Abstract
Neonates suffer unduly from infections, and also respond sub-optimally to most commonly used vaccines. However, a CD8 T cell response can be elicited in neonates if the antigen is introduced into the cytoplasm of antigen presenting cells (APC). Listeria monocytogenes (Lm) targets the cytoplasm of APC, and is a strong CD8 and CD4 Th1 promoting vaccine vehicle in adult mice. We hypothesized that an attenuated strain of Lm would be safe and induce long-lasting protective immunity even in neonates. We found that neonatal mice immunized only once with the attenuated strain ΔactA-Lm developed robust primary and secondary CD8 and CD4 Th1 responses and were fully protected from lethal challenge with virulent wildtype Lm without the need for a booster immunization. Furthermore, ΔactA-Lm expressing a heterologous recombinant antigen induced a strong CD8 and Th1 memory response to that antigen. Based on these data, we propose that ΔactA-Lm or derivatives thereof might serve as a vaccine vehicle for neonatal immunization.
Keywords: Bacterial, T cells, Antigen Presentation/Processing, Memory, Vaccination
Introduction
Approximately 2.5 million neonates and infants die annually from infection, marking this as the time of life most burdened by infectious diseases (1, 2). Newborns are especially prone to infections for which cell-mediated immunity (CMI) is protective (1, 3). Neonates and infants also have a reduced or suboptimal capacity to mount an effective CMI in response to most vaccines (1, 3–5). However, introducing a vaccine antigen into the cytoplasm of antigen presenting cells (APC) induces a significant CD8 CTL response even in neonates (3, 6, 7). Contrary to the induction of CTLs, Th1 (IFN-γ producing) CD4 T cell responses have not only been difficult to induce in the neonate, but appear even more difficult to sustain (4, 5, 8, 9).
Listeria monocytogenes (Lm) is a Gram-positive microbe that resides primarily in the intra-cytoplasmic compartment of host cells, including the primary APCs such as macrophages and dendritic cells. Furthermore, Lm possesses biologically strong Th1 adjuvant characteristics, making it an attractive vehicle for vaccinations (10, 11). Recombinant attenuated strains of Lm induce specific immunity even in the presence of pre-existing immunity to the Lm vehicle (10) , making Lm an ideal candidate vector for neonatal vaccination in the face of pre-existing maternal immunity. But Lm is also a serious pathogen in neonatal life, precluding its use for vaccination without further study. We hypothesized that an attenuated strain of Lm could trigger an antigen-specific and protective memory immune response in the neonate. We found that Lm mutants with the targeted deletion in the virulence determinant ActA (ΔactA-Lm) induce a strong primary and secondary Th1 CD4 and CD8 T cell response in neonatally immunized mice, and provide sterilizing protection from lethal challenge after only a single immunization. Furthermore, ΔactA-Lm expressing a heterologous antigen allowed induction of an antigen-specific memory T cell response to the heterologous antigen, suggesting attenuated strains of Lm might serve as a vehicle for neonatal vaccination.
Materials and Methods
Mice
Because Lm class I immunodominant peptides have been described only in the murine H-2d haplotype and class II immunodominant peptides only in the H-2b haplotype, we used neonatal and 6 week old adult F1 mice (H-2b × H-2d) derived from matings between C57BL/6 (H-2b) and C57B10.D2 (H-2d) mice (12) . In preliminary experiments, there was no difference in the Lm-specific T cell responses in C57BL/6 (H-2b) or C57B10.D2 (H-2d) compared to F1 (H-2b × H-2d) mice for the respective immunodominant epitopes (data not shown). As our experiments were meant to provide insight into mechanisms possibly underlying the human neonate’s reduced response to vaccines, we chose the 5–7 day old murine pup, as they are the closest to human neonates with respect to the maturational status of their immune system (1).
Bacterial Strains and Infection
Wild-type Lm strain 10403s, the ΔactA strain DPL1942, and Lm-OVA were kindly provided by Drs. D. Portnoy (University of California, Berkeley, CA), N. Freitag (Seattle Biomedical Research Institute, Seattle, WA), and Dr. H. Shen (University of Pennsylvania, Philadelphia, PA), respectively. ΔactA-Lm-OVA was constructed from Lm-OVA by cloning ~500 bp fragments of the actA gene into the HindIII/Kpn1 sites of the temperature sensitive plasmid pKSV7 using the following primers: upstream flanking region, forward primer 5’ aagcttgcagcgaccgatagcgaag 3’, reverse primer 5’ gaattccgctgcgctatccgatgg 3’, downstream flanking region, forward primer 5’ gaattcgttaagtccaaaggtatcg 3’, reverse primer 5’ ggtacctaaagagaacacgccaatag 3’ (underlined sequences indicate introduced restriction sites). All strains were grown as described (15). Briefly, Lm were grown to mid log phase (OD600 0.1) at 37°C, diluted in 100 µl of saline and injected i.p. into mice for immunization, or i.v. in 200 µl of saline for challenge. The number of Lm in lysates of infected spleens and livers was determined as described (15), with a lower limit of detection of 20 CFU and 30 CFU per organ for spleen and liver respectively. All experiments were performed under Institutional Animal Care and Use Committee approved protocols.
Enumeration of antigen-specific T cells
Intracellular cytokine staining was performed as described (15), using reagents from BD PharMingen (Mountain View, CA). Briefly, splenocyte suspensions were prepared by homogenizing spleens between two sterile glass slides, subjected to RBC lysis, and filtered through a 70 µm mesh. 2.0 × 106splenocytes were incubated for five hours in 200 µl of RPMI 1640 medium supplemented with 10% FCS (Hyclone, Logan UT), L-glutamine, penicillin, and streptomycin in the presence of the indicated Lm- or OVA-specific peptide (10−6 M), and GolgiStop . Cells were stained for 30 minutes on ice with FITC-labeled CD3 and either PerCP-labeled anti-CD8 or anti-CD4. For surface tetramer staining, tetramers (NIAID Tetramer Facility) specific for the LLO91–99 or P60217–225 epitope were used. To detect intracellular cytokine production, cells were permeabilized with Cytoperm solution, and stained with APC-labeled anti-IFN-γ and PE-labeled anti-IL-4 for 30 minutes at room temperature after surface staining as described above. Stained cells were acquired on a FACSCanto flow cytometer (BD Biosciences, Mountain View, CA), and analyzed using FlowJo software (TreeStar, San Carlos, CA).
