Abstract
Octopus, squid and cuttlefish are renowned for rapid adaptive coloration that is used for a wide range of communication and camouflage. Structural coloration plays a key role in augmenting the skin patterning that is produced largely by neurally controlled pigmented chromatophore organs. While most iridescence and white scattering is produced by passive reflectance or diffusion, some iridophores in squid are actively controlled via a unique cholinergic, non-synaptic neural system. We review the recent anatomical and experimental evidence regarding the mechanisms of reflection and diffusion of light by the different cell types (iridophores and leucophores) of various cephalopod species. The structures that are responsible for the optical effects of some iridophores and leucophores have recently been shown to be proteins. Optical interactions with the overlying pigmented chromatophores are complex, and the recent measurements are presented and synthesized. Polarized light reflected from iridophores can be passed through the chromatophores, thus enabling the use of a discrete communication channel, because cephalopods are especially sensitive to polarized light. We illustrate how structural coloration contributes to the overall appearance of the cephalopods during intra- and interspecific behavioural interactions including camouflage.
Keywords: iridescence, multilayer reflector, light diffusion, leucophore
1. Introduction
Animals can be extraordinarily colourful. There are innumerable examples in both the vertebrate and invertebrate worlds, and, while in some animals these colours function to camouflage their owners, in others they have clear functions in signalling, and some may even perform both functions simultaneously.
Colour is produced by either a structural or pigmentary medium. Structural coloration involves materials that are themselves colourless—the colours are created by coherent scattering. A pigmented material has selective absorbance properties that determine the spectral reflectance of the incident light.
Cephalopods (squid, cuttlefish, octopus) show an impressive repertoire of body patterns for camouflage and signalling, despite their apparent colour blindness (Holmes 1940; Brown & Brown 1958; Packard & Hochberg 1977; Hanlon 1982; Moynihan 1985; Hanlon & Messenger 1988, 1996; Marshall & Messenger 1996; Hanlon & Shashar 2003; Mäthger et al. 2006; but see examples of cephalopod with colour vision, e.g. Kito et al. 1992; Michinomae et al. 1994). What is even more impressive is their ability to almost instantaneously change colour and pattern. This is mediated by the dual action of thousands of chromatophores, which are small pigmented organs (grouped into two or three colour classes depending on the species: red; yellow/orange; and brown/black), and structural reflector cells (iridophores and leucophores) (Williams 1909; Schäfer 1937; Cloney & Brocco 1983; Messenger 2001; figure 1). The appearance of the animal thus depends on which skin elements affect the light incident on the skin. Light may be reflected by either chromatophores or structural reflectors, or a combination of both, and it is the physiological changeability of the chromatophores and iridophores that enables these animals to produce such a wide repertoire of optical effects.
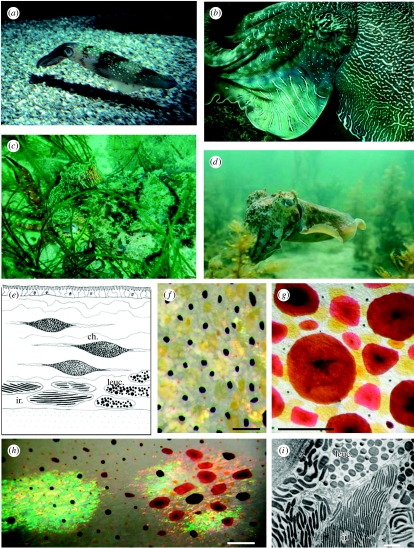
Figure 1.
(a) Iridescent spots in the squid Loligo pealeii. (b) Blue–green iridescence and white scattering leucophore stripes in cuttlefish (Sepia apama). (c) Camouflaged S. apama with pink iridescent arms and white markings caused by leucophores. (d) White leucophores in S. apama. (e) Skin in cross section showing the location of chromatophores (ch.) and structural reflectors (ir., iridophores; leuc., leucophores) in cephalopods. (f) Close-up of cuttlefish skin (Sepia officinalis) showing chromatophores (yellow, expanded; dark brown, partially retracted; orange, retracted) and white leucophores. Scale bar, 1 mm. (g) Brown, red and yellow chromatophores of squid (L. pealeii). Scale bar, 1 mm. (h) Combination of chromatophores and iridophores to illustrate the range of colours. Scale bar, 1 mm. (i) Electron micrograph showing iridophore plates (ir.) and spherical leucophores (leuc.) of cuttlefish (S. officinalis) skin. Scale bar, 1 μm (image courtesy of Alan Kuzirian).
A chromatophore consists of a pigment-containing sac that has dozens of radial muscles attached to its periphery. These muscles are innervated directly by the brain, and by contracting and relaxing the chromatophore muscles the pigment sac increases or decreases in area (Florey 1969) in less than a second (Hill & Solandt 1935). Chromatophore size varies among cephalopods. In squid, such as Loligo plei, an expanded chromatophore may be up to 1.5 mm in diameter, while a retracted chromatophore may be barely visible to the naked eye, measuring as little as a tenth of a millimetre (Hanlon 1982; Mäthger & Hanlon 2007). By selectively expanding and retracting distinct groups of chromatophores, cephalopods can produce an array of patterns, such as bands, stripes and spots.
In this review, we deal mainly with the other optical system that interacts with light—the different types of structural reflectors. Various cell arrangements have been identified and described in the cephalopod literature: (i) those that produce iridescence, a working definition of which would be the production of rainbow-like colours or a metallic sheen, and (ii) those that produce scattering broadband diffuse reflectance, the most striking of which are the distinct white body markings of many cephalopod species.
2. Iridescence
There has been some confusion about the various terms that have been introduced in the literature to describe cephalopod skin structures that produce iridescence. For example, Cloney & Brocco (1983) used the term ‘reflector cell’ (containing plates that are arranged into distinct groups, called reflectosomes) to describe the cells that produce iridescence by thin-film interference, whereas ‘iridocytes’ were described as cells that function by diffracting light. Iridescent cells whose optical mechanisms were unclear were termed ‘iridophores’ following these authors' definition; iridophores contain a number of iridosomal plates that are grouped into iridosomes. These plates (along with the spaces separating them) are ultimately responsible for the observed iridescence. Since the 1980s, there have been several studies looking at the ultrastructure, optical mechanisms and composition of iridescent cells in cephalopods, some of which have revealed inconsistencies with the definitions of Cloney & Brocco (1983). For example, in their paper, the iridescent cells of squid were described as iridocytes, implying that they function as diffraction gratings. This has not been confirmed—on the contrary, all existing papers show evidence that squid reflectors are multilayer reflectors, i.e. they reflect light by thin-film interference, which would warrant the term reflector cell. However, in the more recent literature, these cells are consistently referred to as iridophores. This review will be consistent with these recent publications insofar as iridophores are defined as the cells that produce iridescence.
In this paper, we deal mainly with multilayer reflectance, rather than diffraction, because to date, there is no convincing evidence that diffraction is responsible for iridescence in cephalopod skin. Diffraction gratings differ from multilayer reflectors, in that the structures producing iridescence are oriented in the same plane as the surface. They are characterized by an orderly array of ridges that are ‘engraved’ into the surface. Diffraction gratings produce spectra on the left and right sides of the zero order (the direction of the incident light beam) and have been shown to be the basis of iridescence in some beetles, wasps, ostracod crustaceans and butterflies (Hinton & Gibbs 1969; Parker 1995; Brink & Lee 1999). Certainly, considering the large number of modern cephalopod species (over 700) (Hanlon & Messenger 1996), it may nevertheless be possible that both diffraction gratings and multilayer reflectors play a role in creating iridescence, but evidence thus far suggests that only multilayer reflectors are involved.
2.1 ‘Spectral’ iridescence
Cephalopods have iridophores in many parts of the body and they have precise arrangements that may signify specific functions. Squid generally have only iridophores, i.e. they do not have the broadband reflecting leucophores found in octopus and cuttlefish (some species of the squid genus Sepioteuthis may be an exception; see §3). In most parts of the body (e.g. mantle and head), the iridophores are located in a distinct layer beneath the pigmented chromatophores (Williams 1909; Schäfer 1937; Mirow 1972a,b; Hanlon 1982; Cloney & Brocco 1983). Iridophores are colourless cells that vary in size but are generally smaller than 1 mm (Mirow 1972b; Cooper et al. 1990). They contain stacks of thin plates that reflect light by thin-film interference (Denton & Land 1971; Land 1972; Mäthger & Denton 2001; Mäthger et al. 2004). Light reflected from a multilayer reflector is almost always coloured, as long as two prerequisites are met: (i) there is a difference in the refractive index between the plates and the spaces separating them, and (ii) the plates and spaces have a specific thickness for the constructive interference of light to occur. The mechanism of reflectance is the same as that of coloured soap bubbles. If the soap film (or multilayer plate) is very thin, shorter wavelengths are reflected, e.g. blue light; if it is thicker, longer wavelengths, such as yellow and red, are reflected (Boys 1959; Huxley 1968).
Multilayer reflectors have distinct optical features, the most obvious of which is the effect of changing the angle of illumination/observation on the spectrum of the reflected light. The more oblique the angle, the shorter the peak wavelengths of the reflected light, i.e. a multilayer reflector that appears red at near-normal viewing angles will appear first yellow, then green and blue at increasingly oblique angles (figure 2a). This may seem counterintuitive, and, for the interested reader, we point out that the book by Boys (1959) has a beautifully written section on this optical phenomenon. Furthermore, at around Brewster's angle (angle at which maximum linear polarization occurs), the reflected light is highly polarized (figure 2a), an interesting property that may have some behavioural function because cephalopods have the ability to detect polarized light (see below).
