Abstract
Accumulating evidence suggests that top-down processes, reflected by frontal-midline theta-band (4-8 Hz) electroencephalogram (EEG) oscillations, strengthen the activation of a memory set during short-term memory (STM) retention. In addition, the amplitude of posterior alpha-band (8-13 Hz) oscillations during STM retention is thought to reflect a mechanism that protects fragile STM activations from interference by gating bottom-up sensory inputs. The present study addressed two important questions about these phenomena. First, why have previous studies not consistently found memory set-size effects on frontal-midline theta? Second, how does posterior alpha participate in STM retention? To answer these questions, the present study examined large-scale network connectivity during STM retention by computing EEG wavelet coherence during the retention period of a modified Sternberg task using visually-presented letters as stimuli. The results showed (a) increasing theta-band coherence between frontal-midline and left temporal-parietal sites with increasing memory load, and (b) increasing alpha-band coherence between midline parietal and left temporal/parietal sites with increasing memory load. These findings support the view that theta-band coherence, rather than amplitude, is the key factor in selective top-down strengthening of the memory set and demonstrate that posterior alpha-band oscillations associated with sensory gating are involved in STM retention by participating in the STM network.
Keywords: EEG, coherence, short-term memory, working memory, alpha oscillations, theta oscillations, sensory gating
1. Introduction
Short-term memory (STM) is the temporary retention of information that is no longer available in the environment. A central question of STM research is how this information is retained once the perceptual inputs are no longer present. One developing view is that STM retention consists of the temporary activation of long-term memory (LTM) representations (Jonides et al., 2008). According to the neural instantiation of this view, frontal cortex selectively activates LTM representations in posterior cortex (Jonides et al., 2008; Ruchkin et al., 2003) and that STM is, in effect, an emergent property of such frontal-posterior interactions (Postle, 2006; for a related view, see Yoon et al., 2006). An increase in the magnitude of theta-band (4-8 Hz) oscillations measured over frontal-midline cortex (Sauseng et al., 2007) and increased frontal-posterior theta coherence (Sarnthein et al., 1998) are thought to reflect the top-down processes which serve to strengthen transiently-activated LTM representations in the face of interference or decay.
If STM consists of the transient activation of LTM representations, and if such LTM representations can also be activated by perceptual inputs, then insulating these representations from bottom-up inputs should be an important feature of STM retention (Postle, 2005). One manifestation of such sensory gating (i.e., reduction of sensory inputs) is believed to be the phenomenon of load-related increases in the amplitude of alpha-band (8-13 Hz) oscillations measured over posterior cortex (Jensen et al., 2002; Klimesch et al., 1999; Schack and Klimesch, 2002). Studies comparing EEG power and hemodynamic measures of neural activity have found alpha to be inversely correlated with neural activity (e.g., Kounios et al., 2006; Laufs et al., 2003), thus supporting the notion that alpha reflects an inhibitory process that, in this case, is understood to reflect the gating of visual inputs (Jung-Beeman et al., 2004; Worden et al., 2000). Recent studies have localized the STM load effect on alpha to the parieto-occipital sulcus, an area associated with visual attention rather than STM retention or other non-perceptual processes, thereby supporting the sensory-gating hypothesis (Tuladhar et al., 2007; van Dijk et al., 2008).
The two processes of top-down activation reflected by frontal-midline theta and sensory gating reflected by posterior alpha hypothetically represent complimentary mechanisms supporting STM retention by increasing the signal-to-noise ratio of the STM retention set. While this view is consistent with a number of previous empirical findings, at least two important questions remain.
First, why are increases in STM load not always accompanied by increases in the magnitude of frontal-midline theta during the retention period? Several studies have shown evidence of load effects on frontal-midline theta during retention (Jensen and Tesche, 2002; Michels et al., 2008); however, not all studies have yielded the same result (Jensen et al., 2002; Raghavachari et al., 2001).
Second, how is the posterior-alpha sensory-gating mechanism controlled? If posterior alpha increases with STM load, then some mechanism must detect load increases (or some other index related to the fragility of STM activations, such as increasing noise) and communicate the need to gate potentially interfering perceptual inputs. A recent fMRI study using interfering stimuli during STM retention suggests that dorsolateral prefrontal cortex (PFC) may play a role in the top-down control of sensory gating (Postle, 2005).
Most EEG studies of short-term memory have focused on EEG power, which is a function of both the number of neurons participating in the relevant neuronal assembly and the synchrony of their individual oscillations. Less studied are the interactions among the neurons in an assembly. The present study addresses the two questions mentioned above by examining the large-scale neuronal networks associated with frontal-midline theta and posterior alpha during STM retention.
