Abstract
Muscle-specific tyrosine-kinase-antibody-positive myasthenia gravis (MuSK-MG) has emerged as a distinct entity since 2001. This disease has been reported worldwide, but with varying rates among patients with generalized acetylcholine-receptor-antibody-negative MG. MuSK-MG was detected in approximately 37% of generalized acetylcholine receptor antibody-negative MG. MuSK-MG patients were predominantly female with more prominent facial and bulbar involvement and more frequent crises. Disease onset tended to be earlier. Patients tended to have a relatively poor edrophonium response but showed prominent decrement in the repetitive nerve stimulation test in the facial muscles. Patients were more likely to display poor tolerance of, or a lack of improvement with, anticholinesterase agents. Somewhat better response was observed with steroids and plasma exchange. Most were managed successfully with aggressive immunomodulatory therapies, although a higher proportion of MuSK-MG patients had a refractory course when compared with other forms of generalized MG. I present here an up-to-date overview on MuSK-MG based on our experience at the University of Alabama at Birmingham and the existing literature.
Keywords: myasthenia gravis, muscle-specific tyrosine-kinase-antibody, seronegative myasthenia gravis
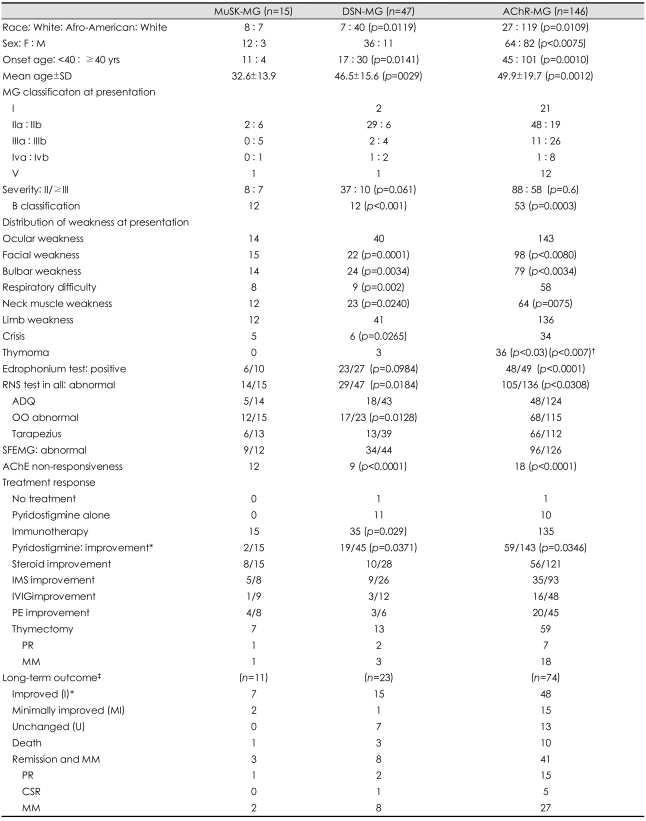
In 1960, Simpson suggested that myasthenia gravis (MG) might be an autoimmune disease.1 This was on the basis of his observation that autoimmune thyroid disease, rheumatoid arthritis, and lupus erythematosis were found more often in MG patients. Sixteen years later, in 1976, the autoimmune mechanism of MG was firmly established when Lindstrom et al. showed that 85% of MG patients had measurable serum antibodies to human muscle acetylcholine receptors (AChRs).2 Twenty-five years later, another serum antibody, antibody to muscle-specific receptor tyrosine kinase (MuSK-Ab) was reported in 70% of patients with generalized seronegative MG.3 In this review, up-to-date information on the clinical, electrophysiological and laboratory features of MuSK-Ab-positive MG (MuSK-MG) will be presented on the basis of experience with 235 cases of MG at the University of Alabama at Birmingham (UAB) and from the current literature (Table 1).
Table 1.
Clinical and laboratory features in MuSK-MG, DSN-MG, and AChR-MG Generalized MG
*Good or excellent responses, †Compared with DSN-MG, ‡Follow-up period: ≥3 yr. Mean Follow-up period: 10.6±8.0 yr in MuSK-MG, 8.5±5.5 yr in DSN-MG. 9.8±5.9 yr in AChR-MG. CSR: complete stable remission, IMS: immunosuppressive therapy MM: minimal manifestation, PE: plasma exchange, PR: pharmacological resmission.
Seronegative Myasthenia Gravis
The general impression has been that there is not much difference between seropositive and seronegative MG except for the presence of the AChR-Ab in the serum, and therapeutic approaches have been identical in the two groups. Surprisingly, there have been only two fully published reports and one abstract supporting this view. Soliven et al. found no difference in the clinical features or therapeutic response in 25 seronegative and 120 seropositive cases and concluded that the absence of serum AChR-Ab did not preclude favorable responses to thymectomy or plasmapheresis.4 However, they found that the rate of abnormal findings in the repetitive nerve stimulation (RNS) test and single-fiber electromyography (SFEMG) was significantly lower in seronegative MG. In an analysis of clinical and electrophysiological features in 38 seronegative and 102 seropositive MG patients, Oh found that seronegative MG represented significantly milder forms (ocular and class 1 MG) compared with seropositive MG, and that RNS abnormalities were significantly less common in seronegative MG.5 The rate of abnormality on the SFEMG was not significantly different between the two groups. Sanders et al stated in an abstract that seronegative cases represent milder MG restricted to the ocular muscles.6 Thus, these studies supported the general impression that, though AChR-Ab was not found, seronegative MG represented a milder form of autoimmune MG. Mossman et al. injected immunoglobulin from seronegative patients intraperitoneally into mice and found significantly impaired neuromuscular transmission despite absent binding of antibody to AChR and a minimal loss of AChR.7 This study conclusively showed that seronegative MG is an autoimmune disease but is immunologically and physiologically distinct from seropositive MG. However, the detection of an antibody responsible for seronegative MG was elusive until 2001, when the antibody to MuSK-Ab was first reported in patients with generalized seronegative MG.3
MuSK and MuSK Induced Experimental Allergic Autoimmune Myasthenia Gravis
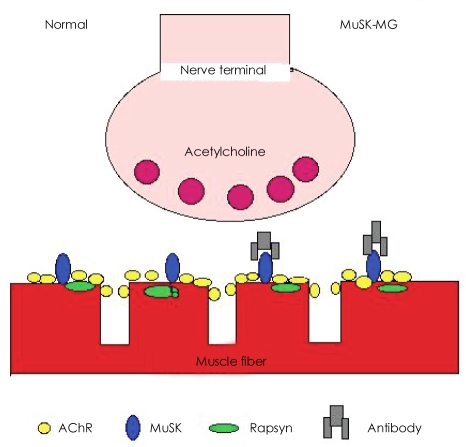
MuSK, one of many receptors near the AChR (Fig. 1), is critical for the synaptic clustering of AChRs during fetal development, and it remains important for the maturation and/or maintenance of the adult neuromuscular synapse in postnatal life. MuSK is activated by agrin, which is released from motor neurons, and induces AChR clustering at the postsynaptic membrane. The pathogenic significance of MuSK-Ab in MuSK-MG was questioned initially because end-plate AChR density in patients remains well preserved.8,9 However, recent studies unequivocally demonstrated that MuSK-Ab can cause MG. Active immunization of mice and rabbits with the extracellular domain of MuSK protein led to EAMG and associated changes in neuromuscular junctions.10,11 Passive immunization of mice with IgG from MuSK MG patients induced EAMG and further demonstrated marked reduction of postsynaptic AChR staining by fluorescent-alpha-bungarotoxin.12 EAMG mice showed weakness as well as decremental (23-47%) response in the repetitive nerve stimulation test.10,12 Jha et al.10 also demonstrated that MuSK sera inhibit agrin-induced AChR aggregation and hypothesized that MG can be caused by blocking the agrin signaling pathway alone or in combination with other factors. According to this hypothesis, a patient's MuSK-Ab causes synaptic impairment by blocking MuSK signaling in the postsynaptic membrane, producing MG. This hypothesis is most commonly accepted as a mechanism of reduced neuromuscular transmission in MuSK-MG.