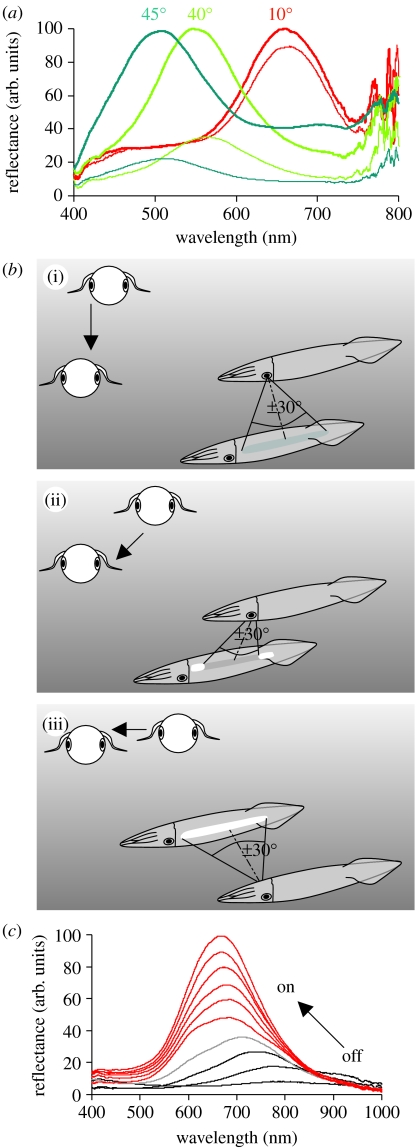
Figure 2.
(a) Spectral reflectance of iridophores (L. pealeii) at different angles of incidence and planes of polarization, showing that with increasing angle of incidence (i.e. 40° and 45°) the reflected light shifts towards the shorter end of the spectrum and becomes polarized. Two reflectance spectra are shown for each angle of incidence: the spectrum reflected in the plane parallel to the plane of incidence and the perpendicular plane of incidence. At oblique angles (40° and 45°), the spectral reflectance in the perpendicular plane is much reduced in comparison with the parallel plane, indicating that the reflected light is linearly polarized. (b) The visibility of the ‘red’ stripe of squid from different orientations taking into account the light distribution in the sea. (i) An observer looking down on a squid will not see any iridescence. (ii) An observer looking down at a squid from a 45° angle will see iridescence from the most anterior and posterior ends of the stripe. (iii) An observer looking directly from the side will see strong iridescence from the entire stripe. See Mäthger & Denton (2001) for more details (modified from Mäthger & Denton 2001). (c) Acetylcholine (1 mM) changes iridescence from non-reflective (black lines, reflectance in IR) through various IR steps (black and grey lines) to red reflective in the squid L. pealeii. Measurements taken at 15 s intervals.
Some species of squid (such as Loligo vulgaris, Loligo forbesii, Loligo pealeii, Alloteuthis subulata, Sepioteuthis australis, Loliolus noctiluca, Euprymna tasmanica, Todaropsis eblanae and probably others) appear to have prominent red-reflecting iridophore stripes arranged longitudinally on each side of the mantle (figure 2a,b). These are arranged in either distinct stripes or ‘splotches’. Mäthger & Denton (2001) suggested that these iridophores may aid in communication between individuals of a school. Although the availability of daylight in the red parts of the spectrum is much reduced at the depths at which these squid live, the majority of the reflective plates of these iridophores are oriented approximately parallel to the skin surface, which means that shorter wavelengths (green and blue) are reflected in a horizontal direction. This would make the stripes highly visible when viewed horizontally, because green and blue are the most prominent wavelengths found in the light environments these animals inhabit (such as the example given in figure 2b, calculated for a depth of 19 m; Mäthger & Denton 2001). Furthermore, the patterns change dramatically with direction and movement of the squid, so that they may be used by squid to coordinate the movements of individuals of a school.
Squid are able to change their iridescence depending on behavioural context, showing iridescence especially during agonistic encounters (Hanlon 1982). In vitro, iridescence is changed by the topical application of acetylcholine (ACh) acting on muscarinic cholinergic receptors (Hanlon 1982; Cooper & Hanlon 1986; Cooper et al. 1990; Hanlon et al. 1990; Mäthger et al. 2004). However, in contrast to the chromatophores that can change within a fraction of a second, iridophore reflectance changes take longer, e.g. several seconds to minutes. In L. pealeii, the reflected wavelengths have been shown to shift by over 100 nm (Mäthger & Hanlon 2007), from non-reflective to red and orange after the application of ACh. The reflectance changes in vitro typically take several seconds (up to 1–2 min). Interestingly, in L. pealeii, the collar region (anterior end) of the mantle has highly reflective iridophores in a tightly packed arrangement, and this group has been shown to reflect in the IR parts of the spectrum (approx. 800 nm) when they appear non-reflective to the human eye (figure 2c).
To date, it is not known exactly how this wavelength change is achieved. Recent evidence has provided confirmation that the plates of squid iridophores are made up of proteins called reflectins (Crookes et al. 2004); see also Cooper et al. (1990), who pointed out that a protein state change (affecting refractive index) combined with a change in the thickness of plates could explain the observed changes in reflectance.
The squid reflectin protein has received interest from researchers in the fields of materials science and optical nanotechnology. For example, Kramer et al. (2007) showed that reflectin proteins have self-assembling properties and that they can be processed into thin films, photonic grating structures and fibres that could find various applications in society.
Another way of changing iridescence is by selectively expanding and retracting the overlying chromatophores. Since chromatophores are innervated directly from the brain, this effect can be immediate. Chromatophores are generally located in a distinct layer above the iridophores and, by selective expansion, they can either change the reflected spectrum of the iridescence (figure 3a–e; see also Mäthger & Hanlon 2007), create contrast against which iridescence is viewed (see, for example, the blue iridescent rings of the blue-ringed octopus; figure 4b) or block it altogether, such as a brown chromatophore expanding over reflective iridophores (figure 3f).
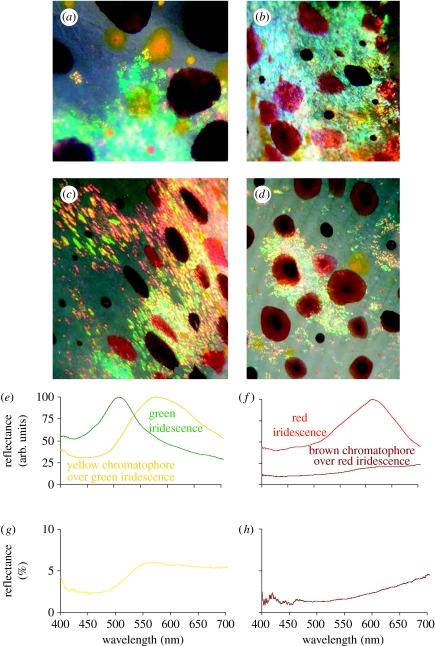
Figure 3.
(a–d) Close-up images of L. pealeii skin showing chromatophores and iridophores. Chromatophores can be used to modulate iridescence. Light reflected from iridophores filtered through (e) a yellow and (f) a brown chromatophore. Reflectance spectra of (g) yellow and (h) brown chromatophore.
Figure 4.
(a) Blue-ringed octopus, Hapalochlaena lunulata, well camouflaged in a laboratory tank. Note the muted iridescent blue rings. (b) Bright blue iridescence is typically seen when a blue-ringed octopus flashes its rings. (c) Electron micrograph of the blue rings, showing closely packed iridophore plates (scale bar, 1 μm).
Some of the aforementioned squid (L. vulgaris, L. pealeii, A. subulata and L. noctiluca) also have a blue reflective stripe along the side of the mantle (see Mäthger & Denton 2001). These iridophores are best seen when observed and illuminated from above the animal (rather than from a horizontal direction where the ‘red’ stripes are visible), and we speculate that they may function in ways similar to the red stripes described above: since the reflectance pattern and intensity change with the movements of the animal, other members of a school may be able to use this information to coordinate their movements.
In all pelagic squid studied so far (namely L. vulgaris, L. pealeii and L. noctiluca), the ventral iridophores reflect red light, when viewed and illuminated from the side (see Mäthger & Denton 2001). The iridophores making up the ventral side are densely packed and their flat surfaces make an angle of approximately 60° with the skin surface. Owing to this orientation, when the iridophores are viewed at oblique angles of incidence (such as from below), the light reflected from the iridophores comes from the inside of the mantle (see fig. 16 in Mäthger & Denton 2001). This may have an interesting function. Midwater animals are most visible when seen from below against the background of the strongest radiance in the sea. Any downwelling light that is absorbed by the animal cannot be replaced by the reflections of light from any other direction (bioluminescence is the only solution to this problem). Being transparent makes a midwater animal less likely to be detected by the predators lurking below, and in comparison with other muscular animals, such as fishes, cephalopods can be very transparent. However, total transparency is impossible because the internal organs (e.g. ink sac, digestive tracts) cannot be made transparent. The dorsal mantle iridophores transmit a great portion of the incoming light into the mantle cavity. The light that enters the mantle cavity then falls on the ventral iridophores obliquely and is channelled downwards. This may help mitigate shadows cast to an observer below the animal (Mäthger & Denton 2001).
Benthic cephalopods, such as Sepia officinalis and E. tasmanica, also have densely packed iridophores lining their ventral mantle, but instead of reflecting red light as the above squid species, they strongly reflect green light when viewed and illuminated side-on. In E. tasmanica, for example, the iridophores lie at an angle of approximately 50° with the skin surface. Sepia and Euprymna are much more opaque than most pelagic cephalopods, and it appears unlikely that any considerable amount of light enters the mantle cavity to be channelled downwards in the manner described above, so their function is unknown.
The blue-ringed octopus is well known for its potentially deadly bites and flashing blue rings. It was previously suggested that this blue iridescence may be the result of the Tyndall scattering (Herring 1994). However, we found that ultrastructural analysis of the rings and lines of Hapalochlaena fasciata (the blue-lined octopus) reveals densely packed reflective plates (up to approx. 30 in one iridophore) grouped together in a parallel arrangement, suggesting that the blue iridescence is caused by multilayer constructive interference (figure 4), which would warrant the term reflector cell following Cloney & Brocco's (1983) definition (however, see above for the reasoning behind calling iridescent cells iridophores). Spectrometer measurements have confirmed this (data not shown). Each iridophore appears to be oriented at a different angle relative to the surface of the skin, and this correlates well with the optical appearance of the rings: the blue iridescence is visible from a wide range of angles. The reflective plates have thicknesses of approximately 70 nm. This appears to be the thickness required for this stack to act as an ideal quarter-wave reflector for which λmax=4nd, where n is the refractive index and d is the actual thickness of the plate. Assuming a refractive index of 1.59 (Kramer et al. 2007), the wavelength of maximum reflectance of the blue-ringed iridophores would be at 445 nm.
