Abstract
In animals, iridescence is generated by the interaction of light with biological tissues that are nanostructured to produce thin films or diffraction gratings. Uniquely among animal visual signals, the study of iridescent coloration contributes to biological and physical sciences by enhancing our understanding of the evolution of communication strategies, and by providing insights into physical optics and inspiring biomimetic technologies useful to humans. Iridescent colours are found in a broad diversity of animal taxa ranging from diminutive marine copepods to terrestrial insects and birds. Iridescent coloration has received a surge of research interest of late, and studies have focused on both characterizing the nanostructures responsible for producing iridescence and identifying the behavioural functions of iridescent colours. In this paper, we begin with a brief description of colour production mechanisms in animals and provide a general overview of the taxonomic distribution of iridescent colours. We then highlight unique properties of iridescent signals and review the proposed functions of iridescent coloration, focusing, in particular, on the ways in which iridescent colours allow animals to communicate with conspecifics and avoid predators. We conclude with a brief overview of non-communicative functions of iridescence in animals. Despite the vast amount of recent work on animal iridescence, our review reveals that many proposed functions of iridescent coloration remain virtually unexplored, and this area is clearly ripe for future research.
Keywords: iridescence, behaviour, function, communication, sexual selection, predation
1. Introduction
Animal coloration strategies have long been a topic of interest to naturalists and philosophers, and even featured prominently in Aristotle's Historia animalium, written more than 2000 years ago (Lennox 2001). In the last few centuries, scientists began to explore in earnest how animal colours were produced (e.g. Newton 1952), and to elucidate their possible functions (e.g. Darwin 1859, 1871; Wallace 1889). Recent reviews have highlighted a striking surge in contemporary research on both the form and function of visual signals in animals (e.g. Caro 2005; Berthier 2006; Hill & McGraw 2006a,b; Ladich et al. 2006). One particular type of coloration, iridescent coloration, produces some of the most spectacular visual displays found in animals, including the sparkling elytra of beetles, the shimmering scales of butterfly wings and the flashing gorgets of hummingbirds. Iridescence appears to play an important role in intraspecific communication and, especially, sexual selection. Intriguingly, iridescent colours may also help animals avoid predation, and may serve non-communicative functions such as enhancing vision, repelling water or strengthening integumentary tissues. Our objective is to review these functional aspects of iridescent coloration in animals. In particular, we briefly describe mechanisms of colour production in animals, provide a general overview of the taxonomic distribution of iridescent colours, highlight unique properties of iridescent coloration and review the various means by which iridescence may function in visual communication. We also identify a number of non-communicative functions of iridescence, and conclude by suggesting promising avenues for future research.
Iridescence is a visual characteristic attributed to surfaces that change in colour with viewing angle. The term derives from the Latin and Greek ‘iris’, meaning rainbow, and also refers to Greek goddess Iris, who is the personification of the rainbow and a messenger to the gods (Barnhart & Steinmetz 1988). As a consequence of their changeable nature and the diversity of hues they can produce, iridescent colours are variously described as rainbow-like, nacreous, opalescent, shimmering, metallic or sparkling. The natural world abounds with examples of iridescent surfaces, from abiotic iridescence produced by minerals such as opals to biotic iridescence produced in all manner of living organisms including algae, plants and animals (Vukusic 2004). In addition, we as humans encounter artificial iridescent phenomena on a daily basis, from iridescent films in soap bubbles and oil slicks to iridescent coatings and photonic devices, some of which have been inspired by natural photonic structures (Parker & Townley 2007).
2. Mechanisms of colour production in animals
Although the term iridescence characterizes a visual phenomenon, all iridescent colours are produced by similar underlying mechanisms, which are perhaps best understood in the general context of colour production in animals. We broadly interpret the term colour to mean those electromagnetic wavelengths that are visible by animals (between 300 and 700 nm), although we recognize that there exists tremendous variation in visual sensitivities across different species, particularly at the upper and lower bounds of that range (Land & Nilsson 2002; Bowmaker 2008). With the exception of bioluminescence, all animal colours are produced by one of two primary mechanisms, or by a combination of these mechanisms: (i) pigmentary coloration and (ii) structural coloration. Pigmentary colours are produced by the deposition of pigments that interact with light on a molecular level to absorb certain wavelengths. For example, carotenoid pigments absorb shorter wavelengths and allow longer wavelengths to be transmitted or reflected, depending on the composition of the surrounding material, thereby resulting in red, orange or yellow colours in animals (Fox 1976). By contrast, melanin pigments exhibit high absorbance across all visible wavelengths, with increasing absorbance at shorter wavelengths, resulting in black or brown colours (Fox 1976).
Structural colours are produced by the physical interaction between light and nanometre-scale variation in the integumentary tissues of some animals. The mechanisms responsible for producing structural colour have been summarized in several excellent reviews (e.g. Fox & Vevers 1960; Land 1972; Fox 1976; Srinivasarao 1999; Vukusic & Sambles 2003; Prum 2006; Bagnara et al. 2007; Kinoshita et al. 2008; Mäthger et al. 2009; Seago et al. 2009; Shawkey et al. 2009), and we describe them only briefly here. When light encounters boundaries between media that differ in refractive index, structural coloration can be produced by interference, diffraction or scattering. Both interference and diffraction can produce iridescent colours that change in appearance with viewing geometry, and are characterized by single or multiple reflectance maxima. Interference colours are produced when light interacts at boundaries of media with different refractive indices, where, depending on the dimensions of the media, some wavelengths constructively interfere to produce brilliant colours, while the remaining wavelengths destructively interfere (Prum 2006; Kinoshita et al. 2008). Interference-based colours can be produced by optical materials arranged in simple thin films or in multilayer reflectors. Diffraction gratings are reflective surfaces with regularly ordered parallel grooves or depressions that disperse different wavelengths of light in different directions, which, in turn, depends on the periodicity of the grating and its relation with incident wavelengths (Srinivasarao 1999). Colour-producing nanostructures that are arranged in a crystalline pattern can also produce iridescence through diffraction following Bragg's law (Prum 2006). Diffraction and interference mechanisms can be combined to produce complex optical effects, as in the scales of some butterfly wings (Kinoshita et al. 2008).
Some forms of scattering produce non-iridescent structural colours that tend to reflect maximally at shorter wavelengths ranging from ultraviolet to turquoise, although there are examples of long-wavelength structural colours produced by scattering (Prum 2006). Non-iridescent structural colours in animals were long thought to be produced by incoherent scattering mechanisms such as Rayleigh scattering and Tyndall scattering (e.g. Fox & Vevers 1960; Fox 1976). However, recent work by Prum and colleagues suggests that many, if not most, non-iridescent structural colours in animals are produced by the constructive interference of light (Prum 2006; Kinoshita et al. 2008). Thus, although iridescent and non-iridescent structural colours were assumed to involve fundamentally different mechanisms, it is becoming increasingly clear that both types of colour can result from coherent scattering, and that the main difference between them results from differences in the organization of their colour-producing nanostructures (Prum 2006; Kinoshita et al. 2008). Because non-iridescent structural colours are produced by quasi-ordered arrays instead of layered or crystalline structures, they tend to be less saturated, more diffuse and unaffected by viewing geometry. Nevertheless, it is sometimes difficult to distinguish between iridescent and non-iridescent colours, since structural colours often involve multiple scales of organization (Kinoshita & Yoshioka 2005). It should also be noted that incoherent scattering can produce whiteness by scattering all visible wavelengths (Prum 2006). For the purposes of this review, we interpret iridescence in its broadest sense, meaning colours that change in hue or intensity with viewing geometry.
3. Taxonomic distribution of iridescence in animals
In this section, we provide a general overview of the taxonomic distribution of iridescent colours. We do not aim to be comprehensive, as there are many more iridescent animals than can be described here, and probably many more whose iridescent properties remain to be characterized. Rather, we wish to highlight the diversity of animals known to produce iridescent colours (see also Fox & Vevers 1960; Fox 1976; Berthier 2006). Iridescent coloration is broadly distributed in the animal kingdom and appears to have evolved independently in a number of different taxonomic groups. Iridescence is a relatively common feature in some groups of invertebrates, particularly arthropods and molluscs. In crustaceans, iridescent multilayer reflectors are found in sapphrinid copepods (Chae & Nishida 1994), Ovalipes decapods (Parker et al. 1998), Limnadia clam shrimps (Spinicaudata) and Tanais tennicornis (Tanaidacea; Parker 2000). In some crustaceans, iridescence is produced through diffraction gratings on setae, setules and other body regions (Parker 1995, 2000). Some polychaete worms also produce iridescence through diffraction gratings (Parker 2000) and arrays of photonic crystals (Parker et al. 2001). Comb jellies (Ctenophora) employ densely packed cilia in their comb rows to produce iridescent photonic crystals that change colours as their combs beat (Welch et al. 2005). Among arachnids, jumping spiders are particularly colourful, with several species exhibiting pronounced sexual dichromatism and iridescent coloration (Lim & Li 2006b; Land et al. 2007; Taylor & McGraw 2007).
In insects, iridescence commonly occurs on the wings and bodies of flies (Diptera; Fox & Vevers 1960), wasps and bees (Hymenoptera; e.g. Sarrazin et al. 2008). Cuckoo wasps and orchid bees are particularly colourful examples. The wings of dragonflies and damselflies (Odonata) are often iridescent (Vukusic et al. 2004), and their thoracic and abdominal epicuticles can produce iridescence in some species (e.g. Fitzstephens & Getty 2000). The scales on the wings of many butterflies and moths (Lepidoptera) produce striking iridescent colours by interference and diffraction, and have been the focus of many studies on the mechanisms and function of iridescence (e.g. Ghiradella et al. 1972; Miaoulis & Heilman 1998; Vukusic et al. 1999, 2009; Plattner 2004; Kemp & Macedonia 2006; Rutowski et al. 2007; Ingram & Parker 2008; Poladian et al. 2009; Shawkey et al. 2009; Vukusic & Stavenga 2009). The pupae of some butterflies also have iridescent markings that change colour as the adult butterfly develops (Steinbrecht et al. 1985). The elytra of many beetles (Coleoptera) are famous for their iridescent properties, including those of jewel-like scarab beetles (e.g. Vulinec 1997; Parker 2000; Schultz 2001; Kurachi et al. 2002; Galusha et al. 2008; Seago et al. 2009). Some species of grasshopper (Orthoptera) and leaf-footed bugs (Hemiptera) also produce iridescent colours.
In molluscs, the Cephalopoda (squid, cuttlefish and octopi) are well known for brilliant coloration that changes dynamically across social and behavioural contexts. Iridophores (changeable iridescent cells) in the skin underlie chromatophores (changeable pigmented organs), and their combined effects produce reflectance patterns that encompass the entire range of the visible spectrum (reviewed in Mäthger et al. 2009). The insides of shells of many Gastropoda and Bivalvia molluscs are composed of iridescent nacre (Jackson et al. 1988; Smith et al. 1999), and the outsides of some shells also have iridescent markings (Brink et al. 2002; Brink & van der Berg 2005). Some bivalves have iridescent mantles, including giant clams (Tridacna spp.; Griffiths et al. 1992) and flame scallops (Lima scabra), a quality that makes them popular in the marine aquarium trade.