Fig. 1.
Simplitied illustration of neuromuscular junction in normal and MuSK-MG. MuSK-MG: muscle-specific tyrosine kinase antibody positive-MG.
Pathological Studies
Thymoma was not detected in most series in MuSK-MG patients. Thymoma was found in four patients in thymectomized histology. A 5-mm thymoma was found in a patient with oculo-bulbar MG.13 In Lavrnic's series, thymoma was found in two of 17 thymus glands removed by thymectomy.14 Evoli found a small nodular thymoma in one of 17 thymectomized patients.15 Thus, thymoma appears to be exceedingly rare. Thymic hyperplasia (lymphoid hyperplasia) was reported in 19 (26%) of 73 cases (Table 2). In the present study, thymoma was not seen in any cases of MuSK-MG in contrast to three patients with double seronegative (DSN)-MG and 13 cases of AChR-MG.
Table 2.
Prevalence and other demographic features of MuSK-MG
*Generalized MG, †All MG SNGMG, seronetative generalized MG.
I, ocular MG. SPGMG: seropositive generalized MG, MuSK-MG: muscle-specific tyrosine kinase antibody positive-MG.
In contrast to AChR-MG, the thymus gland in MuSK-MG is usually normal or demonstrates mild alterations. Lymphoid hyperplasia is seen rarely, being observed in 19 of 73 cases (Table 2). Two studies reported detailed thymic histology studies comparing MuSK-MG patients with AChR-MG and DSN-MG and with healthy controls, using morphometry with immunohistochemical or immunofluorescent staining.16,17 Both studies emphasized the lack of a germinal center or lymphocytic infiltrates in MuSK-MG, in contrast to the changes typically seen in the majority of AChR-MG patients, and about half the DSN-MG patients had histological changes similar to those in AChR-MG patients. These findings indicate probable differences in the pathogenic mechanisms between MuSK-MG and AChR-MG, and suggest that thymectomy may not be of value in the MuSK-MG group but may of value in DSN-MG.
Muscle biopsy usually showed non-specific atrophy. Sanders reported non-specific atrophy in deltoid muscle biopsies in four patients18 Padua found nonspecific atrophy and an increased variability of fiber diameter in seven of 11 biopsied patients19 A more detailed comparison was made in muscle biopsy findings between six MuSK-MG and seven AChR-MG patients by an Italian group. Myopathic changes {mini-cores and ragged red fibers (mitochondrial aggregates)} were common in MuSK-MG patients, whereas fiber type grouping (neurogenic finding) and atrophy were more frequently found in AChR-MG patients.20 Farrugia et al.21 found significant muscle atrophy and fatty replacement in facial and bulbar muscles in MuSK-MG which were not found in AChR-MG patients. They also found a correlation between the duration of prednisone treatment and tongue atrophy with high signal in the MRI scan.
Intercostal muscle biopsy from a 33-year-old man who was MuSK-Ab-positive with longstanding facial, bulbar, and respiratory weakness showed no significant reduction in AChR or MuSK expression compared to control samples.8 On electron microscopy, the structure of the nerve terminals and junctional folds was well-maintained, although postsynaptic density was mildly reduced as a result of simplification of some endplates. Endplate e1ectrophysiologic studies did demonstrate reduced miniature endplate potential amplitudes and currents, as observed in AChR-MG. Similarly, biceps muscle biopsies from eight patients from Japan with MuSK-MG failed to show a significant reduction in AChR or alterations to postsynaptic morphology.22 Immunoglobulin and complement deposition was scant in these reports.8,22 These findings suggest that the pathogenic mechanism of neuromuscular transmission defect in MuSK-MG is different from that in AChR-MG and is not through complement-mediated AChR defect. This finding was anticipated because MuSK antibodies are predominantly immunoglobulin G4-subclass that does not activate complement.23
Prevalence of Muscle-Specific Receptor Tyrosine Kinase-Myasthenia Gravis
Essentially all patients included in various series from North America, Europe, and Asia have had seronegative generalized MG, although there are three case reports of patients with pure ocular symptoms.24-26 Follow-up in one case is less than one year, too short to establish a definite diagnosis of ocular MG, and it is possible that this patient will generalize at a later time.24 In the second case, the patient had ocular symptoms for two years and at least is qualified as ocular MG.25 The third patient had external ophthalmoplegia for 12 years, and MRI of the orbits revealed severe wasting of all extraocular muscles, except for the inferior oblique muscle.26 If we use two years as the minimal observational period for the diagnosis of ocular MG, then MuSK-Ab was positive in at least two cases of ocular MG so far. MuSK-Ab was negative in all of 89 ocular MG cases in six large series including our own.18,23,28-31 MuSK-Ab was negative in all 407 AChR-MG cases in five series including our own (Table 2).29,31-34 The "MuSK-Ab" previously reported in AChR-MG by Ohta was subsequently found to be antibodies to alkaline phosphatase.35,36 One exception is a Swedish study which reported a positive MuSK-Ab in five (36%) of 14 seronegative patients and in five (14%) of 36 AChR-Ab positive patients.37 Because of the atypical features of this study, this report was excluded from further analysis in this review. At UAB, MuSK-Ab was negative in all of 13 tested cases of ocular MG (one AChR-Ab positive) and in all of 16 tested AChR-Ab positive cases.
Although 70% of patients with seronegative generalized MG from Oxford were found to have MuSK-Ab initially,3 the frequency of MuSK-Ab in later series has ranged from 0% in Norway38 to 49% in Turkey,39 with a mean frequency of approximately 37% (Table 1).40 Considering the relatively uniform rate of AChR-Ab positive rates in the range of 62-90% (mean 77%) worldwide, this vast regional or racial difference in the positive rate of MuSK-Ab is striking (Table 2). In Europe, MuSK-Ab rates seem to be lower in the northern latitudes. However, this trend was not observed in the United States or Asia. The most striking racial difference was observed between whites and African-Americans (AA) in the United States. Oh et al.41 reported a significantly higher positive rate (50%) of MuSK-Ab in AA compared with that (17%) in whites in Alabama, USA. This difference was also observed in three different institutions in the USA, confirming that this racial difference is genuine.42 These data suggest that there are differences in the rates of positive MuSK-Ab among patients with AChR-Ab-negative generalized MG worldwide, perhaps reflecting a biological or genetic susceptibility factor.
Demographic Characteristics
A marked female predominance (74%) is widely observed (Table 1 and 2) in all large series including our own. In four reports, all patients identified have been women. Disease onset is significantly earlier than for other MG populations with a mean age of onset at 32.6 years, but it still ranges from the first through the seventh decade in large series. The present study demonstrates that there are more patients with MuSK MG with disease onset prior to 40 years of age than in other groups, but the mean age of onset between AChR-MG and DSN-MG groups did not differ considerably (Table 1). The late onset is most prominent in the AChR-MG groups.
