noted that the 1,25D-resistant variant of HL60 cells, 40AF cells [31], presents a model for such mechanistic studies, as their response to the combination of 1,25D, carnosic acid and SB 202190 (the DCS cocktail) were similar to the responses of the difficult-to-differentiate M1 subtype AML cells (Fig 1). As previously noted, CML cells respond to 1,25D without the enhancers, but differentiation increases when the enhancers are added, as also in some cases of M1 AML (Fig 1, A and B). In contrast, patient blood samples obtained from patients with ALL showed no response to 1,25D or DCS cocktail in this system (data not shown).
Fig. 1.
Vitamin D resistance can be abrogated by DCS “cocktail” in primary leukemia blasts (A-C) and a model cell line 40AF (D). Monocytic cell differentiation is demonstrated by flow cytometry of cell markers CD11b/CD14 and MSE staining. Cells were exposed to 10 nM 1,25D (denoted as D), 10 μM carnosic acid (CA or C), 5 μM SB202190 (SB or S), various combinations of these compounds, or a triple combination of DCS. (A) CML patient's blasts treated ex vivo for 168 h. (B) AML M1 patient's blasts treated for 184 h. (C) Another AML M1 patient's blasts treated for 96 h. Panels (A)-(C) show enhanced cell differentiation in patient's blasts following 1,25D and DCS treatments. Note that DCS induces the highest levels of differentiation markers. (D) 40AF cells treated for 48 h. The resistance of 40AF cells to 1,25D-induced differentiation can be partly reversed by the addition of the DCS “cocktail”.
3.2. Knock-down of JNK2p54 using siRNA enhances the expression of differentiation markers in 40AF cells
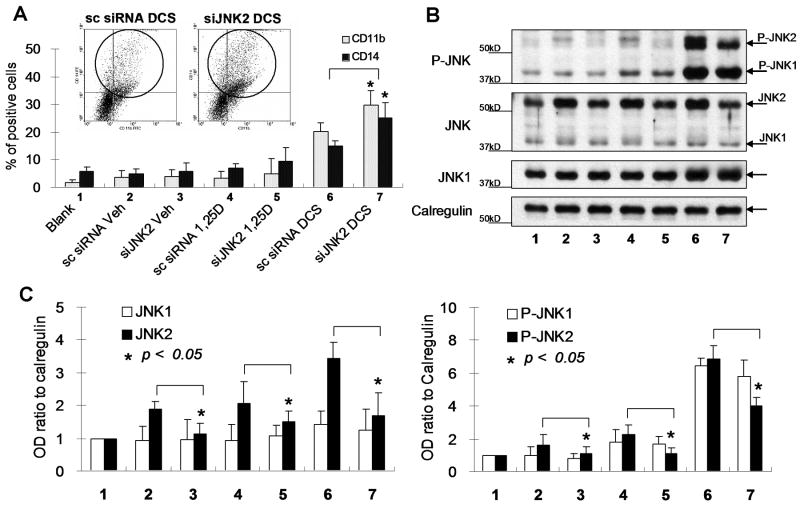
One of the known participating pathways in 1,25D-induced differentiation of AML cells is signaling by JNK [13, 23]. We therefore investigated its role in the reversal of 1,25D resistance by DCS cocktail using the model 40AF cells. Interestingly, exposure of these cells to siRNA specific for JNK2 resulted in increased differentiation, which was statistically significant in the DCS-treated cells (Fig 2A). Partial knock-down of JNK2 was confirmed by Western analysis of the cellular levels of JNK proteins, which showed a significant decrease in JNK2, but not JNK1, both total and phosphorylated forms (Fig 2, B and C). This suggests that JNK2 has a negative role in monocytic differentiation of 1,25D-resistant myeloid leukemia cells.
Fig. 2.
