Abstract
The purpose of the present study was to compare muscular strength of knee extensors and arm flexor muscles of cardiac patients (n = 638) and healthy controls (n = 961) in different age groups. Isometric torques were measured in a sitting position with the elbow, hip, and knee flexed to 900. For statistical analysis, age groups were pooled in decades from the age of 30 to 90 years. Additionally, the influence of physical lifestyle prior to disease on muscular strength was obtained in the patients. For statistical analysis three-way ANOVA (factors age, gender, and physical activity level) was used.
Both in patients and in controls a significant age-dependent decline in maximal torque could be observed for arm flexors and knee extensors. Maximal leg extensor muscle showed statistically significant differences between healthy controls and cardiac patients as well as between subgroups of patients: Physically inactive patients showed lowest torques (male: 148 ± 18 Nm; female: 82 ± 25 Nm) while highest values were measured in control subjects (male: 167 ± 16 Nm; female: 93 ± 17 Nm). In contrast, arm flexor muscles did not show any significant influence of health status or sports history.
This qualitative difference between weight-bearing leg muscles and the muscle group of the upper extremity suggest that lower skeletal muscle strength in heart patients is mainly a consequence of selective disuse of leg muscles rather than any pathological skeletal muscle metabolism. Since a certain level of skeletal muscle strength is a prerequisite to cope with everyday activities, strength training is recommended as an important part of cardiac rehabilitation.
Keywords: isometric torque, ageing, heart disease, rehabilitation
Introduction
For some decades physical training has been recommended as a mainstay of non-pharmacological treatment in cardiac patients. In the beginning of cardiac rehabilitation, physical activities and clinical research concentrated on aerobic exercises such as walking, swimming, or cycling although all activities of daily living require a certain level of muscle strength 1. Since the 90th, clinical research also focussed on peripheral skeletal muscle function, especially in those patients suffering from chronic heart failure (CHF). It could be established that the daily activities of these patients are limited not only by reduced endurance capacity but also by strength deficits 2, 3, 4, 5, 6, 7, 8. In CHF patients, Harrington et al. 9 found a significant reduction in maximal quadriceps strength per muscular cross section area. In line with this finding, Okada et al. 10 showed that in comparison to healthy controls muscle strength was lower even after correction for muscle size although the contractile protein function per se appeared not to be altered in CHF patients. The authors speculated that a decreased myosin content may be responsible for muscular weakness.
Surprisingly little is known about skeletal muscle strength in cardiac patients suffering from other diseases than CHF. Ghroubi et al. 11 found a significant reduced maximal torque in coronary artery disease (CAD) patients as compared to age matched healthy subjects for quadriceps (-28%) and hamstring (-22%) muscles. In contrast, Gayda et al. 12 found no significant differences between CAD patients and controls with respect to absolute maximal quadriceps torque. Unfortunately, both studies used relatively small sample sizes of 30 and 25 subjects, respectively.
However, beside any disease specific alteration in muscular constitution one important contributor to muscular strength may be the physical activity of patients since it is well known that physical activities lead to morphological adaptation of skeletal muscles. Following activities including high loads such as weight bearing activities, muscle fibres react with hypertrophy, an increased myofilament density, and other morphological changes. As a result, muscle strength increases (for review see Folland and Williams 13). Vice versa, unloading of skeletal muscles leads to muscular weakness and atrophy 14.
Thus, the goals of the present study were I) to compare muscular strength in cardiac patients and healthy controls, II) to analyse the influence of age on muscular strength and III) to assess the relationship between sports history and muscular strength in cardiac patients.
Methods
Overall study design
The data used for this cross-sectional analysis were obtained from the FITCAP study (Fitness in Cardiac Patients), a prospective multi-centre training trial including patients of 10 cardiac rehabilitation centres in Germany. During a three week stationary rehabilitation phase, patients performed endurance and strength training three times per week each. Endurance training consisted of cycle ergometry and strength training included exercises with both weight machines (lower body exercises, Tonus Sports & Reha ltd.) and with elastic bands (upper and lower body exercises, Thera-Band®). Strength training was performed using the intermittend training mode as described by Baum et al. 15. During the subsequent 18 months patients received written strength training advices using elastic band exercises and their individual endurance training program. Patients re-entered the hospital after 3, 6, 12 and 18 month for further examinations. The present paper deals with maximal muscle torques of the upper and lower extremity at the final stage of the stationary rehabilitation. Other results of this study will be presented later. Prior to recruitment of subjects the study protocol was approved by the ethic committee of the German Sports University.