Cytokine production
The concentration of IL-4 and IL-13 in the supernatants of splenocyte cultures was determined 72 h after peptide stimulation by ELISA using reagents from R&D Systems (Minneapolis, MN) as previously described (15).
Statistics
The differences in geometric mean CFUs, the percentages and numbers of activated splenocytes, and cytokine concentrations were evaluated using the Student t test, with p < 0.05 taken as statistically significant.
Results
Attenuated strains of Lm are safe in neonates
The listerial actA gene is required for polymerization of host actin into filaments, which allows cell-to-cell spread using components of the host cell’s actin cytoskeleton. The ΔactA strain of Lm carries an in-frame deletion in the actA gene (ΔactA-Lm), is unable to spread from cell-to-cell, and displays a three order of magnitude decrease in virulence without interfering with immunogenicity (10, 13). We and others have shown that ΔactA-Lm was safe in MyD88- deficient or IFN-γ-deficient adult mice, which similar to neonates are highly susceptible to Lm infection (14–17) . We found that neonatal mice survived high-dose infection with ΔactA-Lm without any sign of disease: While the LD50 of neonates for wild-type Lm is ~ 101 CFU, we found that the LD50 for ΔactA- Lm is between 106 and 107 CFU. Examination of spleen and liver homogenates of neonatal or adult mice 7 days after vaccination with up to 106 CFU ΔactA- Lm revealed no recoverable bacteria. Thus, ΔactA-Lm was safe and well tolerated in neonates.
Neonates mount a protective immune response similar to adults
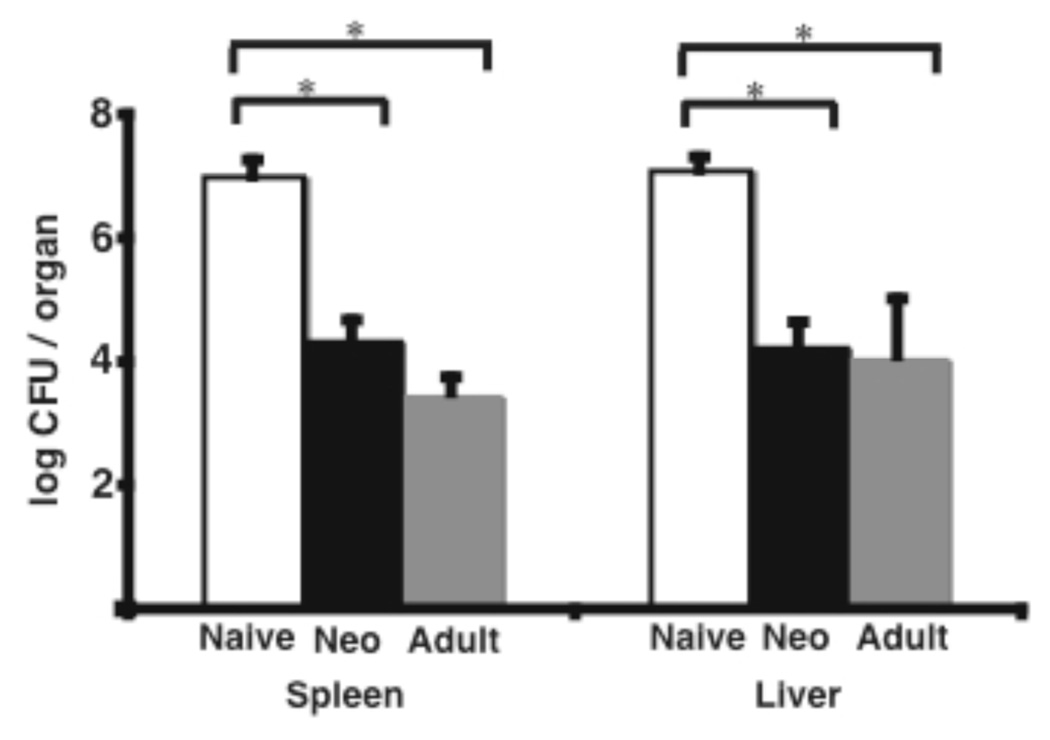
Immunization of adult mice with ΔactA-Lm confers protection against secondary Lm infection (18) . To address if ΔactA-Lm does so in neonates as well, mice were immunized with 104 CFU ΔactA-Lm at 5 days of age (neonatal immunization) or at 6 weeks of age (adult immunization). Both groups of mice were challenged 3 months later with an inoculum of wild type Lm that would be lethal for naïve adult mice (105 CFU). Three days after challenge, the numbers of Lm in the spleens and livers of mice immunized as neonates were dramatically reduced compared with naive age matched controls (Fig. 1). Similar to mice immunized as adults, all neonatally immunized mice remained healthy, survived, and eventually cleared the infection completely by 10 days post-challenge. In contrast, bacterial replication continued in naive control mice, which became moribund, and had to be sacrificed by day 4. Thus, neonatal immunization, like adult immunization resulted in protective sterilizing immunity to subsequent challenge with wild-type Lm.
Figure 1.
Neonatally immunized mice were protected from wild-type Lm challenge to the same degree as mice immunized as adults. Mice immunized with 1×104 CFU i.p. of ΔactA-Lm on day 5 of life (Neo, black bar) or at 6 weeks of age (Adult, grey bar), or age matched non-immune (Naïve, white bar) mice, were infected i.v. with 1×105 CFU of wild-type Lm three months after immunization. Lm CFUs in the spleens or livers of mice 3 days after infection were determined and shown is the mean from a total of 12 mice per group combined from three separate experiments. Bar = SE; * = statistically significant with p < 0.05.