Clinical Phenotype
Myasthenic weakness in MuSK-MG tends to be more severe and refractory to treatment than that observed in patients with other forms of generalized MG.40 An Italian series observed more MuSK-MG patients than DSN-MG patients who were classified as severe by the MG Foundation of America (MGFA) clinical classifications. Pasnoor et al.19 found III-V MGFA classification in 55% of 53 cases. Using the Quantitative MG (QMG) scoring system, Stickler et al.43 found maximum QMG scores to be significantly higher in 20 MuSK-MG patients than in 72 with AChR-MG. The present study showed clearly that MuSK-MG patients have more severe forms of MG compared with the DSN-MG group but not with the AChR-MG group.
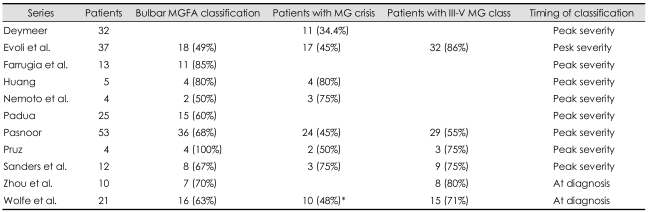
Almost all series showed that the bulbar form of MG is common in MuSK-MG (Table 3). Our study and that of Deymeer et al.39 showed that the "bulbar classification" is significantly more common compared with AChR-MG and DSN-MG groups (Table 1 and 3).
Table 3.
Frequency of bulbar MGFA classificdation, crisis, and III-V MG class in MuSK-MG
*At severity.
M GFA: Myasthenia Gravis Foundation of America, MuSK-MG: muscle-specific tyrosine kinase antibody positive-MG.
There is some evidence that myasthenic crisis is also more common (35-80%) in patients with MuSK-MG (Table 1 and 3). Nemoto et al.32 found this to be true in comparison with AChR-MG patients, whereas our study verified it only in comparison with DSN-MG patients.
Attempts to relate disease severity with MuSK-Ab concentration have met with varying success. However, in the largest analysis of 83 serum samples from 40 patients, there was a correlation between antibody levels and disease severity, measured as a function of both clinical classification and QMG.44 Furthermore, in a subgroup of 14 patients measured both before and after treatment, immunosuppressive therapy significantly reduced antibody titers. No appreciable changes were seen after thymectomy. Ohta also observed a good correlation between in the MuSK-Ab titer and QMG score in serial studies of 12 individuals.33
Three main patterns of generalized disease have been observed in MuSK-MG, two of which may be helpful in distinguishing these patients from AChR-MG patients.18,40 The most classical and common pattern is characterized by rapidly progressing bulbar weakness with dysphonia (nasal voice), dysphagia, and chewing difficulty. These patients remain the most severely affected throughout the course of the disease. Ocular symptoms (diplopia and ptosis) are usually present but mild. Involvement of limbs was relatively less severe and inconsistent. In three series, limb weakness was absent in almost 50-68% of cases.29,33,34 In the present series, limb weakness was present in 80% of patients. Facial weakness, followed by bulbar and ocular weakness, was observed in all patients, and was a distinguishing feature from AChR-MG or DSN-MG. Respiratory difficulty was observed in one-half the patients. Profound facial and tongue atrophy has been described as one of the characteristic findings in MuSK-MG by the Oxford group.21 This was a common clinical feature, confirmed by magnetic resonance imaging, and was observed in seven of 15 MuSK-MG patients.21 Padua19 also reported tongue atrophy in 11 (44%) of 25 cases. This atrophy appears to be a feature of longstanding disease managed with chronic corticosteroids. Though profound facial and tongue atrophy is claimed as a unique feature of MuSK-MG, it has rarely been reported in other series: in only three of 17 cases in Lavric's series,14 in three of 53 cases in Pasnoor's series,45 and in one case in our series. She had had MG longer than 18 years, but not on steroid.
The second pattern is notable for focal predominant neck, shoulder, and respiratory involvement with little or delayed oculo-bulbar weakness. In Sanders' series, this was the most common pattern seen in seven of 12 MuSK-MG patients. Padua34 reported one patient with neck muscle weakness, and Kostera-Prusczyki et al had one patient with neck weakness and respiratory difficulty. Spengos et al.46 also reported a case of "dropped head syndrome" as a prominent clinical feature in MuSK-MG with thymic hyperplasia. These patients may present a diagnostic challenge because MG is not usually considered as a diagnostic possibility. In three cases, Stickler and Padua19,43 were able to make the diagnosis of MG by SFEMG in cervical paraspinal muscles as the only abnormality when the extensor digitorum communis (EDC) and frontalis muscle SFEMG were normal. These cases emphasize the need for the SFEMG in a clinically involved muscle. The third pattern is indistinguishable from AChR-MG.
Neonatal MG was reported in three infants who were born to MuSK-MG mothers.45,47,48 Two had a classical transient neonatal MG lasting 3 weeks.45,48 However, the other infant had a more prolonged recovery with residual symptoms of occasional swallowing difficulty, intermittent ptosis, and stridor when weeping at 20 months.47
Unusual MuSK-MG cases included vocal cord paralysis in two MuSK-MG cases,50,51 and one patient with MG and Morvan's syndrome who had positive AChR, MuSK, and voltage gated potassium channel (VGKC) antibodies.56
In the present series, the clinical features are compared with those in two other types of MG. Clearly, the bulbar classification is significantly common in MuSK-MG. Respiratory weakness and crisis are significantly common in MuSK-MG only compared with DSN-MG. As for muscle strength evaluation, facial, bulbar and neck weakness are significantly common in MuSK-MG. Limb weakness is also common. Thus, the present series is comparable with other series with regard to the clinical features of MuSK-MG. The second phenotype was not observed in the present series. Pasnoor et al. had a similar esxperience.
Diagnostic Features
Edrophonium and neostigmine tests
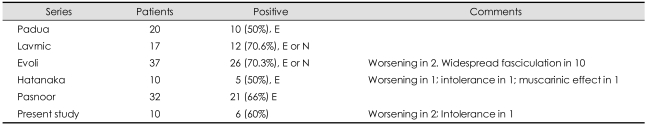
Diagnostic testing with either edrophonium or neostigmine injection was positive in 50-70% of MuSK-Ab-positive cases in the large series (Table 4).14,19,29,45,52 Two series with edrophonium or neostigmine injection reported a higher (70%) positive rate,14-29 while two series with edrophonium alone reported a lower (50%) rate.19,52 It is possible that neostigmine injection might have played a role in the higher positive rate by preventing cholinergic side effects with atropine, which is usually given with the neostigmine test. Hatanaka et al.52 reported that positive edrophonium testing is significantly less common in the MuSK-MG group than in either the AChR-MG or DSN-MG group. The most striking finding with edrophonium testing in MuSK-MG is that it may worsen patients' myasthenic symptoms (hypersensitivity) or precipitate nicotinic (intolerance) and muscarinic side effects, such as increased weakness, widespread fasciculation, severe stomach cramping, or diarrhea. Hatanaka et al.52 observed this in three of 10 cases: worsening of myasthenic symptoms (hypersensitivity) in one, intolerance of edrophonium in one, and severe muscarinic effect in one. Evoli et al.29 also observed worsening of myasthenic symptoms in one case and widespread fasciculation in ten patients. The low percentage of positive edrophonium testing may reflect the poor responsiveness to anticholinesterase treatment which has been reported in MuSK-MG.53 More than 70% of MuSK-MG patients were nonresponsive to anticholinesterase therapy, a significantly higher proportion than for other MG populations.52 Repetitive discharges of compound muscle action potential (CMAP) on low-frequency stimulation triggered by moderate doses of pyridostigmine and edrophonium 10 mg with worsening of myasthenic symptoms were reported in a patient with MuSK-MG and may be a useful indicator of poor tolerance.54
Table 4.