Eligibility criteria
Inclusion criteria of patients were males and females aged 30 to 90 years, who underwent stationary rehabilitation due to cardiac disease and who met the following recommendations of the German Federation for Cardiovascular Prevention and Rehabilitation for the participation of strength training 16: good cardiac performance capacity ( > 1,4 watt / kg body weight) and no ST segment depression. Exclusion criteria were unstable angina pectoris, severe arrhythmias, psychoneurotic disorders, severe pulmonary diseases, severe physical disabilities, and participation in another trial. Participants were informed about study objectives, risks and benefits. Patients entered the study after written consent was obtained. They were allowed to cease study participation at any time.
Subjects
638 cardiac patients (540 male, 98 female) participated in the study. According to the primary diagnosis, 48,7 % were patients after myocardial infarction and 43,5 % suffered from coronary heart disease. The remaining 7,8 % of patients suffered from left ventricular hypertrophy, cardiomyopathy, and congestive heart failure. In addition, the maximal isometric torque of knee extensor and arm flexor muscles were determined in 961 healthy subjects (414 male, 547 female). They were recruited by chance during public health promoting lectures with respect to healthy nutrition and the effects of regular sports activities. Those visitors interested in strength testings gave informations about their experiences with strength training and their health status. For the purpose of the present study, inclusion criteria were that they did not suffer from cardiovascular diseases and that they had no experience in regular strength training. The anthropometric characteristics of all participants are presented in table 1.
Table 1.
Anthropometric data of subjects (mean ± SD).
| males | females | |||
|---|---|---|---|---|
| controls | patients | controls | patients | |
| age group 30 - 39 years | ||||
| number | 62 | 33 | 58 | 2 |
| weight (kg) | 83,7 ± 12,3 | 93,7 ± 18,4 | 70,3 ± 12,2 | 58,5 ± 10,6 |
| height (cm) | 181 ± 6,3 | 180 ± 7,1 | 168 ± 6,1 | 159 ± 12,0 |
| BMI (kg x m-2) | 24,1 ± 6,7 | 28,9 ± 4,7 | 24,8 ± 4,4 | 23,2 ± 0,7 |
| age group 40 - 49 years | ||||
| number | 69 | 122 | 62 | 19 |
| weight (kg) | 87,2 ± 15,5 | 88 ± 13,3 | 67 ± 12,5 | 71,1 ± 12,9 |
| height (cm) | 180 ± 6,2 | 179 ± 7,1 | 167 ± 6,5 | 167 ± 10,6 |
| BMI (kg x m-2) | 27,1 ± 4,9 | 27,2 ± 4,6 | 23,7 ± 5,2 | 25,6 ± 4,7 |
| age group 50 - 59 years | ||||
| number | 92 | 186 | 106 | 19 |
| weight (kg) | 84,4 ± 13,1 | 85,1 ± 13,4 | 69,6 ± 12,0 | 67,4 ± 11,3 |
| height (cm) | 178 ± 6,4 | 176 ± 9,9 | 166 ± 6,1 | 163 ± 5,3 |
| BMI (kg x m-2) | 26,8 ± 3,6 | 27,8 ± 8,1 | 25,3 ± 4,2 | 25,3 ± 4,2 |
| age group 60 - 69 years | ||||
| number | 108 | 155 | 127 | 46 |
| weight (kg) | 80,5 ± 11,5 | 81,7 ± 11,8 | 68,7 ± 11,9 | 74,5 ± 12,1 |
| height (cm) | 176 ± 6,1 | 176 ± 6,4 | 164 ± 5,6 | 165 ± 6,4 |
| BMI (kg x m-2) | 25,9 ± 4,1 | 26,5 ± 3,5 | 25,2 ± 4,9 | 27,6 ± 4,6 |
| age group 70 - 79 years | ||||
| number | 63 | 43 | 102 | 11 |
| weight (kg) | 79,4 ± 11,0 | 80,5 ± 10,3 | 67,8 ± 11,2 | 62,8 ± 10,4 |
| height (cm) | 173 ± 6,5 | 172 ± 13,3 | 162 ± 7,6 | 160 ± 4,4 |
| BMI (kg x m-2) | 26,4 ± 3,1 | 28,2 ± 12,1 | 25,9 ± 3,8 | 24,6 ± 4,2 |
| age group 80 - 89 years | ||||
| number | 20 | 1 | 92 | 1 |