Among vertebrates, the evolution of iridescence has apparently been confined to a few select groups. In many vertebrates, iridescence is produced by specialized cells that are also called iridophores, although there are structural and functional differences between these and invertebrate iridophores (Bagnara et al. 2007). These cells usually contain a basal melanin layer and stacked reflecting platelets that are several layers thick (Bagnara et al. 2007). Iridescence produced by iridophores is common in fishes and can result in a variety of colours, including the silvery iridescence produced by many species and some of the brilliant colours displayed by reef fishes (e.g. Denton 1970; Denton & Land 1971; Kasukawa et al. 1987; Lythgoe & Shand 1989; Goda et al. 1994; Goda & Fujii 1998; Marshall et al. 2003). In reptiles and amphibians, structural colours are also produced by iridophores, and their green coloration usually results from the combination of a blue structural colour and a yellow pigment (e.g. Bagnara et al. 1968; Bagnara & Hadley 1973; Macedonia et al. 2000). Although these colours are often described as non-iridescent, some authors have noted iridescent features such as changes in colour with angle of observation (e.g. Rohrlich & Porter 1972; Morrison et al. 1995). Furthermore, in Sceloporus and Urosaurus lizards, the brick-shaped reflecting platelets found in iridophores are organized in discrete layers, and measured reflectance spectra match those predicted from thin-film models based on the dimensions and refractive indices of those layers (Morrison 1995; Morrison et al. 1995, 1996). Nevertheless, most structural colours produced by reptiles and amphibians are not strongly iridescent, perhaps as a consequence of irregularities in microstructural organization at larger spatial scales, such as the orientation of iridophores relative to the surface of the skin (Kobelt & Linsenmair 1992). Despite these ambiguities, a number of herpetile species are unquestionably iridescent. For example, many snakes have an iridescent sheen to their coloration (Fox 1976), and some snakes are highly iridescent, including sunbeam snakes (Xenopeltis unicolor), rainbow boas (Epicrates cenchria) and indigo snakes (Drymarchon corais). In indigo snakes, iridescence is produced by a diffraction grating at junctions between rows of cells (Monroe & Monroe 1968). Some skinks, such as the rainbow skink (Lampropholis delicata), also change in colour with viewing geometry, although the mechanism responsible remains to be described. Some anurans also possess distinctly iridescent coloration (e.g. Kobelt & Linsenmair 1992).
In birds, the nanostructural organization of keratin, melanin and air in feather barbules can produce iridescent coloration through thin films, multilayer reflectors or photonic crystals (e.g. Greenewalt et al. 1960; Durrer & Villiger 1966, 1970; Land 1972; Zi et al. 2003; Prum 2006). Iridescence is broadly distributed throughout Aves, and appears to have evolved independently in a number of different groups (Prum 2006). Iridescence is very unusual in mammals, although the fur of golden moles produces an iridescent sheen, a phenomenon for which this group of small mammals is named (Fox & Vevers 1960; Kuyper 1985).
Many animals, both vertebrate and invertebrate, have iridescent eyes (e.g. Fox & Vevers 1960; Denton & Land 1971; Fox 1976; Land & Nilsson 2002). Many nocturnal animals also possess a reflective structure called the tapetum lucidum in their eyes. This structure, which produces eyeshine in nocturnal species, is highly iridescent (Fox & Vevers 1960; Fox 1976; Parker 2000). In addition, a number of bioluminescent species use iridescence as a complement to their light-producing organs (photophores), which allows them to adjust the intensity, directionality or spectral quality of their bioluminescent signals (Herring 1994, 2000).
Despite the cursory nature of this taxonomic overview, it is clear that iridescence has evolved multiple times in groups of different organisms. The diverse ecologies and life histories of species that exploit iridescent coloration, from ocean-dwelling copepods to lek-mating birds, already hint at the multiplicity of functions that might be served by iridescence. Although some functions of iridescent coloration are shared with other mechanisms of colour production, others probably derive from unique optical properties of iridescent signals.
4. Unique features of iridescent visual signals
4.1 Directionality
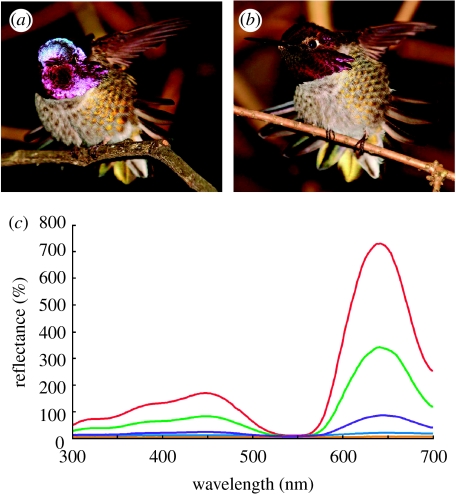
Iridescent colours are by definition highly directional. Changes in viewing geometry can dramatically alter the appearance of iridescent colours, producing considerable changes in hue, intensity or both (e.g. Huxley 1968; Land 1972; Osorio & Ham 2002; Vukusic et al. 2004; Kinoshita et al. 2008; figure 1). Changes in intensity are often particularly noticeable, as iridescent colours usually appear brilliant and saturated at optimal viewing geometries. When the viewing geometry changes, however, the iridescence can disappear entirely, leaving visible only the colour produced by underlying pigments, which are often black melanins (e.g. Osorio & Ham 2002; figure 1). Animals could exploit this feature of iridescence in a number of ways. First, the directionality of iridescent colours might allow animals to direct their signals at intended receivers, such as prospective mates or rivals. This not only enables animals to target and engage particular individuals, but also to avoid unnecessary conflict with nearby conspecifics (Bradbury & Vehrencamp 1998). Indeed, many species seem to have evolved ultrastructural modifications that increase the directionality of iridescent signals, such as angled feather barbules in birds (Osorio & Ham 2002). A related benefit of directional signals is that they might allow animals to be conspicuous to their intended signal receivers while remaining relatively inconspicuous to potential predators. Just as animals might use directionality to avoid confronting certain conspecifics, they could use directionality to avoid drawing the attention of predators by orienting so that their iridescence appears brilliant to intended signal receivers while appearing dull to predators. Another consequence of directionality is that many iridescent colours appear much more brilliant in direct rather than diffuse light (Osorio & Ham 2002), and their overall intensity depends on the total amount of light available. Since light environments vary according to weather, habitat and time of day (Endler 1993, 1997), animals could seek out particular light environments for performing conspicuous displays and favour others when trying to remain cryptic (e.g. Endler & Théry 1996; Schultz 2001; §5.6.3).
Figure 1.
(a,b) Photographs of and (c) reflectance spectra from Anna's hummingbird (Calypte anna). (a) Anna's hummingbird facing the observer at nearly optimal viewing geometry, showing highly iridescent gorget and crown feathers (photo by Camden Hackworth). (b) The same hummingbird turned away from the observer, with iridescence greatly reduced at this viewing geometry (photo by Camden Hackworth). (c) Spectral reflectance from a single Anna's hummingbird gorget feather measured with fixed probe and light source and the feather rotated for maximum reflectance (red line), and then rotated away by 5° (green line), 10° (violet line), 15° (blue line) and 20° (orange line). The orange line overlaps the x-axis because the feather is nearly perfectly black when rotated away from maximum reflectance by only 20°. Spectral reflectance was calculated relative to an MgO white standard. Note that reflectance values are well over 100% owing to the high reflectivity and specularity of these feathers in comparison with the diffuse white standard.
Directionality also allows animals to produce rapid flashes of colour. These flashes could incorporate changes in hue, intensity or both. Such flashes may be used in intraspecific displays, such as the ‘chatter-sway’ display of male Anna's hummingbirds (Calypte anna), where iridescent gorget and crown feathers are erected and the head is turned back and forth, causing the feathers to flash at the receiver (Stiles 1982; figure 1). Iridescent coloration on the surface of butterfly wings is also thought to function as an intraspecific communication signal that flashes on and off during flight (e.g. Vukusic et al. 1999). Such bright flashes of colour could also be used as predator deterrent signals as described in §5.8.2. To take full advantage of signal directionality, animals should evolve behaviours and postures that enhance or reduce the conspicuousness of their iridescent signals, depending on the context. The directionality of iridescent structures has also favoured their evolution as complements to photophores in bioluminescent organisms (reviewed in Herring 2000), allowing these animals to direct their bioluminescent signals towards intended receivers.
4.2 Maximizing conspicuousness
Another unique feature of iridescent colours is that, depending on the underlying mechanism and viewing geometry, they can be exceptionally bright and saturated to a degree that is not usually achieved by pigment-based colours (e.g. Vukusic et al. 1999; Osorio & Ham 2002). Indeed, iridescent reflectance spectra often exceed 100 per cent reflectance when measured relative to a diffuse white standard (e.g. Osorio & Ham 2002; figure 1c).
To produce conspicuous visual signals, animals must use colours that generate a high degree of contrast against the background (Endler 1990). Because iridescent colours are so bright and saturated, they can produce highly conspicuous signals that generate high chromatic and achromatic visual contrast against natural backgrounds, which are usually darker and less saturated (e.g. bark, vegetation, gravel; Endler 1980; Endler & Théry 1996; Endler et al. 2005). Dark iridescent colours, such as blue-black iridescence, may also produce signals that generate high achromatic contrast by being darker than the visual background (e.g. Doucet et al. 2007). Another means of enhancing signal conspicuousness involves producing high within-pattern contrast by pairing colours that together generate high chromatic or achromatic contrast (Endler 1990). This can be accomplished in a number of ways with iridescent colours. First, animals can use pairs of highly saturated iridescent colours that peak at different wavelengths to generate high chromatic contrast; such pairings of bright iridescent colours are common in hummingbirds (e.g. Greenewalt et al. 1960), peafowl (e.g. Loyau et al. 2007), beetles (e.g. Seago et al. 2009) and butterflies (e.g. Prum et al. 2006). Second, animals can combine saturated iridescent colours with saturated pigment-based colours to produce high chromatic contrast, and such combinations are found in many reef fishes (e.g. Marshall 2000; Losey 2003), butterflies (e.g. Wijnen et al. 2007) and birds such as trogons (e.g. Espinosa de los Monteros 1998). Third, bright, saturated iridescent colours can be combined with dark pigment-based colours to simultaneously maximize both chromatic and achromatic contrasts. For example, in many butterflies, bright iridescent colours are delineated by dark pigment-based colours that enhance spectral contrast (e.g. Stavenga et al. 2004). Similarly, many birds of paradise have modified feathers that produce dramatic iridescent displays (Frith & Beehler 1998), and these are often surrounded by pigmented black feathers that presumably enhance the conspicuousness of the signal. Furthermore, Théry et al. (2008) recently found that in the horned beetle Coprophanaeus lancifer, bright, iridescent pronotum coloration produces high visual contrast with the dark non-iridescent horn. Finally, dark, blue-black iridescence can be combined with bright colours such as white and yellow to maximize both chromatic and achromatic contrasts, as in Corapipo manakins (e.g. Endler & Théry 1996; Doucet et al. 2007). Because iridescent colours can generate high visual contrast, they are particularly useful in contexts that require conspicuous signals, such as intraspecific communication, warning coloration or flash and startle displays.