Results of edrophonium test
The present study showed a 60% positive rate on the edrophonium test in ten tested cases. Compared with AChR-MG and DSN-MG patients, MuSK-MG patients had a significantly low positive rate. Among four negative cases, three developed worsening of myasthenic symptoms and one, intolerance with cholinergic (muscarinic) symptoms with the edrophonium test.
Repetitive nerve stimulation
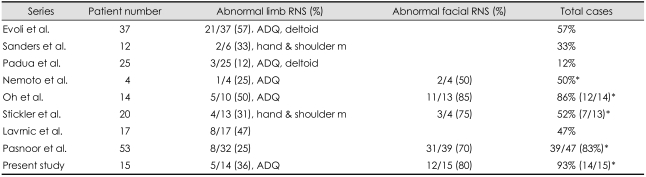
Earlier series suggested that repetitive nerve stimulation (RNS) had a relatively low yield in MuSK-MG patients (Table 5).18,29 For instance, RNS of limb muscles was abnormal in 57% of patients with MuSK-MG versus 78% of DSN-MG patients.29 Sanders reported a decremental response in two (33%) of six patients.18 Padua observed abnormal decremental response in the RNS test in the limb muscles only in three (12%) of 25 MuSK-MG patients. On the contrary,19 Oh's study, based on cases at two University Centers, showed the highest diagnostic yield in MuSK-MG (86%) compared with AChR-MG (81%) or DSN-MG (55%).55 This was mostly due to the higher rate of abnormality in the facial muscles and was confirmed by subsequent studies (Fig. 2).43,45 The earlier series did not do the RNS test on the facial or trapezius muscles.18,29
Table 5.
Abnormal rate in the repetitive nerve stimulation (RNS) test in the various muscles
*All three muscle testing: limb, trapezius, and facial muscle.
ADQ: abductor digiti quinti.
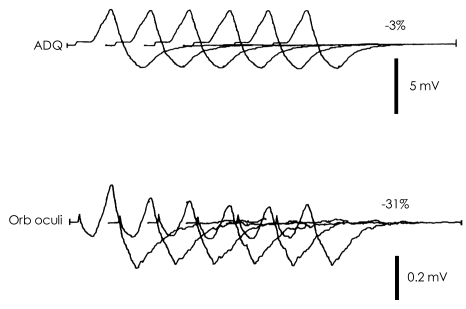
Fig. 2.
Classical repetitive nerve stimulation response in MuSK-MG. A remarkable decremental (31%) response in the orbicularis oculi (Orb oculi) muscle and normal response (3%) in the abductor digiti quinti (ADQ) muscle. Low CMAP amplitude is also noted in Orb oculi muscle. MuSK-MG: muscle-specific tyrosine kinase antibody positive-MG, CMAP: compound muscle action potential.
Oh's study also showed that percentage decrements were of greater magnitude in MuSK-MG patients in the facial muscles than in the other two groups, but a greater abnormality was observed in AChR-MG group in the abductor digiti quinti (ADQ) muscles.55 Including facial muscles in RNS protocols is important when evaluating MG patients who are potentially MuSK-seropositive. Facial RNS abnormalities reflect the propensity for cranial muscle involvement in this population. The present study at UAB showed the same findings as noted in the previous report.
Single-fiber electromyography
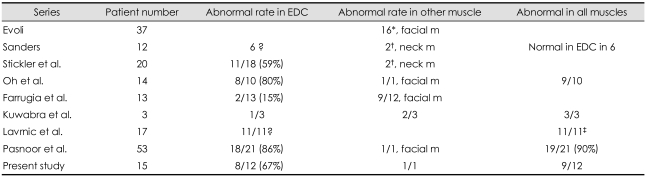
SFEMG provided critical information for diagnosis of many MuSK-MG patients in the earlier series when the RNS test on the limb muscles was normal (Table 6). In the MuSK-Ab-positive series by Evoli et al.29 SFEMG of facial muscles was utilized to confirm the diagnosis of MG for cases with normal RNS. SFEMG was abnormal in all of 16 patients who underwent the procedure. Sanders et al. found SFEMG of the EDC to be normal in eight (67%) of 12 cases.18 Investigation of the neck extensor muscles in two cases and the frontalis and deltoid in one case detected abnormal jitter in three of the patients with normal EDC studies.
Table 6.
Abnormal rate in SFEMG
*Abnormal SFEMG in all cases with normal RNS test, †2 cases, SFEMG in EDC and frontalis muscles was normal, ‡Did not specify which muscle.
SFEMG: single-fiber electromyography, EDC: extensor digitorum communis.
In analogous fashion to RNS abnormalities, SFEMG of limb muscles was reported to have a relatively low yield in MuSK-MG (Table 6). In several studies, the percentage of MuSK-MG patients with abnormal jitter on EDC recording was significantly lower than for either AChR-MG or DSN-MG patients.32,43,56,57 In the latter two populations, SFEMG of the EDC was abnormal at least in 80% of the time. EDC fiber pairs displaying abnormal jitter or blocking tended to be lower in MuSK-MG patients.19 Farrugia et al.57 found abnormal jitter in only two of 13 patients using the concentric needle for the SFEMG test on the EDC muscle, but abnormal jitter in the facial muscles in 66% of cases. Kuwabra et al.55 found abnormal jitter in one of three cases in the EDC using the stimulation SFEMG.56 Oh et al.55 and Pasnoor et al.45 however, confirmed abnormal jitter in the EDC in nearly all tested MuSK-MG patients. Oh et al.55 did not observe any obvious difference between MuSK-MG, AChR-MG and DSN-MG groups. The present study also showed 67% abnormality in the EDC muscles on the SFEMG. In one patient with normal SFEMG in the EDC muscle, the frontalis muscle showed abnormal jitter. In three other cases, the facial muscles were not checked. The reason for this difference between our series and prior reports is most likely due to the frequency of involvement of limb muscles. Limb weakness was absent in 68% of cases in Evoli's series29 and in 11 of 12 cases in Sanders' series.18 In our study, in contrast, limb weakness was observed in 80% of cases.55 Thus, as a group, our patients had a greater degree of upper-limb involvement. Unlike those of Sanders et al.18 our patients tended to have more generalized impairments (Table 1) with weakness extending beyond ocular, oropharyngeal, neck extensor, and respiratory muscles.
As a whole in MuSK-MG, SFEMG of more proximal muscles including the deltoid, frontalis, orbicularis oculi, or neck extensors may be markedly abnormal in patients with normal EDC jitter.18,56,57 Thus, it is important to do the SFEMG in the affected muscles in MuSK-MG patients if the EDC muscle is normal.