| weight (kg) | 73 ± 12,7 | 64 | 64,5 ± 13,4 | 52,2 |
| height (cm) | 170 ± 5,4 | 160 | 160 ± 6,0 | 164 |
| BMI (kg x m-2) | 25,1 ± 3,7 | 25 | 24,8 ± 3,5 | 19,4 |
| all patients | ||||
| number | 414 | 540 | 547 | 98 |
| age (years) | 59,2 ± 14,3 | 58,8 ± 13,7 | 59,4 ± 13,9 | 58,9 ± 17,3 |
| weight (kg) | 81,4 ± 6,3 | 82,2 ± 6,8 | 68,0 ± 5,9 | 64,4 ± 5,8 |
| height (cm) | 176 ± 3,1 | 174 ± 4,3 | 165 ± 3,2 | 163 ± 7,7 |
| BMI (kg x m-2) | 25,9 ± 1,2 | 27,3 ± 1,5 | 24,9 ± 1,7 | 24,3 ± 3,2 |
Maximal isometric torque
Maximal isometric torques (MIT) were determined within the last four days of a three week stationary rehabilitation program. MIT was obtained from knee extensors and elbow flexors of both sides at a joint angle of 90o. To this end, subjects were placed on a chair with a vertically mounted backrest. The knee and hip were flexed to 90o. A non-elastic band was wrapped around the ankle joint and coupled to a force transducer (Digimax®), fixed at the backside of the chair. The lever arm was determined as the distance between the middle of the band and the knee joint. Subjects were instructed to avoid Valsalva manoeuvres and to increase contraction force gradually to the maximum during the initial two to three seconds. Each muscle group was tested two times with a resting interval of about 30 s in between. The best of both trials was used for further computation. Prior to statistical computations, the individual mean of the left and right side was calculated and used for further evaluation. In addition to the absolute values, results were expressed as maximal torque divided by body weight (relative maximal torque).
Statistics
Statistical analyses were performed using SPSSTM 14.0. Data are given as means and standard error (SE; in figures) or standard deviation (SD, in tables and text). Maximal torque of leg extensors and arm flexors were investigated by three-way ANOVA (factors “age”, “sex”, and “sport experience”). The LSD-Test was used for post-hoc comparisons. Significance level was chosen as p < 0.05.
Subjects were divided into three groups according to their sport experience:
1. Patients which never participated in regular sports activities prior to hospitalisation (20,7 %), 2. patients which participated in regular sports activities but stopped exercising for at least two years prior to hospitalisation (43,1 %), and 3. patients which participated in regular sports activities immediately until hospitalisation (36,2 %).
Regular sports activities was definded as at least one hour per week of vigorous sports such as jogging, cycling, or soccer.
Age related subgroup analysis was performed for the six groups 30-39 years, 40-49 years, 50-59 years, 60-69 years, 70-79 years, and 80 - 89 years. For further computations, age groups were individually taken into account if the number of subjects exceeded or equaled 10.
Results
General effects of gender and age
Patients and controls of both sexes showed a significant age-related reduction in muscular maximal torque. The age effect persists when torques are related to body weight. Both in healthy controls and in cardiac patients men yielded significantly higher torques then women. The percentages of torques in women in relation to the torques of corresponding men groups are presented in table 2.
Table 2.
Percentages of muscular maximal torques of females in relation to male subjects of identical age groups (mean ± SD; ** p < 0,01).