EEG coherence, the frequency-specific correlation between oscillations at spatially-separated electrodes, enables the characterization and quantification of (direct or indirect) communication between brain regions (Ruchkin, 2005). The application of a time-frequency technique such as the wavelet transform allows the coherence of non-stationary signals to be computed making the detection of temporal covariance possible (Lachaux, 2002; Saab et al., 2005). Computing wavelet coherence therefore enables the elucidation of frequency-dependent, large-scale, transient, cortical integration between brain regions.
This report describes new wavelet coherence analyses of posterior alpha and frontal-midline theta oscillations from an experiment originally reported by Jensen et al. (2002). The experiment used a modified Sternberg task with memory sets of 2, 4, and 6 letters in order to examine the effect of load on the STM network while other task requirements were held constant. As previously reported, posterior alpha was the dominant rhythm recorded in his experiment; the amplitude of these oscillations increased parametrically with memory load. In contrast, there were no systematic load effects on the magnitude of theta or other frequency bands (for details of the procedure and the original analyses, see Jensen et al., 2002). The goal of the current analyses was to elucidate patterns of connectivity involving the frontal theta network thought to reflect top-down activation and the posterior alpha response thought to reflect the sensory gating.
2. Results
As the parietal midline site (Pz) demonstrated the most salient alpha magnitude effect in this experiment (Jensen et al., 2002) and the frontal-midline site (Fz) is a known locus of frontal-midline theta during STM and other tasks, the coherent relationships of all other electrodes to electrodes Pz and Fz were calculated for the theta (4.0 - 7. 5 Hz) and alpha (8.0 - 13 Hz) frequency bands, respectively, across the 2.8 s retention period. Visual inspection of the coherence subtraction wavelets revealed no load effects in other frequency bands. Mean EEG coherence values for each subject were converted to z-scores (across electrodes, separately for each frequency band) prior to subjecting them to statistical analysis. Pz and Fz coherence data were analyzed separately by five-way ANOVAs with factors FREQUENCY (theta, alpha), LOAD (2, 4, and 6 letters), ANTERIOR-POSTERIOR (4 levels), HEMISPHERE (left, right), and DORSAL-VENTRAL (2 levels). The 16-electrode array used for both ANOVAs included: F3/4, F7/8, C3/4, T7/8, P3/4, P7/8, P9/10, and PI1/2. Greenhouse-Geisser corrected values are reported where appropriate.
To elucidate the specific effects of increasing set size (S) on the spatial patterns of coherence, follow-up two-tailed, paired-sample t-tests comparing conditions S2 with S4, S4 with S6, and S2 with S6 were calculated for each of the 28 Pz-electrode and Fz-electrode pairs (excluding electrooculogram electrodes and the right-mastoid electrode). Topographic coherence maps were then plotted showing the scalp distribution of these t-scores. These maps only show regions in which the t-scores lie in the top or bottom 2.5% of the t-distribution.
The Fz ANOVA revealed a significant interaction among factors FREQUENCY, LOAD, ANTERIOR-POSTERIOR, and HEMISPHERE (F6/54 = 80.0, p < .001) and a significant interaction among factors FREQUENCY, LOAD, ANTERIOR-POSTERIOR, and DORSAL-VENTRAL (F6/54 = 6.1, p = .003). Follow-up t-tests for each of the 28 electrode-Fz pairs revealed that these ANOVA results were driven, in part, by an increase in Fz theta coherence with left hemisphere (LH) parietal and temporal electrodes as set-size increased (Figure 1). (There were no corresponding load effects on theta power that could have artifactually caused these load effects on coherence.) For the S4 minus S2 comparison, maximal coherence was between Fz and left parietal electrodes. For the high-load S6 minus S4 comparison, the coherence was maximal between Fz and the left-temporal area. The overall S6 minus S2 comparison revealed a combined increase between Fz and left parietal and left temporal electrodes.
Figure 1.