Tyndall scattering may nevertheless be a method of producing the blue iridescence in some cephalopod species. For example, Octopus bimaculatus has two characteristic blue rings (often referred to as ‘ocelli’) on each side of its head, near the eyes. This has been attributed to the Tyndall scattering from fine purine granules (Fox & Vevers 1960; Fox 1976; Packard & Hochberg 1977). There may also be other squid species in which the Tyndall scattering produces the blue iridescence. Herring (1994) suggested that the blue flashes of the squid Onychia caribbaea are produced by the expansion of dark chromatophores beneath a blue-reflecting Tyndall scatterer.
2.2 Iridophore polarization and optical enhancement of chromatophores
For human observers, creating colour is probably the most notable aspect of cephalopod iridophores for our eyes. However, when viewed at oblique angles, cephalopod iridophores also polarize light (Shashar & Hanlon 1997; Mäthger 2001; Shashar et al. 2001; Mäthger & Hanlon 2006; Chiou et al. 2007). Light is polarized most strongly at Brewster's angle (θ), which is simply defined as follows: tan θ=n1/n2, where n1 and n2 are the refractive indices of the two media (e.g. going from a watery surrounding, n=1.33, to protein, n=1.59, Brewster's angle is approx. 50°).
For cephalopods, this may have a useful behavioural function. Cephalopods have a rhabdomeric visual system that allows them to detect linearly polarized light (Moody & Parriss 1960; Shashar & Cronin 1996; Shashar et al. 1998, 2002), and it is therefore conceivable that the polarized aspect of iridescence may have communication functions (Cronin et al. 2003; Boal et al. 2004). This has also been termed a ‘hidden communication channel’ because cephalopod predators, such as teleost fish, sharks and marine mammals, are believed not to be polarization sensitive and would therefore not be able to perceive this sort of visual information (Shashar et al. 1996; Land & Nilsson 2002; Boal et al. 2004; Mäthger & Hanlon 2006).
In cephalopod skin, polarization is a ‘side product’ of iridescence, and iridescence can be very conspicuous. This is where having a variety of optical structures in the skin is beneficial. In a study on L. pealeii, it was shown that the polarized aspect of iridescence is maintained after passing through the overlying pigmented chromatophores, which produce the most visible and dynamically changeable aspect of camouflage patterns in cephalopods (Mäthger & Hanlon 2006, 2007). Cephalopods are polarization sensitive and can regulate polarization via skin iridescence (Shashar & Hanlon 1997; Mäthger & Denton 2001; Chiou et al. 2007). Two behavioural functions come to mind. First, it is conceivable that they could send polarized signals to conspecifics while using chromatophores to regulate the potentially conspicuous iridescence and stay camouflaged to fishes or mammalian predators, most of which are probably not polarization sensitive (Land & Nilsson 2002). Second, they could use polarization signals during intense intraspecific interactions (sexual selection); Boal et al. (2004) found that females showed more polarization signals than males and also changed their behaviour in response to polarized patterns. Boal et al.'s results suggest (but did not prove) that females may use polarized signals as a source of information about conspecifics (e.g. species, sex, location, size).
Iridophores are generally located just beneath the pigmented chromatophores (there are some exceptions to this) and the final appearance of the animal is frequently the result of the optical interactions between iridophores and chromatophores. Chromatophores are very thin when expanded and can filter light reflected from the iridophores; or, turning this idea around, iridophores can enhance the chromatophores' appearance. Mäthger & Hanlon (2007) showed evidence that three colour classes of pigments (yellow, red and brown), combined with a single type of reflective cell (with dynamic iridescence), produce colours that encompass the whole of the visible spectrum, enabling the extreme visual diversity for dynamic camouflage and signalling.
3. Broadband reflectance and scattering
3.1 Broadband ‘silvery’ reflectors
Cephalopods have very striking silvery iridescence around their eyes (figure 5a,b). This was studied in detail for A. subulata and L. vulgaris and it was shown that, unlike the iridophores described above, the reflected wavelengths do not change with angle of incidence/view (Mäthger 2001) and the reflected light is not polarized at oblique angles (figure 5c). When viewed closely, the skin shows some variations in colour on a scale of a few micrometres (figure 5b). Some parts reflect red, others green and blue and the reflective properties of each of these microscopic parts are typical of multilayer reflectors: with increasing angle of incidence, the reflected wavelengths move towards the shorter end of the spectrum and become polarized. When viewed from a distance, however, the various coloured parts merge together and the net effect is the reflectance of unpolarized white light. Ultrastructurally, we can see that the stacking sequence of the plates and spaces is responsible for the silvery appearance (figure 5d). The plates are long (more than 10 μm) and wavy with varying thicknesses (ranging from 80 to 130 nm) and irregular spaces between them, which facilitates this broadband reflectance. This natural pointillistic approach to creating colour can also be seen in Ribbonfish (Trichiurus spp.), whose silvery surface is covered with fine streaks that show a variety of colours when examined microscopically (Nicol & Van Baalen 1968).
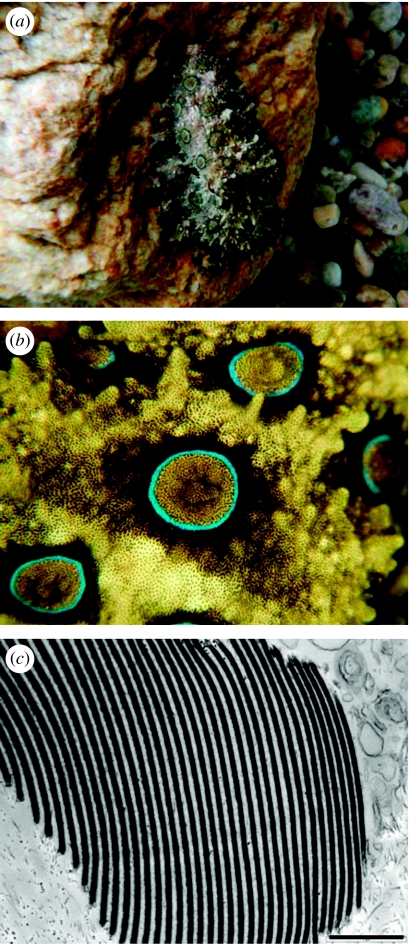
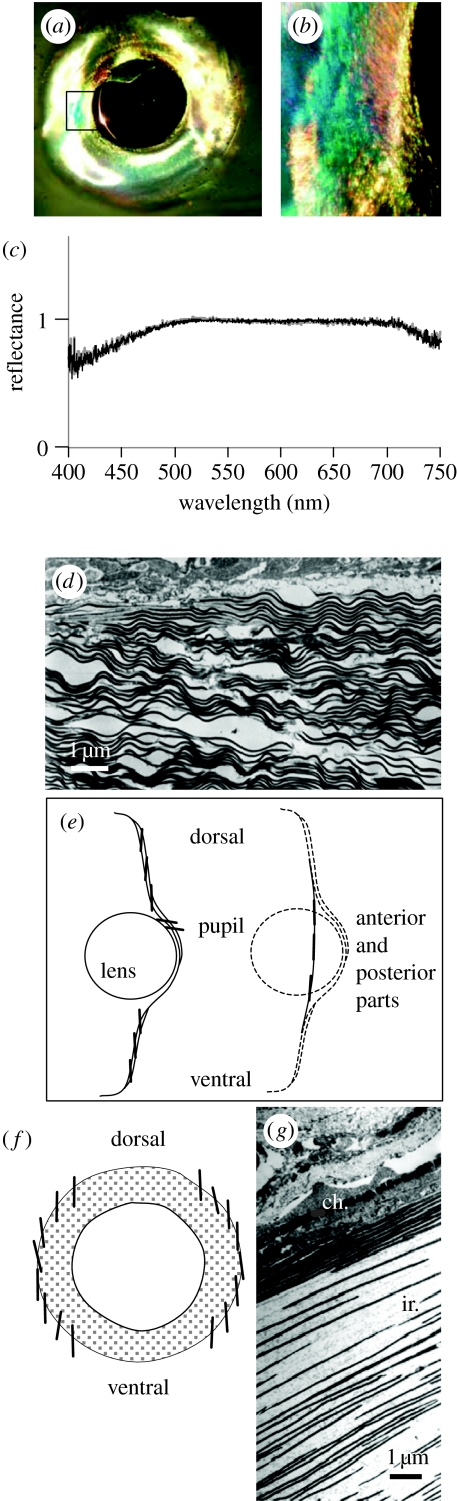
Figure 5.
(a) Silvery iridescence around the eyes of squid (L. pealeii). (b) Close-up of the section highlighted in (a) showing variations in spectral reflectance. (c) Spectral reflectance on both planes of polarization at 45° incidence (black and grey lines), showing that the light reflected from silvery reflectors is not polarized. (d) Electron micrograph of silvery reflectors in cross section, showing wavy arrangement of reflective plates. (e) Orientation of the reflective plates of silvery eyes obtained using the techniques described in the text. The reflective plates (short, thick black lines) are tilted towards the vertical, much like the reflectors on the scales of the silvery fish. (f) Orientation of the reflective plates of golden silvery stripe along the lateral side of the oceanic squid Todaropsis. Reflective plates are also oriented towards the vertical. (g) Electron micrograph of Todaropsis golden silvery stripe, showing a chromatophore (chr.) and iridophore plates (ir.).