Knock-down of JNK2 using siRNA enhances the expression of differentiation markers in 40AF cells. The 40AF cells were transfected with JNK2 siRNA (siJNK2) or scrambled siRNA (sc siRNA) as control, then after 24 h the cells were exposed to 1, 25D or DCS as indicated in Fig.1 for 48 h. Control samples were untreated cells (Blank) and Vehicle-treated cells (Veh). The numbered groups indicated in (B) and (C) are the same as (A). (A) Flow cytometric determinations of CD11b and CD14 show a significant increase of differentiation in 40AF cells in which JNK2 levels were reduced by siJNK2, and then treated with DCS, compared to scrambled siRNA control (lanes 6 and 7). Insets illustrate an individual flow cytometry experiment (the X axis represents CD11b, the Y axis is CD14), bar graphs show means+/-SE, n=3, * p<0.05). (B) Western blots show a decrease of total JNK2 protein level (50-60%), and a marked decrease of P-JNK2, but no significant change of JNK1 and P-JNK1 protein levels. (C) The OD ratios of total JNK2, JNK1, P-JNK2 and P-JNK1 were normalized to Calregulin, the loading control.
3.3. The expression c-Jun/AP-1 transcription factors increases after knock-down of JNK2 in 40AF cells
Examination of the down-stream targets of JNK signaling following partial KD of JNK2 produced further unexpected observations. The principal components of the AP-1 transcription factor, c-Jun and ATF-2 [29], as well as JunB, were up-regulated when JNK2 levels were reduced by siRNA (Fig 3A). This occurred in the absence of siJNK2-induced changes in the levels of receptor for 1,25D, VDR, or its transcription factor partner, RXRα (Fig 3A), suggesting a mechanism independent of the initial signal provided by 1,25D. Interestingly, the exposure to DCS cocktail did increase VDR abundance irrespective of JNK2 knockdown (Fig 3A, lanes 5 and 6), reinforcing the view that JNK2 participates only in the downstream events initiated by 1,25D.
Fig. 3.
The expression c-Jun/AP-1 transcription factors increases after knock-down of JNK2 in 40AF cells. (A) Protein levels in whole cell extracts determined by western blots of P-c-Jun, c-Jun, JunB and ATF2, but not of VDR and RXRα showed increases after the knock-down of JNK2 using siJNK2 in DCS-treated 40AF cells (compare lane 5 with lane 6). OD ratios of signal intensity, normalized to the control, are shown under each band. (B) Western blots of cytoplasm/membrane (C/M) and nuclear (N) fractions showing the levels of JNK2, JNK1, P-JNK, as well as of the downstream transcription factors, after JNK2 knock-down. To assess the purity of the fractions, the cytoplasmic component glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and the nuclear constituent histone H3 are also shown.
Since transcription factors function in the cell nucleus, although they can be activated by phosphorylation either in the cytoplasm or the nucleus, we examined the effect of siJNK2 on the localization of the members of the JNK pathway in these cell compartments. We noted that cytoplasmic form of JNK2 increased after DCS treatment (Fig 3B, top panel, lanes 1 vs 5), but was reduced by siJNK2 (lane7), while there was a less marked reduction of the nuclear JNK2, which had slower electrophoretic mobility (Fig 3B, top panel). JNK1 total and phosphorylated protein also increased following DCS treatment of the cells (Fig 3B, second and third panels from top), and as anticipated, siJNK2 did not affect the cytoplasmic levels of P-JNK1. However, unexpectedly, transfection of siJNK2 slightly increased nuclear levels of P-JNK1 while the lower nuclear P-JNK2 levels confirmed its knock-down (lanes 6 vs 8). The levels of known JNK downstream targets, P-c-Jun, P-ATF2 and JunB, also increased in the nuclei of siJNK2-treated cells (Fig 3B). In addition, the level of C/EBP/β, a transcription factor linked to monocytic differentiation [5, 33], also increased in the nuclei of siJNK2 treated cells (Fig 3B).