4.3 Short-wavelength colours
Another important feature of iridescent signals, as well as other structural colours, is that they provide animals with the ability to produce colours reflecting maximally or secondarily at short wavelengths ranging from blue to ultraviolet (e.g. Bennett et al. 1997; Doucet & Montgomerie 2003a; Lim & Li 2006b; Prum 2006; Kemp 2008; figure 1c). Blue pigments are rare in animals, and only a few invertebrate species are known to use blue pigments for coloration (Fox 1976). Among vertebrates, only two species of callionymid fishes have been found to produce blue integumentary colour using pigments (Bagnara et al. 2007). Despite this, the visual systems of most animals are sensitive to blue wavelengths, and many species can also detect ultraviolet wavelengths (e.g. Hamdorf et al. 1971; Silberglied 1979; Hawryshyn 1992; Fleishman et al. 1993; Cuthill 2006; Lim & Li 2006a; Bowmaker 2008). As a consequence, the short-wavelength region of the spectrum must be considered in the production of either cryptic or conspicuous signals. For example, iridescent colours may allow animals to more closely match the reflectance of their visual background for camouflage (Endler 1978), or provide strong chromatic contrast when combined with long-wavelength colours to produce conspicuous signals used in courtship or as warning coloration (Endler 1988). Some species might also use short-wavelength colours as a private communication channel if their primary predators lack UV vision (e.g. Endler 1991; Cummings et al. 2003).
4.4 Environmental variation
Another feature that sets iridescent colours apart from pigment-based colours is that their hue, saturation and brightness are directly dependent on the dimensions and refractive indices of the colour-producing nanostructures. Nanometre-scale differences in either of these characteristics can cause dramatic variation in colour both across species (e.g. Denton 1970; Prum 2006; Prum et al. 2006; Shawkey et al. 2006) and within species (e.g. Kobelt & Linsenmair 1992; Fitzstephens & Getty 2000; Doucet et al. 2006; Kemp et al. 2006). At an intraspecific level, this feature of iridescence has important implications because it might allow an animal to alter its coloration in response to changes in its environment. These changes might be driven by abiotic factors such as in fishes, where corneal iridescence changes in response to light availability, which may enhance visual sensitivity in low light conditions (e.g. Shand 1988). In the reed frog (Hyperolius viridiflavus), dry season increases in temperature induce changes in iridescence that result in higher overall reflectance, which is thought to help the frogs thermoregulate (Kobelt & Linsenmair 1992).
Alternatively, colour-producing nanostructures might change in response to an animal's physiological state. In black-winged damselflies (Calopteryx maculata), for example, males with high fat stores are iridescent blue, whereas leaner males are green (Fitzstephens & Getty 2000). Fatter males achieve bluer coloration through compression of the chitin and melanin layers that produce iridescence (Fitzstephens & Getty 2000). Even in species where colour-producing nanostructures remain fixed after development, physiological stress during nanostructure development could potentially alter the dimensions, configuration or regularity of these structures. Indeed, a number of studies suggest that iridescent coloration is associated with variation in condition (§5.6.1), and one experimental study has demonstrated a direct connection between developmental stress, nanostructural architecture and iridescent colour in a butterfly (Kemp et al. 2006). In addition, the structures responsible for producing iridescent colours are often located on or near the surface of animals, and structural colours might therefore be more susceptible to wear and tear than pigment-based colours, as suggested by patterns of barbule breakage in birds (Fitzpatrick 1998) and age-dependent scale damage in butterflies (Kemp 2006a). These characteristics of iridescent colours highlight their suitability as indicators of age or quality in some species. However, we still need a better understanding of the development of iridescent colours to determine whether and how developmental stress might affect the colour-producing nanostructures. For example, does developmental stress limit the total amount of colour-producing nanostructural material? Does stress result in limitation of a condition-dependent building block of the nanostructure, such as melanin in bird feathers (McGraw 2008)? Or does it simply affect the regularity of the nanostructural architecture? Future studies should also evaluate whether iridescent colours really are more susceptible to external damage by comparing damage-induced variation in colour across colours produced by different mechanisms.
5. Iridescence and visual communication
Iridescent colours probably serve the same primary functions ascribed to other coloration strategies used by animals: namely, to communicate with conspecifics and to avoid predation. However, the unique optical and structural properties of iridescent colours may be exploited for specialized forms of visual communication, or may even serve entirely non-communicative functions. In the following sections, we summarize these various functions, reviewing evidence from the published literature where available.
5.1 Species recognition
To communicate with conspecifics, individuals must recognize members of their own species, and this can be accomplished by iridescent coloration. A series of experiments performed by Silberglied & Taylor (1978) demonstrated that UV iridescent coloration functions as a species isolation mechanism in both male and female Colias butterflies. In guppies (Poecilia reticulata), males in low-predation populations have more iridescent spots (among other traits) than males in high-predation populations, and females prefer males from their own populations (Endler & Houde 1995). Such population divergence in appearance and preference has important implications, since it may eventually lead to reproductive isolation and speciation (Endler & Houde 1995). Although species recognition is undoubtedly an important function of iridescent coloration in animals, as has been proposed for a number of species (e.g. Silberglied & Taylor 1973; Rutowski 1977; Chae & Nishida 1994; Kinoshita et al. 2002), there have been few explicit tests of this hypothesis.
5.2 Sex recognition
Within the context of intraspecific communication, iridescence may allow animals to identify the sex of conspecifics. Sexual dichromatism is a common feature of iridescent signals in birds (e.g. Owens & Hartley 1998), fishes (e.g. Kodric-Brown 1998), butterflies (e.g. Silberglied 1979; Penz & DeVries 2002), jumping spiders (e.g. Lim & Li 2006b) and many other animals. While sexual dimorphism often evolves for reasons other than signalling sexual identity, there is certainly much potential for iridescence to function as a signal of sex recognition, although only a few studies have addressed this idea. Experiments have demonstrated that male Eurema lisa butterflies can distinguish females from conspecific males on the basis of UV iridescence (Rutowski 1977), and UV iridescence also appears to function in sex recognition in Colias butterflies (Silberglied & Taylor 1978). Similarly, male Heliconius cydno butterflies appear to use polarized iridescence to recognize females (Sweeney et al. 2003). In the jumping spider Cosmophasis umbratica, males have a prominent iridescent UV reflectance peak that is absent in females (Lim & Li 2006b). In full-spectrum light, males will perform agonistic displays towards each other, but will rapidly switch to courtship displays in the absence of UV light (Lim & Li 2006a). Females, on the other hand, ignore males in the absence of UV light, suggesting that the presence of a UV reflectance peak is used as a sex recognition mechanism in both sexes (Lim et al. 2007). Although sex recognition is not a common focus of functional studies of iridescence, further experiments may demonstrate the use of iridescent colours as sex recognition traits in other species where the sexes differ in iridescent coloration.
5.3 Age
Iridescent reflectance may encode information about the age of the signaller. In birds, many species exhibit delayed plumage maturation, where males remain in juvenile plumage for one or more years after reaching reproductive maturity (e.g. Rohwer et al. 1980; Lyon & Montgomerie 1986), and this phenomenon is exhibited by a number of species with iridescent plumage (e.g. Stutchbury 1991; Doucet et al. 2006). In addition, iridescent plumage may vary with age in species without delayed plumage maturation, or where individuals have already achieved their definitive (adult) plumage (Komdeur et al. 2005; Madsen et al. 2007; Bitton & Dawson 2008). In butterflies, the intensity of UV iridescence tends to decrease with age, and this is probably a function of the wear and tear experienced by the scales producing the iridescent coloration (Kemp 2006a, 2008; Kemp & Macedonia 2006). Age-based variation in iridescence has also been demonstrated in jumping spiders (Lim & Li 2007) and guppies P. reticulata (Miller & Brooks 2005), and may yet be uncovered in many other species.
5.4 Mate choice
A growing number of studies suggest that iridescence plays an important role in mate choice. In the butterfly Eurema hecabe, males with intact UV reflectance experience greater copulation success than males with experimentally reduced UV reflectance, and in naturally occurring copulations, males with more UV reflectance copulated with larger females (Kemp 2008). Similar preferences for UV iridescence were documented in the butterflies Hypolimnas bolina (Kemp 2007), H. misippus (Stride 1958), H. cydno (Sweeney et al. 2003) and Colias eurytheme (Papke et al. 2007). Iridescence also appears to function in mate choice in the guppy (Kodric-Brown & Johnson 2002). Among birds, a recent study found that in peacocks (Pavo cristatus), both the brightness of eyespots and their degree of iridescence affected male reproductive success, suggesting that iridescence per se is an important mate choice cue (Loyau et al. 2007). A mate choice function of iridescent coloration has been implicated in several other avian species, including European starlings (Sturnus vulgaris; Bennett et al. 1997), tree swallows (Tachycineta bicolor; Bitton et al. 2007) and mallards (Anas platyrhynchos; Omland 1996a,b). Despite compelling evidence that iridescent coloration appears to be an important mate choice cue in some species, a few studies have also failed to detect an influence of iridescence on mate choice (e.g. Mateos & Carranza 1995; Perrier et al. 2002; Kemp 2006b).
5.5 Agonistic interactions
Iridescent colours appear to mediate agonistic intrasexual encounters in a number of species. As mentioned previously, in the jumping spider C. umbratica, UV iridescence is restricted to males and emphasized by behavioural displays during male–male agonistic encounters when UV light is available, but not in the absence of UV light (Lim & Li 2006a). In the black-winged damselfly, iridescent blue-green coloration is an accurate predictor of territorial status (Fitzstephens & Getty 2000). By contrast, iridescent coloration does not appear to function as a territorial signal in the butterfly H. bolina (Rutowski 1992; Kemp & Macedonia 2006), and its use may be restricted to mate choice in this species (Kemp 2007). Vulinec (1997) suggested that the iridescent prothoracic shield of Phanaeus vindex horned dung beetles may serve to highlight the size of the horn, facilitating male–male assessment during agonistic interactions, but this hypothesis remains to be tested. Squid (Loligo plei and Lolliguncula brevis) and cuttlefish (Sepia officinalis) dynamically alter their iridescent patterns in various social contexts, and display iridescent colours during aggressive encounters (Hanlon 1982; Hanlon et al. 1990; Shashar et al. 1996). In purple martins (Progne subis), adult males have blue-black iridescent plumage, whereas subadults have a mixture of blue-black and brown feathers (Stutchbury 1991). Subadult males that were experimentally dyed black to mimic adult males acquired breeding territories faster than control subadults. However, because the dye used resulted in darker, but not iridescent, adult-like coloration, the exact role played by iridescence remains to be determined in this species. In ring-necked pheasants (Phasianus colchicus), males with experimentally dulled plumage suffered more aggressive attacks than control males (Mateos & Carranza 1997). Iridescence has also been linked to aggressive female feeding strategies in hummingbirds; iridescent gorgets are usually reduced or lacking entirely in females, with the exception of those species in which females also defend feeding territories (e.g. Pitelka 1942; Wolf & Stiles 1970); this may explain female polychromatism in tourmaline sunangels, Heliangelus exortis (Bleiweiss 1985).
Although these studies suggest a role for iridescent coloration in agonistic signalling, most do not quantify variation in iridescent coloration using spectrophotometric techniques, or examine patterns of territory ownership rather than observing agonistic interactions between individuals. Thus, there is a great deal more to learn about the role of iridescence in mediating agonistic interactions.