Neonates mount a primary T cell response similar to adults
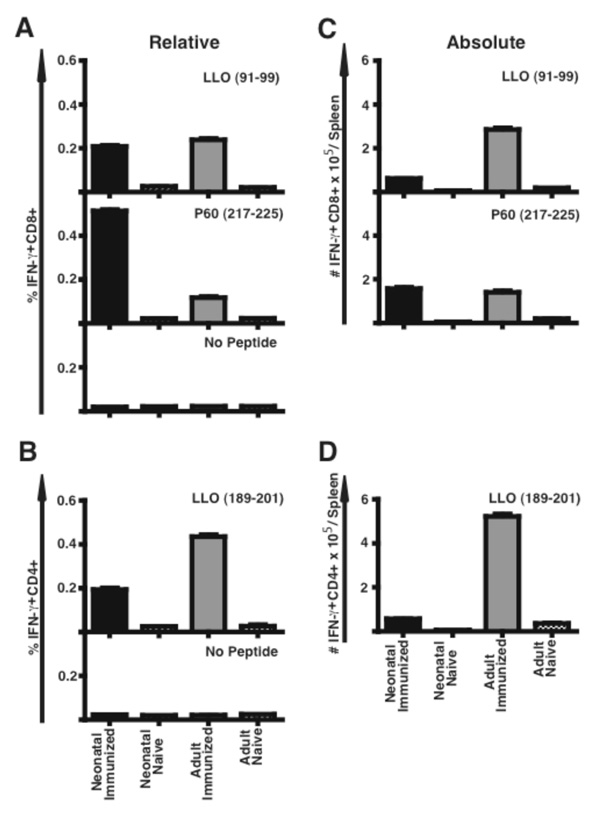
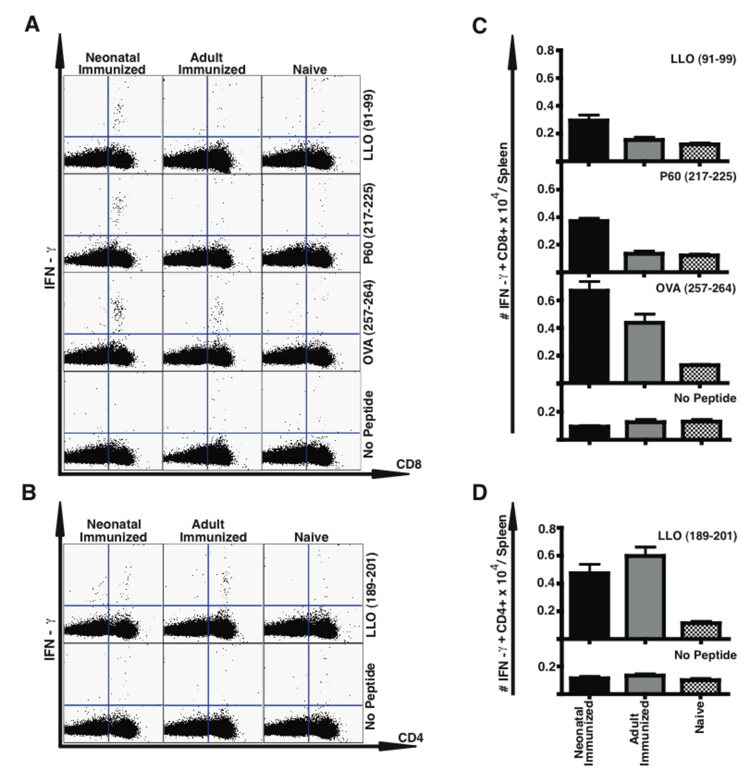
To our knowledge, there are no published reports on the successful induction of antigen-specific CD8 or CD4 primary T cells in response to Lm infection in the neonate. Seven days after immunization with ΔactA-Lm, we enumerated Lm-specific CD8 and CD4 T cells in neonatal and adult mice by intracellular IFN-γ (a marker for a Th1-type response) and IL-4 (a marker for a Th2-type response) staining of splenocytes after re-stimulation in vitro with Lm-specific MHC class I listeriolysin O (LLO)91–99, P60217–225, P60449–457, and mpl84–192, or MHC class II LLO189–201 -restricted peptides (Fig. 2). The neonate generated similar percentages of LLO91–99 specific CD8 T cells compared to adult mice, and threefold higher percentages of P60217–225 specific CD8 T cells than adult mice. This response could be detected by intracellular IFN-γ staining (Fig. 2), or by surface staining with tetramers specific for LLO or P60 (data not shown). Detection of the subdominant epitopes P60449–457 and MPl84–92 was similarly low in both adult and neonate (data not shown). A substantial percentage of neonatal CD4 T cells produced IFN-γ in response to LLO189–201 peptide stimulation, although twofold less than adult cells. With only ~30 × 106 cells/spleen in the 12 day old, but ~120 × 106 cells/spleen in the adult, the absolute numbers of LLO specific CD8 (Fig. 2 C) and CD4 (Fig. 2 D) T cells per spleen were lower in mice immunized as neonate vs. adult, but the absolute numbers of P60-specific CD8 T cells were equivalent (Fig. 2C). Neither neonatally nor adult immunized mice displayed a CD4 Th2 type response as measured by IL-4 production in a flow cytometric assay or IL-4 or IL-13 production by ELISA (data not shown). Thus, neonates similar to adults were able to mount a CD8 and CD4 Th1 primary response.
Figure 2.