The orientation of the reflectors relative to the surface of the skin offers a clue to the possible function of this iridescence (figure 5e). These measurements were obtained following the methods described originally by Denton & Nicol (1962). A piece of silver foil is placed adjacent to the reflective area under investigation, and, using a goniometer, the difference in angle between the light reflected from the foil (which equals the skin surface) and the reflector (which may or may not be angled with respect to the skin surface) is measured. These measurements show that the reflective plates are tilted towards the true vertical. Figure 5e shows that the reflectors in the dorsal and ventral areas are oriented at an angle with respect to the skin surface, while those in the anterior and posterior areas lie parallel. This arrangement would give close to perfect camouflage, much like that in silvery fish: the reflectors reflect light as a mirror suspended vertically in the sea. The orientation of the reflective plates is such that the light reflected from almost any angle equals the intensity of the background light against which the animal is viewed (Denton & Nicol 1962, 1965, 1966; Denton 1970; Denton & Land 1971; Denton et al. 1972). The organization of the plates furthermore minimizes the polarization of the reflected light, which reduces the squid's chances of being detected by the predators that are polarization sensitive.
Cephalopods have pupils that respond to the changes in light levels as well as under specific behavioural contexts. In squid (L. vulgaris, L. pealeii, A. subulata), these pupils are lined with silvery iridophores that are tilted towards the horizontal. The iridophores are weakly reflective when the pupil covers the eye but reflect strongly when the pupil is open, for example during low light levels or aggressive encounters. In such situations, the iridophores may function to prevent strong downwelling light from entering the eye. The pupil of Octopus vulgaris is also lined with silvery iridophores (Froesch & Messenger 1978), although the plates are much shorter (less than 3 μm in length) and do not have the wavy structure as those of squid.
Longley (1917) found that the internal organs of some transparent fishes (e.g. Coralliozetus cardonae) are camouflaged by the principle of countershading. This is also true of the ink sac of cephalopods: the dorsal area is black; the sides are silvery; and the ventral area is white (Denton 1970; Denton & Land 1971; Mäthger 2001). In A. subulata, the silvery iridophores of the ink sac reflect light in a way similar to that of the iridophores around the eyes and their structural arrangement is also similar, with plates ranging in thickness from 85 to 120 nm with irregular spaces between the wavy plates (Mäthger 2001).
At least some cephalopods from the families Ommastrephidae, Sepiolidae and Pyroteuthidae (e.g. T. eblanae, Sthenoteuthis pteropus, Ommastrephes bartrami, Heteroteuthis dispar and Pterygioteuthis microlampas) have a very striking silvery golden stripe that runs along each side of the mantle and head. As is common in silvery fish, the iridophores of this stripe are tilted towards the vertical (figure 5f), suggesting an obvious role in camouflage. The iridophores of this stripe are very densely packed and, unlike other iridophores, no single iridophore cell can be distinguished by eye. Figure 5g shows that the plates are the thickest (approx. 140 nm) nearer the skin surface and become thinner farther from the skin surface (to as thin as 70 nm) (Mäthger 2001).
3.2 Broadband reflectance from photophore reflectors
In habitats with ample supply of daylight, such as the coastal areas and the upper layers of the sea, camouflage and communication signals can be created by reflecting light. However, at night and at greater depths to which daylight does not penetrate, this is nearly impossible, and, instead, many animals, such as crustaceans, cephalopods and fishes, use luminescence for camouflage and communication, either through intracellular mechanisms, extracellular excretions or symbiosis with bioluminescent bacteria (e.g. Nicol 1960; Herring 1988, 2000). Bioluminescence is particularly widespread in the marine environment, but it is by no means restricted to it. Some terrestrial organisms, such as some fungi, bacteria and insects emit light, although their luminescent structures are generally quite simple, lacking the additional optical components (such as reflectors, lenses and filters) that control the optical characteristics of the more complex light-emitting photophores in some cephalopods and fishes (Harvey 1952; Lloyd 1975, 1978; Lall et al. 1980; Herring 1988, 2000; Wilson & Hastings 1998). However, particularly in insects, luminescence behaviour may be very elaborate: glow-worms and fireflies are known to point their light organs in specific directions to attract mates or lure prey. This makes up for the lack of directionality in their light organs. At the same time, male/female signalling dialogues may be extremely complex (Lloyd 1975, 1978).
Cephalopods and fishes have many photophores that can be highly directional and have very complex structures (e.g. Chun 1910; Nicol 1960; Robison & Young 1981, Herring 1988, 2000; Young & Bennett 1988; Johnsen et al. 1999a,b). Although there is an impressive anatomical variety in cephalopod photophores, especially with respect to the occurrence and position of lenses, filters and reflectors, the common theme among cephalopods is a thick layer of reflective plates at the base of the photophore. Acting together, in ventral photophores, these optical structures help to channel and direct light downwards for effective countershading (e.g. when viewed from below against the more brightly lit downwelling light; see Arnold et al. 1974; Herring et al. 1981; Herring 1985, 1988, 2000; McFall-Ngai & Montgomery 1990). Cephalopod photophore reflectors differ in their composition and appearance from diffuse to highly specular. In the midwater squid Abralia and Watasenia, both of which have several different types of photophore, the simplest ones have only a basal concave reflector, formed of a highly organized multilayer of collagen rods that reflect specific wavelengths (Young & Arnold 1982; Young & Bennett 1988; see also reflectin protein in Euprymna photophores, Crookes et al. 2004). Collagen fibres also provide the main reflector in the photophores of Stauroteuthis syrtensis, Vampyroteuthis infernalis and Spirula spirula, as well as being primarily responsible for the silvery appearance of the main body wall of the last species (Herring et al. 1981, 1994; Johnsen et al. 1999b). Some of the more complex photophores (lensed and filtered) have additional areas with reflective tissue, such as light guides and filters (Herring 1988; Young & Bennett 1988). Photophores often have a pearly iridescent appearance that can result from irregularity in thickness and organization of reflective plates, similar to the silvery reflectors around the eyes of cephalopods (described above). In the shallow-water Hawaian bobtail squid Euprymna, for example, the reflective plates appear to be of various thicknesses (average 100 nm) and orientations (McFall-Ngai & Montgomery 1990). Some mesopelagic squid, such as Liocranchia, have highly wavy reflective plates; in Sandalops and Megalocranchia, the reflective plates are highly organized into stacks but the overall orientation differs between stacks (Herring et al. 2002). Both arrangements could be the cause of the photophore's ‘pearly’ appearance. These basal reflectors reflect light back through the photophore for more efficient light emission. Reflectors can also play a role in tuning the wavelength of the emitted light. In the squid Pterygioteuthis, the reflective plates are spaced and oriented so that they reflect only blue light vertically downwards (Arnold et al. 1974). The spectral characteristics of light production are determined by the luminescence chemistry, but the photophore emission can be modified by spectrally selective filters and reflectors that alter the bandwidth. Interestingly, some squid are able to regulate their bioluminescence emission spectra to match the spectral composition of downwelling light. Young & Mencher (1980) showed that, in the squid Abraliopsis and Abralia, the changes in water temperature experienced during diel vertical migration alter the spectral emission of light from their photophores, resulting in a match with the downwelling light spectrum, which is different during day and night. This may be achieved in the following way: the photophore iridophores are surrounded by muscle tissue, and the tensional forces of the muscles could affect the spacing of the plates, which in turn could affect the spectral composition of the emitted light (Arnold et al. 1974; Young & Arnold 1982). There appears to be no evidence to suggest that the photophore iridophores are physiologically active as the iridophores in the mantle skin of many squid (see above), but to our knowledge this has not been tested.
3.3 Diffuse white scattering
The body patterns that cephalopods use for camouflage and communication show a wide variety of contrast and brightness levels. In addition to iridophores, cuttlefish and octopus have another structural reflector type that creates bright white patterns, shapes and spots. These reflectors are called leucophores (‘white cells’) and they are made up of spherical assemblages called leucosomes (Froesch & Messenger 1978; Cloney & Brocco 1983). Leucophores reflect the ambient wavelengths of light (i.e. they look white in white light, red in red light, blue in blue light, etc.) and are found in high density in specific skin areas (zebra stripes, fin spots, fin line and various other skin components) that can be bright white depending on the body patterning that is shown (figure 6a). Squid generally do not have leucophores (Sepioteuthis lessoniana and Sepioteuthis sepioidea may be exceptions).
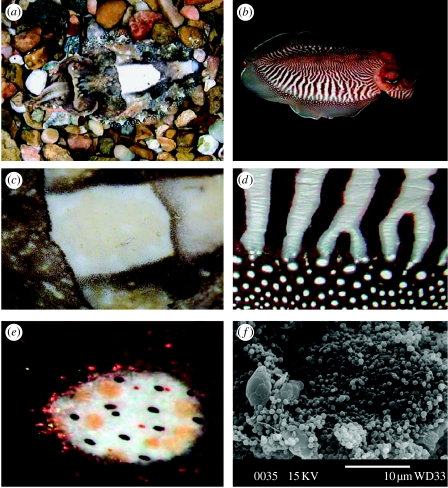
Figure 6.
Whiteness in cuttlefish is created by scattering of light from leucophores. (a) Disruptive pattern, (b) zebra pattern, (c) close-up of white square, (d) close-up of zebra pattern, (e) close-up of white finspot, created by leucophores. Note the chromatophores in the superficial layer; chromatophores can modulate whiteness. (f) Scanning electron micrograph of leucophores showing spherical arrangement of leucosomes (courtesy of Alan Kuzirian).
Mäthger et al. (submitted) show that the leucophores of cuttlefish, S. officinalis, have the optical properties of a perfect diffuser, appearing equally bright from all angles of view and that they reflect light at consistently high levels (up to 70% at wavelengths from 300 to 900 nm). The light-scattering elements of leucophores are spheres (leucosomes) that range in size from 250 to 1250 nm in diameter (figure 6b) and are composed of mucoproteins—highly sulphated and weakly acidic mucopolysaccharides (they stain negatively with antibodies to reflectin, which is the light-reflecting protein in squid skin).