5.6 Mechanisms of sexual selection
The examples highlighted above suggest that in at least some species, sexual selection has played an important role in the evolution of iridescent coloration by mediating either intrasexual aggression or mate choice (Darwin 1871). Why are iridescent colours preferred in mate choice and important in intrasexual encounters? Even within the boundaries of sexual selection, a number of non-mutually exclusive mechanisms could have favoured the evolution of iridescent visual signals, and we review these briefly here.
5.6.1 Honest signalling models
Honest advertisement models of sexual selection posit that certain traits are favoured as sexually selected signals because they honestly reveal some aspects of individual quality (Zahavi 1975; Kodric-Brown & Brown 1984; Grafen 1990; Getty 2006). To preserve honesty and prevent cheating in this kind of signalling system, there must be some cost associated with producing or maintaining the signal. The signal honestly reveals quality because only high-quality individuals can afford to pay the higher cost of increased signalling (Zahavi 1975; Kodric-Brown & Brown 1984; Grafen 1990; Getty 2006). Under this scenario, high-quality individuals should display the most elaborate ornaments, and prospective mates or rivals should use ornament elaboration to gauge the quality of the displaying individual. Rivals could evaluate ornaments to decide whether or not to engage in more escalated contests over resources such as mates or territories, and prospective mates could use ornaments to evaluate the genetic or phenotypic quality of potential sires, and thereby obtain information about the indirect or direct benefits they might accrue by choosing to copulate with, or establish a long-term partnership with, a particular individual (Andersson 1994).
A number of recent studies on iridescent coloration have focused explicitly on testing honest indicator models of sexual selection. For example, Kemp and colleagues found that in the butterfly C. eurytheme, experimentally induced nutrient stress and thermal stress affected the brightness and angular visibility of the iridescent UV component of male dorsal coloration, demonstrating that iridescent coloration is condition dependent in this species (Kemp et al. 2006; Kemp & Rutowski 2007). In another experimental study, Lim & Li (2007) showed that starvation affected iridescent UV coloration in the jumping spider C. umbratica. Similarly, Fitzstephens & Getty (2000) found that in black-winged damselflies, wild males with higher fat stores were bluer, and captive males placed on a high food diet had bluer iridescent coloration than males placed on a low food diet. Vulinec (1997) proposed that the iridescent coloration of certain dung beetles might serve as an indicator trait by revealing the presence of kleptoparasitic flies, which would be conspicuous against the iridescent pronotum coloration, although this hypothesis remains to be tested. In birds, a large comparative analysis supported several predictions relating to the honest signalling potential of iridescent plumage (Fitzpatrick 1998). In two experimental studies, McGraw et al. (2002) found that nutritional stress affected the iridescent coloration, but not the melanin pigmentation, of brown-headed cowbirds (Molothrus ater), and Hill et al. (2005) showed that experimental infection with parasites influenced iridescent coloration in wild turkeys (Meleagris gallopavo). Several other studies provide correlational evidence that iridescent coloration covaries with individual health or condition in birds (Doucet 2002; Møller & Petrie 2002; Doucet & Montgomerie 2003a,b; Costa & Macedo 2005; Bitton et al. 2008).
As discussed in §4.4, iridescent colours may be well suited to function as condition-dependent signals because of the direct relationship between the dimension and organization of colour-producing nanostructures and the colours they generate. Thus, individual health or condition might relate to colour if condition can directly affect the dimensions of colour-producing nanostructures, such as in black-winged damselflies (Fitzstephens & Getty 2000), or if it can indirectly affect an animal's ability to produce nanostructures of ideal dimensions or organization. Alternatively, individuals in poor condition might develop fewer colour-producing structures, such as feather barbules, or might suffer more surface damage to colour-producing body regions.
5.6.2 Amplifier traits
Iridescent colours might also function as amplifiers. Amplifiers are traits that do not themselves signal quality, but instead amplify perceived differences in signals of quality and thereby enhance the resolution power of quality assessments (Hasson 1989, 1991). Although no study has focused specifically on determining whether iridescent colours might function as amplifiers, the iridescent pronotum of Phanaeus dung beetles might be a good candidate trait. Phanaeus dung beetles have a dark horn, which contrasts starkly against the bright iridescence of their pronotum. The size of these horns is important in mate choice and male–male interactions (Emlen et al. 2007), and Vulinec (1997) proposed that pronotum iridescence might enhance the assessment of horn size in this group. Iridescent colours might also function as amplifiers when they are paired with contrasting colours as described in §§4.2 and 4.3, although the reverse is also possible, with non-iridescent colours serving to amplify iridescent traits.
5.6.3 Sensory drive and receiver biases
The evolution of iridescent colours might also be influenced by the sensory ecology of signal receivers (Endler & Basolo 1998). Under a sensory drive scenario, iridescent colours might be favoured if they transmit more effectively in the signalling environment used by a particular species (Endler & Basolo 1998). Under a receiver bias scenario, iridescent colours might be favoured by sexual selection if they stimulate a bias in the female's sensory system (Endler & Basolo 1998). A recent study by Douglas et al. (2007) provides strong evidence for the influence of sensory drive in butterfly coloration. In this large comparative analysis, species exhibiting polarized iridescence were significantly more likely to occupy forested habitats (Douglas et al. 2007). These findings suggest that complex light environments found in forests (Endler 1993, 1997) and the absence of polarization in most forest backgrounds (Sweeney et al. 2003) may favour the evolution of polarized light signals (Douglas et al. 2007). Multi-species studies in birds, including iridescent species, also support the influence of light environments on signal evolution (Endler & Théry 1996; McNaught & Owens 2002; Doucet et al. 2007). By contrast, a recent comparative analysis across five species of Corapipo manakins suggests that sensory drive does not explain evolutionary changes in coloration or display site preferences in this group, although males of one species did prefer to display in particular subsets of the light environment (Anciães & Prum 2008), corroborating earlier findings in this species (Théry & Vehrencamp 1995). As further support for the influence of sensory drive on the evolution of iridescence, female guppies in low-predation environments preferred males with blue or silver iridescence, as did females in habitats with higher transmission of short wavelengths relative to long wavelengths (Endler & Houde 1995). Finally, among iridescent sapphrinid copepods, different species are found at different depths in the water column, and variation in the dominant hue of each species corresponds to the wavelengths available at that depth, supporting an influence of sensory drive (Chae & Nishida 1995).
5.6.4 An integrative perspective on mechanisms of sexual selection
As indicated earlier, the mechanisms of sexual selection we describe above are not mutually exclusive. In fact, these mechanisms are likely to operate in concert to shape the evolution of sexually selected traits and mating preferences for those traits (Kokko et al. 2003). For instance, it is easy to imagine a situation where a trait exploiting a female sensory bias might experience stronger selection if it also revealed some aspect of a male's genetic quality. Similarly, sensory drive and amplifier mechanisms should be good complements to indicator mechanisms, since they would allow females to more effectively detect potential mates and assess their quality. Finally, the Fisherian runaway process, where a genetic correlation between an attractive trait and a preference for that trait drives the rapid evolution of the trait beyond its naturally selected optimum (Fisher 1930), probably plays an important role in the evolution of many sexually selected traits, whether or not they are associated with individual quality (Kokko et al. 2003).
5.7 Orientation, schooling and flocking behaviour
Iridescent coloration may also function in intraspecific communication by helping animals to coordinate group movements. In particular, iridescent colours may facilitate orientation while moving in schools or flocks as a direct consequence of their directionality. In squid, for example, the appearance of iridescent stripes, which are directional and polarized, changes with relative orientation, and individuals could use this information to adjust their position in a school (Mäthger & Denton 2001). Denton (1970) also proposed that in shoaling silvery fishes, individuals sometimes swim on their sides to temporarily make themselves more visible by disrupting the camouflage described in §5.8.1. We hypothesize that iridescent colours might be generally useful in any situation where animals engage in visually coordinated group movements. For example, theoretical models and empirical studies have shown that animals moving in large coordinated groups, such as schools of fishes or flocks of birds, adjust their speeds and positions by following simple rules including moving away from very near neighbours, moving in the same direction as nearby individuals and avoiding becoming isolated (Sumpter 2006). The directionality of iridescent colours could emphasize distances and orientations of neighbours and thereby help to synchronize collective movement behaviour. Many species of ducks have iridescent patches of colour on their wings called specula that might also be helpful in coordinating flight movements. To our knowledge, the hypothesis that iridescent coloration is used in orientation and schooling or flocking has not been empirically tested.
5.8 Avoiding predators
5.8.1 Mimicry and camouflage
Although this proposition may at first seem counter-intuitive, iridescent colours could play an important role in escaping or avoiding predation. In principle, mimicry and camouflage do not constitute true communication, since the intention is to avoid detection by predators (Bradbury & Vehrencamp 1998); nevertheless, we include this section here because the other forms of predator avoidance described below do rely on communication. One means of avoiding predators is through mimicry. Iridescent green leaf beetles and iridescent spiders, for example, may escape the notice of predators by resembling droplets of water or dew on leaves (Crowson 1981; Jackson 1986; Jackson & Hallas 1986; Vulinec 1997). Perhaps more commonly, iridescent animals may escape predation through camouflage. In some species of tiger beetles, minute areas of different iridescent colours blend together by partitive mixing when viewed from a distance, resulting in an unsaturated appearance that matches the visual background of the beetles (Schultz 1986, 2001; Schultz & Bernard 1989; Seago et al. 2009). Similarly, in the beetle Chlorophila obscuripennis, the surface of the multilayer structure responsible for producing its iridescent coloration is modulated such that blue iridescence is produced by small, regularly spaced indentations, and green iridescence is produced by ridges surrounding the indentations (Liu et al. 2008). The overall appearance resulting from colour mixing is a dull bluish green that may provide camouflage against the green leaf background occupied by these beetles (Liu et al. 2008). The use of iridophores in camouflage has been extensively described in cephalopods, and may also occur in other molluscs. Silvery iridophores around the eyes, ink sac and sides of the mantle of some cephalopods provide excellent countershading (Mäthger et al. 2009), and iridophores are used in conjunction with chromatophores to create nearly any colour in the visible spectrum (Mäthger & Hanlon 2007), providing effective camouflage in a colourful underwater environment. Polarized visual signals produced by iridophores may also provide a form of intraspecific communication in cephalopods that is invisible to their primary predators and may therefore function as a private communication channel (Shashar et al. 1996; Mäthger & Hanlon 2006). The iridescent patterns in the mantles of giant clams (Tridacna spp.) may provide camouflage against the colourful, high-contrast background of coral reefs (Griffiths et al. 1992).
Among vertebrates, the silver iridescence produced by iridophores in the skin and eyes of fishes has been proposed to function in underwater camouflage (Denton 1970; Lythgoe 1975; Lythgoe & Shand 1989). Light in water is scattered such that beyond a certain depth, it becomes vertically symmetrical, regardless of the position of the sun (Jerlov 1976). This phenomenon occurs at depths of 300 m or more in clear water on a sunny day, but can occur at shallower depths in turbid water or on cloudy days (Denton et al. 1972; Jerlov 1976). In addition, unless other organisms are nearby, the colour of the water column often forms the entire visual background at these depths. In a light field of this kind, a vertically oriented mirror would provide perfect camouflage from all directions except directly above or below. The silvery scales of many fishes are thought to function as vertical mirrors, and the vertical arrangement of scales appears to be preserved despite the ellipsoid shape of fish bodies through slight adjustments in the orientation of individual scales (Denton 1970). Even among brightly coloured reef fishes, iridescent colours may provide camouflage against colourful corals (Marshall 2000). Iridophores paired with photophores may also help marine animals to direct bioluminescent light downwards to provide effective countershading (Herring 2000). The structural colours produced by reptiles and amphibians, when combined with pigments contained in surrounding xanthophores and melanophores, produce green and brown colours that surely provide camouflage against vegetated backgrounds. Although most of these are non-iridescent structural colours, some herpetile iridophores with iridescent properties may function in this way.