Needle EMG
With conventional EMG, a myopathic pattern of small-amplitude, short-duration (SASD) motor unit potential (MUP) has been observed in three series. Padua observed SASD MUPs in 44% of MuSK-MG patients in contrast to 26% of DSN-MG patients.19 Sanders18 reported scattered fibrillations and SASD MUPs in five patients. Using quantitative EMG in 13 patients with MuSK-MG, Farrugia et al.58 found a significant decrease in the mean duration of MUP in MuSK-MG as well as in AChR-MG, and a myopathic EMG pattern in 50% of MuSK-MG and 40% of AChR-MG patients, and concluded that the facial atrophy seen in some MuSK-MG patients is of myopathic origin, resulting from either musclefiber shrinkage or loss of muscle fibers from motor units. In our study, all three tested patients showed SASD MUPs and two showed scattered fibrillation potentials.55 One of two had peripheral neuropathy accounting for the fibrillation. It appears that SASD MUPs are common and fibrillation not uncommon in MuSK-MG. Such needle EMG abnormalities are unusual for MG. A myopathic EMG pattern was observed in only 33-38% of MG cases in other series.58,59 Fibrillation potentials were reported to be extremely rare in MG, but no exact figure was reported.
Response to Treatment
Anticholinesterase agents
The clinical response to anticholinesterase agents (anti-ChE) in MuSK-MG has generally been unsatisfactory, with improvement in a minority of patients. Unresponsiveness to standard pyridostigmine dosing was reported in an early series,18,29 and this observation was confirmed in later reports.14,15,52,53 Intolerance manifested by severe muscatinic and nicotinic side-effects was common.14 Worsening of myasthenic symptoms was also reported with anti-ChE with cholinergic crisis in a few patients.
In a series of 14 patients with MuSK-MG at two United States (US) University Centers, only three of 14 benefited from pyridostigmine.52 Anticholinesterase nonresponsiveness was noted in the remaining 11 patients, classified as 4 with no improvement, 4 intolerant as a result of cholinergic side-effect, and 3 hypersensitive with worsening of myasthenic symptoms. Nonresponsiveness to anticholinesterase agents was significantly more common in MuSK-MG patients than in AChR-MG or DSN-MG patients.52 In another series, 30% of MuSK-MG patients responded to pyridostigmine and continued it long term, a lower percentage than for patients who were seronegative.28 Evoli et al.15 in the latest review reported mild or no benefit in 70% of cases and a satisfactory response in 21% of 57 cases. They observed frequent appearance of cholinergic side effects, and cholinergic crisis in 9% of cases. Pasnoor et al.45 found that only eight (15%) of 51 patients responded to pyridostigmine, whereas 15 had intolerable sideeffects, and ten worsened. The present study showed non-responsiveness in 80% of cases and long term benefit in 13% of cases. Hypersensitivity to anti-AChE was noted in the RNS test by an extra repetitive discharge.54 This electrophysiological feature correlates with clinical deterioration and may be a useful indicator of the adverse potential of anticholinesterase agents in select patients.
Thymectomy
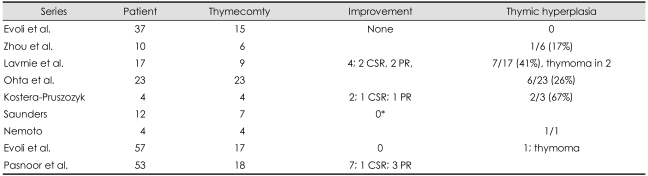
Most studies do not report clinical benefit from thymectomy for patients with MuSK MG (Table 7). Some centers do not recommend the procedure for this population, on a theoretical basis in regard to thymus pathology, as discussed above.17 Seven patients followed for at least 8 months after surgery did not appear to benefit.18 An eight-months follow-up period is insufficient to assess the final benefit of thymectomy. Neither did 17 patients in another series, most of whom remained dependent on chronic immunosuppressive therapy and showed no better outcome than those who did not undergo surgery.29 Some reported remission in a few patients: four of 9 thymectomized patients in Lavric's series14 and two of 4 thymectomized patients in another series.34,45 In the present study, one of five patients achieved pharmacological remission. Thus, these data seem to argue against performing thymectomy.
Table 7.
Improvement rate and rate of thymic hyperplasia
*Followed for 8 months. No improvement.
CSR: complete stable remission, PR: pharma cological remission.
Immunosuppressive therapy
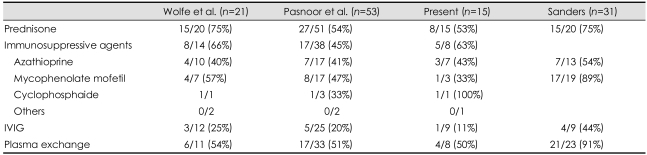
Because of the disease severity and poor response to anti-AChE-Is, 95% to 100% of MuSK-MG patients require immunosuppressive treatment.53 In contrast to anticholinesterase agents, MuSK-MG patients for the most part have a favorable response to immunosuppressive therapy (Table 8).53 However, this is usually achieved with various combinations of immunosuppressive agents. A variety of agents have been used, including corticosteroids, azathioprine, cyclosporine, mycophenolate mofetil, cyclophosphamide, and rituximab.53,61-63 Zou et al.28 reported that various combinations of immunosuppressive therapy produced improvement in virtually all patients. However, this is not a common experience by others. Evoli et al.15 reported refractory disease (repeated exacerbation on high-dose immunosuppressive agents) in 23% of 57 cases. In 20% of cases, no change or minimal improvement was noted in the present series. Though Sanders60 reported the best improvement results (89%) with mycophenolate mofetil among immunosuppressive (IS) agents, the general consensus is that the best improvement was achieved with high-dose prednisone.14,15,53 The response rates to various immunomodulatory therapies from four U.S.-based series are summarized in Table 7. In refractory cases, highdose cyclophosphamide (50 mg/kg daily intravenously for four days) has been used safely and effectively with no symptom recurrence for 1.5 to 3.5 years.61,63 Similarly, rituximab was effective and well-tolerated in a patient who had been refractory, with disease stabilization for 12 months after initiation.62 The present study showed improvement (excellent or good) in 53% of patients with steroid and in 50% of patients with other immunosuppressive agents.
Table 8.
Improvement rate to immunotherapy in MuSK-MG
IVIG: Intravenous immunoglobulin, MuSK-MG: muscle-specific tyrosine kinase antibody positive-MG.
Plasma exchange
In uniform fashion across studies, plasma exchange has had a favorable effect on MuSK-MG, usually with dramatic improvement15,53 and showing at least transient benefit in patients refractory to other interventions15 In three U.S. series, the response rates to plasma exchange ranged from 51% to 91% (Table 8). In the present series, plasma exchange (PE) brought improvement in 50% of cases.
Intravenous immunoglobulin
There seems to be some disagreement concerning the response rate to intravenous immunoglobulin (IVIG) in MuSK-MG patients. Evoli15 reported that MuSK-MG patients responded well to PE and IVIG. No detailed data were available in the report. According to four U.S. reports, IVIG appears to be less effective in patients with MuSK-MG than other immunomodulatory therapies, showing a favorable response in 19% and 44% of patients (Table 8)18,45,53 In Wolfe's series, IVIG was more effective in AChR-MG than in MuSK-MG.53 Pasnoor et al.45 reported an improvement in 5 (25%) of 25 treated cases. Still, two Japanese women dependent on plasma exchange who were unresponsive to thymectomy, corticosteroids, and tacrolimus demonstrated both clinical and electrophysiological improvement three days after initiation of IV-IG.64 The present study showed IVIG effectiveness in one of 9 treated patients, confirming the U.S. experiences.