Neonatally immunized mice develop an Lm-specific primary CD8 and CD4 T cell response. Spleen cells were obtained from the indicated mice 7 days after infection with ΔactA-Lm, and stimulated with the indicated MHC class I- or class II-restricted Lm-specific peptides prior to analysis of intracellular cytokine expression by flow cytometry. Shown is the mean percentage (A, B) or total number/spleen (C, D) of CD8 (A, C) or CD4 (B, D) IFN-γ producing splenocytes. For each group the percentage of unstimulated IFN-γ producing control cells is shown for CD4 or CD8, and the numbers of these cells were subtracted from the absolute numbers of antigen-specific cells shown (C, D). These data represent five mice per group from two combined experiments. Bar = SE.
Neonates mount a secondary T cell response similar to adults
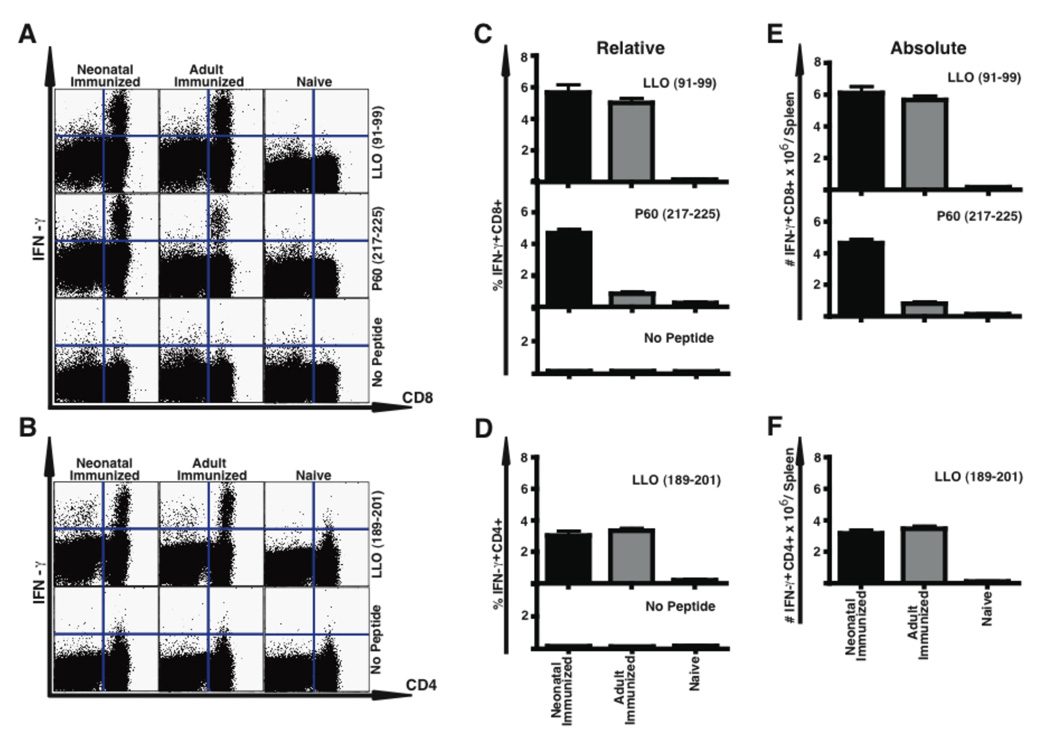
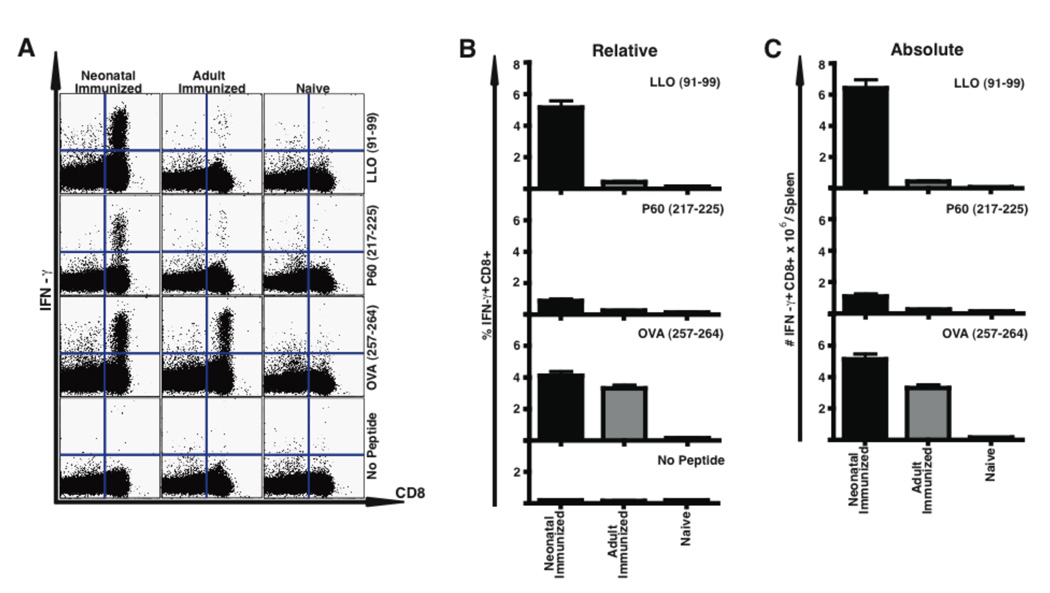
Sterilizing immunity to Lm in adults is mediated by antigen-specific T cells. Our data suggested that neonatal immunization with ΔactA-Lm induced T cell responses similar to adults. To test this directly, we immunized neonates and adults and three months later enumerated Lm-specific CD8 and CD4 T cells by intracellular IFN-γ staining 5 days after secondary infection (Fig. 3). Neonatally and adult immunized mice generated equivalent relative and absolute numbers of LLO91–99 specific CD8 T cells. Neonatally immunized mice mounted an approximately threefold higher P60217–225 -specific CD8 T cell response than mice immunized as adults. This finding was confirmed using LLO- and P60-specific tetramers (data not shown). Detection of the subdominant epitopes P60449–457 and MPl84–92 again was similarly low in both adult and neonatally immunized mice (data not shown). Contrary to what was seen for the primary CD4 T cell response, neonatally immunized mice mounted as effective a CD4 memory response as mice immunized as adults. Importantly, there was no reversion to a Th2 phenotype, since in response to Lm peptide stimulation neither in mice immunized as neonates nor as adults were IL-4 producing CD4 T cells observed by flow cytometry, and both neonatal or adult CD4 T cells made similar low levels of IL-4 or IL-13 as measured by ELISA (data not shown). Neonates thus mount a sustained CD4 Th1 response along with a strong CD8 T cell memory response.