| controls | cardiac patients | |||
|---|---|---|---|---|
| arm flexor muscles | leg extensor muscles | arm flexor muscles | leg extensor muscles | |
| abs. max. torque | 56 ± 3,4 ** | 59 ± 2,7 ** | 53 ± 2,3 ** | 60 ± 2,8 ** |
| rel. max. torque | 67 ± 4,2 ** | 72 ± 2,5 ** | 64 ± 3,6 ** | 73 ± 6,9 ** |
Leg extensor muscles
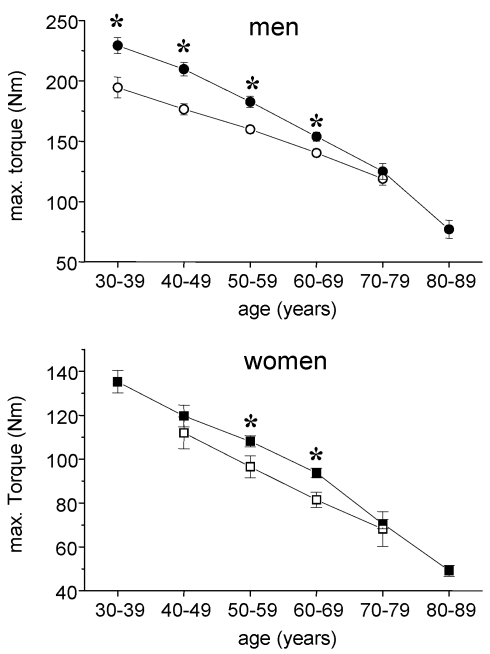
In healthy men the maximal isometric torque of leg extensor muscles ranged from 229 ± 50,1 Nm in the 30-39 year group to 77 ± 30 Nm in the 80-89 year group corresponding to relative torques of 2,77 ± 0,61 Nm x kg-1 and 1,08 ± 0,32 Nm x kg-1, respectively. In male cardiac patients, 195 ± 49 Nm corresponding to 2,10 ± 0,44 Nm x kg-1 could be measured within the age group 30-39 years. The post hoc test showed a significant difference between control subjects and cardiac patients for all ages except the age group 70-79 years (Fig. 1). A similar pattern at lower levels was found in women. The female control group showed a continuous age related decline from 135 ± 37 Nm (30 - 39 years) to 49 ± 20 Nm (80 - 89 years). Female cardiac patients did not reach control values in each age group with significant differences for the groups 50-59 years and 60-69 years.
Fig 1.
Absolute max. isometric torques of knee extensor muscles in cardiac patients (open symbols) and healthy subjects (closed symbols). Circles, males, squares = females; mean ± SE; * p < 0,05.
Arm flexor muscles
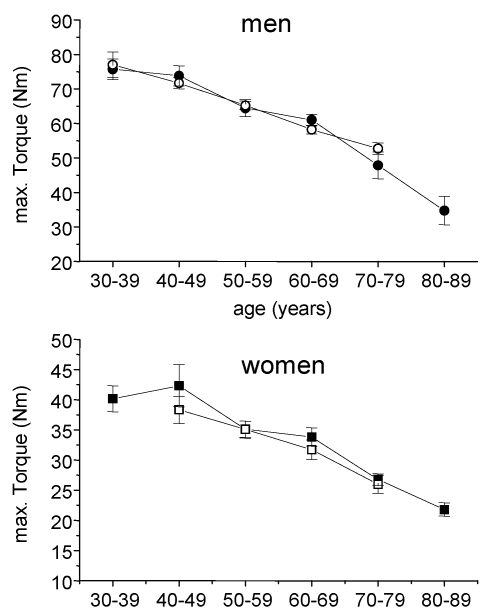
In healthy 30-39 years old controls the maximal isometric torque of arm flexor muscles amounted to 75 ± 14,0 and 40 ± 7,2 Nm in males and females, respectively. In the oldest age group a nearly 50 % reduction could be observed leading to 35 ± 16,3 Nm in man and 22 ± 8,4 Nm in women. Within the age groups there were no statistical significant difference between healthy controls and cardiac patients (Fig. 2).
Fig 2.
Absolute max. isometric torques of arm flexor muscles in cardiac patients (open symbols) and healthy subjects (closed symbols). Circles, males, squares = females; mean ± SE.