Prognosis
Though MuSK-MG is hard to treat, the general impression is that the outcome of MuSK-MG patients is on a par with that for other MG populations and that response to immunosuppression is similar.28,39,45 However, the maintenance dose of corticosteroids in one series was significantly higher in MuSK-MG patients (30 mg/48 hours) than in AChR-MG (18 mg for 2 days) or DSN-MG patients (10 mg for 2 days).39
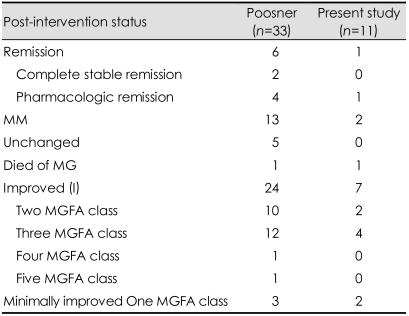
When comparing therapeutic results in MuSK-MG and AChR-MG in meta-analysis, Evoli found significant differences in remission rates.15 In patients with MuSK-MG, the percentage of remission ranged from 10 to 35% (mean, 22%) and was significantly lower than that reported in AChR-MG (24-58%, mean, 38%)(p=0.005). In the same study, the mean rates of complete stable remission (CSR) were 7.5% in MuSK-MG and 16% in AChR-MG (p=0.01). Pasnoor45 reported remission in six (18%) of 33 patients who were followed longer than three years: CSR in two and pharma cological remission (PR) in four (Table 9). The present study did show a significantly higher remission rate (55%) in AChR-MG than in MuSK-MG (27%). However, there was no significant difference between the response in MuSK-MG versus DSN-MG groups.
Table 9.
Outcome of long-term (<3 years' follow-up) outcome
In a preliminary report, a higher percentage of MuSK-MG than AChR-MG patients were resistant to immunosuppressive medication.65 Furthermore, poor MGFA post-intervention status (unchanged or worse) was observed in 22% of patients who were MuSK-Ab-positive, a proportion that was 1.5 to 2 times higher than other populations, but this did not reach statistical significance.39 With a mean follow-up period of eight years, Wolfe's studies demonstrated a poor post-intervention status in four patients (19%), and the present study, a poor post-intervention status in two (20%) cases.
An unstable clinical course in the first few years after onset with periodic cranial, bulbar, respiratory, and limb exacerbations requiring plasma exchange was common, observed in approximately 30% of patients.29 Nevertheless, with aggressive therapy most MuSK-MG patients ultimately fare well. In larger series, including our own, at least three-fourths of patients are classified as improved, with minimal manifestations, or as in remission on post-intervention status.45 However, remission rate is low and permanent weakness with some facial and bulbar muscles was evident in 30% of cases.15
References
- 1.Simpson JA. Myasthenia gravis: a new hypothesis. Scott Med J. 1960;5:419–436. doi: 10.1177/003693307702200305. [DOI] [PubMed] [Google Scholar]
- 2.Lindstrom JM, Seybold ME, Lennon VA, Whittingham S, Duane DD. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology. 1976;26:1054–1059. doi: 10.1212/wnl.26.11.1054. [DOI] [PubMed] [Google Scholar]
- 3.Hoch W, McConville J, Helms S, Newsom-Davis J, Melms A, Vincent A. Auto-antibodies to the receptor tyrosine kinsase MuSK in patients with myasthenia gravis, without acetylcholine receptor antibodies. Nat Med. 2001;7:365–368. doi: 10.1038/85520. [DOI] [PubMed] [Google Scholar]
- 4.Soliven BC, Lange DJ, Penn AS, Younger D, Jeretzki A, 3rd, Lovelace RE, et al. Seronegative maysthenia gravis. Neurology. 1988;38:514–517. doi: 10.1212/wnl.38.4.514. [DOI] [PubMed] [Google Scholar]
- 5.Oh SJ. Electrophysiological characteristics in seronegative myasthenia gravis. Ann N Y Acad Sci. 1993;681:584–587. doi: 10.1111/j.1749-6632.1993.tb22948.x. [DOI] [PubMed] [Google Scholar]
- 6.Sanders DB, Howard JF, Massey JM, Mihovilovic M, Ollanow CW. Seronegative myasthenia gravis. Ann Neurol. 1987;22:126. [Google Scholar]
- 7.Mossman S, Vincent A, Newsom-Davis J. Myasthenia gravis without acetylcholine-receptor antibody: a distinct disease entity. Lancet. 1986;1:116–119. doi: 10.1016/s0140-6736(86)92259-2. [DOI] [PubMed] [Google Scholar]
- 8.Selcen D, Fukuda T, Shen XM, Engel AG. Are MuSK antibodies the primary cause of myasthenic symptoms? Neurology. 2004;62:1945–1950. doi: 10.1212/01.wnl.0000128048.23930.1d. [DOI] [PubMed] [Google Scholar]
- 9.Shiraishi H, Motomura M, Yoshimura T, Fukudome T, Fukuda T, Nakao Y, et al. Acetylcholine receptors loss and postsynptic damage in MuSK antibody-positive myasthenia gravis. Ann Neurol. 2005;57:289–293. doi: 10.1002/ana.20341. [DOI] [PubMed] [Google Scholar]
- 10.Jha S, Xu K, Maruta T, Oshima M, Mosier DR, Atassi MZ, et al. Myasthenia gravis induced in mice by immunization with the recombinanat extracellular domain of rat muscle-specific kinase (MuSK) J Neuroimmunol. 2006;175:107–117. doi: 10.1016/j.jneuroim.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Shigemoto K, Kubo S, Maryyama N, Hato N, Yamada H, Jie C, et al. Induction of myasthenia by immunization against muscle-specific kinase. J Clin Invest. 2006;116:1016–1024. doi: 10.1172/JCI21545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole RN, Reddel SW, Gervásio OL, Phillips WD. Anti-MuSK patient antibodies disrupt the mouse neuromuscular junction. Ann Neurol. 2008;63:782–789. doi: 10.1002/ana.21371. [DOI] [PubMed] [Google Scholar]
- 13.Saka E, Topcuoglu MA, Akkaya B, Galati A, Onal MZ, Vincent A. Thymus changes in anti-MuSK-positive and- negative myasthenia gravis. Neurology. 2005;65:782–783. doi: 10.1212/wnl.65.5.782. [DOI] [PubMed] [Google Scholar]
- 14.Lavrnic D, Losen M, Vujic A, De Baets M, Hajdukovic LJ, Stojanovic V, et al. The features of myasthenia gravis with autoantibodies to MuSK. J Neurol Neurosurg Psychiatry. 2005;76:1099–1102. doi: 10.1136/jnnp.2004.052415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evoli A, Bianchi MR, Riso R, Minicuci GM, Batocchi AP, Servidei S, et al. Response to therapy in myasthenia gravis with anti-MuSK antibodies. Ann N Y Acad Sci. 2008;1132:76–83. doi: 10.1196/annals.1405.012. [DOI] [PubMed] [Google Scholar]
- 16.Lauriola L, Ranelletti F, Maggiano N, Guerriero M, Punzi C, Marsili F, et al. Thymius changes in anti-MuSK-positive and -negative myasthenia gravis. Neurology. 2005;64:536–538. doi: 10.1212/01.WNL.0000150587.71497.B6. [DOI] [PubMed] [Google Scholar]
