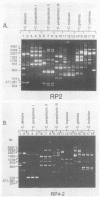
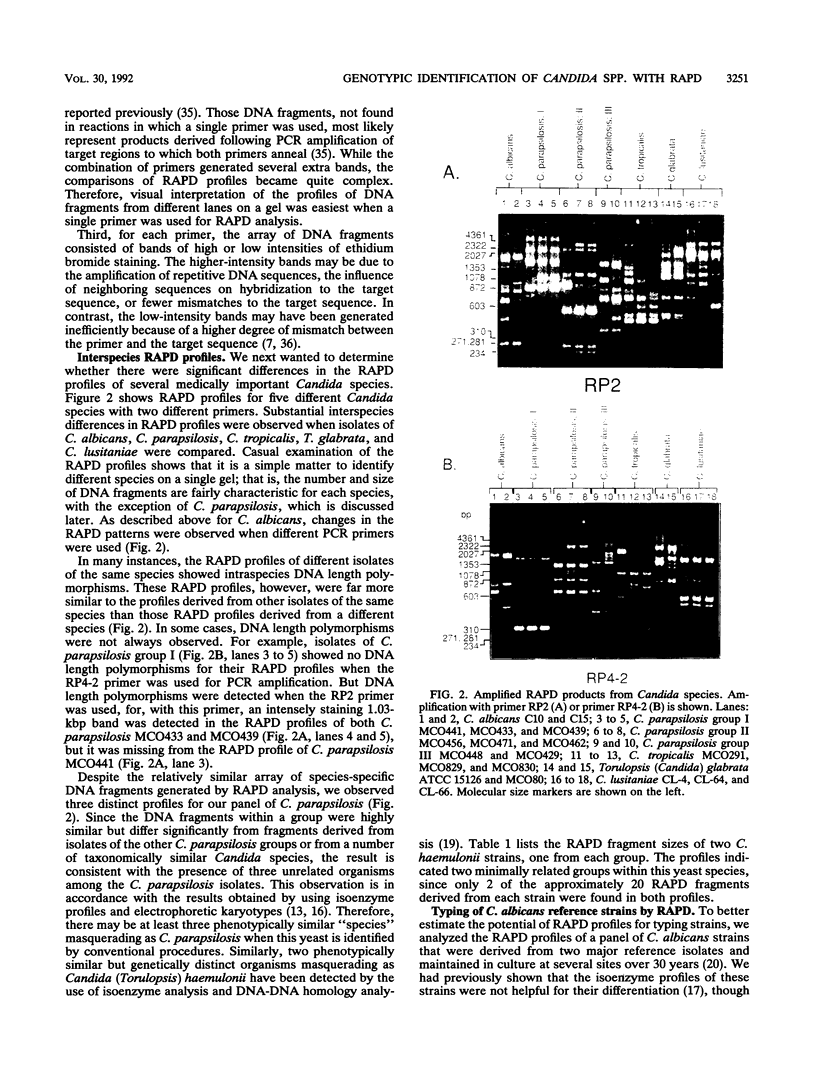
Abstract
Random amplified polymorphic DNA (RAPD) was used to better characterize the genotypic relatedness among medically important Candida species. By using short oligomer primers (10-mers) with arbitrarily chosen sequences in the polymerase chain reaction, distinctive and reproducible sets of polymerase chain reaction products were observed for isolates of C. albicans, C. lusitaniae, C. tropicalis, and Torulopsis (Candida) glabrata. The RAPD analysis differentiated a physiologically homogeneous panel of C. parapsilosis into three distinct groups and showed genetic diversity within C. haemulonii. Intraspecies DNA-length polymorphisms were seen for RAPD profiles derived from different isolates of each species. Analysis of RAPDs from a panel of C. albicans, which included 16 laboratory derivatives of two reference strains, showed that the profiles of unrelated strains differed and that the derivatives of each reference strain were identifiable. Minor differences in the RAPD profiles, suggestive of mutations that had occurred during the long-term maintenance of the strains, were detected. Because of its ease and reliability, RAPD analysis should be useful in providing genotypic characters for taxonomic descriptions, for confirming the identities of stock isolates, for typing Candida species in epidemiologic investigations, and for use in the rapid identification of pathogenic fungi.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee S. N., Emori T. G., Culver D. H., Gaynes R. P., Jarvis W. R., Horan T., Edwards J. R., Tolson J., Henderson T., Martone W. J. Secular trends in nosocomial primary bloodstream infections in the United States, 1980-1989. National Nosocomial Infections Surveillance System. Am J Med. 1991 Sep 16;91(3B):86S–89S. doi: 10.1016/0002-9343(91)90349-3. [DOI] [PubMed] [Google Scholar]
- Berger S. A. Lack of precision in commercial identification systems: correction using Bayesian analysis. J Appl Bacteriol. 1990 Mar;68(3):285–288. doi: 10.1111/j.1365-2672.1990.tb02576.x. [DOI] [PubMed] [Google Scholar]
- Crowhurst R. N., Hawthorne B. T., Rikkerink E. H., Templeton M. D. Differentiation of Fusarium solani f. sp. cucurbitae races 1 and 2 by random amplification of polymorphic DNA. Curr Genet. 1991 Nov;20(5):391–396. doi: 10.1007/BF00317067. [DOI] [PubMed] [Google Scholar]
- Doebbeling B. N., Hollis R. J., Isenberg H. D., Wenzel R. P., Pfaller M. A. Restriction fragment analysis of a Candida tropicalis outbreak of sternal wound infections. J Clin Microbiol. 1991 Jun;29(6):1268–1270. doi: 10.1128/jcm.29.6.1268-1270.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin P. H., Annis S. L. Rapid identification of genetic variation and pathotype of Leptosphaeria maculans by random amplified polymorphic DNA assay. Appl Environ Microbiol. 1991 Sep;57(9):2482–2486. doi: 10.1128/aem.57.9.2482-2486.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter P. R., Fraser C. A. Application of a numerical index of discriminatory power to a comparison of four physiochemical typing methods for Candida albicans. J Clin Microbiol. 1989 Oct;27(10):2156–2160. doi: 10.1128/jcm.27.10.2156-2160.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg H. D., Tucci V., Cintron F., Singer C., Weinstein G. S., Tyras D. H. Single-source outbreak of Candida tropicalis complicating coronary bypass surgery. J Clin Microbiol. 1989 Nov;27(11):2426–2428. doi: 10.1128/jcm.27.11.2426-2428.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E. D., Dong M. W. Rapid analysis and purification of polymerase chain reaction products by high-performance liquid chromatography. Biotechniques. 1990 May;8(5):546–555. [PubMed] [Google Scholar]