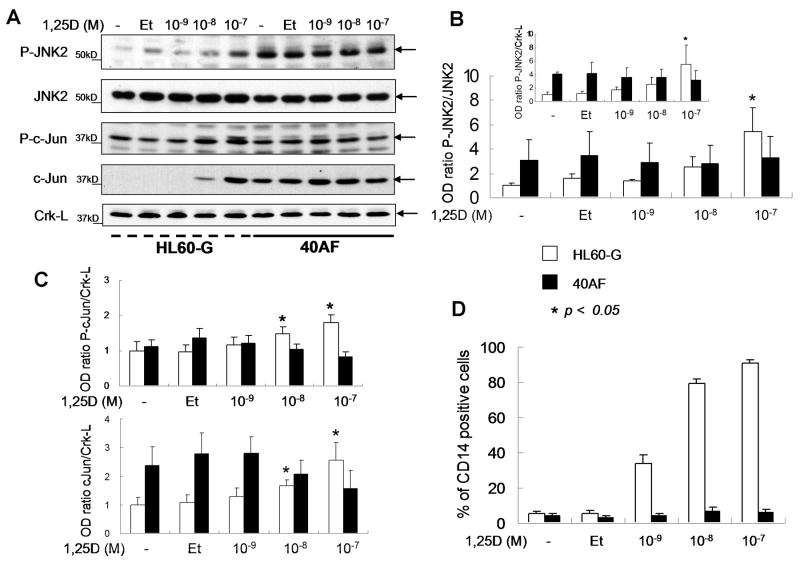
These findings indicate that JNK2 functions to limit the activation of the transcription factors linked to monocytic differentiation of myeloid leukemia cells. This is further supported by data which show that 40AF cells have high constitutive levels of activated JNK2 (P-JNK2), while its levels in the 1,25D-sensitive HL60-G cells are very low, and increase only in the presence of pharmacological concentration (10-7 M) of 1,25D (Fig 4). Thus, the increase in P-JNK2 in 1, 25D-treated sensitive cells may represent a protective reaction to the presence of excessively high concentration of the vitamin-hormone.
Fig. 4.
Higher expression of P-JNK2 is observed in 40AF cells compared to HL60-G cells, while exposure to 1,25D increases the expression of CD14 in 1,25D-sensitive HL60G cells, but not in 1,25D-resistant 40AF cells. (A) Western blots showing levels of P-JNK2, JNK2, P-c-Jun and c-Jun in HL60-G and 40AF cells exposed to increasing concentrations of 1,25D for 48 h. Crk-L is shown as a loading control. Both untreated (-) and ethanol-treated (Et) groups were used as negative controls. In panels B and C the means+/-SE of the OD ratios compared to “Et” control from 3 similar experiments are shown (* p<0.05). (D) Flow cytometric determinations of the monocyte-specific marker CD14 are shown following the exposure of HL60G and 40AF cells to increasing concentrations of 1,25D.
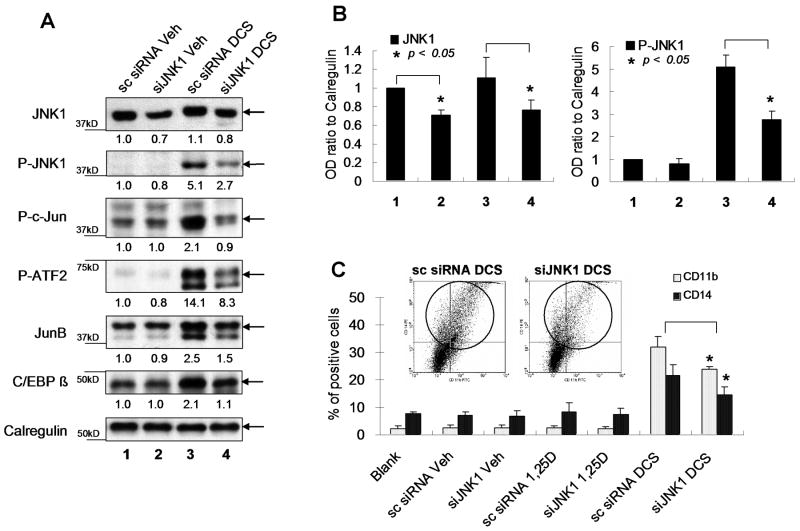
3.4. Partial knock-down of JNK1 expression by siRNA reduces DCS-induced differentiation of 40AF cells and the levels of transcription factors P-c-Jun, P-ATF2, JunB and C/EBP β
To document the contrasting effects of JNK1 and JNK2 on monocytic differentiation, we also used siJNK1 in this system. Although we were able to obtain only a partial KD of JNK1 (Fig 5A), it was clear that the downstream transcription factors and differentiation were reduced by the KD (Fig 5). These data suggest that the activation, by phosphorylation, of downstream transcription factors is exquisitely dependent on the threshold levels of JNK1. This is clearly illustrated by the markedly increased level of phosphorylated ATF2 by DCS (Fig 5A, lane 3) in comparison with only a slight increase in total ATF2 protein (Fig 3A, lane 5), and by the reduction of the phosphorylation of ATF2 by siJNK1.