Relationship between sports history and muscular torque in cardiac patients
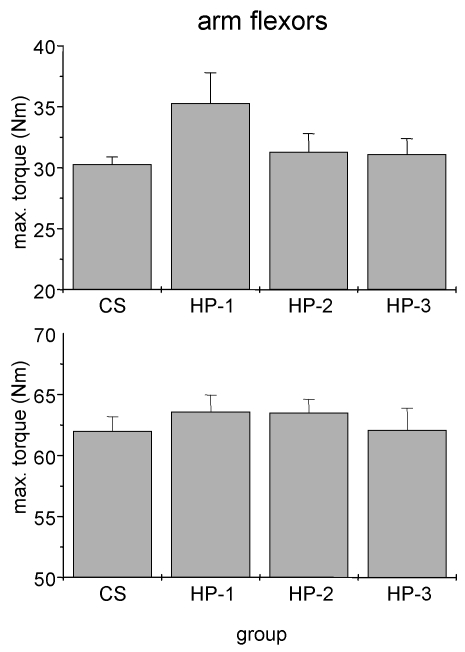
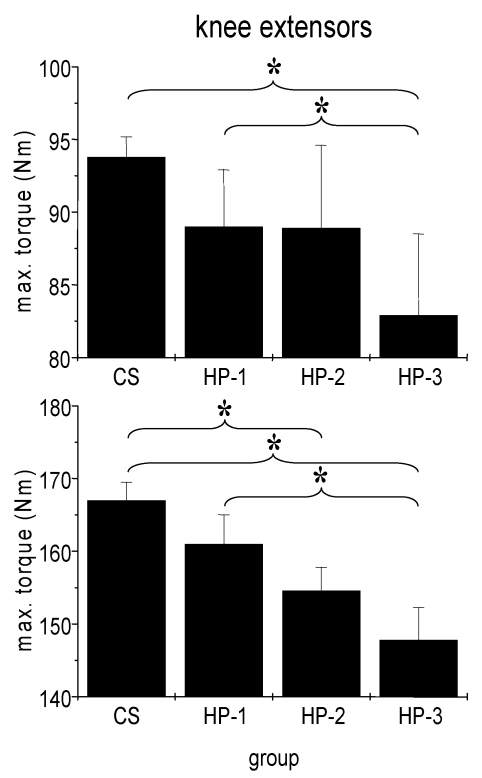
We failed to detect any influence of sports history on MIT of arm flexor muscles (Fig. 3). In particular the comparison between healthy adults and the three subgroups of cardiac patients yielded no significant difference. By contrast, both man and women showed a significant influence of sports history on MIT of leg extensor muscles with the lowest torque in patients which never exercised regularly (Fig. 4).
Fig 3.
Absolute isometric torques of arm flexor muscles of control subjects (CS) and heart patients (HP-1 = regular sports activities until disease; HP-2 = former regularly active but sports activities ceased at least two years before disease; HP-3 = never been regularly active before disease). Mean ± SE.
Fig 4.
Absolute isometric torques of knee extensor muscles of control subjects (CS) and heart patients (HP-1 = regular sports activities until disease; HP-2 = former regularly active but sports activities ceased at least two years before disease; HP-3 = never been regularly active before disease). Mean ± SE; * p < 0,05.
Discussion
The main findings of the present study are
a qualitative difference in leg and arm muscle torque: leg muscle torque was significantly lower in cardiac patients when compared to healthy controls while no difference could be detected for the arm muscle. Furthermore, the subgroup analysis in cardiac patients yielded a significant influence of sports history on leg but not on arm muscle torque, and
in both cardiac patients and healthy controls muscle torque decreased strongly with age.
Gayda et al 12 investigated the maximal isometric torque in 16 patients suffering from coronary artery disease and in 9 age-matched healthy control subjects. They found nearly identical absolute values of about 230 Nm for leg extensor muscles. The authors concluded that no difference exists between muscle strength of cardiac patients and controls. However, beside the limited number of subjects, both groups differed in body weight (means of 74 kg for controls and 85 kg for patients). Computing the relative maximal torques of their subjects yields a difference of about 16 %, which is in line with our findings. Since leg extensor muscles have to deal with the whole body weight during everyday activities, the relative torque appears to be superior when comparing performance capacity.