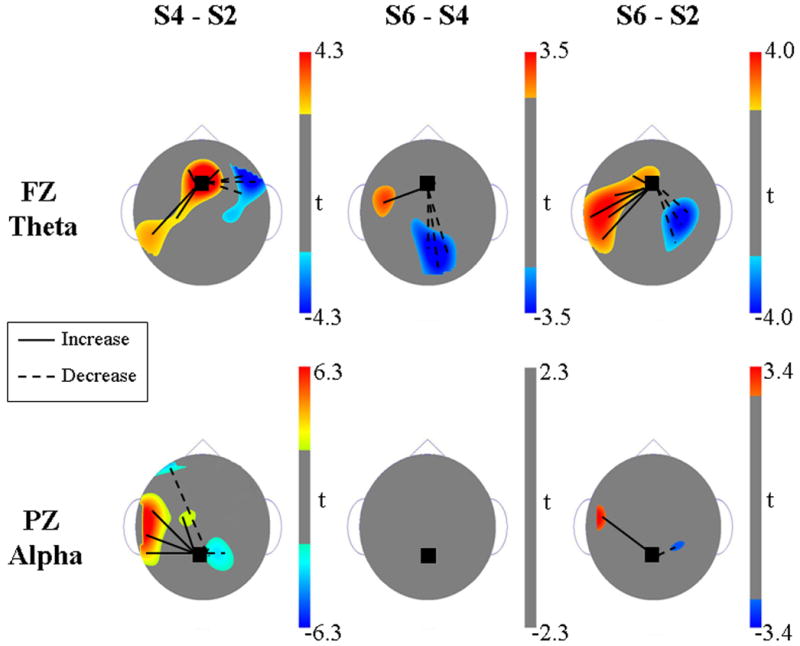
Topographic t-maps illustrating statistically significant increases (solid line) and decreases (dotted line) in EEG coherence for the S4 vs. S2, S6 vs. S4, and S6 vs. S2 comparisons. Included for orientation are rounded ears to the right and left sides and a triangle nose at the top of each map. Top row: changes in theta (4 – 7.75 Hz) coherence with frontal-midline electrode Fz. Bottom row: changes in alpha (8 – 13 Hz) coherence with parietal-midline electrode Pz. Changes in coherence between signals at two electrodes signify a change in the relationship between the two regions but do not indicate causality. These maps only show regions in which the t-scores lie outside of the ranges that correspond to the top or bottom 2.5% of the t-distribution.
Coherence subtraction wavelets showed that although increases in theta coherence were constrained to the retention period, the effect did not extend across the entire 2.8 s retention interval in any of the three comparisons (S4-S2, S6-S4, or S6-S2). For the high-load S6 minus S4 comparison, theta-coherence activity occurred in the second half of the retention period and appeared to consist of two relatively brief bursts (Figure 2a). The earlier one was centered around 2,000 ms after stimulus onset at a frequency of approximately 4 Hz. The second one centered around 2,600 ms after stimulus onset at a frequency of approximately 6 Hz. In addition to the LH frontal-parietal increases in theta coherence, a decrease in coherence was observed between Fz and primarily right-hemisphere frontal, parietal, and occipital electrodes.
Figure 2.
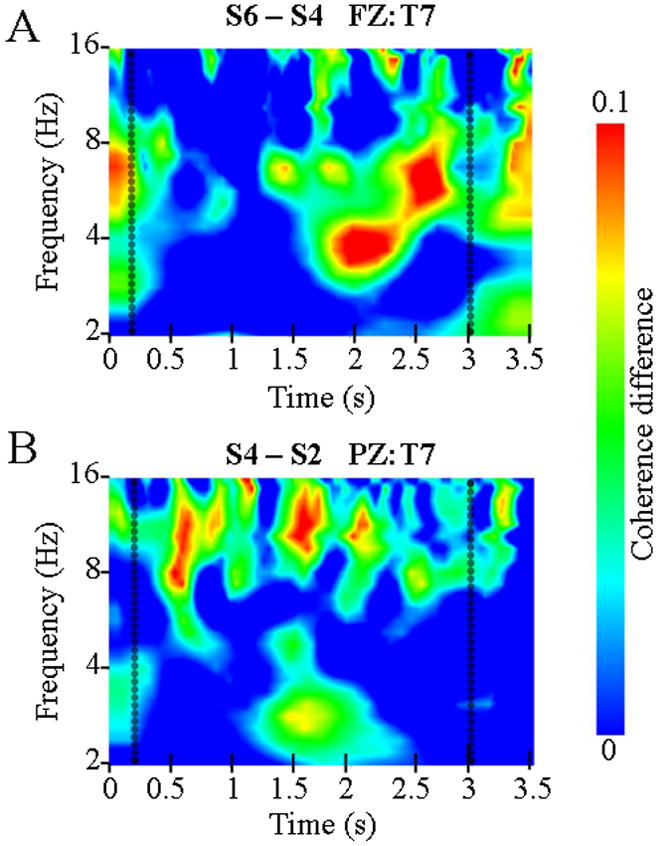
Time-frequency wavelets illustrating increases in coherence between representative electrodes during the retention period. At t = 0 s the memory set is presented for 0.2 s and at t = 3 the probe appears. (A) Increase in coherence between frontal-midline electrode Fz and T7 in the S6 minus S4 subtraction. (B) Increase in coherence between parietal-midline electrode Pz and left-temporal electrode T7 in the S4 minus S2 subtraction.