Of course, being cryptic might also benefit potential predators, if it allows them to approach prey without being detected. Many predatory fishes also appear to use their silvery scales as vertical mirrors designed for camouflage (Denton 1970).
5.8.2 Predator deterrence
In some species, iridescent colours can produce bright flashes of colour that might briefly startle a potential predator and thereby increase the prey's probability of escape, or inhibit the predator's ability to quickly judge the exact position of a potential prey item (Hinton 1973). As described in §4, the multilayered arrays that produce iridescent colours can reflect a great deal of incident light at certain viewing angles, creating glare that can flash on and off. This bright flash of colour can function as a startle display, whereby a potential prey surprises a predator by quickly changing its colour, posture or behaviour (Edmunds 1974). Such a mechanism may operate when shoals of fishes, suddenly surprised by a predator, disperse rapidly, with individuals rolling and flitting in different directions and producing bright flashes from their normally inconspicuous silver iridescence (Denton 1970). The sudden display of eyespots and other conspicuous patterns in insects, including iridescent colours, may similarly function as startle displays (Sargent 1990).
In a second mechanism of predator deterrence, iridescent colours may hinder the ability of the predator to judge the prey's position. If a prey animal is stationary, subtle shifts in its position, or the position of the predator as it approaches the prey, could dramatically reduce the prey's brightness and cause the predator to lose sight of the prey (Robinson 1969). Moreover, changes in colour caused by the movement of iridescent animals, either through normal locomotion or during predator escape, could hinder a predator's ability to pinpoint the prey's exact location in an attempted strike (Robinson 1969). This distraction or flash mechanism has been proposed as a function of iridescence in dung beetles (Vulinec 1997) and tiger beetles (Schultz 1986, 2001; Acorn 1988). In butterflies, iridescent wing coloration might produce a flash and conceal effect during flight that could confuse predators (Clench 1966). Flame scallops display rapidly flashing blue iridescence on their mantle, which can only be observed when their shell is open (M.G. Meadows 2005, personal observations), and this may deter predators from attacking the exposed mantle tissue. Predator deterrence using iridescent coloration has received little attention in vertebrates, although the crest display of royal flycatchers, Onychorhyncus coronatus, may function as a startle mechanism. In this species, both males and females possess elaborate and vibrantly coloured crests with iridescent blue tips. These crests can be concealed or displayed at will, and individuals of both sexes perform crest displays in various contexts, including when being handled by potential predators such as humans (e.g. Graves 1990; M. G. Meadows 2006, personal observations).
5.8.3 Warning coloration
Iridescent colours may also serve to warn predators of the potential unpalatability, or even toxicity, of potential prey items (Cott 1940). Tiger beetles release defence compounds (Pearson et al. 1988), and the conspicuous iridescence displayed by some species might serve as aposematic warning coloration (Schultz 2001). Iridescence might also be aposematic in butterflies (e.g. Bowers & Larin 1989), gyrinid beetles (Hinton & Gibbs 1971) and frogs (Summers 2003). One particularly striking example of possible aposematism involves a Panamanian tortoise beetle (Charidotella egregia). This species rapidly changes from iridescent gold to a matte red colour when disturbed, and this dramatic and reversible colour change may be aposematic, although its function remains poorly understood (Vigneron et al. 2007).
Once aposematic colours evolve, it is not uncommon for non-toxic species to mimic the coloration of toxic species (Batesian mimicry) or for sympatric toxic species to converge on a particular coloration pattern (Müllerian mimicry; Cott 1940), and both of these mechanisms could therefore lead to the evolution of iridescent displays. The evolution of iridescent coloration through mimicry has been proposed for tiger beetles (Acorn 1988; Owens & Hartley 1998). One species of fishes, the bluestriped fangblenny (Plagiotremus rhinorhynchos), also uses iridescence in aggressive mimicry. These fish mimic juvenile cleaner fish, but rather than cleaning the client fish, they attack them by removing scales and dermal tissue (Cheney et al. 2008).
The studies outlined above suggest that iridescent colours may help animals avoid predation through camouflage, warning coloration and flash or startle displays. Regrettably, very little empirical work has been devoted to testing this hypothesis and future research is needed in this area.
6. Non-communicative functions of iridescence
Because iridescent colours are so striking in appearance, it is tempting to ascribe a communicative function to all forms of iridescence. Yet, the evolution of iridescence predates the evolution of eyes, thereby highlighting the fact that not all iridescent colours function in communication (Parker 1998, 2000). Several non-communicative functions of iridescence have been proposed, and in some cases demonstrated, and we review these briefly in the following sections. It is also worth noting that iridescence could evolve for a non-communicative function and then be co-opted for visual signalling, and vice versa.
6.1 Thermoregulation
Whether or not iridescence functions in thermoregulation—either through heat absorption or heat dispersion—has been a matter of debate over the past decade. Some researchers maintain that reflectance by iridescent structures must decrease absorption of solar radiation (e.g. Kobelt & Linsenmair 1992; Koon & Crawford 2000; Biró et al. 2003), whereas others suggest that air spaces between structures that create iridescent colours are heat collectors, and that iridescence is used for warming (e.g. Heilman & Miaoulis 1994; Miaoulis & Heilman 1998; Tada et al. 1998). Wasserthal (1975) concluded that only 15 per cent of the basal portion of butterflies' wings contributes to thermoregulation, and that haemolymph from the wings contributes little to body temperature regulation, casting doubt on the claim that iridescent butterfly wings either enhance or reduce solar heat absorption. In an experimental study, Schultz & Hadley (1987) found no evidence of differences in thermoregulation between iridescent and non-iridescent tiger beetles. By contrast, in the reed frog, iridophores are thought to function as radiance reflectors in the heat of the dry season (Kobelt & Linsenmair 1992). Interestingly, the Hercules beetle, Dynastes hercules, changes colour from iridescent olive green to black with changes in humidity (Hinton & Jarman 1972; Rassart et al. 2008). When humidity is low, a spongy internal structure produces predominantly olive green iridescent coloration in the elytra. When humidity increases, water enters the internal structure through cracks in the waxy cuticle, causing the air spaces in the spongy layer to fill with water and the elytra to appear black. Hinton & Jarman (1973) proposed the function of the colour change to be thermoregulatory or camouflaging, although this has not been empirically tested. While the function of this phenomenon is unknown for this beetle, humans could use similar biomimetic technology to detect environmental changes (e.g. Potyrailo et al. 2007; Rassart et al. 2008).
6.2 Friction reduction
Iridescent structures may serve to reduce friction in burrowing organisms. Several independent lineages of fossorial or semifossorial snakes have iridescent scales, and many of these have regularly arranged ridges (Gower 2003). Observing that these snakes often appear clean of dirt even when excavated from wet soil, researchers have suggested that the microridges resulting in iridescent coloration are adaptations for friction reduction as the snakes move through the soil (Gans & Baic 1977; Gower 2003), although this hypothesis has yet to be empirically tested. Fossorial golden moles have iridescent fur (Kuyper 1985), and the structures that cause the fur to appear iridescent may serve a similar function. Some beetles that live under bark also have microsculptured cuticles that are iridescent, possibly as a by-product of friction reduction (Crowson 1981; Vulinec 1997; Seago et al. 2009).
6.3 Water repellency
In addition to reducing friction, Gans & Baic (1977) and Gower (2003) postulated that the ridges that make snake scales iridescent are water repellent. Drops of water on the skin observed by microscopy do not spread. Although not studying iridescent insects per se, Wagner et al. (1996) noted that insect wings with papillae spacing similar to the ridge spacing on the snake scales were less wettable compared with smooth wings. Microridges on plant leaves have also been shown to reduce wettability (Barthlott & Neinhuis 1997).
6.4 Strengthening
Although some iridescent structures may be particularly susceptible to damage (§4.3), others may be particularly strong. The diffraction gratings that create iridescent coloration in some fossorial snakes and beetles (Hinton 1969) provide examples. In fact, 49 Myr-old fossilized beetles have been discovered with their iridescent structures still intact (Parker & McKenzie 2003). The iridescent nacre of mollusc shells is made up of aragonite platelets with an organic ‘mortar’, producing a structure that is very strong (Jackson et al. 1988; Smith et al. 1999). Iridescent bird feathers may also be stronger than some non-iridescent or white feathers. Iridescent feathers commonly involve the pigment melanin as a structural element, and melanized feathers are sometimes stronger than unmelanized feathers (reviewed in Bonser 1995; Butler & Johnson 2004).
6.5 Photoprotection and vision enhancement
Fishes and cephalopods may use corneal and pupillary iridophores to limit the amount of downwelling solar radiation entering the eye. Several species of fishes have corneal iridophores that are positioned so that light from above is reflected without reducing the amount of less bright sidewelling light entering the eye (Douglas & Marshall 1999). Furthermore, the coloration of corneal iridescence changes with ambient illumination, disappearing or shifting to shorter wavelengths in the dark (Douglas & Marshall 1999; Siebeck et al. 2003), presumably to increase photon capture when light levels are low. The pupils of some squid and the common octopus, Octopus vulgaris, are lined with iridophores that may also function to limit the amount of light entering the eye (Froesch & Messenger 1978; Mäthger et al. 2009).
Iridescent bands on the cornea of horse and deer flies caused by reflecting multilayers may serve as spectral filters for the underlying photoreceptors and thereby enhance colour discrimination. Direct physiological evidence supporting this hypothesis has not yet been obtained (Stavenga 2002).
While corneal iridescence reduces light absorbed by the photoreceptors, the tapetum lucidum does the opposite. Iridescent tapeta lucida produce eyeshine in the eyes of many nocturnal animals (e.g. arthropods, some birds, many mammals and fishes) and function to reflect light that has passed through the retina back through a second time for maximal stimulation of photoreceptors (reviewed in Fox 1976; Douglas & Marshall 1999).
7. Conclusions
Iridescent colours have evolved in many different animal taxa and serve a diversity of functions. Although we have made significant strides in understanding the form and function of iridescence in recent years, research on this topic lags in a number of important areas. Among the best-understood aspects of iridescence are the mechanisms responsible for producing these colours. Inspired in part by technological advances and a new focus on biomimetic technologies, researchers have characterized the nanostructural architectures responsible for producing some of the most spectacular visual phenomena found in nature. Even in this area, however, more research is needed. In addition to ongoing characterizations of iridescent mechanisms in new species, we encourage future work on the evolution of nanostructural architecture among closely related species, the relative importance of organization at different spatial scales and the development of biological nanostructures as it relates to intraspecific variation in colour.