4. Conclusions
In cephalopods, colour change is mediated by two types of skin components: pigmented chromatophores and structurally reflecting cells (iridophores and leucophores). Iridophores are the cells that are made up of stacks of thin protein plates that function as multilayer reflectors, whereas leucophores contain spherical protein assemblages that scatter light equally well throughout the visible, IR and UV parts of the spectrum.
In this paper, we have shown that iridescence in cephalopods spans the whole of the visible spectrum, including the near-IR. Biologically, IR reflectance most likely plays little or no role in cephalopod behaviour because IR is absorbed rapidly by sea water. Furthermore, most cephalopods studied thus far appear to be colour blind, having only one visual pigment, with the absorbance peak generally in the blue–green part of the spectrum (Brown & Brown 1958; Bellingham et al. 1998; but see Kito et al. 1992; Michinomae et al. 1994). Therefore, reflectance in the IR is most likely irrelevant for vision in these animals. Interestingly, in the cephalopod species discussed in this paper, there has so far been no evidence for iridescence in the UV parts of the spectrum, but considering the diversity of cephalopod skin coloration, and the fact that some fish predators can see UV, this possibility should not be ruled out.
Leucophores create the white skin areas that are part of various camouflage and signalling body patterns of cuttlefish and octopus. They provide a backdrop against which chromatophores (and iridophores) can create highly contrasting patterns, such as those typical of the light skin components of disruptive coloration in cuttlefish (Hanlon & Messenger 1988; Chiao & Hanlon 2001; Barbosa et al. 2007; Kelman et al. 2007; figure 6a). Leucophores are not physiologically active (as some iridophores are), they do not polarize light and they look equally bright from all angles of view. Leucophores reflect the ambient wavelengths of light (they look red in red light, blue in blue light, white in white, etc.), which may aid both wavelength and intensity matching at least at a localized level in the skin (Froesch & Messenger 1978). This may be particularly useful in the habitats in which shorter (blue and green) wavelengths predominate, primarily those of greater depths (Jerlov 1976).
Two clear functions of structural coloration in cephalopods emerge: (i) camouflage and (ii) signalling/communication.
4.1 Camouflage
Many iridophores are oriented in precise ways to facilitate reflectance in specific directions. Iridophores provide a range of wavelengths that complement the yellow, red and brown pigments in the chromatophores, so that camouflage can encompass the entire visible spectrum. Additionally, some squid are able to regulate the intensity of light reflected from iridophores, and matching the intensities of the ambient light field is a key step towards achieving effective camouflage. Furthermore, the silvery iridescence that is found around the eyes, the ink sac and the sides of the mantle suggests a role in camouflage by acting as vertically oriented mirrors. The reflective plates are tilted towards the vertical and they maximally reflect the incident light, much in the same way as silvery fish (e.g. Denton & Nicol 1962; Denton 1970; Denton & Land 1971).
Leucophores also play a role in camouflage: they provide light areas that may facilitate both background matching (by resembling specific light objects in the background) and disruptive coloration (by visually breaking the body into distinct objects of high-contrasting patches) (Cott 1940; Hanlon & Messenger 1988; Hanlon et al. 2009).
4.2 Signalling/communication
The optical properties of the structural reflectors provide cephalopods with an excellent means of communication. Optical signals, both intra- and interspecific, can vary dramatically from the zebra stripes of cuttlefish (the light stripes are created by leucophores; figure 6b) to the flashing blue iridescent rings of the blue-ringed octopus (figure 4). Some iridescent areas are strongly polarized at certain angles (e.g. Shashar & Hanlon 1997; Mäthger & Hanlon 2006; Chiou et al. 2007) and, since cephalopods have the ability to detect polarized light, this may have behavioural functions (e.g. Cronin et al. 2003; Boal et al. 2004). It has been shown that cuttlefish take advantage of their polarization vision when hunting for silvery fish whose scales polarize light (Shashar et al. 2000), so that it is conceivable that polarization may be used in various signalling aspects of cephalopod behaviour (Boal et al. 2004).
Squid are commonly found in large schools. The communication of the movements of individuals in a school is crucial for maintaining the integrity of a school and iridescence may play such a role. The various iridescent stripes and splotches in squid (e.g. Hanlon 1982; Hanlon et al. 1999; Mäthger & Denton 2001) have characteristic optical features that differ depending on where the onlooker is positioned, so that it is possible that the squid may use their iridescent markings to communicate changes in swimming direction, etc. (Mäthger & Denton 2001).
Thus far, there is no optical evidence that cephalopod iridescence may be caused by diffraction gratings. In a short communication, Hanlon et al. (1983), showed that the iridophore plates of some squid are oriented ‘edge on’, i.e. with their short ends facing the skin surface, in some ways resembling the face of a diffraction grating. By contrast, Mäthger & Denton (2001) subsequently showed that iridophores with reflective plates of this orientation, as commonly observed in the iridophores of the lateral and ventral mantle skin, nevertheless act as multilayer reflectors. However, considering how few species have been studied in detail, compared with the diversity of the class Cephalopoda, it should not be ruled out that diffraction gratings may be a mechanism of iridescence in some cephalopod species.
Iridescence is widespread in the animal kingdom and its function is not always associated with signalling or camouflage (e.g. mother of pearl, in which the iridescent layer on the interior of the mollusc shell provides structural strength; Barthelat et al. 2007) but for many species it is. Some of the most exotic iridescent patterns can be seen in a great number of butterfly species (e.g. Ghiradella 1991; Vukusic et al. 1999, 2000; Sweeney et al. 2003; Vukusic & Sambles 2003; Stavenga et al. 2004) and the plumage and skin of many birds (Prum et al. 1998; Vorobyev et al. 1998; Cuthill et al. 1999; Osorio & Ham 2002; Prum & Torres 2003). The feathers of the male peacocks and birds of paradise (Frith & Beehler 1998; Zi et al. 2003) are superb examples of how nature has built complex optical devices for achieving special visual effects.
Many marine vertebrate and invertebrate species—mantis shrimp and coral reef fish are particularly remarkable—have incorporated iridescent structures into their body markings whose function it is to attract females, warn intruders or aid in blending into the visual background (Kasukawa et al. 1987; Fujii et al. 1989; Lythgoe & Shand 1989a; Fujii 1993; Herring 1994; Marshall 2000; Marshall et al. 2003; Mazel et al. 2004; Siebeck 2004; Chiou et al. 2005; R. Caldwell 2008, personal communication). Arachnids also have iridescent markings, some of which are most prominent in the UV parts of the spectrum (Oxford & Gillespie 1998; Lim & Li 2007; Taylor & McGraw 2007).
Most animals have available to them only one body pattern that may undergo seasonal or ontogenetic changes but more often stays the same throughout the animal's life (Cott 1940; Edmunds 1974; Booth 1990). Changeable iridescence is not common in the animal kingdom, presumably because of the physical challenge of creating such devices. Squid are one of a few known animals that have changeable iridescence. In vertebrates, iridescence changes occur: most have not been well quantified, and those that have been generally appear to take minutes, hours or even days (e.g. fishes: Lythgoe & Shand 1982, 1989b, Kasukawa et al. 1987, Fujii et al. 1989; lizards: Hadley & Oldman 1969, Taylor & Hadley 1970, Morrison et al. 1996; tree frogs: Stegen et al. 2004). One known exception may be the paradise whiptail (Pentapodus paradiseus), a tropical fish whose reflective changes are very fast (i.e. fraction of a second (Mäthger et al. 2003). Billfish are also reported to have fast reflective changes, but this has not been quantified to the best of our knowledge (e.g. Davie 1990; Fritsches et al. 2000).
By studying animal structural coloration, we gain insight into how nature has solved the problem of creating optical devices with specific functions in ecological contexts. The impressive repertoire of cephalopod colour change reaches far beyond the field of biology. In recent years, cephalopod iridophores and chromatophores have received interest from materials scientists who aim to model the optical properties of these structures and create synthetic materials with similar characteristics for various applications in optical nanotechnology (e.g. Crookes et al. 2004; Kramer et al. 2007; Sutherland et al. 2008a,b; Vaia & Baur 2008).
In summary, studying animal structural coloration is mesmerizing not only because of the sheer beauty that is created by the microscopic assemblies of reflective materials with highly precise arrangements and orientations, but also because of what we can learn from these biophotonic structures in our efforts to produce electronic visual displays as well as various kinds of paints and coatings (e.g. Vaia & Baur 2008). Despite the recent attention to cephalopod structural coloration, many questions remain unanswered. For example, what are the behavioural functions of iridescent signals? What other structural light reflectors are present in the diverse class Cephalopoda? Cephalopods have made their way into all the world's oceans, including the tropics and polar waters, and even to the depths exceeding 3000 m (Hanlon & Messenger 1996), so we are likely to find more fascinating structural reflectors in the future.
Acknowledgments
We thank Malcolm Clarke for providing some of the specimens that L.M.M. used during her PhD. Sir E. J. Denton was L.M.M.'s PhD mentor and he was involved in much of the characterization of iridescence of squid presented in this paper. We would also like to thank Morley Stone for continuous stimulating discussions of these topics. L.M.M. gratefully acknowledges funding from the Gottlieb Daimler- and Karl Benz-Foundation (PhD) and the Royal Society (postdoctoral fellowship in Australia). We are also grateful to Peter Herring and an anonymous reviewer for their constructive comments that greatly improved this manuscript.
Footnotes
One contribution of 13 to a Theme Supplement ‘Iridescence: more than meets the eye’.