- 17.Leite MI, Strobel P, Jones M, Micklem K, Moritz R, Gold R, et al. Fewer thymic changes in MuSK antibody-positive than in MuSK antibody-negative MG. Ann Neurol. 2005;57:444–448. doi: 10.1002/ana.20386. [DOI] [PubMed] [Google Scholar]
- 18.Sanders DB, El-Salem K, Massey JM, McConville J, Vincent A. Clinical aspects of MuSK antibody positive seronegative MG. Neurology. 2003;60:1978–1980. doi: 10.1212/01.wnl.0000065882.63904.53. [DOI] [PubMed] [Google Scholar]
- 19.Padua V, Tenali P, Aprile I, Caliandro P, Bartoccioni E, Evoli A. Seronegative myasthenia gravis: comparison of neurophysiological picture in MuSK+ and MuSK- patients. Eur J Neurol. 2006;13:273–276. doi: 10.1111/j.1468-1331.2006.01214.x. [DOI] [PubMed] [Google Scholar]
- 20.Martignago S, Fanin M, Albertini E, Pegoraro E, Angelini C. Muscle histopathology in myasthenia gravis with antibodies against MuSK and AChR. Neuropathol and Appl Neurobiol. 2009;35:103–110. doi: 10.1111/j.1365-2990.2008.00965.x. [DOI] [PubMed] [Google Scholar]
- 21.Farrugia ME, Robson MD, Clover L, Anslow P, Newsom-Davis J, Kennett R, et al. MRI and clinical studies of facial and bulbar involvement in MuSK antobody-associated myasthenia gravis. Brain. 2006;129:1481–1492. doi: 10.1093/brain/awl095. [DOI] [PubMed] [Google Scholar]
- 22.Shirashi H, Motomura M, Yoshimura T, Fukudome T, Fukuda T, Nakao Y, et al. Acetylcholine receptors loss and postsynaptic damage in MuSK antibody positrive myasthenia gravis. Ann Neurol. 2005;57:289–293. doi: 10.1002/ana.20341. [DOI] [PubMed] [Google Scholar]
- 23.McConville J, Farrugia ME, Beeson D, Kishore U, Metcalfe R, Newsom-Davis J, et al. Detection and characterization of MuSK antibodies in seronegative myasthenia gravis. Ann Neurol. 2004;55:580–584. doi: 10.1002/ana.20061. [DOI] [PubMed] [Google Scholar]
- 24.Caress JB, Hunt CH, Batish SD. Anti-MuSK myasthenia gravis presenting with purely ocular findings. Arch Neurol. 2005;62:1002–1003. doi: 10.1001/archneur.62.6.1002. [DOI] [PubMed] [Google Scholar]
- 25.Hanisch E, Eger K, Zierz S. MuSk-antibody positive pure ocular myasthenia gravis. J Neurol. 2006;253:659–660. doi: 10.1007/s00415-005-0032-8. [DOI] [PubMed] [Google Scholar]
- 26.Chan JW, Orrison WW. Ocular myathenia: a rare presentation with MuSK antibody and bilateral extraocular muscle atrophy. Br J Ophthalmol. 2007;91:842–843. doi: 10.1136/bjo.2006.108498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kupersmith MJ, Latkany R, Homel P. Developmen of generalized disease at 2 years in patients with ocular myasthenia gravis. Arch Neurol. 2003;60:243–248. doi: 10.1001/archneur.60.2.243. [DOI] [PubMed] [Google Scholar]
- 28.Zhou L, McConville J, Chaudhry V, Adams RN, Skolasky RL, Vincent A, et al. Clinical comparison of muscle-specific tyrosine kinase (MuSK) antibody-positive and -negative myasthenic patients. Muscle Nerve. 2004;30:55–60. doi: 10.1002/mus.20069. [DOI] [PubMed] [Google Scholar]
- 29.Evoli A, Tonali PA, Padua L, Monaco ML, Scuderi F, Batocchi AP, et al. Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain. 2003;126:2304–2311. doi: 10.1093/brain/awg223. [DOI] [PubMed] [Google Scholar]
- 30.Lee JY, Sung JJ, Cho JY, Oh DH, Kim HJ, Park JH, et al. MusK antibody-positive seronegative myasthenia gravis in Korea. J Clin Neurosci. 2006;13:353–355. doi: 10.1016/j.jocn.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 31.NiKs EH, Kuks JB, Verschuuren JJ. Epidemiology of myathenia gravis with anti-muscle specific kinase antibodies in The Netherlands. J Neurol Neurosurg Psychiatry. 2007;78:417–418. doi: 10.1136/jnnp.2006.102517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nemoto Y, Kuwabara S, Misawa S, Kawaguchi N, Hattori T, Takamori M, et al. Patterns and severity of neuromuscular transmission failure in seronegative myasthenia gravis. J Neurol Neurosurg Psychiatry. 2005;76:714–718. doi: 10.1136/jnnp.2004.043125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohta K, Shigemoto K, Fujinami A, Maruyama N, Konishi T, Ohta M. Clinical and experimental features of MuSK antibody positive MG in Japan. Eur J Neurol. 2007;14:1029–1034. doi: 10.1111/j.1468-1331.2007.01870.x. [DOI] [PubMed] [Google Scholar]
- 34.Kostera-Pruszczyk A, Kamińska A, Dutkiewicz M, Emeryk-Szajewska B, Strugalska-Cynowska MH, Vincent A, et al. MuSK-positive myasthenia gravis is rare in the Polish population. Eur J Neurol. 2008;15:720–724. doi: 10.1111/j.1468-1331.2008.02176.x. [DOI] [PubMed] [Google Scholar]
- 35.Ohta K, Shigemoto K, Kubo S, Maruyama N, Abe Y, Ueda N, et al. MuSk antibodies in AChR Ab-seropositive MG vs AChR Ab-seronegative MG. Neurology. 2004;62:2132–2133. doi: 10.1212/01.wnl.0000129274.12702.92. [DOI] [PubMed] [Google Scholar]
- 36.Ohta K, Shigemoto K, Kubo S, Maruyama N, Abe Y, Ueda N, et al. MuSK Ab described in seropositive MG sera found to be Ab to alkaline phosphatase. Neurology. 2004;65:1988. doi: 10.1212/01.wnl.0000188881.46043.44. [DOI] [PubMed] [Google Scholar]
- 37.Rostedt Punga A, Ahlqvist K, Bartoccioni E, Scuderi F, Marino M, Suomalainen A, et al. Neurophysiological and mitochondrial abnormalities in MuSK antibody seropositive myasthenia gravis compared to other immunological subtypes. Clin Neurophysiol. 2006;117:1434–1443. doi: 10.1016/j.clinph.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 38.Romi F, Aarli JA, Gilhus NE. Seronegative myasthenia gravis: disease severity and prognosis. Eur J Neurol. 2005;12:413–418. doi: 10.1111/j.1468-1331.2005.01137.x. [DOI] [PubMed] [Google Scholar]
- 39.Deymeer F, Gungor-Tuncer O, Yilmaz V, Parman Y, Serdaroglu P, Ozdemir C, et al. Clinical comparison of anti-MuSK- vs antiAChR-positive and seronegative myasthenia gravis. Neurology. 2007;68:609–611. doi: 10.1212/01.wnl.0000254620.45529.97. [DOI] [PubMed] [Google Scholar]
- 40.Wolfe GI, Oh SJ. Clinical phenotype of muscle-specific tyrosine kinase-antibody-positive myasthenia gravis. Ann N Y Acad Sci. 2008;1132:71–75. doi: 10.1196/annals.1405.005. [DOI] [PubMed] [Google Scholar]