Fig. 5.
Partial knock-down of JNK1 with siRNA reduces the expression of differentiation markers in 40AF cells, and the levels of transcription factors P-c-Jun, P-ATF2, JunB and C/EBP β. The 40AF cells were transfected with JNK1 siRNA (siJNK1) or scrambled siRNA, and after 24 h the cells were exposed for 48h to vehicle, 1, 25D or DCS as indicated in Fig. 1. (A) Western blots showing that the reduction of JNK1 protein level in whole cell extracts was about 30%, and a dramatic reduction of P-JNK1 level, when JNK1 was knocked down using siRNA in DCS-treated 40AF cells. The levels of down-stream transcription factors were also reduced. The OD ratios normalized to calregulin are shown under each band. (B). Quantitation of OD ratios of JNK1 and P-JNK1 in three experiments, means+/-SE. The numbered groups indicated here are the same as (A). (C) Flow cytometric determination of CD11b/CD14 shows decreases after JNK1 knock-down in DCS-treated 40AF cells. The inset shows an illustration of key data from one experiment (the X axis represents CD11b, the Y axis is CD14), while the bar chart shows the means+/-SE of this and additional experiments (* p<0.05).
Further, a direct comparison of nuclear levels of AP-1 transcription factors showed the differential alterations induced by siJNK2 and siJNK1. Knock-down of JNK1 resulted in the expected reduction in the nuclear transcription factors (Fig 6A), while an opposite effect was seen after JNK2 knock-down. As also noted in Fig 3B, JNK2 KD resulted in increased nuclear levels of P-JNK1, which may explain the increases in phosphorylated c-Jun and ATF-2 in cells treated with siJNK2 (Fig 6B).
Fig. 6.
Direct comparison of nuclear levels of transcription factors shows differential alterations induced by siJNK1 and siJNK2. (A) siJNK1 reduced the levels of P-JNK1 and of the activated AP-1 transcription factors c-Jun, ATF-2 and JunB in the nuclear compartment. Phosphorylation of JunB protein was assessed by its reduced gel mobility. (B) siJNK2 reduced the level of nuclear P-JNK2, but not of P-JNK1 or of the downstream transcription factors, which show higher levels following knock-down of JNK2. Western blots of P-JNK and transcription factors from nuclear fraction are shown in each panel. The OD ratios normalized to calregulin are indicated under each band.
4. Discussion
The major novel finding of this investigation is that JNK2 antagonizes the signaling of differentiation by the JNK pathway in myeloid leukemia cells that are resistant to 1,25D when it is a sole differentiating agent. The data also suggest that the constitutively high level of activated JNK2 in 40AF cells contributes to the resistance of 40AF cells to 1,25D-induced differentiation. Further, and perhaps of wider significance, is the dramatic contrast between the biological consequences of the reduction of the protein levels of JNK2 (Fig 2), and the consequences of reducing the level of JNK1 (Fig 5) in this cell system.
Another intriguing aspect of cellular signaling uncovered by this investigation is that the localization of P-JNK1 in non-differentiated 40AF cells is principally cytoplasmic, while P-JNK2 is predominantly found in the cell nuclei (Fig 3B), suggesting that these isoforms may be subject to different intracellular trafficking controls. Alternatively, it is also possible that different upstream kinases can phosphorylate these isoforms; a cytoplasmic kinase for JNK1, such as a RACK1-anchored protein kinase C [34], and an exclusively nuclear kinase for JNK2. It was also noted that in parallel with activated JNK1, activated c-Jun was undetectable in the nuclei of undifferentiated 40AF cells (Fig 3B, lanes 2 and 4), but exposure to DCS increased nuclear localization of both P-JNK1 and P-c-Jun (Fig 3B, lanes 6 and 8), but no such parallel was observed between P-JNK2 and P-c-Jun. This, and the increase in c-Jun and P-c-Jun levels following exposure of the cells to siJNK2, suggests that JNK2 may target c-Jun for degradation, as described in other cell types [35, 36].
This study adds to the growing list of cellular systems in which JNK1 and JNK2 have been shown to differentially regulate their targets, even though they also have overlapping functions. For instance, the JNK protein kinase isoforms interact selectively with transcription factors [37], and the TATA binding protein [38], and have distinct roles in regulating JNK activity and cell proliferation [35, 39]. In particular, JNK2 is considered to be a negative regulator of cellular proliferation [40], although this has been questioned [41].