References
- Arnold J.M., Young R.E., King M.V. Ultrastructure of a cephalopod photophore. II. Iridophores as reflectors and transmitters. Biol. Bull. 1974;147:522–534. doi: 10.2307/1540737. [DOI] [PubMed] [Google Scholar]
- Barbosa A., Mäthger L.M., Chubb C., Florio C., Chiao C.-C., Hanlon R.T. Disruptive coloration in cuttlefish: a visual perception mechanism that regulates ontogenetic adjustment of skin patterning. J. Exp. Biol. 2007;210:1139–1147. doi: 10.1242/jeb.02741. [DOI] [PubMed] [Google Scholar]
- Barthelat F., Tang H., Zavattieri P.D., Li C.-M., Espinosa H.D. On the mechanics of mother-of-pearl: a key feature in the material hierarchical structure. J. Mech. Phys. Solids. 2007;55:306–337. doi: 10.1016/j.jmps.2006.07.007. [DOI] [Google Scholar]
- Bellingham J., Morris A.G., Hunt D.M. The rhodopsin gene of the cuttlefish Sepia officinalis: sequence and spectral tuning. J. Exp. Biol. 1998;201:2299–2306. doi: 10.1242/jeb.201.15.2299. [DOI] [PubMed] [Google Scholar]
- Boal J.G., Shashar N., Grable M.M., Vaughan K.H., Loew E.R., Hanlon R.T. Behavioral evidence for intraspecific signaling with achromatic and polarized light by cuttlefish (Mollusca: Cephalopoda) Behaviour. 2004;141:837–861. doi: 10.1163/1568539042265662. [DOI] [Google Scholar]
- Booth C.L. Evolutionary significance of ontogenetic colour change in animals. Biol. J. Linn. Soc. 1990;40:125–163. doi: 10.1111/j.1095-8312.1990.tb01973.x. [DOI] [Google Scholar]
- Boys C.V. Dover Publications, Inc; New York, NY: 1959. Soap bubbles—their colors and forces which mold them. [Google Scholar]
- Brink D.J., Lee M.E. Confined blue iridescence by a diffracting microstructure: an optical investigation of the Cynandra Opis butterfly. Appl. Opt. 1999;38:5282–5289. doi: 10.1364/AO.38.005282. [DOI] [PubMed] [Google Scholar]
- Brown P.K., Brown P.S. Visual pigments of the octopus and cuttlefish. Nature. 1958;182:1288–1290. doi: 10.1038/1821288a0. [DOI] [PubMed] [Google Scholar]
- Chiao C.-C., Hanlon R.T. Cuttlefish camouflage: visual perception of size, contrast and number of white squares on artificial checkerboard substrata initiates disruptive coloration. J. Exp. Biol. 2001;204:2119–2125. doi: 10.1242/jeb.204.12.2119. [DOI] [PubMed] [Google Scholar]
- Chiou T.-H., Cronin T.W., Caldwell R.L., Marshall N.J. Biological polarized light reflectors in stomatopod crustaceans. Proc. Soc. Photo Opt. Instrum. Eng. 2005;5888:58881B. doi: 10.1117/12.613117. [DOI] [Google Scholar]
- Chiou T.-H., Mäthger L.M., Hanlon R.T., Cronin T.W. Spectral and spatial properties of polarized light reflections from the arms of squid (Loligo pealeii) and cuttlefish (Sepia officinalis L.) J. Exp. Biol. 2007;210:3624–3635. doi: 10.1242/jeb.006932. [DOI] [PubMed] [Google Scholar]
- Chun C. Die Cephalopoden. Wiss Ergebn. Dt. Tiefsee-Exped Valdivia. 1910;18:1–552. [Google Scholar]
- Cloney R.A., Brocco S.L. Chromatophore organs, reflector cells, iridocytes and leucophores in cephalopods. Am. Zool. 1983;23:581–592. doi: 10.1093/icb/23.3.581. [DOI] [Google Scholar]
- Cooper K.M., Hanlon R.T. Correlation of iridescence with changes in iridophore platelet ultrastructure in the squid Lolliguncula brevis. J. Exp. Biol. 1986;121:451–455. doi: 10.1242/jeb.121.1.451. [DOI] [PubMed] [Google Scholar]
- Cooper K.M., Hanlon R.T., Budelmann B.U. Physiological color-change in squid iridophores. II. Ultrastructural mechanisms in Lolliguncula brevis. Cell Tissue Res. 1990;259:15–24. doi: 10.1007/BF00571425. [DOI] [PubMed] [Google Scholar]
- Cott H.B. Methuen & Co., Ltd; London, UK: 1940. Adaptive coloration in animals. [Google Scholar]
- Cronin T.W., Shashar N., Caldwell R.L., Marshall N.J., Cheroske A.G., Chiou T.-H. Polarization vision and its role in biological signaling. Integr. Comp. Biol. 2003;43:549–558. doi: 10.1093/icb/43.4.549. [DOI] [PubMed] [Google Scholar]
- Crookes W.J., Ding L., Huang Q.L., Kimbell J.R., Horwitz J., McFall-Ngai M.J. Reflectins: the unusual proteins of squid reflective tissues. Science. 2004;303:235–238. doi: 10.1126/science.1091288. [DOI] [PubMed] [Google Scholar]
- Cuthill I.C., Bennett A.T.D., Partridge J.C., Maier E.J. Plumage reflectance and the objective assessment of avian sexual dichromatism. Am. Nat. 1999;160:183–200. doi: 10.1086/303160. [DOI] [PubMed] [Google Scholar]
- Davie P.S. Massey University; Palmerston North, New Zealand: 1990. Pacific marlins: anatomy and physiology. [Google Scholar]
- Denton E.J. On the organization of reflecting surfaces in some marine animals. Phil. Trans. R. Soc. B. 1970;258:285–313. doi: 10.1098/rstb.1970.0037. [DOI] [PubMed] [Google Scholar]
- Denton E.J., Land M.F. Mechanism of reflexion in silvery layers of fish and cephalopods. Proc. R. Soc. Lond. A. 1971;178:43–61. doi: 10.1098/rspb.1971.0051. [DOI] [PubMed] [Google Scholar]
- Denton E.J., Nicol J.A.C. Why fishes have silvery sides; and a method of measuring reflectivity. J. Physiol. Lond. 1962;165:13P–15P. [Google Scholar]
- Denton E.J., Nicol J.A.C. Studies on reflexion of light from silvery surfaces of fishes, with special reference to the bleak, Alburnus alburnus. J. Mar. Biol. Assoc. UK. 1965;45:683–703. [Google Scholar]
- Denton E.J., Nicol J.A.C. A survey of reflectivity in silvery teleosts. J. Mar. Biol. Assoc. UK. 1966;46:685–722. [Google Scholar]
- Denton E.J., Gilpin-Brown J.B., Wright P.G. The angular distribution of the light produced by some mesopelagic fish in relation to their camouflage. Proc. R. Soc. Lond. B. 1972;182:145–158. doi: 10.1098/rspb.1972.0071. [DOI] [Google Scholar]
- Edmunds M. Longman Group Ltd; Harlow, UK: 1974. Defence in animals—a survey of anti-predator defences. [Google Scholar]
- Florey E. Ultrastructure and function of cephalopod chromatophores. Am. Zool. 1969;9:429–442. doi: 10.1093/icb/9.2.429. [DOI] [PubMed] [Google Scholar]
- Fox D.L. University of California Press; Berkeley, CA: 1976. Animal biochromes and structural colours. [Google Scholar]
- Fox H.M., Vevers G. Sidgwick and Jackson; London, UK: 1960. The nature of animal colours. [Google Scholar]
- Frith C.B., Beehler B.M. Oxford University Press; Oxford, UK: 1998. The birds of paradise. [Google Scholar]
- Fritsches K.A., Partridge J.C., Pettigrew J.D., Marshall N.J. Colour vision in billfish. Phil. Trans. R. Soc. B. 2000;355:1253–1256. doi: 10.1098/rstb.2000.0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froesch D., Messenger J.B. On leucophores and the chromatic unit of Octopus vulgaris. J. Zool. Lond. 1978;186:163–173. [Google Scholar]
- Fujii R. Coloration and chromatophores. In: Evans D.H., editor. The physiology of fishes. CRC Press; Boca Raton, FL: 1993. pp. 535–562. [Google Scholar]
- Fujii R., Kasukawa H., Miyaji K. Mechanisms of skin coloration and its changes in the blue-green damselfish, Chromis viridis. Zool. Sci. 1989;6:477–486. [Google Scholar]
- Ghiradella H. Light and colour of the wing: structural colours in butterflies and moths. Appl. Opt. 1991;30:3492–3500. doi: 10.1364/AO.30.003492. [DOI] [PubMed] [Google Scholar]
- Hadley M.E., Oldman J.M.G. Physiological color changes in reptiles. Am. Zool. 1969;9:489–504. doi: 10.1093/icb/9.2.489. [DOI] [PubMed] [Google Scholar]
- Hanlon R.T. The functional organization of chromatophores and iridescent cells in the body patterning of Loligo plei (Cephalopoda: Myopsida) Malacologia. 1982;23:89–119. [Google Scholar]
- Hanlon R.T., Messenger J.B. Adaptive coloration in young cuttlefish (Sepia officinalis L.): the morphology and development of body patterns and their relation to behaviour. Phil. Trans. R. Soc. B. 1988;320:437–487. doi: 10.1098/rstb.1988.0087. [DOI] [Google Scholar]
- Hanlon R.T., Messenger J.B. Cambridge University Press; Cambridge, UK: 1996. Cephalopod behaviour. [Google Scholar]
- Hanlon R.T., Shashar N. Aspects of the sensory ecology of cephalopods. In: Collin S.P., Marshall N.J., editors. Sensory processing in the aquatic environment. Springer; Heidelberg, Germany: 2003. pp. 266–282. [Google Scholar]
- Hanlon R.T., Cooper K.M., Cloney R.A. Do iridophores of the squid mantle reflect light or diffract light in the production of structural colors? Am. Malacol. Bull. 1983;2:91. [Google Scholar]