- 41.Oh SJ, Morgan MB, Lu L, Hatanaka Y, Hemmi S, Young A, et al. Racial differences in myasthenia gravis in Alabama. Muscle Nerve. 2009;39:328–332. doi: 10.1002/mus.21191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burns T, Wolfe GI, Nations S, Trivedi J, Phillips LT, Phillips L, et al. Variable MuSK antibody positive rates among seronegative MG in the United States (Abstract) Neurology. 2008;70(suppl 1):A302. [Google Scholar]
- 43.Stickler DE, Massey JM, Sanders DB. MuSK-antibody positive myasthenia gravis: clinical and electrodiagnostic patterns. Clin Neurophysiol. 2005;116:2065–2068. doi: 10.1016/j.clinph.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Bartoccioni E, Scuderi F, Minicuci GM, Mario M, Ciarffa F, Evoli A. Anti-MuSK antibodies: correlation with myasthenia gravis severity. Neurology. 2006;67:505–507. doi: 10.1212/01.wnl.0000228225.23349.5d. [DOI] [PubMed] [Google Scholar]
- 45.Pasnoor M, Wolfe G, Nations S, Trivedi J, Barohn RJ, Hervbelin L, et al. Clinical findings in MuSK-antibody positive myasthenia gravis. A U.S. Experience. Muscle Nerve. 2009 doi: 10.1002/mus.21533. (In Press) [DOI] [PubMed] [Google Scholar]
- 46.Spengos K, Vassilopoulou S, Papadimas G, Tsivqoulis G, Karandreas N, Zambelis T, et al. Dropped head syndrome as prominent clinical feature in MuSK-positive myathenia gravis with thymus hyperplasia. Neuromuscul Disord. 2008;18:175–177. doi: 10.1016/j.nmd.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 47.Béhin A, Mayer M, Kassis-Makhoul B, Jugie M, Espil-Taris C, Ferrer X, et al. Severe neonatal myasthenia due to materanal anti-MuSK antibodeies. Neuromuscul Disord. 2008;18:443–446. doi: 10.1016/j.nmd.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Niks EH, Verrips A, Semmekrot BA, Prick MJ, Vincent A, van Tol MJ, et al. A transient neonatal myasthenic syndrome with anti-musk antibodies. Neurology. 2008;70:1215–1216. doi: 10.1212/01.wnl.0000307751.20968.f1. [DOI] [PubMed] [Google Scholar]
- 49.Sylva M, van der Kooi AJ, Grolman W. Dyspnoea due to vocal fold abduiction paresis in anti-MuSK myasthenia gravis. J Neurol Neurosurg Psychiatry. 2008;79:1083–1084. doi: 10.1136/jnnp.2007.135319. [DOI] [PubMed] [Google Scholar]
- 50.Hara K, Mashima T, Matsuda A, Tanaka K, Tomita M, Shiraishi H, et al. Vocal cord paralysis in myasthenia gravis with anti-MuSK antibodies. Neurology. 2007;68:621–622. doi: 10.1212/01.wnl.0000254617.15644.f4. [DOI] [PubMed] [Google Scholar]
- 51.Díaz-Manera J, Rojas-García R, Gallardo E, Juárez C, Martínez-Domeño A, Martínez-ramírez S, et al. Antibodies to AChR, Musk and VGKC in a patient with myasthenia gravis and Morvan's syndrome. Nat Clin Pract Neurol. 2007;3:405–410. doi: 10.1038/ncpneuro0526. [DOI] [PubMed] [Google Scholar]
- 52.Hatanaka Y, Hemmi A, Morgan MB, Scheufele ML, Claussen GC, Wolfe GI, et al. Nonresponsiveness to anticholinesterase agents in patients with MuSK-antibody-positive MG. Neurology. 2005;65:1508–1509. doi: 10.1212/01.wnl.0000183145.91579.74. [DOI] [PubMed] [Google Scholar]
- 53.Wolfe GI, Trivedi JR, Oh SJ. Clinical review of Muscle-Specific Tyrosine Kinase-Antibody positive myasthenia gravis. Jr Clin Neuromuscular Dis. 2007;8:217–224. [Google Scholar]
- 54.Punga AR, Flink R, Askmark H, Stålberg EV. Cholinergic neuromuscular hyperactivity in patients with myasthenia gravis seropositive for MuSK antibody. Muscle Nerve. 2006;34:111–115. doi: 10.1002/mus.20515. [DOI] [PubMed] [Google Scholar]
- 55.Oh SJ, Hatanaká Y, Hemmi S, Young AM, Scheufele ML, Nations SP, et al. Repetitive nerve stimulation of facial muscles in MuSK antibody positive myasthenia gravis. Muscle Nerve. 2006;33:500–504. doi: 10.1002/mus.20498. [DOI] [PubMed] [Google Scholar]
- 56.Kuwarbara S, Nemoto Y, Misawa S, Takahashi H, Kawaguchi N, Hattori T. Anti-MuSK positive myasthenia gravis: neuromuscular transmission failure in facial and limb muscles. Acta Neurol Scand. 2007;115:126–128. doi: 10.1111/j.1600-0404.2006.00721.x. [DOI] [PubMed] [Google Scholar]
- 57.Farrugia ME, Kennett RP, Newsom-Davis J, Hilton-Jones D, Vincent A. Single-fiber electromyography in limb and facial muscles in muscle-specific kinase antibody and acetylcholine receptor antibody myasthenia gravis. Muscle Nerve. 2006;33:568–570. doi: 10.1002/mus.20491. [DOI] [PubMed] [Google Scholar]
- 58.Farrugia ME, Kennett RP, Hilton-Jones D, Newsom-Davis J, Vincent A. Quantitative EMG of facial muscles in myasthenia patients with MuSK antibodies. Clin Neurophysiol. 2007;118:269–277. doi: 10.1016/j.clinph.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 59.Odabasi Z, Kuruoglu R, Oh SJ. Turns-ampliutude analysis and motor unit potential analysis in myasthenia gravis. Acta Neurol Scand. 2000;101:315–320. doi: 10.1034/j.1600-0404.2000.90303.x. [DOI] [PubMed] [Google Scholar]
- 60.Sanders D, Massey J, Juel V. Muscle antibody positive myasthenia gravis: response to treatment in 31 patients. Neurology (asbtract) 2007;68:A299. [Google Scholar]
- 61.Drachman DB, Jones RJ, Brodsky RA. Treatment of refractory myasthenia: "rebooting" with high-dose cyclophosphamide. Ann Neurol. 2003;5:29–34. doi: 10.1002/ana.10400. [DOI] [PubMed] [Google Scholar]
- 62.Hain B, Jordan K, Deschauer M, Zierz S. Successful treatment of MuSK antibody-positive myasthenia gravis with rituximab. Muscle Nerve. 2006;33:575–580. doi: 10.1002/mus.20479. [DOI] [PubMed] [Google Scholar]
- 63.Hain B, Jordan K, Deschauer M, Zierz S. Successful treatment of MuSK antibody-positive myasthenia gravis with rituximab. Muscle Nerve. 2006;33:575–580. doi: 10.1002/mus.20479. [DOI] [PubMed] [Google Scholar]
- 64.Takahashi H, Kawaguchi N, Nemoto Y, Hattori T. High-dose intravenous immunoglobulin for the treatment of MuSK antibody-positive seronegative myasthenia gravis. J Neurol Sci. 2006;247:239–241. doi: 10.1016/j.jns.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 65.Diaz-Manera J, Rajas-Garcia R, Juarez C, Pradas J, Gallardo E, Illa I. Are immunosuppressors as effective in MuSK+ MG as in AChR+ MG patients? Evaluation of 150 myashenic patients treated with the same protocol (abstract) Neurology. 2007;68:A300. [Google Scholar]