- Kemker B. J., Lehmann P. F., Lee J. W., Walsh T. J. Distinction of deep versus superficial clinical and nonclinical isolates of Trichosporon beigelii by isoenzymes and restriction fragment length polymorphisms of rDNA generated by polymerase chain reaction. J Clin Microbiol. 1991 Aug;29(8):1677–1683. doi: 10.1128/jcm.29.8.1677-1683.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann P. F., Hsiao C. B., Salkin I. F. Protein and enzyme electrophoresis profiles of selected Candida species. J Clin Microbiol. 1989 Mar;27(3):400–404. doi: 10.1128/jcm.27.3.400-404.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann P. F., Wu L. C., Mackenzie D. W. Isoenzyme changes in Candida albicans during domestication. J Clin Microbiol. 1991 Nov;29(11):2623–2625. doi: 10.1128/jcm.29.11.2623-2625.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie D. W., Odds F. C. Non-identity and authentication of two major reference strains of Candida albicans. J Med Vet Mycol. 1991;29(4):255–261. doi: 10.1080/02681219180000371. [DOI] [PubMed] [Google Scholar]
- Magee B. B., D'Souza T. M., Magee P. T. Strain and species identification by restriction fragment length polymorphisms in the ribosomal DNA repeat of Candida species. J Bacteriol. 1987 Apr;169(4):1639–1643. doi: 10.1128/jb.169.4.1639-1643.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason M. M., Lasker B. A., Riggsby W. S. Molecular probe for identification of medically important Candida species and Torulopsis glabrata. J Clin Microbiol. 1987 Mar;25(3):563–566. doi: 10.1128/jcm.25.3.563-566.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz W. G., Khazan U., Jabra-Rizk M. A., Wu L. C., Osterhout G. J., Lehmann P. F. Strain delineation and epidemiology of Candida (Clavispora) lusitaniae. J Clin Microbiol. 1992 Feb;30(2):449–454. doi: 10.1128/jcm.30.2.449-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer W., Koch A., Niemann C., Beyermann B., Epplen J. T., Börner T. Differentiation of species and strains among filamentous fungi by DNA fingerprinting. Curr Genet. 1991 Mar;19(3):239–242. doi: 10.1007/BF00336493. [DOI] [PubMed] [Google Scholar]
- Schaberg D. R., Culver D. H., Gaynes R. P. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991 Sep 16;91(3B):72S–75S. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- Scherer S., Stevens D. A. Application of DNA typing methods to epidemiology and taxonomy of Candida species. J Clin Microbiol. 1987 Apr;25(4):675–679. doi: 10.1128/jcm.25.4.675-679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N. D., Biernat J., McManus J., Köster H. Polymer support oligonucleotide synthesis XVIII: use of beta-cyanoethyl-N,N-dialkylamino-/N-morpholino phosphoramidite of deoxynucleosides for the synthesis of DNA fragments simplifying deprotection and isolation of the final product. Nucleic Acids Res. 1984 Jun 11;12(11):4539–4557. doi: 10.1093/nar/12.11.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J., McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990 Dec 25;18(24):7213–7218. doi: 10.1093/nar/18.24.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J., McClelland M. Genomic fingerprinting using arbitrarily primed PCR and a matrix of pairwise combinations of primers. Nucleic Acids Res. 1991 Oct 11;19(19):5275–5279. doi: 10.1093/nar/19.19.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J., McClelland M. Genomic fingerprints produced by PCR with consensus tRNA gene primers. Nucleic Acids Res. 1991 Feb 25;19(4):861–866. doi: 10.1093/nar/19.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Kubelik A. R., Livak K. J., Rafalski J. A., Tingey S. V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990 Nov 25;18(22):6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]