Our review highlights unique features of iridescent colours, such as their capacity to be highly directional, remarkably bright and saturated, maximally reflective at a broad range of wavelengths and sensitive to environmental stress. Interestingly, these features of iridescence are often taken for granted, and few studies have sought to identify and quantify differences between iridescent colours and other mechanisms of colour production. Such comparisons would be particularly informative in a comparative context, where researchers could examine whether certain ecological or life-history traits appear to favour the evolution of iridescent coloration, and whether certain colour-producing mechanisms appear to serve particular functions. Intraspecific studies would also be useful in this area, especially studies that evaluate whether animals use specific behaviours or movements to enhance or reduce the conspicuousness of their iridescent colours in a context-dependent manner (e.g. when courting, foraging, escaping predation).
The greater part of our review focuses on the function of iridescence in visual communication. Some functions of iridescence, such as its use in mate choice and intrasexual interactions, have received a considerable amount of research attention. Nevertheless, many of these studies were conducted prior to the availability of objective colour quantification tools, and most research has been restricted to a small number of model species. Other communicative functions of iridescence, including its use in predator deterrence or avoidance, remain virtually unstudied. We encourage more correlational and experimental work in this area, although we recognize that experimental studies will be challenging and will require innovative approaches, since spectrally realistic manipulations of iridescent coloration will be difficult to achieve. Iridescent colours that do not function in visual communication have received only limited research attention, perhaps because they are not as common as iridescent visual signals. The continued study of iridescent coloration will contribute to our understanding of the evolution of communication strategies in animals.
Acknowledgments
We are grateful to the organizers of the conference ‘Iridescence: more than meets the eye’ for logistical support (see Meadows et al. 2009), the Frontiers in Life Sciences programme at Arizona State University for conference funding and other conference attendees, including R. T. Hanlon, for stimulating discussions and suggestions. We thank M. Abdellah for help with researching this topic. During the preparation of this manuscript, S.M.D. was supported by an NSERC Discovery grant and University Faculty Award, and M.G.M. was supported by an NSF Graduate Research Fellowship.
Footnotes
One contribution of 13 to a Theme Supplement ‘Iridescence: more than meets the eye’.
References
- Acorn J.H. Mimetic tiger beetles and the puzzle of cicindelid coloration (Coleoptera: Cicindelidae) Coleopt. Bull. 1988;42:28–33. [Google Scholar]
- Anciães M., Prum R.O. Manakin display and visiting behaviour: a comparative test of sensory drive. Anim. Behav. 2008;75:783–790. doi: 10.1016/j.anbehav.2007.06.013. [DOI] [Google Scholar]
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Bagnara J.T., Hadley M.E. Prentice-Hall; Englewood Cliffs, NJ: 1973. Chromatophores and color change. The comparative physiology of animal pigmentation. [Google Scholar]
- Bagnara J.T., Taylor J.D., Handley M.E. The dermal chromatophore unit. J. Cell Biol. 1968;38:67–69. doi: 10.1083/jcb.38.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnara J.T., Fernandez P.J., Fujii K. On the blue coloration of vertebrates. Pigment Cell Res. 2007;20:14–26. doi: 10.1111/j.1600-0749.2006.00360.x. [DOI] [PubMed] [Google Scholar]
- Barnhart R.K., Steinmetz S. H. W. Wilson Co; Bronx, NY: 1988. Barnhart dictionary of etymology. [Google Scholar]
- Barthlott W., Neinhuis C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta. 1997;202:1–8. doi: 10.1007/s004250050096. [DOI] [Google Scholar]
- Bennett A.T.D., Cuthill I.C., Partridge J.C., Lunau K. Ultraviolet plumage colors predict mate preferences in starlings. Proc. Natl Acad. Sci. USA. 1997;94:8618–8621. doi: 10.1073/pnas.94.16.8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthier S. Springer; New York, NY: 2006. Iridescences: the physical colors of insects. [Google Scholar]
- Biró L.P., et al. Role of photonic-crystal-type structures in the thermal regulation of a lycaenid butterfly sister species pair. Phys. Rev. E. 2003;67:021907. doi: 10.1103/PhysRevE.67.021907. [DOI] [PubMed] [Google Scholar]
- Bitton P.P., Dawson R.D. Age-related differences in plumage characteristics of male tree swallows Tachycineta bicolor: hue and brightness signal different aspects of individual quality. J. Avian Biol. 2008;39:446–452. [Google Scholar]
- Bitton P.P., O'Brien E.L., Dawson R.D. Plumage brightness and age predict extrapair fertilization success of male tree swallows, Tachycineta bicolor. Anim. Behav. 2007;74:1777–1784. doi: 10.1016/j.anbehav.2007.03.018. [DOI] [Google Scholar]
- Bitton P.P., Dawson R.D., Ochs C.L. Plumage characteristics, reproductive investment and assortative mating in tree swallows Tachycineta bicolor. Behav. Ecol. Sociobiol. 2008;62:1543–1550. doi: 10.1007/s00265-008-0583-7. [DOI] [Google Scholar]
- Bleiweiss R. Iridescent polychromatism in a female hummingbird—is it related to feeding strategies? Auk. 1985;102:701–713. [Google Scholar]
- Bonser R.H.C. Melanin and the abrasion resistance of feathers. Condor. 1995;97:590–591. doi: 10.2307/1369048. [DOI] [Google Scholar]
- Bowers M.D., Larin Z. Acquired chemical defense in the lycaenid butterfly, Eumaeus atala. J. Chem. Ecol. 1989;15:1133–1146. doi: 10.1007/BF01014817. [DOI] [PubMed] [Google Scholar]
- Bowmaker J.K. Evolution of vertebrate visual pigments. Vision Res. 2008;48:2022–2041. doi: 10.1016/j.visres.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Bradbury J.W., Vehrencamp S.L. Sinauer Associates; Sunderland, MA: 1998. Principles of animal communication. [Google Scholar]
- Brink D.J., van der Berg N.G. An investigation of green iridescence on the mollusc Patella granatina. J. Phys. D: Appl. Phys. 2005;38:338–343. doi: 10.1088/0022-3727/38/2/019. [DOI] [Google Scholar]
- Brink D.J., van der Berg N.G., Botha A.J. Iridescent colors on seashells: an optical and structural investigation of Helcion pruinosus. Appl. Opt. 2002;41:717–722. doi: 10.1364/AO.41.000717. [DOI] [PubMed] [Google Scholar]
- Butler M.W., Johnson A.S. Are melanized feather barbs stronger? J. Exp. Biol. 2004;207:285–293. doi: 10.1242/jeb.00746. [DOI] [PubMed] [Google Scholar]
- Caro T. The adaptive significance of coloration in mammals. Bioscience. 2005;55:125–136. doi: 10.1641/0006-3568(2005)055%5B0125:TASOCI%5D2.0.CO;2. [DOI] [Google Scholar]
- Chae J., Nishida S. Integumental ultrastructure and color patterns of the iridescent copepods of the family Sapphrinidae (Copepoda: Poecilostomatoida) Mar. Biol. 1994;119:205–210. doi: 10.1007/BF00349558. [DOI] [Google Scholar]
- Chae J., Nishida S. Vertical distribution and diel migration in the iridescent copepods of the family Sapphirinidae—a unique example of reverse migration. Mar. Ecol. Prog. Ser. 1995;119:111–124. doi: 10.3354/meps119111. [DOI] [Google Scholar]
- Cheney K.L., Grutter A.S., Marshall N.J. Facultative mimicry: cues for colour change and colour accuracy in a coral reef fish. Proc. R. Soc. B. 2008;270:117–122. doi: 10.1098/rspb.2007.0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clench H.K. Behavioral thermoregulation in butterflies. Ecology. 1966;47:1021–1034. doi: 10.2307/1935649. [DOI] [Google Scholar]
- Costa F.J.V., Macedo R.H. Coccidian oocyst parasitism in the blue-black grassquit: influence on secondary sex ornaments and body condition. Anim. Behav. 2005;70:1401–1409. doi: 10.1016/j.anbehav.2005.03.024. [DOI] [Google Scholar]
- Cott H.B. Methuen; London, UK: 1940. Adaptive coloration in animals. [Google Scholar]
- Crowson R.A. Academic Press; New York, NY: 1981. The biology of the Coleoptera. [Google Scholar]
- Cummings M.E., Rosenthal G.G., Ryan M.J. A private communication channel in visual communication. Proc. R. Soc. B. 2003;270:897–904. doi: 10.1098/rspb.2003.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthill I.C. Color perception. In: Hill G.E., McGraw K.J., editors. Bird coloration. Mechanisms and measurements. vol. 1. Harvard University Press; Cambridge, MA: 2006. pp. 3–40. [Google Scholar]
- Darwin C. Murray; London, UK: 1859. On the origin of species by means of natural selection. [Google Scholar]
- Darwin C. Murray; London, UK: 1871. The descent of man and selection in relation to sex. [Google Scholar]
- Denton E.J. On the organization of reflecting surfaces in some marine animals. Phil. Trans. R. Soc. B. 1970;258:285–313. doi: 10.1098/rstb.1970.0037. [DOI] [PubMed] [Google Scholar]
- Denton E.J., Land M.F. Mechanism of reflexion in silvery layers of fish and cephalopods. Proc. R. Soc. B. 1971;178:43–61. doi: 10.1098/rspb.1971.0051. [DOI] [PubMed] [Google Scholar]
- Denton E.J., Gilpin-Brown J.B., Wright P.G. The angular distribution of the light produced by some mesoplagic fish in relation to their camouflage. Proc. R. Soc. B. 1972;182:145–158. doi: 10.1098/rspb.1972.0071. [DOI] [Google Scholar]