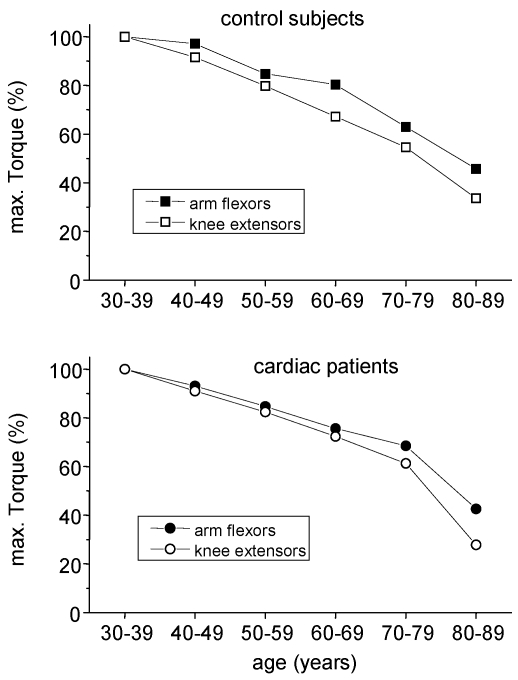
The decreases in skeletal muscle torque with age are in line with other cross-sectional findings 8, 10. Those reductions in strength originate mainly from two sources: The biological aging by itself and a reduction of daily physical activity with increasing age. Regular stimuli are needed to maintain muscle mass and torque. From bed rest studies 14, 17 and immobilisation experiments 18 it is known that reduced muscular activities lead to a decline in skeletal muscle mass and strength. During daily activities, arm and leg muscles are normally exposed to different tasks: While leg muscles have to move the whole body weight, arm muscles almost serve for more sophisticated tasks. So, even without any explicit strength training, leg muscles of physically active people are exposed to high loads throughout everyday tasks such as walking or stair climbing. On the other hand, arm muscles are usually not exposed to high loads throughout life span. Therefore, in order to roughly distinguish the influence of biological aging and physical inactivity on the age-related reduction in muscular strength, arm flexor muscle torques may serve as an indicator of ageing effects while leg muscle torque reflects the combined effect of ageing and physical activity. If this hypothesis holds, a stronger decline of maximal torque has to be expected for leg muscles. Indeed, this was seen in both healthy controls and cardiac patients, which is shown for men in fig. 5.
Fig 5.
Age related changes in maximal torques of arm flexor and knee extensor muscles in males. Torques of the age group 30 - 39 years were taken for comparison.
As a consequence, the obtained difference in leg muscle torque between healthy controls and cardiac patients not necessarily indicates a pathological skeletal muscle metabolism in patients as proposed by Coats et al. 19. The obtained sequence of maximal leg muscle torque may simply reflect the overall physical activity in the long run and/or an incomplete rehabilitation in spite of the three week stationary program. The identity of arm torques within the certain age groups is in line with this hypothesis since any systemic skeletal muscle pathology should have worsened skeletal muscle strength of the whole body.
Strength training may be regarded as critical for patients due to elevated blood pressures during exercise or a subsequently sudden drop in blood pressure after cessation of exercise. The medical safety of strength training can be improved by the intermittend method described by Baum et al. 15: Compared to traditional strength training the use of short relaxation periods after each concentric-eccentric cycle leads to significantly reduced blood pressure increases even at loads of 70 % to 80 % of the one repetition maximum.
Limitations of the study
We have no information about sports activities of the control subjects except that they were not involved in strength training. However, physical activity measures based on self-reported questionnaires just modest correlate with objective measures 28, 29. We can not exclude that some control subjects may have underestimated their experience with strength training. On the other hand it has to be considered that subjects were recruited during health promoting lectures. These events may primarily be attractive for those, who wants to be active but actually follow a more sedentary life style. Alltogether, it can be expected that there is no important bias due to the recruitment process of control subjects.
The data of cardiac patients were obtained at the final stage of a 3-week stationary program including strength and endurance training. Therefore, pre-rehabilitation values will even be lower than those of the actual study. Furthermore, due to the selection process the present findings probably overestimate the muscular performance capacity of cardiac patients: Beside the inclusion criteria all patients had to agree to exercise on a regular basis for 18 month after stationary rehabilitation, which tends to discourage patients with poor fitness and only minor interest in physical activities. This assumption is supported by the facts that just 20,7 % of our patients had no experience in regular sports activities prior to the disease.
Implications for cardiac rehabilitation
The significant lower leg muscle torques in patients compared to control subjects of identical age groups were obtained although patients already completed the stationary rehabilitation program including strength training.
According to the threshold theory proposed by Buchner et al. 30 people start to have difficulties in functional tasks beneath a critical level of muscular strength. In younger stages, the actual level will be well above the critical figure but the risk increases with age. In this regard, a prospective study over five years yielded that the initial muscle strength was a strong predictor for the onset of a dependent way of living 31. Since most cardiac patients are at middle or higher ages, the present results give strong support to the concept that strengthening of skeletal muscles - especially of weight bearing muscles - has to be considered as a longlasting equivalent part of cardiac rehabilitation beside endurance training 20, 21, 22, 23, 24, 25, 26, 27, 32.