- Hanlon R.T., Cooper K.M., Budelmann B.U., Pappas T.C. Physiological color-change in squid iridophores. I. Behavior, morphology and pharmacology in Lolliguncula brevis. Cell Tissue Res. 1990;259:3–14. doi: 10.1007/BF00571424. [DOI] [PubMed] [Google Scholar]
- Hanlon R.T., Maxwell M.R., Shashar N., Loew E.R., Boyle K.L. An ethogram of body patterning behavior in the biomedically and commercially valuable squid Loligo pealei off Cape Cod, Massachusetts. Biol. Bull. 1999;197:49–62. doi: 10.2307/1542996. [DOI] [PubMed] [Google Scholar]
- Hanlon R.T., Chiao C., Mäthger L., Barbosa A., Buresch K.C., Chubb C. Cephalopod dynamic camouflage: bridging the continuum between background matching and disruptive coloration. Phil. Trans. R. Soc. B. 2009;364:429–437. doi: 10.1098/rstb.2008.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey E.N. Academic Press; New York, NY: 1952. Bioluminescence. [Google Scholar]
- Herring P.J. How to survive in the dark: bioluminescence in the deep sea. Symp. Soc. Exp. Biol. 1985;39:323–350. [PubMed] [Google Scholar]
- Herring P.J. The mollusca, form and function. vol. 11. Academic Press; San Diego, CA: 1988. Luminescent organs; pp. 449–489. [Google Scholar]
- Herring P.J. Reflective systems in aquatic animals. Comp. Biochem. Physiol. A. 1994;109:513–546. doi: 10.1016/0300-9629(94)90192-9. [DOI] [Google Scholar]
- Herring P.J. Bioluminescent signals and the role of reflectors. J. Opt. A Pure Appl. Opt. 2000;2:R29–R38. doi: 10.1088/1464-4258/2/6/202. [DOI] [Google Scholar]
- Herring P.J., Clarke M.R., Boletzky S.V., Ryan K.P. The light organs of Sepiola atlantica and Spirula spirula (mollusca: Cephalopoda): bacterial and intrinsic systems in the order Sepioidea. J. Mar. Biol. Assoc. UK. 1981;61:901–916. [Google Scholar]
- Herring P.J., Dilly P.N., Cope C. The bioluminescent organs of the deep-sea cephalopod Vampyroteuthis infernalis (Cephalopoda: Vampyromorpha) J. Zool. Lond. 1994;233:45–55. [Google Scholar]
- Herring P.J., Dilly P.N., Cope C. The photophores of the squid family Cranchiidae (Cephalopoda: Oegopsida) J. Zool. Lond. 2002;258:73–90. [Google Scholar]
- Hill A.V., Solandt D.Y. Myograms from the chromatophores of Sepia. J. Physiol. Lond. 1935;83:13–14. [Google Scholar]
- Hinton H.E., Gibbs D.F. Diffraction gratings in phalacrid beetles. Nature. 1969;221:953–954. doi: 10.1038/221953a0. [DOI] [Google Scholar]
- Holmes W. The colour changes and colour patterns of Sepia officinalis L. Proc. Zool. Soc. Lond. A. 1940;110:2–35. [Google Scholar]
- Huxley A.F. A theoretical treatment of the reflexion of light by multi-layer structures. J. Exp. Biol. 1968;48:227–245. [Google Scholar]
- Jerlov N.G. Elsevier; Amsterdam, The Netherlands: 1976. Marine optics. [Google Scholar]
- Johnsen S., Balser E.J., Fisher E.D., Widder E.A. Bioluminescence in the deep-sea cirrate octopod Stauroteuthis syrtensis Verrill (Mollusca: Cephalopoda) Biol. Bull. 1999a;197:26–39. doi: 10.2307/1542994. [DOI] [PubMed] [Google Scholar]
- Johnsen S., Balser E.J., Widder E.A. Light-emitting suckers in an octopus. Nature. 1999b;398:113–114. doi: 10.1038/18131. [DOI] [Google Scholar]
- Kasukawa H., Oshima N., Fujii R. Mechanism of light reflection in blue damselfish motile iridophore. Zool. Sci. 1987;4:243–257. [Google Scholar]
- Kelman E.J., Baddeley R.J., Shohet A.J., Osorio D. Perception of visual texture and the expression of disruptive camouflage by the cuttlefish, Sepia officinalis. Proc. R. Soc. B. 2007;274:1369–1375. doi: 10.1098/rspb.2007.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito Y., Narita K., Seidou M., Michinomae M., Yoshihara K., Partridge J.C., Herring P.J. A blue-sensitive visual pigment based on 4-hydroxyretinal is found widely in mesopelagic cephalopods. In: Rigaud J.L., editor. Structures and functions of retinal proteins. vol. 221. Colloque INSERM/Jhon Libbey Eurotext Ltd; Montrouge, France: 1992. pp. 411–414. [Google Scholar]
- Kramer R.M., Crookes-Goodson W.J., Naik R.R. The self-organizing properties of squid reflectin protein. Nat. Mater. 2007;6:533–538. doi: 10.1038/nmat1930. [DOI] [PubMed] [Google Scholar]
- Lall A.B., Seliger H.H., Biggley W.H., Lloyd J.E. Ecology and colors of firefly bioluminescence. Science. 1980;210:560–562. doi: 10.1126/science.210.4469.560. [DOI] [PubMed] [Google Scholar]
- Land M.F. The physics and biology of animal reflectors. Prog. Biophys. Mol. Biol. 1972;24:75–106. doi: 10.1016/0079-6107(72)90004-1. [DOI] [PubMed] [Google Scholar]
- Land M.F., Nilsson D.E. Oxford University Press; Oxford, UK: 2002. Animal eyes. [Google Scholar]
- Lim M.L.M., Li D. Effects of age and feeding history on structure-based UV ornaments of a jumping spider (Araneae: Salticidae) Proc. R. Soc. B. 2007;274:569–575. doi: 10.1098/rspb.2006.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd J.E. Aggressive mimicry in Photuris fireflies: signal repertoires by femmes fatales. Science. 1975;187:588–591. doi: 10.1126/science.187.4175.452. [DOI] [PubMed] [Google Scholar]
- Lloyd J.E. Insect bioluminescence. In: Herring P.J., editor. Bioluminescence in action. Academic Press; London, UK: 1978. pp. 241–272. [Google Scholar]
- Longley W.H. Studies upon the biological significance of animal coloration. I. The colors and color changes of West Indian reef-fishes. J. Exp. Biol. 1917;1:533–601. [Google Scholar]
- Lythgoe J.N., Shand J. Changes in spectral reflexions from the iridophores of the neon tetra. J. Physiol. Lond. 1982;325:23–34. doi: 10.1113/jphysiol.1982.sp014132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lythgoe J.N., Shand J. The structural basis for iridescent colour changes in dermal and corneal iridophores in fish. J. Exp. Biol. 1989a;141:313–325. [Google Scholar]
- Lythgoe J.N., Shand J. The structural basis for iridescent colour changes in dermal and corneal iridophores in fish. J. Exp. Biol. 1989b;141:313–325. [Google Scholar]
- Marshall N.J. Communication and camouflage with the same ‘bright’ colours in reef fishes. Phil. Trans. R. Soc. B. 2000;355:1243–1248. doi: 10.1098/rstb.2000.0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall N.J., Messenger J.B. Colour-blind camouflage. Nature. 1996;382:408–409. doi: 10.1038/382408b0. [DOI] [Google Scholar]
- Marshall N.J., Jennings K.J., McFarland W.N., Loew E.R., Losey G.S. Visual biology of Hawaiian coral reef fishes. II. Colors of Hawaiian coral reef fish. Copeia. 2003;3:455–466. doi: 10.1643/01-055. [DOI] [Google Scholar]
- Mäthger, L. M. 2001 A study of the properties and functions of light reflectors in squid. PhD, University of Sheffield, Sheffield, UK.
- Mäthger L.M., Denton E.J. Reflective properties of iridophores and fluorescent ‘eyespots’ in the loliginid squid Alloteuthis subulata and Loligo vulgaris. J. Exp. Biol. 2001;204:2103–2118. doi: 10.1242/jeb.204.12.2103. [DOI] [PubMed] [Google Scholar]
- Mäthger L.M., Hanlon R.T. Anatomical basis for camouflaged polarized light communication in squid. Biol. Lett. 2006;2:494–496. doi: 10.1098/rsbl.2006.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäthger L.M., Hanlon R.T. Malleable skin coloration in cephalopods: selective reflectance, transmission and absorbance of light by chromatophores and iridophores. Cell Tissue Res. 2007;329:179–186. doi: 10.1007/s00441-007-0384-8. [DOI] [PubMed] [Google Scholar]
- Mäthger L.M., Land M.F., Siebeck U.E., Marshall N.J. Rapid colour changes in multilayer reflecting stripes in the Paradise Whiptail, Pentapodus paradiseus. J. Exp. Biol. 2003;206:3607–3613. doi: 10.1242/jeb.00599. [DOI] [PubMed] [Google Scholar]
- Mäthger L.M., Collins T.F.T., Lima P.A. The role of muscarinic receptors and intracellular Ca2+ in the spectral reflectivity changes of squid iridophores. J. Exp. Biol. 2004;207:1759–1769. doi: 10.1242/jeb.00955. [DOI] [PubMed] [Google Scholar]
- Mäthger L.M., Barbosa A., Miner S., Hanlon R.T. Color blindness and contrast perception in cuttlefish (Sepia officinalis) determined by a visual sensorimotor assay. Vision Res. 2006;46:1746–1753. doi: 10.1016/j.visres.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Mäthger, L. M., Kuzirian, A. M. & Hanlon, R. T. Submitted. Exceptional bright white diffusion by cuttlefish skin.