In the system studied here, P-JNK2 appears to be a negative regulator of monocytic differentiation, and the mechanism is consistent with the proposal that JNK2 competes with JNK1 for phosphorylation by upstream kinases, and thus interferes with JNK1 phosphorylation [35], although other explanations are also possible. We noted that the exposure of 40AF cells to siJNK2 resulted in the expected decrease in nuclear P-JNK2, but that there was also an increase in nuclear P-JNK1 (Fig 6B), that could be explained by reduced competition from the JNK2 protein for activation by upstream kinases. Thus, the increased level of JNK2 in 40AF cells can contribute to their resistance to 1,25D, and consequently JNK2 may be a potential target for the design of anti-leukemia drugs, which can complement differentiation therapy with 1,25D.
Acknowledgments
We thank Dr. Milan Uskokovic for the gift of 1,25D, and Dr. Xuening Wang for comments on the manuscript. Support was provided by NIH grants from the National Cancer Institute (RO1-CA 044722-18 and RO1-CA 117942-2).
Footnotes
Conflict of interest statement: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lian JB, Javed A, Zaidi SK, Lengner C, Montecino M, van Wijnen AJ, Stein JL, Stein GS. Regulatory controls for osteoblast growth and differentiation: role of Runx/Cbfa/AML factors. Crit Rev Eukaryot Gene Expr. 2004;14:1–41. [PubMed] [Google Scholar]
- 2.Pui JC, Allman D, Xu L, DeRocco S, Karnell FG, Bakkour S, Lee JY, Kadesch T, Hardy RR, Aster JC, Pear WS. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11:299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- 3.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–96. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Studzinski GP. Raf-1 signaling is required for the later stages of 1,25-dihydroxyvitamin D3-induced differentiation of HL60 cells but is not mediated by the MEK/ERK module. J Cell Physiol. 2006;209:253–60. doi: 10.1002/jcp.20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji Y, Studzinski GP. Retinoblastoma protein and CCAAT/enhancer-binding protein beta are required for 1,25-dihydroxyvitamin D3-induced monocytic differentiation of HL60 cells. Cancer Res. 2004;64:370–7. doi: 10.1158/0008-5472.can-03-3029. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Studzinski GP. Inhibition of p38MAP kinase potentiates the JNK/SAPK pathway and AP-1 activity in monocytic but not in macrophage or granulocytic differentiation of HL60 cells. J Cell Biochem. 2001;82:68–77. doi: 10.1002/jcb.1141. [DOI] [PubMed] [Google Scholar]
- 7.Sitrin MD, Bissonnette M, Bolt MJ, Wali R, Khare S, Scaglione-Sewell B, Skarosi S, Brasitus TA. Rapid effects of 1,25(OH)2 vitamin D3 on signal transduction systems in colonic cells. Steroids. 1999;64:137–42. doi: 10.1016/s0039-128x(98)00102-0. [DOI] [PubMed] [Google Scholar]
- 8.Bikle DD, Ng D, Tu CL, Oda Y, Xie Z. Calcium- and vitamin D-regulated keratinocyte differentiation. Mol Cell Endocrinol. 2001;177:161–71. doi: 10.1016/s0303-7207(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 9.Xie Z, Bikle DD. The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-gamma1 activation and human keratinocyte differentiation. J Biol Chem. 2007;282:8695–703. doi: 10.1074/jbc.M609135200. [DOI] [PubMed] [Google Scholar]
- 10.Marcinkowska E, Kutner A. Side-chain modified vitamin D analogs require activation of both PI 3-K and erk1,2 signal transduction pathways to induce differentiation of human promyelocytic leukemia cells. Acta Biochim Pol. 2002;49:393–406. [PubMed] [Google Scholar]
- 11.Hmama Z, Nandan D, Sly L, Knutson KL, Herrera-Velit P, Reiner NE. 1alpha,25-dihydroxyvitamin D(3)-induced myeloid cell differentiation is regulated by a vitamin D receptor-phosphatidylinositol 3-kinase signaling complex. J Exp Med. 1999;190:1583–94. doi: 10.1084/jem.190.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Zhang J, Studzinski GP. AKT pathway is activated by 1, 25-dihydroxyvitamin D3 and participates in its anti-apoptotic effect and cell cycle control in differentiating HL60 cells. Cell Cycle. 2006;5:447–51. doi: 10.4161/cc.5.4.2467. [DOI] [PubMed] [Google Scholar]