Figure 3.
Neonatally immunized mice develop an Lm-specific secondary CD8 and CD4 T cell response. Mice immunized with 1×104 CFU i.p. of ΔactA-Lm on day 5 of life (Neonate) or at 6 weeks of age (Adult) were infected i.v. with 1×105 CFU of wild-type Lm three months after immunization. Spleen cells were obtained from indicated mice 5 days after infection along with age matched non-immune (Naïve) splenocytes, and stimulated with the indicated MHC class I- or class II-restricted Lm-specific peptides prior to analysis of intracellular cytokine expression by flow cytometry. Shown in (A) for CD8 and in (B) for CD4 are examples of the flow cytometric analysis, and in the other panels the mean percentage (C, D) and total number/spleen (E, F) of CD8 (C, E) or CD4 (D, F) IFN-γ producing splenocytes are shown. For each group unstimulated IFN-γ producing controls are shown in the examples, as well as in the summary graphs for CD4 or CD8, and the numbers of these cells were subtracted from the absolute numbers of antigen-specific cells shown (C–F). The data in C–F represent five mice per group from two combined experiments. Bar = SE.
The neonate and adult display similar primary immune response kinetics to ΔactA-Lm expressing heterologous antigen
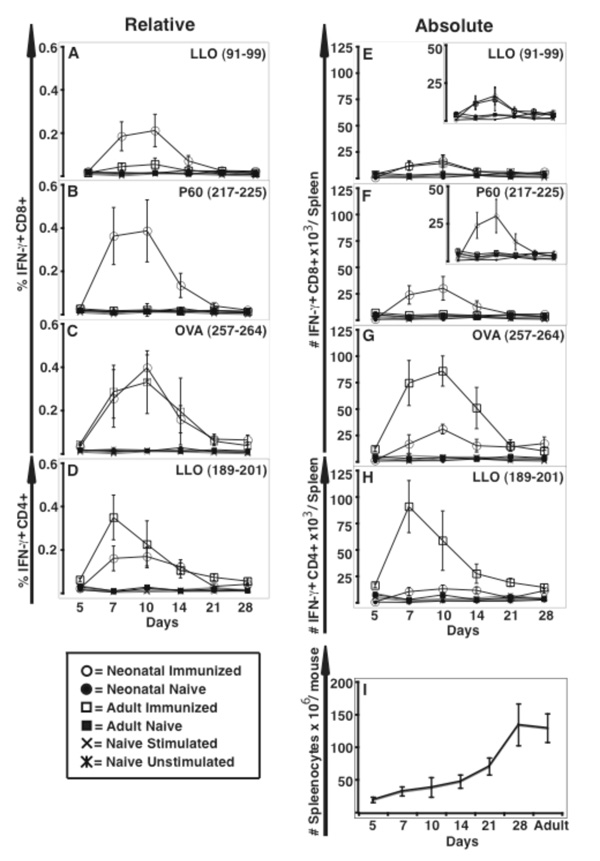
Given its ability to induce a strong, sustained protective Th1 memory response, we asked if ΔactA-Lm could function as a vaccine vehicle in the neonate. To test this we generated ΔactA-Lm-OVA, which expresses the known dominant MHC-I restricted CD8 T cell epitope of ovalbumin, and immunized neonates and adults with this recombinant strain. As shown in Figure 4, the adult CD8 T cell primary response was skewed to the now dominant OVA257–264 epitope of ovalbumin, with the endogenous Lm LLO91–99 and P60217–225 CD8 T cell response detected only at background levels. Contrary to the adult, the ΔactA-Lm-OVA immunized neonate developed not only a substantial OVA257–264 CD8 T cell response, but maintained a substantial fraction of LLO91–99 and P60217–225 reactive CD8 T cells. Expansion, peak, and contraction of antigen-specific T cells followed similar kinetics in mice immunized as adults or as neonates (Figure 4). The peak absolute number of OVA257–264 specific CD8 T cells was lower in mice immunized as neonate vs. adult (Figure 4-G). But the peak absolute numbers of LLO91–99 were equal (Figure 4-E), and the peak absolute numbers of P60217–225 (Figure 4-F) specific CD8 T cells were even higher in the neonatally immunized mice at all time points examined. While the total number of cells per spleen not unexpectedly differs between 12 day old and ~8 week old mice (see Figure 4-I), there was no significant difference in total cell number per spleen between immunized or naïve mice within each age group (n = 12 per group; data not shown). At 28 days after immunization, when the contraction phase was complete and neonatally or adult immunized mice had equivalent total spleen cell numbers, neonatally immunized mice contained low but equivalent if not greater absolute numbers of antigen-specific CD8 and CD4 memory T cells as compared to adult immune mice (Figure 5).
Figure 4.
Neonatally immunized mice develop an Lm- and OVA-specific primary CD8 T cell response. Spleen cells were obtained from the indicated mice 5-, 7-, 10-, 14, 21-, or 28- days after infection with ΔactA-Lm-OVA, and stimulated with the indicated MHC class I-restricted Lm- or OVA-specific peptides prior to analysis of intracellular cytokine expression by flow cytometry. Shown are the mean percentage (A–D) and total number/spleen (E–H) of CD8 (A–C, E–G) or CD4 (D, H) IFN-γ producing splenocytes. Naïve age-matched mice and unstimulated controls are included in each graph. The insert in (E) and (F) show the same data, but on a smaller scale than the main Figure for better visualization. (I) shows the total number of splenocytes for each age group. These data represent five to ten mice per age group from two combined experiments. Bar = SE.