The Pz ANOVA revealed a significant five-way interaction among factors FREQUENCY, LOAD, ANTERIOR-POSTERIOR, HEMISPHERE, and DORSAL-VENTRAL (F6/54 = 74.96, p = .000). Follow-up t-tests conducted for each of the 28 electrode-Pz pairs revealed an increase in alpha coherence between Pz and left hemisphere temporal and parietal electrodes that was apparent in the S4 minus S2 and S6 minus S2 comparisons (Figure 1). This increase in alpha coherence was confined to the duration of the retention period (Figure 2b). This effect, though not constant over time, did extend over the retention interval. Alpha also showed a reduction in coherence between Pz and left and right anterior frontal and right parietal electrodes when the set-size was increased from 2 to 4.
3. Discussion
In the current study, the effect of STM load on frontal and parietal functional connectivity during the retention period of a modified Sternberg task was investigated using time-frequency coherence analysis. The results show that frontal-midline theta is involved in the response of the STM network to increasing load. It had been predicted in the initial report (Jensen et al., 2002) that theta amplitude would increase as memory load increased. This hypothesis was based on previous reports which demonstrated increased theta power in high-load STM conditions (e.g., Gevins et al., 1997; Klimesch et al., 1999). Jensen et al., (2002) predicted that varying load parametrically from 2 to 6 items would thus reveal a systematic increase in theta power. Power analyses revealed no such effect. However, the present analysis demonstrates load-related increase in theta coherence between frontal-midline and left temporal/parietal electrodes (Figure 1).
The load-induced enhancement of the frontal-midline theta network suggests that interactions between these regions are more important for STM retention than is the amplitude of frontal-midline theta oscillations, which did not respond to increasing load. The regions implicated in this analysis are consistent with neuroimaging studies that identify frontal and temporal/parietal activations as key areas participating in verbal STM retention (Awh et al., 1996; Paulesu et al., 1993; Smith & Jonides, 1997).
Although coherence between two brain regions does not imply directionality or causality, frontal-midline theta observed during demanding cognitive tasks has been shown to originate in the anterior cingulate and surrounding cortex (Gevins et al., 1997; Onton et al., 2005). Such anterior cingulate activity is thought to reflect executive functions associated with mental effort (Kahana et al., 2001), monitoring of cognitive conflict (Botvinick et al., 2004), and top-down control (Onton et al., 2005). Thus, the frontal-posterior interactions demonstrated in the current study are consistent with the notion of top-down processing to strengthen the activation of the memory set as set-size increases. Decreases in frontal-theta coherence with right-hemisphere regions as set-size increases suggest that in order to accommodate a demanding memory load, frontal influence to stabilize representations in relevant LH regions is accompanied by reduction in communication with potentially interfering RH areas.
That there was no increase in theta power, yet an increase in theta coherence with memory load is also consistent with models suggesting that theta oscillations serve as a timing mechanism during STM retention (Jensen and Lisman, 1998; Onton et al., 2005). According to the Lisman and Idiart model (1995) of nested oscillatory subcycles, individual items of a memory set are represented by synchronous neuronal firing in the gamma range (approximately 40 Hz) which is organized and refreshed at the theta cycle. According to this view, the magnitude of theta oscillations would not necessarily vary with the size of the memory set. However, a successful timing mechanism would rely on the entrainment of relevant neural networks into coherent theta oscillations.
The current results also show that alpha coherence increases between parietal midline and LH temporal and parietal regions thought to be involved in verbal STM retention (Figure 1). These findings indicate that posterior alpha oscillations contribute to STM retention, supporting the view that task-related alpha does not simply represent a passive suppression of activity in cortical regions unrelated to the task, but is instead an active inhibition process supporting STM retention.
Although increases in posterior alpha power are understood to reflect the gating of bottom-up visual inputs which could disrupt retention of the memory set (Cooper et al., 2003; Jokisch and Jensen, 2007; Jung-Beeman et al., 2004; Tuladhar et al., 2007;), it still remains unclear precisely how such sensory inputs are inhibited. It has been suggested that the entrainment of neurons into the 8 – 13 Hz alpha rhythm effectively prevents the transmission of perceptual information from occipital to temporal regions (Tuladhar et al., 2007). This functional gating may result from a GABAergic inhibitory feedback mechanism which generates alpha cycles (van Dijk et al., 2008), thus reducing the gain of the visual dorsal stream (Jokisch and Jensen, 2007) and decreasing the likelihood of interference from extraneous processes in high-demand situations.