- 13.Wang Q, Wang X, Studzinski GP. Jun N-terminal kinase pathway enhances signaling of monocytic differentiation of human leukemia cells induced by 1,25-dihydroxyvitamin D3. J Cell Biochem. 2003;89:1087–101. doi: 10.1002/jcb.10595. [DOI] [PubMed] [Google Scholar]
- 14.Ji Y, Kutner A, Verstuyf A, Verlinden L, Studzinski GP. Derivatives of vitamins D2 and D3 activate three MAPK pathways and upregulate pRb expression in differentiating HL60 cells. Cell Cycle. 2002;1:410–5. doi: 10.4161/cc.1.6.269. [DOI] [PubMed] [Google Scholar]
- 15.Hughes PJ, Brown G. 1Alpha,25-dihydroxyvitamin D3-mediated stimulation of steroid sulphatase activity in myeloid leukaemic cell lines requires VDRnuc-mediated activation of the RAS/RAF/ERK-MAP kinase signalling pathway. J Cell Biochem. 2006;98:590–617. doi: 10.1002/jcb.20787. [DOI] [PubMed] [Google Scholar]
- 16.Beno DW, Brady LM, Bissonnette M, Davis BH. Protein kinase C and mitogen-activated protein kinase are required for 1,25-dihydroxyvitamin D3-stimulated Egr induction. J Biol Chem. 1995;270:3642–7. doi: 10.1074/jbc.270.8.3642. [DOI] [PubMed] [Google Scholar]
- 17.Narayanan R, Sepulveda VA, Falzon M, Weigel NL. The functional consequences of cross-talk between the vitamin D receptor and ERK signaling pathways are cell-specific. J Biol Chem. 2004;279:47298–310. doi: 10.1074/jbc.M404101200. [DOI] [PubMed] [Google Scholar]
- 18.Marcinkowska E. Evidence that activation of MEK1,2/erk1,2 signal transduction pathway is necessary for calcitriol-induced differentiation of HL-60 cells. Anticancer Res. 2001;21:499–504. [PubMed] [Google Scholar]
- 19.Aubert J, Belmonte N, Dani C. Role of pathways for signal transducers and activators of transcription, and mitogen-activated protein kinase in adipocyte differentiation. Cell Mol Life Sci. 1999;56:538–42. doi: 10.1007/s000180050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumura I, Kanakura Y. Molecular control of megakaryopoiesis and thrombopoiesis. Int J Hematol. 2002;75:473–83. doi: 10.1007/BF02982109. [DOI] [PubMed] [Google Scholar]
- 21.Frebel K, Wiese S. Signalling molecules essential for neuronal survival and differentiation. Biochem Soc Trans. 2006;34:1287–90. doi: 10.1042/BST0341287. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Gocek E, Liu CG, Studzinski GP. MicroRNAs181 regulate the expression of p27Kip1 in human myeloid leukemia cells induced to differentiate by 1,25-dihydroxyvitamin D3. Cell Cycle. 2009 doi: 10.4161/cc.8.5.7870. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Q, Harrison JS, Uskokovic M, Kutner A, Studzinski GP. Translational study of vitamin D differentiation therapy of myeloid leukemia: effects of the combination with a p38 MAPK inhibitor and an antioxidant. Leukemia. 2005;19:1812–7. doi: 10.1038/sj.leu.2403916. [DOI] [PubMed] [Google Scholar]
- 24.Campbell MJ, Adorini L. The vitamin D receptor as a therapeutic target. Expert Opin Ther Targets. 2006;10:735–48. doi: 10.1517/14728222.10.5.735. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Studzinski GP. Activation of extracellular signal-regulated kinases (ERKs) defines the first phase of 1,25-dihydroxyvitamin D3-induced differentiation of HL60 cells. J Cell Biochem. 2001;80:471–82. doi: 10.1002/1097-4644(20010315)80:4<471::aid-jcb1001>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 26.Studzinski GP, Garay E, Patel R, Zhang J, Wang X. Vitamin D receptor signaling of monocytic differentiation in human leukemia cells: role of MAPK pathways in transcription factor activation. Curr Top Med Chem. 2006;6:1267–71. doi: 10.2174/156802606777864935. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, Salman H, Danilenko M, Studzinski GP. Cooperation between antioxidants and 1,25-dihydroxyvitamin D3 in induction of leukemia HL60 cell differentiation through the JNK/AP-1/Egr-1 pathway. J Cell Physiol. 2005;204:964–74. doi: 10.1002/jcp.20355. [DOI] [PubMed] [Google Scholar]