Acknowledgments
This study was supported by a grant of Pfizer Pharma GmbH. The authors gratefully acknowledge the cooperativeness of all subjects and the excellent technical help of our clinical staffs enrolled in the study.
References
- 1.Buchner DM, Larson BE, Wagner EH. et al. Evidence of a non-linear relationship between leg strength and gait speed. Age Ageing. 1996;25:386–91. doi: 10.1093/ageing/25.5.386. [DOI] [PubMed] [Google Scholar]
- 2.Anker SD, Swan JW, Volterrani M. et al. The influence of muscle mass, strength, fatigability, and blood flow on exercise capacity in cachectic and non-cachectic patients with chronic heart failure. Eur Heart J. 1997;18:259–69. doi: 10.1093/oxfordjournals.eurheartj.a015229. [DOI] [PubMed] [Google Scholar]
- 3.Buller NP, Jones D, Poole-Wilson PA. Direct measurement of skeletal muscle fatigue in patients with chronic heart failure. Br Heart J. 1991;65:20–4. doi: 10.1136/hrt.65.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drexler H, Riede U, Münzel T. et al. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992 May;85:1751–9. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- 5.Mancini DM, Walter G, Reichek N. et al. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992;85:1364–73. doi: 10.1161/01.cir.85.4.1364. [DOI] [PubMed] [Google Scholar]
- 6.Meyer K. Resistance exercise in chronic heart failure -landmark studies and implications for practice. Clin Invest Med. 2006;29:166–9. [PubMed] [Google Scholar]
- 7.Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation. 1990;81:518–27. doi: 10.1161/01.cir.81.2.518. [DOI] [PubMed] [Google Scholar]
- 8.Volterrani M, Clark AL, Ludman PF. et al. Predictors of exercise capacity in chronic heart failure. Eur Heart J. 1994;15:801–9. doi: 10.1093/oxfordjournals.eurheartj.a060588. [DOI] [PubMed] [Google Scholar]
- 9.Harrington D, Anker SD, Chua TP. et al. Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. J Am Coll Cardiol. 1997;30:1758–64. doi: 10.1016/s0735-1097(97)00381-1. [DOI] [PubMed] [Google Scholar]
- 10.Okada Y, Toth MJ, Vanburen P. Skeletal Muscle Contractile Protein Function is preserved in Human Heart Failure. J Appl Physiol. 2008;104:952–7. doi: 10.1152/japplphysiol.01072.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghroubi S, Chaari M, Elleuch H. et al. The isokinetic assessment of peripheral muscle function in patients with coronary artery disease: correlations with cardiorespiratory capacity. Ann Readapt Med Phys. 2007;50:295–301. doi: 10.1016/j.annrmp.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Gayda M, Merzouk A, Choquet D, Ahmaidi S. Assessment of skeletal muscle fatigue in men with coronary artery disease using surface electromyography during isometric contraction of quadriceps muscles. Arch Phys Med Rehabil. 2005;86:210–5. doi: 10.1016/j.apmr.2004.07.351. [DOI] [PubMed] [Google Scholar]
- 13.Folland JP, Williams AG. The adaptation to strength training: Morphological and neurological contributions to increased strength. Sports Med. 2007;37:145–68. doi: 10.2165/00007256-200737020-00004. [DOI] [PubMed] [Google Scholar]
- 14.Berg HE, Eiken O, Miklavcic L, Mekjavic IB. Hip, thigh and calf muscle atrophy and bone loss after 5-week bedrest inactivity. Eur J Apl Physiol. 2007;99:183–9. doi: 10.1007/s00421-006-0346-y. [DOI] [PubMed] [Google Scholar]
- 15.Baum K, Rüther Th, Essfeld D. Reduction in blood pressure response during strength training through intermittend muscle relaxations. Int J Sports Med. 2003;24:441–5. doi: 10.1055/s-2003-41172. [DOI] [PubMed] [Google Scholar]
- 16.Bjarnason-Wehrens B, Mayer-Berger W, Meister E.R, Baum K, Hambrecht R, Gielen S. Recommendations for resistance exercise in cardiac rehabilitation. Recommendations of the German Federation for Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil. 2004;11:352–61. doi: 10.1097/01.hjr.0000137692.36013.27. [DOI] [PubMed] [Google Scholar]