It is also possible that posterior alpha plays an even more direct role in STM maintenance. Alpha inhibition theory has recently been extended to include an active role as an inhibition-timing mechanism during STM retention. Klimesch et al. (2007) proposed that alpha oscillations serve as an inhibitory filter which reduces the transmission of information, thus resulting in the selective processing necessary for STM retention. The phase-setting of this inhibitory alpha-filter may be a timing mechanism which coordinates the processes of probe encoding and retrieval in STM tasks (Klimesch et al., 2007; Schack & Klimesch, 2002). In addition, it has been proposed that alpha oscillations may reflect a state of internal attention (Cooper et al., 2006) or serve an active role in sustaining the representation of a memory set during STM retention, as inferred from the increase in cross-frequency phase synchronization between alpha and higher-frequency bands during demanding working memory tasks (Palva & Palva, 2007).
Interestingly, although the amplitude of posterior alpha oscillations increased with set size from S2 to S4 (see Jensen et al., 2002), neither alpha coherence nor alpha amplitude increased significantly between the parietal-midline electrode Pz and LH temporal/parietal areas from S4 to S6. The lack of additional increase in alpha amplitude or coherence between posterior and temporal-parietal regions from S4 to S6 implies a limitation, either physical or functional, on additional visual gating. Such a limitation on visual gating makes sense because an unchecked increase in inhibition could potentially block all information processing. That the functional limit of this gating mechanism approximately corresponds to an STM load of 4 items is consistent with growing evidence suggesting that the capacity of STM is 4 items (Cowan, 2000; Jonides et al., 2008), with more complex strategies, such as verbal rehearsal, underlying retention beyond this capacity limit (Jonides et al., 2008).
Extensive research has attempted to identify the origins of top-down control exerted during STM retention. Lesion studies and STM tasks involving the presentation of distracting stimuli indicate that the prefrontal cortex (PFC) functions to successfully minimize the influence of irrelevant or interfering stimuli during retention (e.g. Chao & Knight, 1995; Jha et al., 2004; Postle 2005). However, it is not known whether this is achieved through selective processing of task-relevant information, the gating of task-irrrelevant information, or both (for review, Postle, 2006; Smith and Jonides, 1999). A functional magnetic resonance imaging (fMRI) study using interfering stimuli during STM retention suggests that lateral PFC plays a role in the top-down control of sensory gating (Postle, 2005). It is possible that one mechanism of this sensory gating is through the recruitment of the inhibitory posterior alpha system. Another fMRI study of retention found that interfering stimuli disrupt functional connectivity between prefrontal cortex (PFC) and posterior association cortex, thus implying that the PFC selectively codes relevant stimuli which is actively maintained through functional connections (Yoon et al., 2006). A manifestation of this selective connectivity during verbal STM retention may be the load-related theta coherence between frontal midline and temporal-parietal electrodes. A recent study reported by Dolcos and colleagues (2007) supports the notion that both selective processing and sensory gating are orchestrated by different regions of the PFC; the dorsolateral PFC being involved in the encoding and maintenance of a memory set while ventrolateral PFC is involved in the inhibition of distracting stimuli.
Finally, though these results have isolated important aspects of network dynamics supporting STM retention, neither these results nor the earlier report of Jensen et al. (2002), seem to directly point to a signal that reflects retention itself. Increased theta coherence between the frontal-midline (Fz) and left-temporal (T7) electrodes occurred primarily during the second half of the retention interval (Figure 2a), a finding more consistent with the notion that extra top-down influence is required to compensate for increased load than the idea that this theta coherence reflects retention itself. Alpha coherence between the left-temporal and parietal-midline (Pz) electrodes extended over the retention interval (Figure 2b), though not at a constant level. However, the alpha-coherence effect was only observed in the S4 minus S2 subtraction and not the S6 minus S4 subtraction (Figure 1) which is inconsistent with the possibility that alpha coherence itself reflects memory-set retention. Instead, this result, together with earlier alpha studies cited above, suggests that attenuated bottom-up sensory input with increased load is either required or helpful during the whole retention period.