- 28.Garay E, Donnelly R, Wang X, Studzinski GP. Resistance to 1,25D-induced differentiation in human acute myeloid leukemia HL60-40AF cells is associated with reduced transcriptional activity and nuclear localization of the vitamin D receptor. J Cell Physiol. 2007;213:816–25. doi: 10.1002/jcp.21150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Studzinski GP. The requirement for and changing composition of the activating protein-1 transcription factor during differentiation of human leukemia HL60 cells induced by 1,25-dihydroxyvitamin D3. Cancer Res. 2006;66:4402–9. doi: 10.1158/0008-5472.CAN-05-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Studzinski GP, Reddy KB, Hill HZ, Bhandal AK. Potentiation of 1-beta-D-arabinofuranosylcytosine cytotoxicity to HL-60 cells by 1,25-dihydroxyvitamin D3 correlates with reduced rate of maturation of DNA replication intermediates. Cancer Res. 1991;51:3451–5. [PubMed] [Google Scholar]
- 31.Wajchmann HJ, Rathod B, Song S, Xu H, Wang X, Uskokovic MR, Studzinski GP. Loss of deoxcytidine kinase expression and tetraploidization of HL60 cells following long-term culture in 1,25-dihydroxyvitamin D3. Exp Cell Res. 1996;224:312–22. doi: 10.1006/excr.1996.0141. [DOI] [PubMed] [Google Scholar]
- 32.Andrews NC, Faller DV. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991;19:2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcinkowska E, Garay E, Gocek E, Chrobak A, Wang X, Studzinski GP. Regulation of C/EBP beta isoforms by MAPK pathways in HL60 cells induced to differentiate by 1,25-dihydroxyvitamin D3. Exp Cell Res. 2006;312:2054–65. doi: 10.1016/j.yexcr.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez-Bergami P, Habelhah H, Bhoumik A, Zhang W, Wang LH, Ronai Z. RACK1 mediates activation of JNK by protein kinase C. Mol Cell. 2005;19:309–20. doi: 10.1016/j.molcel.2005.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J, Minemoto Y, Lin A. c-Jun N-terminal protein kinase 1 (JNK1), but not JNK2, is essential for tumor necrosis factor alpha-induced c-Jun kinase activation and apoptosis. Mol Cell Biol. 2004;24:10844–56. doi: 10.1128/MCB.24.24.10844-10856.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabapathy K, Hochedlinger K, Nam SY, Bauer A, Karin M, Wagner EF. Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation. Mol Cell. 2004;15:713–25. doi: 10.1016/j.molcel.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 37.Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Derijard B, Davis RJ. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–70. [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong S, Fromm J, Johnson DL. TBP is differentially regulated by c-Jun N-terminal kinase 1 (JNK1) and JNK2 through Elk-1, controlling c-Jun expression and cell proliferation. Mol Cell Biol. 2007;27:54–64. doi: 10.1128/MCB.01365-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hochedlinger K, Wagner EF, Sabapathy K. Differential effects of JNK1 and JNK2 on signal specific induction of apoptosis. Oncogene. 2002;21:2441–5. doi: 10.1038/sj.onc.1205348. [DOI] [PubMed] [Google Scholar]
- 40.Sabapathy K, Wagner EF. JNK2: a negative regulator of cellular proliferation. Cell Cycle. 2004;3:1520–3. doi: 10.4161/cc.3.12.1315. [DOI] [PubMed] [Google Scholar]
- 41.Jaeschke A, Karasarides M, Ventura JJ, Ehrhardt A, Zhang C, Flavell RA, Shokat KM, Davis RJ. JNK2 is a positive regulator of the cJun transcription factor. Mol Cell. 2006;23:899–911. doi: 10.1016/j.molcel.2006.07.028. [DOI] [PubMed] [Google Scholar]