Figure 5.
Neonatally immunized mice develop an Lm- and OVA-specific memory T cell response. Spleen cells were obtained from the indicated mice after completion of the contraction phase at 28 days after infection with ΔactA-Lm-OVA, and stimulated with the indicated MHC class I-restricted Lm- or OVA-specific peptides prior to analysis of intracellular cytokine expression by flow cytometry. Shown in (A) for CD8 and in (B) for CD4 are examples of the flow cytometric analysis; the other panels show the mean total number/spleen of CD8 (C) or CD4 (D) IFN-γ producing splenocytes. For each group unstimulated IFN-γ producing controls are shown in the examples, as well as in the summary graphs for CD4 or CD8. The data represent five mice per group. Bar = SE.
Neonates mount a memory T cell response to ΔactA-Lm expressing a heterologous antigen
Mice immunized with ΔactA-Lm-OVA as neonates or adults were challenged 3 months later with wild-type Lm expressing OVA. Five days after secondary infection, we enumerated antigen-specific T cells by intracellular IFN-γ staining in response to peptide re-stimulation. As shown in Figure 6, the same relative CD8 T cell epitope hierarchy as detected in the primary response was maintained in both adult and neonatally immunized mice, with the adult CD8 T cell memory response completely dominated by the OVA257–264 response, but the neonatally immunized mice displaying not only significant OVA257–264 responses but also LLO91–99 and P60217–225 reactive CD8 T cells responses. In summary, neonates mounted a strong primary and secondary memory CD8 T cell response to the heterologous OVA antigen.
Figure 6.
Neonatally immunized mice develop an Lm- and OVA-specific secondary CD8 T cell response. Mice immunized with 1×104 CFU i.p. of ΔactA-Lm-OVA on day 5 of life (Neonate) or at 6 weeks of age (Adult) were infected i.v. with 1×105 CFU of wild-type Lm-OVA three months after immunization. Spleen cells were obtained from the indicated mice 5 days after infection along with age matched non-immune (Naïve) splenocytes, and stimulated with the indicated MHC class I-restricted Lm- or OVA-specific peptides prior to analysis of intracellular cytokine expression by flow cytometry. Shown in (A) are examples of the flow cytometric analysis. Figures B shows the mean percentage, and Figure C the total number/spleen of CD8 IFN-γ-producing splenocytes. For each group unstimulated IFN-γ producing controls are shown in the examples, as well as in the summary graphs (B), and the numbers of these cells were subtracted from the absolute numbers of antigen-specific cells shown. The data represent five mice per group from two combined experiments. Bar = SE.
Discussion
We set out to test whether the attenuated strain ΔactA-Lm could induce a protective immune response in mice immunized as neonates. In summary, we found that a) immunization with ΔactA-Lm provided long-lasting protection after only one dose given around birth; b) immunization with ΔactA-Lm induced a primary T cell response with similar kinetics in neonatal and adult mice; c) neonatally immunized mice developed robust and sustained Th1 CD4 and CD8 Lm-specific T cell memory responses after only a single round of immunization; and d) ΔactA-Lm expressing a heterologous antigen (ovalbumin) induced a strong antigen specific primary and memory CD8 T cell response. Based on these findings, we propose that ΔactA-Lm (or a derivative thereof) might provide a suitable platform for neonatal vaccination.
Protection against intracellular pathogens appears significantly limited in the newborn. Recent evidence indicates that under appropriate conditions human and murine neonates can mount a detectable CD8 T cell immune response (1–3). The key requirement for a successful induction of neonatal CD8 T cell response in the mouse appears to be the entrance of the antigen into the cytoplasm of APCs (3, 6, 7, 19). Lm fulfills this requirement (10, 11) . We thus hypothesized that Lm might serve as a neonatal vaccine vehicle. Unfortunately, very little is known about the immune response to Listeria in either the murine or the human neonate. While survival of neonatal mice infected with Lm can be increased through pre-treatment with Flt-3 ligand, anti-IL-10 antibody, or CpG adjuvant (20–22) , the natural resistance to Lm infection only slowly increases in murine pups to reach adult levels around 4 weeks of age (23) . To address this increased susceptibility of neonates to Lm infection, a previous study examined a hyper-attenuated auxotrophic strain of Lm, but this strain does not induce a detectable memory CD8 T cell response unless mice are boosted as adults (24) . We have shown that the ΔactA strain of Lm is safe and induces primary as well as secondary CD4 Th1 and CD8 T cell responses in adult MyD88 deficient mice, which are highly susceptible to infection with wildtype Lm (15). We now show that neonatal mice survived high dose of ΔactA-Lm infection without any sign of disease, and cleared the inoculum similar to adult mice. While ΔactA-Lm derivatives have been shown to be safe in adult mice (10, 11), ours is the first demonstration of their safety in neonatal mice.
It is known that neonatal mice and humans develop functional CD8 T cells after viral infection (1, 3), but nothing at all is known about the neonatal primary response to Lm. We show here that neonatal mice, after only a single round of immunization with ΔactA-Lm, developed not only a strong CD8, but also a substantial CD4 Th1 primary T cell response. This, to our knowledge, is the first time a single immunization given to a neonatal mouse was able to induce strong CD8 and CD4 Th1 primary T cell response. More importantly, we found that a single immunization with ΔactA-Lm was sufficient to induce long-lasting protection from challenge with an otherwise lethal inoculum of wild-type Lm. Only the survivors of neonatal mice pre-treated with CpG prior to Lm challenge had previously been shown to develop a protective memory response (20). Our data indicate that ΔactA-Lm activates mechanisms leading to sustained CD4 and CD8 T cell memory responses that are fully functional in the neonatal mouse.