- 17.Alkner BA, Tesch PA. Knee Extensor and Plantar Flexor Muscle Size and Function Following 90 days of Bed Rest With and Without Resistance Exercise. Eur J Appl Physiol. 2004;93:294–305. doi: 10.1007/s00421-004-1172-8. [DOI] [PubMed] [Google Scholar]
- 18.Tesch PA, von Walden F, Gustafsson T. et al. Skeletal muscle proteolysis in response to short-term unloading in humans. J Appl Physiol. 2008;105:902–6. doi: 10.1152/japplphysiol.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coats AJ, Clark AAL, Piepoli M. et al. Symptoms and quality of life in heart failure: the muscle hypothesis. Br Heart J. 1994;72(Suppl 2):36–9. doi: 10.1136/hrt.72.2_suppl.s36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartlo P. Evidence-based application of aerobic and resistance training in patients with congestive heart failure. J Cardiopulm Rehabil Prev. 2007;27:368–75. doi: 10.1097/01.HCR.0000300263.07764.4a. [DOI] [PubMed] [Google Scholar]
- 21.Beniamini Y, Rubenstein JJ, Zaichkowsky LD, Crim MC. Effects of high-intensity strength training on quality-of-life parameters in cardiac rehabilitation patients. Am J Cardiol. 1997;80:841–6. doi: 10.1016/s0002-9149(97)00533-x. [DOI] [PubMed] [Google Scholar]
- 22.Delagardelle C, Feiereisen P, Autier P. et al. Strength/endurance training versus endurance training in congestive heart failure. Med Sci Sports Exerc. 2002;34:1868–72. doi: 10.1097/00005768-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Feiereisen P, Delagardelle C, Vaillant M. et al. Is strength training the more efficient training modality in chronic heart failure? Med Sci Sports Exerc. 2007;39:1910–7. doi: 10.1249/mss.0b013e31814fb545. [DOI] [PubMed] [Google Scholar]
- 24.Fragnoli-Munn K, Savage PD, Ades PA. Combined resistive-aerobic training in older patients with coronary artery disease early after myocardial infarction. J Cardiopulm Rehabil. 1998;18:416–20. doi: 10.1097/00008483-199811000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Gunn E, Smith KM, McKelvie RS, Arthur HM. Exercise and the heart failure patient: aerobic vs strength training-is there a need for both? Prog Cardiovasc Nurs. 2006;21:146–50. doi: 10.1111/j.0889-7204.2006.04678.x. [DOI] [PubMed] [Google Scholar]
- 26.Jankowska EA, Wegrzynowska K, Superlak M. et al. The 12-week progressive quadriceps resistance training improves muscle strength, exercise capacity and quality of life in patients with stable chronic heart failure. Int J Cardiol. 2008;130:36–43. doi: 10.1016/j.ijcard.2007.07.158. [DOI] [PubMed] [Google Scholar]
- 27.Miche E, Roelleke E, Wirtz U. et al. Combined endurance and muscle strength training in female and male patients with chronic heart failure. Clin Res Cardiol. 2008;97:615–22. doi: 10.1007/s00392-008-0660-y. [DOI] [PubMed] [Google Scholar]
- 28.Siconolfi SF, Lasater TM, Snow RC, Carleton RA. Self-reported physical activity compared with maximal oxygen uptake. Am J Epidemiol. 1985;122:101–05. doi: 10.1093/oxfordjournals.aje.a114068. [DOI] [PubMed] [Google Scholar]
- 29.Aadahl M, Kjaer M, Kristensen JH, Mollerup B, Jorgensen T. Self-reported physical activity compared with maximal oxygen uptake in adults. Eur J Cardiovasc Prev Rehabil. 2007;14:422– 8. doi: 10.1097/HJR.0b013e3280128d00. [DOI] [PubMed] [Google Scholar]
- 30.Buchner DM, Larson BE, Wagner EH. et al. Evidence of a non-linear relationship between leg strength and gait speed. Age Ageing. 1996;25:386–91. doi: 10.1093/ageing/25.5.386. [DOI] [PubMed] [Google Scholar]
- 31.Rantanen T, Avlund K, Suominen H. et al. Muscle strength as a predictor of onset of ADL dependence in people aged 75 years. Agin Clin Exp Res. 2002;14:10–5. [PubMed] [Google Scholar]
- 32.Meyer K. Resistance exercise in chronic heart failure-landmark studies and implications for practice. Clin Invest Med. 2006;29(3):166–9. [PubMed] [Google Scholar]