- Doucet S.M. Structural plumage coloration, male body size, and condition in the blue-black grassquit. Condor. 2002;104:30–38. doi: 10.1650/0010-5422(2002)104%5B0030:SPCMBS%5D2.0.CO;2. [DOI] [Google Scholar]
- Doucet S.M., Montgomerie R. Multiple sexual ornaments in satin bowerbirds: ultraviolet plumage and bowers signal different aspects of male quality. Behav. Ecol. 2003a;14:503–509. doi: 10.1093/beheco/arg035. [DOI] [Google Scholar]
- Doucet S.M., Montgomerie R. Structural plumage colour and parasites in satin bowerbirds Ptilonorhynchus violaceus: implications for sexual selection. J. Avian Biol. 2003b;34:237–242. doi: 10.1034/j.1600-048X.2003.03113.x. [DOI] [Google Scholar]
- Doucet S.M., Shawkey M.D., Hill G.E., Montgomerie R. Iridescent plumage in satin bowerbirds: structure, mechanisms, and nanostructural predictors of individual variation in colour. J. Exp. Biol. 2006;209:380–390. doi: 10.1242/jeb.01988. [DOI] [PubMed] [Google Scholar]
- Doucet S.M., Mennill D.J., Hill G.E. The evolution of signal design in manakin plumage ornaments. Am. Nat. 2007;160:S63–S80. doi: 10.1086/510162. [DOI] [PubMed] [Google Scholar]
- Douglas J.M., Cronin T.W., Chiou T.H., Dominy N.J. Light habitats and the role of polarized iridescence in the sensory ecology of neotropical nymphalid butterflies (Lepidoptera: Nymphalidae) J. Exp. Biol. 2007;210:788–799. doi: 10.1242/jeb.02713. [DOI] [PubMed] [Google Scholar]
- Douglas R.H., Marshall N.J. A review of vertebrate and invertebrate ocular filters. In: Archer S.N., Djamgoz M.B.A., Loew E.R., Partridge J.C., Vallerga S., editors. Adaptive mechanisms in the ecology of vision. Springer Publishing Company; New York, NY: 1999. pp. 95–162. [Google Scholar]
- Durrer H., Villiger W. Schillerfarben der Trogoniden. J. Ornithol. 1966;107:1–26. doi: 10.1007/BF01671870. [DOI] [Google Scholar]
- Durrer H., Villiger W. Schillerfarben der Stare (Sturnidae) J. Ornithol. 1970;111:133–153. doi: 10.1007/BF01675592. [DOI] [Google Scholar]
- Edmunds M. Longman Press; New York, NY: 1974. Defence in animals: a survey of anti-predator defences. [Google Scholar]
- Emlen D.J., Lavine L.C., Ewen-Campen B. On the origin and evolutionary diversification of beetle horns. Proc. Natl Acad. Sci. USA. 2007;104:8661–8668. doi: 10.1073/pnas.0701209104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endler J.A. A predator's view of animal color patterns. Evol. Biol. 1978;11:319–364. [Google Scholar]
- Endler J.A. Natural selection on color patterns in Poecilia reticulata. Evolution. 1980;34:76–91. doi: 10.2307/2408316. [DOI] [PubMed] [Google Scholar]
- Endler J.A. Frequency-dependent predation, crypsis and aposematic coloration. Phil. Trans. R. Soc. B. 1988;319:505–523. doi: 10.1098/rstb.1988.0062. [DOI] [PubMed] [Google Scholar]
- Endler J.A. On the measurement and classification of color in studies of animal color patterns. Biol. J. Linn. Soc. 1990;41:315–352. doi: 10.1111/j.1095-8312.1990.tb00839.x. [DOI] [Google Scholar]
- Endler J.A. Variation in the appearance of guppy color patterns to guppies and their predators under different visual conditions. Vision Res. 1991;31:587–608. doi: 10.1016/0042-6989(91)90109-I. [DOI] [PubMed] [Google Scholar]
- Endler J.A. The color of light in forests and its implications. Ecol. Monogr. 1993;63:1–27. doi: 10.2307/2937121. [DOI] [Google Scholar]
- Endler J.A. Light, behavior, and conservation of forest-dwelling organisms. In: Clemmons J.R., Buchholz R., editors. Behavioral approaches to conservation in the wild. Cambridge University Press; Cambridge, UK: 1997. pp. 329–355. [Google Scholar]
- Endler J.A., Basolo A.L. Sensory ecology, receiver biases and sexual selection. Trends Ecol. Evol. 1998;13:415–420. doi: 10.1016/S0169-5347(98)01471-2. [DOI] [PubMed] [Google Scholar]
- Endler J.A., Houde A.E. Geographic variation in female preferences for male traits in Poecilia reticulata. Evolution. 1995;49:456–468. doi: 10.2307/2410270. [DOI] [PubMed] [Google Scholar]
- Endler J.A., Théry M. Interacting effects of lek placement, display behavior, ambient light, and color patterns in three neotropical forest-dwelling birds. Am. Nat. 1996;148:421–452. doi: 10.1086/285934. [DOI] [Google Scholar]
- Endler J.A., Westcott D.A., Madden J.R., Robson T. Animal visual systems and the evolution of color patterns: sensory processing illuminates signal evolution. Evolution. 2005;59:1795–1818. doi: 10.1554/04-669.1. [DOI] [PubMed] [Google Scholar]
- Espinosa de los Monteros A. Phylogenetic relationships among the trogons. Auk. 1998;115:937–954. [Google Scholar]
- Fisher R.A. Clarendon; Oxford, UK: 1930. The genetical theory of natural selection. [Google Scholar]
- Fitzpatrick S. Colour schemes for birds: structural coloration and signals of quality in feathers. Ann. Zool. Fenn. 1998;35:67–77. [Google Scholar]
- Fitzstephens D.M., Getty T. Colour, fat and social status in male damselflies, Calopteryx maculata. Anim. Behav. 2000;60:851–855. doi: 10.1006/anbe.2000.1548. [DOI] [PubMed] [Google Scholar]
- Fleishman L.J., Loew E.R., Leal M. Ultraviolet vision in lizards. Nature. 1993;365:397. doi: 10.1038/365397a0. [DOI] [Google Scholar]
- Fox D.L. University of California Press; Berkeley, CA: 1976. Animal biochromes and structural colors. [Google Scholar]
- Fox H.M., Vevers G. Macmillan; New York, NY: 1960. The nature of animal colors. [Google Scholar]
- Frith C.B., Beehler B.M. Oxford University Press; Oxford, UK: 1998. The birds of paradise. [Google Scholar]
- Froesch D., Messenger J.B. On leucophores and the chromatic unit of Octopus vulgaris. Aust. J. Zool. 1978;186:163–173. [Google Scholar]
- Galusha J.W., Richey L.R., Gardner J.S., Cha J.N., Bartl M.H. Discovery of a diamond-based photonic crystal structure in beetle scales. Phys. Rev. E. 2008;77:050904(R). doi: 10.1103/PhysRevE.77.050904. [DOI] [PubMed] [Google Scholar]
- Gans C., Baic D. Regional specialization of reptile scale surfaces: relation of texture and biologic role. Science. 1977;195:1348–1350. doi: 10.1126/science.195.4284.1348. [DOI] [PubMed] [Google Scholar]
- Getty T. Sexually selected signals are not similar to sports handicaps. Trends Ecol. Evol. 2006;21:83–88. doi: 10.1016/j.tree.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Ghiradella H., Aneshansley D., Eisner T., Silberglied R.E., Hinton H.E. Ultraviolet reflection of a male butterfly: interference color caused by thin-layer elaboration of wing scales. Science. 1972;178:1214–1217. doi: 10.1126/science.178.4066.1214. [DOI] [PubMed] [Google Scholar]
- Goda M., Fujii R. The blue coloration of the common surgeonfish, Paracanthurus hepatus—II. Color revelation and color changes. Zool. Sci. 1998;15:323–333. doi: 10.2108/zsj.15.323. [DOI] [PubMed] [Google Scholar]
- Goda M., Toyohara J., Visconti M.A., Oshima N., Fujii R. The blue coloration of the common surgeonfish, Paracanthurus hepatus. I. Morphological features of chromatophores. Zool. Sci. 1994;11:527–535. [Google Scholar]
- Gower D.J. Scale microornamentation of uropeltid snakes. J. Morphol. 2003;258:249–268. doi: 10.1002/jmor.10147. [DOI] [PubMed] [Google Scholar]
- Grafen A. Biological signals as handicaps. J. Theor. Biol. 1990;144:517–546. doi: 10.1016/S0022-5193(05)80088-8. [DOI] [PubMed] [Google Scholar]
- Graves G.R. Function of crest displays in royal flycatchers (Onychorhynchus) Condor. 1990;92:522–524. doi: 10.2307/1368252. [DOI] [Google Scholar]
- Greenewalt C.H., Brandt W., Friel D.D. Iridescent colors of hummingbird feathers. J. Opt. Soc. Am. 1960;50:1005–1030. doi: 10.1364/JOSA.50.001005. [DOI] [Google Scholar]
- Griffiths D.J., Winsor H., Luong-Van T. Iridophores in the mantle of giant clams. Aust. J. Zool. 1992;40:319–326. doi: 10.1071/ZO9920319. [DOI] [Google Scholar]
- Hamdorf K., Schwemer J., Gogala M. Insect visual pigment sensitive to ultraviolet light. Nature. 1971;231:458–459. doi: 10.1038/231458a0. [DOI] [PubMed] [Google Scholar]
- Hanlon R.T. The functional-organization of chromatophores and iridescent cells in the body patterning of Loligo plei (Cephalopoda, Myopsida) Malacologia. 1982;23:89–119. [Google Scholar]
- Hanlon R.T., Cooper K.M., Budelmann B.U., Pappas T.C. Physiological color change in squid iridophores. I. Behavior, morphology and pharmacology in Lolliguncula brevis. Cell Tissue Res. 1990;259:3–14. doi: 10.1007/BF00571424. [DOI] [PubMed] [Google Scholar]
- Hasson O. Amplifiers and the handicap principle in sexual selection: a different emphasis. Proc. R. Soc. B. 1989;235:383–406. doi: 10.1098/rspb.1989.0006. [DOI] [PubMed] [Google Scholar]
- Hasson O. Sexual displays as amplifiers: practical examples with an emphasis on feather decorations. Behav. Ecol. 1991;2:189–197. doi: 10.1093/beheco/2.3.189. [DOI] [Google Scholar]
- Hawryshyn C.W. Polarization vision in fish. Am. Sci. 1992;80:164–175. [Google Scholar]
- Heilman B.D., Miaoulis I.N. Insect thin films as solar collectors. Appl. Opt. 1994;33:6642–6647. doi: 10.1364/AO.33.006642. [DOI] [PubMed] [Google Scholar]
- Herring P.J. Reflective systems in aquatic animals. Comp. Biochem. Phys. A. 1994;109:513–546. doi: 10.1016/0300-9629(94)90192-9. [DOI] [Google Scholar]
- Herring P.J. Bioluminescent signals and the role of reflectors. J. Opt. A: Pure Appl. Opt. 2000;2:R28–R38. doi: 10.1088/1464-4258/2/6/202. [DOI] [Google Scholar]
- Hill, G. E. & McGraw, K. J. 2006a Bird coloration Mechanisms and measurements, vol. 1. Cambridge, MA: Harvard University Press.
- Hill, G. E. & McGraw, K. J. 2006b Bird coloration Function and evolution, vol. 2. Cambridge, MA: Harvard University Press.