Recombinant Lm has great potential as a vaccine vehicle (10, 11, 25). Importantly for neonatal vaccination, recombinant attenuated strains of Lm induce antigen-specific immunity even in the presence of pre-existing immunity (10), an issue of importance for vaccines where pre-existing maternal immunity might interfere with the vaccine response in the newborn or infant. Based on our data a single immunization with ΔactA-Lm given at birth was sufficient to induce a long-lasting memory response. To our knowledge, this stands in sharp contrast to previously published records, where an adult booster dose was needed to maintain detectable levels of antigen-specific T cell memory (8). We thus reasoned that Lm could be an ideal vector for neonatal or infant vaccination. As a first test, we immunized neonatal and adult mice with ΔactA-Lm-OVA, and assayed the primary and secondary memory T cell response to ovalbumin in parallel to the endogenous Lm antigens. We show here that after only one immunization neonatally immunized mice generate OVA-specific primary and memory CD8 T cells with kinetics and at frequencies similar to adult immunized mice, satisfying the requirement for a potential neonatal vaccine vehicle. To test this hypothesis directly, recombinant antigens from relevant pathogens expressed in ΔactA-Lm would need to be tested in appropriate challenge models. This work is currently in progress.
The T lymphocyte response to pathogenic organisms focuses on a small number of epitopes, resulting in an epitope-specific CTL frequency hierarchy (i.e. immunodominance) (12, 26). We found that the fraction of the CD8 T cell response that was P60217–225- vs. LLO91–99 –specific was approximately threefold greater in mice immunized with ΔactA-Lm as neonates compared to mice immunized as adults. And while LLO91–99 specific CD8 T cells increased to a dominant level in neonatally immunized mice during the secondary response, neonatally immunized mice still harbored approximately threefold more P60217–225 -specific CD8 T cells than adults. This finding resembles what has been described in IFN-γ deficient adult mice (27). Neonates have been described to have reduced IFN-γ production compared to adults (28, 29). IFN-γ is known to induce the expression of molecules involved in MHC class I antigen processing and presentation (e.g. LMP2 and LMP7) in APCs, leading to a change from constitutive proteasomes to immunoproteasomes, which impacts antigen processing (30). Thus reduced IFN-γ production in neonates (and IFN-γ deficient adults) might prevent this switch, resulting in a P60-dominated response. While the reasons for the difference in epitope hierarchy between the adult and neonatally immunized mice are presently not clear, the model described in this study should enable us to identify the mechanisms at the molecular level.
With the possibility to detect a significant primary and secondary immune response in neonatal mice using ΔactA-Lm, we are now in a position to manipulate the factors determining the magnitude, tempo and quality of the immune response and to delineate the mechanisms that lead to differences between the adult and neonate, such as epitope hierarchy. The induction in neonates of long-lived protective immunity without the need for a booster dose suggest ΔactA-Lm, or derivatives thereof, as promising candidates for novel vaccine approaches in the neonatal setting.
Acknowledgements
We thank Brooke Nakhuda for expert technical assistance and animal care and Lisa Xu for outstanding flow cytometry assistance.
Abbreviations used in this paper
- Lm
Listeria monocytogenes
- CMI
cellmediated immunity
Footnotes
This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). the American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. The version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Instituted of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org
This work was supported by National Institutes of Health Grants HD049826 (KO8 to T.R.K), HD043376 (K12 to T.R.K.), HL069503 (RO1 for A.M.H.) and HD018184 (RO1 to C.B.W.). The research of T.R.K. is supported in part by a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund.
References
- 1.Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19:3331–3346. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 2.Marchant A, Goldman M. T cell-mediated immune responses in human newborns: ready to learn? Clin. Exp. Immunol. 2005;141:10–18. doi: 10.1111/j.1365-2249.2005.02799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Lee HH, Bell JJ, Gregg RK, Ellis JS, Gessner A, Zaghouani H. IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity. 2004;20:429–440. doi: 10.1016/s1074-7613(04)00072-x. [DOI] [PubMed] [Google Scholar]
- 5.Adkins B, Bu Y, Guevara P. Murine neonatal CD4+ lymph node cells are highly deficient in the development of antigen-specific Th1 function in adoptive adult hosts. J. Immunol. 2002;169:4998–5004. doi: 10.4049/jimmunol.169.9.4998. [DOI] [PubMed] [Google Scholar]
- 6.Siegrist CA, Saddallah F, Tougne C, Martinez X, Kovarik J, Lambert PH. Induction of neonatal TH1 and CTL responses by live viral vaccines: a role for replication patterns within antigen presenting cells? Vaccine. 1998;16:1473–1478. doi: 10.1016/s0264-410x(98)00111-x. [DOI] [PubMed] [Google Scholar]