- Mazel C.H., Cronin T.W., Caldwell R.L., Marshall N.J. Fluorescent enhancement of signaling in a mantis shrimp. Science. 2004;303:51. doi: 10.1126/science.1089803. [DOI] [PubMed] [Google Scholar]
- McFall-Ngai M.J., Montgomery M.K. The anatomy and morphology of the adult bacterial light organ of Euprymna scolopes Berry (Cephalopoda: Sepiolidae) Biol. Bull. 1990;179:332–339. doi: 10.2307/1542325. [DOI] [PubMed] [Google Scholar]
- Messenger J.B. Cephalopod chromatophores: neurobiology and natural history. Biol. Rev. 2001;76:473–528. doi: 10.1017/s1464793101005772. [DOI] [PubMed] [Google Scholar]
- Michinomae M., Masuda H., Seidou M., Kito Y. Structural basis for wavelength discrimination in the banked retina of the firefly squid Watasenia scintillans. J. Exp. Biol. 1994;193:1–12. doi: 10.1242/jeb.193.1.1. [DOI] [PubMed] [Google Scholar]
- Mirow S. Skin color in the squids Loligo pealii and Loligo opalescens. I. Chromatophores. Z. Zellforsch. 1972a;125:143–175. doi: 10.1007/BF00306786. [DOI] [PubMed] [Google Scholar]
- Mirow S. Skin color in the squids Loligo pealii and Loligo opalescens. II. Iridophores. Z. Zellforsch. 1972b;125:176–190. doi: 10.1007/BF00306787. [DOI] [PubMed] [Google Scholar]
- Moody M.F., Parriss J.R. The visual system of octopus. Discrimination of polarized light by octopus. Nature. 1960;186:839–840. doi: 10.1038/186839a0. [DOI] [PubMed] [Google Scholar]
- Morrison R.L., Sherbrooke W.C., Frost-Mason S.K. Temperature-sensitive, physiologically active iridophores in the lizard Urosaurus ornatus: an ultrastructural analysis of color change. Copeia. 1996;1996:804–812. doi: 10.2307/1447641. [DOI] [Google Scholar]
- Moynihan M. Indiana University Press; Bloomington, IN: 1985. Communication and noncommunication by cephalopods. [Google Scholar]
- Nicol J.A.C. The regulation of light emission in animals. Biol. Rev. 1960;35:1–42. doi: 10.1111/j.1469-185X.1960.tb0321.x. [DOI] [Google Scholar]
- Nicol J.A.C., Van Baalen C. Studies on the reflective layers of fishes. Contrib. Mar. Sci. 1968;13:65–88. [Google Scholar]
- Osorio D., Ham A.D. Spectral reflectance and directional properties of structural coloration in bird plumage. J. Exp. Biol. 2002;205:2017–2027. doi: 10.1242/jeb.205.14.2017. [DOI] [PubMed] [Google Scholar]
- Oxford G.S., Gillespie R.G. Evolution and ecology of spider coloration. Annu. Rev. Entomol. 1998;41:619–643. doi: 10.1146/annurev.ento.43.1.619. [DOI] [PubMed] [Google Scholar]
- Packard A., Hochberg F.G. Skin patterning in Octopus and other genera. Symp. Zool. Soc. Lond. 1977;38:191–231. [Google Scholar]
- Parker A.R. Discovery of functional iridescence and its coevolution with eyes in the phylogeny of Ostracoda (Crustacea) Proc. R. Soc. B. 1995;262:349–355. doi: 10.1098/rspb.1995.0216. [DOI] [Google Scholar]
- Prum R.O., Torres R.H. Structural colouration of avian skin: convergent evolution of coherently scattering dermal collagen arrays. J. Exp. Biol. 2003;206:2409–2429. doi: 10.1242/jeb.00431. [DOI] [PubMed] [Google Scholar]
- Prum R.O., Torres R.H., Williamson S., Dyck J. Coherent light scattering by blue feather barbs. Nature. 1998;396:28–29. doi: 10.1038/23838. [DOI] [Google Scholar]
- Robison B.H., Young R.E. Bioluminescence in pelagic octopods. Pac. Sci. 1981;35:39–44. [Google Scholar]
- Schäfer W. Bau, Entwicklung und Farbentstehung bei den Flitterzellen von Sepia officinalis. Z. Zellforsch. 1937;27:222–245. doi: 10.1007/BF01880083. [DOI] [Google Scholar]
- Shashar N., Cronin T.W. Polarization contrast vision in octopus. J. Exp. Biol. 1996;199:999–1004. doi: 10.1242/jeb.199.4.999. [DOI] [PubMed] [Google Scholar]
- Shashar N., Hanlon R.T. Squids (Loligo pealei and Euprymna scolopes) can exhibit polarized light patterns produced by their skin. Biol. Bull. 1997;193:207–208. doi: 10.1086/BBLv193n2p207. [DOI] [PubMed] [Google Scholar]
- Shashar N., Rutledge P.S., Cronin T.W. Polarization vision in cuttlefish—a concealed communication channel? J. Exp. Biol. 1996;199:2077–2084. doi: 10.1242/jeb.199.9.2077. [DOI] [PubMed] [Google Scholar]
- Shashar N., Hanlon R.T., Petz A.D. Polarization vision helps detect transparent prey. Nature. 1998;393:222–223. doi: 10.1038/30380. [DOI] [Google Scholar]
- Shashar N., Hagan R., Boal J.G., Hanlon R.T. Cuttlefish use polarization sensitivity in predation on silvery fish. Vision Res. 2000;40:71–75. doi: 10.1016/S0042-6989(99)00158-3. [DOI] [PubMed] [Google Scholar]
- Shashar N., Borst D.T., Ament S.A., Saidel W.M., Smolowitz R.M., Hanlon R.T. Polarization reflecting iridophores in the arms of the squid Loligo pealeii. Biol. Bull. 2001;201:267–268. doi: 10.2307/1543358. [DOI] [PubMed] [Google Scholar]
- Shashar N., Milbury C.A., Hanlon R.T. Polarization vision in cephalopods: neuroanatomical and behavioral features that illustrate aspects of form and function. Mar. Freshw. Behav. Physiol. 2002;35:57–68. doi: 10.1080/10236240290025617. [DOI] [Google Scholar]
- Siebeck U.E. Communication in coral reef fish: the role of ultraviolet colour patterns in damselfish territorial behaviour. Anim. Behav. 2004;68:273–282. doi: 10.1016/j.anbehav.2003.11.010. [DOI] [Google Scholar]
- Stavenga D.G., Stowe S., Siebke K., Zeil J., Arikawa K. Butterfly wing colours: scale beads make white pierid wings brighter. Proc. R. Soc. B. 2004;271:1577–1584. doi: 10.1098/rspb.2004.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegen J.C., Gienger C.M., Sun L. The control of color change in the Pacific tree frog, Hyla regilla. Can. J. Zool. 2004;82:889–896. doi: 10.1139/z04-068. [DOI] [Google Scholar]
- Sutherland R.L., Mäthger L.M., Hanlon R.T., Urbas A.M., Stone M.O. Cephalopod coloration model. I. Squid chromatophores and iridophores. J. Opt. Soc. Am. A. 2008a;25:588–599. doi: 10.1364/JOSAA.25.000588. [DOI] [PubMed] [Google Scholar]
- Sutherland R.L., Mäthger L.M., Hanlon R.T., Urbas A.M., Stone M.O. Cephalopod coloration model. II. Multiple layer skin effects. J. Opt. Soc. Am. A. 2008b;25:2044–2054. doi: 10.1364/JOSAA.25.002044. [DOI] [PubMed] [Google Scholar]
- Sweeney A., Jiggins C., Johnsen S. Polarized light as a butterfly mating signal. Nature. 2003;423:31–32. doi: 10.1038/423031a. [DOI] [PubMed] [Google Scholar]
- Taylor J.D., Hadley M.E. Chromatophores and color change in the lizard, Anolis carolinensis. Cell Tissue Res. 1970;104:282–294. doi: 10.1007/BF00309737. [DOI] [PubMed] [Google Scholar]
- Taylor L.A., McGraw K.J. Animal coloration: sexy spider scales. Curr. Biol. 2007;17:R592–R593. doi: 10.1016/j.cub.2007.05.064. [DOI] [PubMed] [Google Scholar]
- Vaia R., Baur J. Adaptive composites. Science. 2008;319:420–421. doi: 10.1126/science.1152931. [DOI] [PubMed] [Google Scholar]
- Vorobyev M., Osorio D., Bennett A.T.D., Marshall N.J., Cuthill I.C. Tetrachromacy, oil droplets and bird plumage colours. J. Comp. Physiol. A. 1998;183:621–633. doi: 10.1007/s003590050286. [DOI] [PubMed] [Google Scholar]
- Vukusic P., Sambles J.R. Photonic structures in biology. Nature. 2003;424:852–855. doi: 10.1038/nature01941. [DOI] [PubMed] [Google Scholar]
- Vukusic P., Sambles J.R., Lawrence C.R., Wootton R.J. Quantified interference and diffraction in single Morpho butterfly scales. Proc. R. Soc. B. 1999;266:1403–1411. doi: 10.1098/rspb.1999.0794. [DOI] [Google Scholar]
- Vukusic P., Sambles J.R., Lawrence C.R. Colour mixing in wing scales of a butterfly. Nature. 2000;404:457. doi: 10.1038/35006561. [DOI] [PubMed] [Google Scholar]
- Williams L. E.J. Brill; Leiden, Holland: 1909. The anatomy of the common squid, Loligo pealii. [Google Scholar]
- Wilson T., Hastings J.W. Bioluminescence. Annu. Rev. Cell Dev. Biol. 1998;14:197–230. doi: 10.1146/annurev.cellbio.14.1.197. [DOI] [PubMed] [Google Scholar]
- Young R.E., Arnold J.M. The functional morphology of a ventral photophore from the mesopelagic squid Abralia trigonura. Malacologia. 1982;23:135–163. [Google Scholar]
- Young R.E., Bennett T.M. The mollusca, paleontology and neontology of cephalopods. vol. 12. Academic Press; San Diego, CA: 1988. Photophore structure and evolution within the Enoploteuthinae (Cephalopoda) pp. 241–251. [Google Scholar]
- Young R.E., Mencher F.M. Bioluminescence in mesopelagic squid: diel color change during counterillumination. Science. 1980;208:1286–1288. doi: 10.1126/science.208.4449.1286. [DOI] [PubMed] [Google Scholar]
- Zi J., Yu X., Li Y., Hu X., Xu C., Wang X., Liu X., Fu R. Coloration strategies on peacock feathers. Proc. Natl Acad. Sci. USA. 2003;100:12 576–12 578. doi: 10.1073/pnas.2133313100. [DOI] [PMC free article] [PubMed] [Google Scholar]