In contrast with results from previous studies suggesting that gamma-band oscillations may reflect a retention mechanism (e.g., Howard et al., 2002; Tallon-Baudry et al., 1998), the present study did not yield a load effect on coherence that extended across the retention period for all contrasts in any frequency band, nor did it yield a monotonic effect of load on power in any frequency band (except for the posterior alpha load effect shown by Jensen et al., 2002). Since gamma-band effects measured at the scalp can have a relatively focused topographic distribution (e.g., Jung-Beeman et al., 2004), it is possible that the comparatively sparse EEG electrode array used in this study missed the critical gamma-band retention signal. Future studies with denser electrode arrays can address this question. Also, gamma oscillations represent a stronger candidate for the organization of items in a memory set than retention itself (Howard et al., 2002; Jensen and Lisman, 1998; Lisman and Idiart, 1995; Tallon-Baudry et al., 1998), a theory supported by reports that gamma effects during STM are not constrained to the retention period (Howard et al., 2002) nor do they exhibit an effect of load (Tallon-Baudry et al., 1998).
Another possibility is that short-range coherence in the left temporal-parietal region may instantiate retention. Unfortunately, physical limitations on the spacing of EEG electrodes and the difficulty in ruling out contributions to short-range coherence from volume conduction make this possibility impossible to test using scalp-recorded EEG.
This analysis has shown that frontal-midline theta and posterior alpha both exhibit demand-induced interactions with LH temporal and parietal regions associated with verbal STM retention. The fact that both frontal-midline theta and parietal-midline alpha exhibit a nexus of coherence in the left temporal/parietal area is indirect evidence, consistent with functional neuroimaging studies, that this region supports retention. These results are consistent with the notion that these interactions represent complimentary mechanisms supporting retention through top-down selective strengthening of memory set representations and gating of potentially interfering bottom-up inputs.
4. Experimental Procedure
4.1. Subjects
Ten subjects (ages 18–35, half male/half female) performed a modified Sternberg (Sternberg, 1966) task while the EEG was recorded. Informed consent was obtained from each subject prior to the study. The study was approved by the Princeton University Institutional Review Board.
4.2. Task
One second after a warning tone, a horizontally arranged list of six consonants was presented simultaneously at the center of a computer monitor (with one blank space between adjacent characters) for 0.2 s. The memory set (S) consisted of the middle 2, 4, or 6 letters, with each unused position (i.e. in the S = 2 or 4 conditions) filled with an ‘X’ (which was never a member of the memory set), except for the middle position which contained a fixation cross throughout the retention interval. Thus, the display was always of the same physical size and the visual content was the same, irrespective of the size of the memory set. After a 2.8 s retention interval with a blank-screen display, the probe replaced the fixation cross and was displayed for the duration of the recognition interval (1 s). Subjects were instructed to press a mouse button as quickly as possible without making errors to indicate when the probe was a member of the memory set. Hand of response was counterbalanced across subjects. Following the recognition interval, subjects were allowed 3,000 ms to relax and blink. There were 25 positive trials (probe on the list) and 25 negative trials (probe not on the list) for each set size, making a total of 150 trials per subject. The trials were presented in random order with respect to set size. The task differed from the original Sternberg paradigm primarily in that: (a) the items were presented all at once rather than sequentially; (b) button presses were required only for positive trials; and (c) no performance feedback was given.
4.3. Data acquisition
EEG epochs were recorded during the encoding, retention, and recognition intervals (6 s per trial) using 32 electrodes placed according to the extended International 10–20 System. The signals were sampled at 250 Hz and bandpass-filtered at 0.01–100 Hz. The reference electrode was placed over the left mastoid. The horizontal and vertical electrooculograms (EOG) were recorded in order to monitor eye-blinks and eye movements.
4.4. EEG wavelet coherence
All EEG analyses were performed with EMSE 5.1 (www.sourcesignal.com). First, eye-blink artifacts in the EEG were corrected offline using an adaptive filter constructed separately for each subject. EEG segments containing other artifacts were detected by visual inspection and excised. Time-frequency coherence wavelets were generated from artifact-free segments for the 2,800 ms interval after stimulus presentation for each subject and memory load (S2, S4, and S6). The coherence wavelets were also averaged across subjects into a grand coherence wavelet for each memory load and each relevant electrode pair. The averaged wavelets were used for the subtraction of the S4 minus S2, S6 minus S4, and S6 minus S2 conditions. These subtraction wavelets allowed the visualization of frequency-specific changes in coherence with set-size over time.
Wavelet coherence is the time-frequency analog of time-domain correlation and it is used to measure how pairs of channels co-vary in both time and frequency.
For channels j and k, coherence is defined by:
Where Y is the complex Morlet wavelet transform (Grossmann & Morlet, 1984) of EEG signal at frequency (α) and time (t). I defines the number of trials, and * denotes complex conjugation.