- 7.Franchini M, Abril C, Schwerdel C, Ruedl C, Ackermann M, Suter M. Protective T-cell-based immunity induced in neonatal mice by a single replicative cycle of herpes simplex virus. J. Virol. 2001;75:83–89. doi: 10.1128/JVI.75.1.83-89.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marchant A, Appay V, Van Der Sande M, Dulphy N, Liesnard C, Kidd M, Kaye S, Ojuola O, Gillespie GM, Vargas Cuero AL, Cerundolo V, Callan M, McAdam KP, Rowland-Jones SL, Donner C, McMichael AJ, Whittle H. Mature CD8(+) T lymphocyte response to viral infection during fetal life. J. Clin. Invest. 2003;111:1747–1755. doi: 10.1172/JCI17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tu W, Chen S, Sharp M, Dekker C, Manganello AM, Tongson EC, Maecker HT, Holmes TH, Wang Z, Kemble G, Adler S, Arvin A, Lewis DB. Persistent and selective deficiency of CD4+ T cell immunity to cytomegalovirus in immunocompetent young children. J. Immunol. 2004;172:3260–3267. doi: 10.4049/jimmunol.172.5.3260. [DOI] [PubMed] [Google Scholar]
- 10.Starks H, Bruhn KW, Shen H, Barry RA, Dubensky TW, Brockstedt D, Hinrichs DJ, Higgins DE, Miller JF, Giedlin M, Bouwer HG. Listeria monocytogenes as a vaccine vector: virulence attenuation or existing antivector immunity does not diminish therapeutic efficacy. J. Immunol. 2004;173:420–427. doi: 10.4049/jimmunol.173.1.420. [DOI] [PubMed] [Google Scholar]
- 11.Brockstedt DG, Giedlin MA, Leong ML, Bahjat KS, Gao Y, Luckett W, Liu W, Cook DN, Portnoy DA, Dubensky TW., Jr Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc. Natl. Acad. Sci. U S A. 2004;101:13832–13837. doi: 10.1073/pnas.0406035101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vijh S, Pamer EG. Immunodominant and subdominant CTL responses to Listeria monocytogenes infection. J. Immunol. 1997;158:3366–3371. [PubMed] [Google Scholar]
- 13.Brundage RA, Smith GA, Camilli A, Theriot JA, Portnoy DA. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proc. Natl. Acad. Sci. U S A. 1993;90:11890–11894. doi: 10.1073/pnas.90.24.11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 15.Way SS, Kollmann TR, Hajjar AM, Wilson CB. Cutting edge: protective cell-mediated immunity to Listeria monocytogenes in the absence of myeloid differentiation factor 88. J. Immunol. 2003;171:533–537. doi: 10.4049/jimmunol.171.2.533. [DOI] [PubMed] [Google Scholar]
- 16.Edelson BT, Unanue ER. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J. Immunol. 2002;169:3869–3875. doi: 10.4049/jimmunol.169.7.3869. [DOI] [PubMed] [Google Scholar]
- 17.Kursar M, Mittrucker HW, Koch M, Kohler A, Herma M, Kaufmann SH. Protective T cell response against intracellular pathogens in the absence of Toll-like receptor signaling via myeloid differentiation factor 88. Int. Immunol. 2004;16:415–421. doi: 10.1093/intimm/dxh047. [DOI] [PubMed] [Google Scholar]
- 18.Way SS, Thompson LJ, Lopes JE, Hajjar AM, Kollmann TR, Freitag NE, Wilson CB. Characterization of flagellin expression and its role in Listeria monocytogenes infection and immunity. Cell. Microbiol. 2004;6:235–242. doi: 10.1046/j.1462-5822.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- 19.Regner M, Martinez X, Belnoue E, Sun CM, Boisgerault F, Lambert PH, Leclerc C, Siegrist CA. Partial activation of neonatal CD11c+ dendritic cells and induction of adult-like CD8+ cytotoxic T cell responses by synthetic microspheres. J. Immunol. 2004;173:2669–2674. doi: 10.4049/jimmunol.173.4.2669. [DOI] [PubMed] [Google Scholar]
- 20.Ito S, Pedras-Vasconcelos J, Klinman DM. CpG oligodeoxynucleotides increase the susceptibility of normal mice to infection by Candida albicans. Infect. Immun. 2005;73:6154–6156. doi: 10.1128/IAI.73.9.6154-6156.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Genovese F, Mancuso G, Cuzzola M, Biondo C, Beninati C, Delfino D, Teti G. Role of IL-10 in a neonatal mouse listeriosis model. J. Immunol. 1999;163:2777–2782. [PubMed] [Google Scholar]
- 22.Vollstedt S, Franchini M, Hefti HP, Odermatt B, O'Keeffe M, Alber G, Glanzmann B, Riesen M, Ackermann M, Suter M. Flt3 ligand-treated neonatal mice have increased innate immunity against intracellular pathogens and efficiently control virus infections. J. Exp. Med. 2003;197:575–584. doi: 10.1084/jem.20021900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohara R, Mitsuyama M, Miyata M, Nomoto K. Ontogeny of macrophage-mediated protection against Listeria monocytogenes. Infect. Immun. 1985;48:763–768. doi: 10.1128/iai.48.3.763-768.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rayevskaya M, Kushnir N, Frankel FR. Safety and immunogenicity in neonatal mice of a hyperattenuated Listeria vaccine directed against human immunodeficiency virus. J. Virol. 2002;76:918–922. doi: 10.1128/JVI.76.2.918-922.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoen C, Stritzker J, Goebel W, Pilgrim S. Bacteria as DNA vaccine carriers for genetic immunization. Int. J. Med. Microbiol. 2004;294:319–335. doi: 10.1016/j.ijmm.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Busch DH, Pilip IM, Vijh S, Pamer EG. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity. 1998;8:353–362. doi: 10.1016/s1074-7613(00)80540-3. [DOI] [PubMed] [Google Scholar]
- 27.Skoberne M, Geginat G. Efficient in vivo presentation of Listeria monocytogenes- derived CD4 and CD8 T cell epitopes in the absence of IFN-gamma. J. Immunol. 2002;168:1854–1860. doi: 10.4049/jimmunol.168.4.1854. [DOI] [PubMed] [Google Scholar]
- 28.Lewis DB, Larsen A, Wilson CB. Reduced interferon-gamma mRNA levels in human neonates. Evidence for an intrinsic T cell deficiency independent of other genes involved in T cell activation. J. Exp. Med. 1986;163:1018–1023. doi: 10.1084/jem.163.4.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson CB, Westall J, Johnston L, Lewis DB, Dower SK, Alpert AR. Decreased production of interferon-gamma by human neonatal cells. Intrinsic and regulatory deficiencies. J. Clin. Invest. 1986;77:860–867. doi: 10.1172/JCI112383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sijts AJ, Neisig A, Neefjes J, Pamer EG. Two Listeria monocytogenes CTL epitopes are processed from the same antigen with different efficiencies. J. Immunol. 1996;156:683–692. [PubMed] [Google Scholar]