In order to ascertain whether any signals picked up by the reference electrode might contaminate computations of coherence between pairs of electrodes, the power and frequency wavelets were inspected for evidence of any signal common to all electrodes (which would suggest that such a signal originated near the reference electrode). This inspection yielded no evidence of any such contaminating signal. In addition, wavelets computed for the actively-recorded right mastoid electrode were virtually flat, indicating that the left-mastoid reference electrode was not picking up any significant activity.
Acknowledgments
The authors thank Jack Gelfand for assistance with experiment implementation and data collection. Preparation of this report was funded by the Drexel University Critical Research Fellowship Award, 2005.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Awh E, Jonides J, Smith EE, Schumacher EH, Eric H, Koeppe RA, Katz S. Dissociation of storage and rehearsal in verbal working memory: Evidence from positron emission topography. Psychol Sci. 1996;7:25–31. [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8(12):539–46. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Human prefrontal regions increase distractibility to irrelevant sensory inputs. NeuroReport. 1995;6:1605–1610. doi: 10.1097/00001756-199508000-00005. [DOI] [PubMed] [Google Scholar]
- Cooper NR, Croft RJ, Dominey SJ, Burgess AP, Gruzelier JH. Paradox lost? Exploring the role of alpha oscillations during externally vs. internally directed attention and the implications for idling and inhibition. Int J Psychophysiol. 2003;47:65–74. doi: 10.1016/s0167-8760(02)00107-1. [DOI] [PubMed] [Google Scholar]
- Cooper NR, Burgess AP, Croft RJ, Gruzelier JH. Investigating evoked and induced electroencephalogram activity in task-related alpha power increases during and internally directed attention task. Neuroreport. 2006;17:205–208. doi: 10.1097/01.wnr.0000198433.29389.54. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci. 2000;24:87–185. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Miller B, Kragel P, Jha A, McCarthy G. Regional brain differences in the effect of distraction during the delay interval of a working memory task. Brain Res. 2007;1152:171–181. doi: 10.1016/j.brainres.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb Cortex. 1997;7(4):374–85. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Grossman A, Morlet J. Decomposition of Hardy functions into square integrable wavelets of constant shape. SIAM Journal of Mathematical Analysis. 1984;15:723–736. [Google Scholar]
- Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schulze-Bonhage A, Kahana MJ. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2002;13(12):1369–74. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. An oscillatory short-term memory buffer model can account for data on the Sternberg task. J of Neurosci. 1998;18(24):10688–10699. doi: 10.1523/JNEUROSCI.18-24-10688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, Lisman JE. Oscillations in the alpha band (9-12 Hz) increase with memory load during retention in a short-term memory task. Cerebral Cortex. 2002;12:882–887. doi: 10.1093/cercor/12.8.877. [DOI] [PubMed] [Google Scholar]
- Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. European J of Neuroscience. 2002;15:1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- Jha AP, Fabian SA, Aguirre GK. The role of prefrontal cortex in resolving distracter interference. Cogn, Affective, and Beh Neurosience. 2004;4(4):517–527. doi: 10.3758/cabn.4.4.517. [DOI] [PubMed] [Google Scholar]
- Jokisch D, Jensen O. Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J Neuro. 2007;27(12):3244–3251. doi: 10.1523/JNEUROSCI.5399-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokisch D, Jensen O. Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J of Neurosci. 2007;27(12):3244–3251. doi: 10.1523/JNEUROSCI.5399-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Lewis RL, Nee DE, Lustig CA, Berman MG, Moore KS. The mind and brain of short-term memory. Annu Rev Psychol. 2008;59:193–224. doi: 10.1146/annurev.psych.59.103006.093615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung-Beeman M, Bowden EM, Haberman J, Frymiare JL, Arambel-Liu S, Greenblatt R, Reber PJ, Kounios J. Neural activity observed in people solving verbal problems with insight. Public Library of Science - Biology. 2004;2:500–510. doi: 10.1371/journal.pbio.0020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana MJ, Seelig D, Madsen JR. Theta returns. Curr Opin Neurobiol. 2001;11(6):739–44. doi: 10.1016/s0959-4388(01)00278-1. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schwaiger J, Auinger P, Winkler TH. ‘Paradoxical’ alpha synchronization in a memory task. Cognitive Brain Research. 1999;7:493–501. doi: 10.1016/s0926-6410(98)00056-1. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kounios J, Frymiare JL, Bowden EM, Fleck JI, Subramaniam K, Parrish TB, Jung-Beeman M. The prepared mind: neural activity prior to problem presentation predicts subsequent solution by sudden insight. Psychol Sci. 2006;17(10):882–90. doi: 10.1111/j.1467-9280.2006.01798.x. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Lutz A, Rudrauf D, Cosmelli D, Le Van Quyen M, Martinerie J, Varela F. Estimating the time-course of coherence between single-trial brain signals: an introduction to wavelet coherence. Neurophysiol Clin. 2002;32(3):157–74. doi: 10.1016/s0987-7053(02)00301-5. [DOI] [PubMed] [Google Scholar]
- Laufs H, Kleinschmidt A, Beyerle A, Eger E, Salek-Haddadi A, Preibisch C, Krakow K. EEG-correlated fMRI of human alpha activity. NeuroImage. 2003;19:1463–1476. doi: 10.1016/s1053-8119(03)00286-6. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Idiart MAP. Storage of 7 +/- 2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–1514. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- Michels L, Moazami-Goudarzi M, Jeanmonod D, Sarnthein J. EEG alpha distinguishes between cuneal and precuneal activation in working memory. NeuroImage. 2008;40:1296–1310. doi: 10.1016/j.neuroimage.2007.12.048. [DOI] [PubMed] [Google Scholar]
- Onton J, Delorme A, Makeig S. Frontal midline EEG dynamics during working memory. NeuroImage. 2005;27:341–356. doi: 10.1016/j.neuroimage.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Palva S, Palva JM. New vistas for α-frequency band oscillations. Trends in Neurosciences. 2007;30(4):150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak SJ. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G. Induced oscillations in the alpha band: functional meaning. Epilepsia. 2003;44(Suppl12):2–8. doi: 10.1111/j.0013-9580.2003.12001.x. [DOI] [PubMed] [Google Scholar]
- Postle BR. Delay-period activity in the prefrontal cortex: One function is sensory gating. J of Cog Neurosci. 2005;17:1679–1690. doi: 10.1162/089892905774589208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR. Working Memory as an Emergent Property of the Mind and Brain. Neuroscience. 2006;139(1):23–38. doi: 10.1016/j.neuroscience.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Kahana M, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, Madsen JR, Lisman JE. Gating of human theta oscillations by a working memory task. Neurosci. 2001;21(9):3175–83. doi: 10.1523/JNEUROSCI.21-09-03175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchkin DS, Grafman J, Cameron K, Berndt RS. Working memory retention systems: a state of activated long-term memory. Behav Brain Sci. 2003;(6):709–28. doi: 10.1017/s0140525x03000165. [DOI] [PubMed] [Google Scholar]
- Ruchkin DS. EEG coherence. Int J Psychophysiology. 2005;57(2):83–85. doi: 10.1016/j.ijpsycho.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Saab R, McKeown MJ, Myers LJ, Abu-Gharbieh R. A Wavelet Based Approach for the Detection of Coupling in EEG Signals. Neural Engineering Conference Proc. 2005:616–620. [Google Scholar]
- Sarnthein J, Petsche H, Rappelsberger P, Shaw GL, von Stein A. Synchronization between prefrontal and posterior association cortex during human working memory. PNAS. 1998;95:7092–7096. doi: 10.1073/pnas.95.12.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schack B, Klimesch W. Frequency characteristics of evoked and oscillatory electroencephalic activity in a human memory scanning task. Neurosci Letters. 2002;331:107–110. doi: 10.1016/s0304-3940(02)00846-7. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Hoppe J, Klimesch W, Gerloff C, Hummel FC. Dissociation of sustained attention from central executive functions: local activity and interregional connectivity in the theta range. Eur J Neurosci. 2007;25(2):587–93. doi: 10.1111/j.1460-9568.2006.05286.x. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Working memory: a view from neuroimaging. Cognit Psychol. 1997;33(1):5–42. doi: 10.1006/cogp.1997.0658. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Sternberg S. High speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced γ-band activity during the delay of a visual short-term memory task in humans. Journal of Neuroscience. 1998;18:4244–4254. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuladhar AM, ter Huurne N, Schoffelen JM, Maris E, Oostenveld R, Jensen O. Parieto-occipital sources account for the increase in alpha activity with working memory load. Human Brain Mapp. 2007;8:785–792. doi: 10.1002/hbm.20306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk H, Schoffelen JM, Oostenveld R, Jensen O. Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. Journal of Neuroscience. 2008;28:1816–1823. doi: 10.1523/JNEUROSCI.1853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. Journal of Neuroscience. 2000;20:1–6. doi: 10.1523/JNEUROSCI.20-06-j0002.2000. RC63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Curtis CE, D'Esposito M. Differential effects of distraction during working memory on delay-period activity in the prefrontal cortex and the visual association cortex. NeuroImage. 2006;29(4):1117–1126. doi: 10.1016/j.neuroimage.2005.08.024. [DOI] [PubMed] [Google Scholar]