- Hill G.E., Doucet S.M., Buchholz R. The effect of coccidial infection on iridescent plumage coloration in wild turkeys. Anim. Behav. 2005;69:387–394. doi: 10.1016/j.anbehav.2004.03.013. [DOI] [Google Scholar]
- Hinton D.E., Jarman G. Physiological colour change in the Hercules beetle. Nature. 1972;238:160–161. doi: 10.1038/238160a0. [DOI] [Google Scholar]
- Hinton H.E. Diffraction gratings in burying beetles (Nicrophorus) Entomologist. 1969;102:185–189. [Google Scholar]
- Hinton H.E. Natural deception. In: Gregory R.L., Gombrich E.E., editors. Illusion in nature and art. Charles Scribener's Sons; New York, NY: 1973. pp. 97–160. [Google Scholar]
- Hinton H.E., Gibbs D.F. Diffraction gratings in gyrinid beetles. J. Insect Physiol. 1971;17:1023–1035. doi: 10.1016/0022-1910(71)90006-0. [DOI] [Google Scholar]
- Hinton H.E., Jarman G. Physiological colour changes in the elytra of the Hercules beetles, Dynastes hercules. J. Insect Physiol. 1973;19:533–549. doi: 10.1016/0022-1910(73)90064-4. [DOI] [Google Scholar]
- Huxley A.F. A theoretical treatment of the reflexion of light by multilayer structures. J. Exp. Biol. 1968;48:227–245. [Google Scholar]
- Ingram A.L., Parker A.R. A review of the diversity and evolution of photonic structures in butterflies, incorporating the work of John Huxley (The Natural History Museum, London from 1961 to 1990) Phil. Trans. R. Soc. B. 2008;363:2465–2480. doi: 10.1098/rstb.2007.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A.P., Vincent J.F.V., Turner R.M. The mechanical design of nacre. Proc. R. Soc. B. 1988;234:415–440. doi: 10.1098/rspb.1988.0056. [DOI] [Google Scholar]
- Jackson R.R. Silk utilization and defensive behavior of Thiania, an iridescent jumping spider (Araneae, Salticidae) from Malaysia. New Zeal. J. Zool. 1986;13:553–561. [Google Scholar]
- Jackson R.R., Hallas S.E.A. Predatory versatility and intraspecific interactions of spartaeine jumping spiders (Araneae: Salticidae): Brettus adonis, B. cingulatus, Cyrba algerina and Phaecius sp. indet. New Zeal. J. Zool. 1986;13:491–520. [Google Scholar]
- Jerlov N.G. Elsevier Scientific Publishing Company; Amersterdam, The Netherlands: 1976. Marine optics. [Google Scholar]
- Kasukawa H., Oshima N., Fujii R. Mechanism of light-reflection in blue damselfish motile iridophore. Zool. Sci. 1987;4:243–257. [Google Scholar]
- Kemp D.J. Heightened phenotypic variation and age-based fading of ultraviolet butterfly wing coloration. Evol. Ecol. Res. 2006a;8:515–527. [Google Scholar]
- Kemp D.J. Ultraviolet ornamentation and male mating success in a high-density assemblage of the butterfly Colias eurytheme. J. Insect Behav. 2006b;19:669–684. doi: 10.1007/s10905-006-9060-1. [DOI] [Google Scholar]
- Kemp D.J. Female butterflies prefer males bearing bright iridescent ornamentation. Proc. R. Soc. B. 2007;274:1043–1047. doi: 10.1098/rspb.2006.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D.J. Female mating biases for bright ultraviolet iridescence in the butterfly Eurema hecabe (Pieridae) Behav. Ecol. 2008;19:1–8. doi: 10.1093/beheco/arm094. [DOI] [Google Scholar]
- Kemp D.J., Macedonia J.M. Structural ultraviolet ornamentation in the butterfly Hypolimnas bolina L. (Nymphalidae): visual, morphological and ecological properties. Aust. J. Zool. 2006;54:235–244. doi: 10.1071/ZO06005. [DOI] [Google Scholar]
- Kemp D.J., Rutowski R.L. Condition dependence, quantitative genetics, and the potential signal content of iridescent ultraviolet butterfly coloration. Evolution. 2007;61:168–183. doi: 10.1111/j.1558-5646.2007.00014.x. [DOI] [PubMed] [Google Scholar]
- Kemp D.J., Vukusic P., Rutowski R.L. Stress-mediated covariance between nano-structural architecture and ultraviolet butterfly coloration. Funct. Ecol. 2006;20:282–289. doi: 10.1111/j.1365-2435.2006.01100.x. [DOI] [Google Scholar]
- Kinoshita S., Yoshioka S. Structural colors in nature: the role of regularity and irregularity in the structure. ChemPhysChem. 2005;6:1442–1459. doi: 10.1002/cphc.200500007. [DOI] [PubMed] [Google Scholar]
- Kinoshita S., Yoshioka S., Kawagoe K. Mechanisms of structural colour in the Morpho butterfly: cooperation of regularity and irregularity in an iridescent scale. Proc. R. Soc. B. 2002;269:1417–1421. doi: 10.1098/rspb.2002.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita S., Yoshioka S., Miyazaki J. Physics of structural colors. Rep. Prog. Phys. 2008;71:1–30. doi: 10.1088/0034-4885/71/7/076401. [DOI] [Google Scholar]
- Kobelt F., Linsenmair K.E. Adaptations of the reed frog Hyperolius viridiflavus (Amphibia, Anura, Hyperoliidae) to its arid environment. VI. The iridophores in the skin as radiation reflectors. J. Comp. Physiol. B. 1992;162:314–326. doi: 10.1007/BF00260758. [DOI] [PubMed] [Google Scholar]
- Kodric-Brown A. Sexual dichromatism and temporary color changes in the reproduction of fishes. Am. Zool. 1998;38:70–81. doi: 10.1093/icb/38.1.70. [DOI] [Google Scholar]
- Kodric-Brown A., Brown J.H. Truth in advertising: the kinds of traits favored by sexual selection. Am. Nat. 1984;124:309–323. doi: 10.1086/284275. [DOI] [Google Scholar]
- Kodric-Brown A., Johnson S.C. Ultraviolet reflectance patterns of male guppies enhance their attractiveness to females. Anim. Behav. 2002;63:391–396. doi: 10.1006/anbe.2001.1917. [DOI] [Google Scholar]
- Kokko H., Brooks R., Jennions M.D., Morley J. The evolution of mate choice and mating biases. Proc. R. Soc. B. 2003;270:653–664. doi: 10.1098/rspb.2002.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komdeur J., Oorebeek M., van Overveld T., Cuthill I.C. Mutual ornamentation, age, and reproductive performance in the European starling. Behav. Ecol. 2005;16:805–817. doi: 10.1093/beheco/ari059. [DOI] [Google Scholar]
- Koon D.W., Crawford A.B. Insect thin films as sun blocks, not solar collectors. Appl. Opt. 2000;39:2496–2498. doi: 10.1364/AO.39.002496. [DOI] [PubMed] [Google Scholar]
- Kurachi M., Takaku Y., Komiya Y., Hariyama T. The origin of extensive colour polymorphism in Plateumaris sericea (Chrysomelidae, Coleoptera) Naturwissenschaften. 2002;89:295–298. doi: 10.1007/s00114-002-0332-0. [DOI] [PubMed] [Google Scholar]
- Kuyper M.A. The ecology of the golden mole Amblysomus hottentotus. Mammal Rev. 1985;15:3–11. doi: 10.1111/j.1365-2907.1985.tb00379.x. [DOI] [Google Scholar]
- Ladich, F., Collin, S. P., Moller, P. & Kapoor, B. G. 2006 Communication in fishes, vol. 2. Enfield, NH: Science Publishers.
- Land M.F. The physics and biology of animal reflectors. Prog. Biophys. Mol. Biol. 1972;24:77–106. doi: 10.1016/0079-6107(72)90004-1. [DOI] [PubMed] [Google Scholar]
- Land M.F., Nilsson D.-E. Oxford University Press; Oxford, UK: 2002. Animal eyes. [Google Scholar]
- Land M.F., Horwood J., Lim M.L.M., Li D.Q. Optics of the ultraviolet reflecting scales of a jumping spider. Proc. R. Soc. B. 2007;274:1583–1589. doi: 10.1098/rspb.2007.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennox J.G.Aristotle: on the parts of animals I–IV2001Oxford University Press; Oxford, UK [Google Scholar]
- Lim M.L.M., Li D.Q. Behavioural evidence of UV sensitivity in jumping spiders (Araneae: Salticidae) J. Comp. Physiol. A. 2006a;192:871–878. doi: 10.1007/s00359-006-0126-5. [DOI] [PubMed] [Google Scholar]
- Lim M.L.M., Li D.Q. Extreme ultraviolet sexual dimorphism in jumping spiders (Araneae: Salticidae) Biol. J. Linn. Soc. 2006b;89:397–406. doi: 10.1111/j.1095-8312.2006.00704.x. [DOI] [Google Scholar]
- Lim M.L.M., Li D.Q. Effects of age and feeding history on structure-based UV ornaments of a jumping spider (Araneae: Salticidae) Proc. R. Soc. B. 2007;274:569–575. doi: 10.1098/rspb.2006.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim M.L.M., Land M.F., Li D. Sex-specific UV and fluorescence signals in jumping spiders. Science. 2007;315:481. doi: 10.1126/science.1134254. [DOI] [PubMed] [Google Scholar]
- Liu F., Yin H.W., Dong B.Q., Qing Y.H., Zhao L., Meyer S., Liu X.H., Zi J., Chen B. Inconspicuous structural coloration in the elytra of beetles Chlorophila obscuripennis (Coleoptera) Phys. Rev. E. 2008;77:012901. doi: 10.1103/PhysRevE.77.012901. [DOI] [PubMed] [Google Scholar]
- Losey G.S., Jr Crypsis and communication functions of UV–visible coloration in two coral reef damselfish, Dascyllus aruanus and D. reticulatus. Anim. Behav. 2003;66:299–307. doi: 10.1006/anbe.2003.2214. [DOI] [Google Scholar]
- Loyau A., Gomez D., Moureau B.T., Théry M., Hart N.S., Saint Jalme M., Bennett A.T.D., Sorci G. Iridescent structurally based coloration of eyespots correlates with mating success in the peacock. Behav. Ecol. 2007;18:1123–1131. doi: 10.1093/beheco/arm088. [DOI] [Google Scholar]
- Lyon B.E., Montgomerie R.D. Delayed plumage maturation in passerine birds: reliable signaling by subordinate males. Evolution. 1986;40:605–615. doi: 10.2307/2408581. [DOI] [PubMed] [Google Scholar]
- Lythgoe J.N. Structure and function of iridescent corneas in teleost fishes. Proc. R. Soc. B. 1975;188:437–457. doi: 10.1098/rspb.1975.0030. [DOI] [PubMed] [Google Scholar]
- Lythgoe J.N., Shand J. The structural basis for iridescent color changes in dermal and corneal iridophores in fish. J. Exp. Biol. 1989;141:313–325. [Google Scholar]
- Macedonia J.M., James S., Wittle L.W., Clark D.L. Skin pigments and coloration in the Jamaican radiation of Anolis lizards. J. Herpetol. 2000;34:99–109. doi: 10.2307/1565245. [DOI] [Google Scholar]
- Madsen V., Dabelsteen T., Osorio D., Osorno J.L. Morphology and ornamentation in male magnificent frigatebirds: variation with age class and mating status. Am. Nat. 2007;169:S93–S111. doi: 10.1086/510096. [DOI] [PubMed] [Google Scholar]
- Marshall N.J. Communication and camouflage with the same ‘bright’ colours in reef fishes. Proc. R. Soc. B. 2000;355:1243–1248. doi: 10.1098/rstb.2000.0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall N.J., Jennings K., McFarland W.N., Loew E.R., Losey G.S. Visual biology of Hawaiian coral reef fishes. II. Colors of Hawaiian coral reef fish. Copeia. 2003;2003:455–466. doi: 10.1643/01-055. [DOI] [Google Scholar]
- Mateos C., Carranza J. Female choice for morphological features of male ring-necked pheasants. Anim. Behav. 1995;49:737–748. doi: 10.1016/0003-3472(95)80206-1. [DOI] [Google Scholar]
- Mateos C., Carranza J. The role of bright plumage in male–male interactions in the ring-necked pheasant. Anim. Behav. 1997;54:1205–1214. doi: 10.1006/anbe.1997.0516. [DOI] [PubMed] [Google Scholar]
- Mäthger L.M., Denton E.J. Reflective properties of iridophores and fluorescent ‘eyespots’ in the loliginid squid Alloteuthis subulata and Loligo vulgaris. J. Exp. Biol. 2001;204:2103–2118. doi: 10.1242/jeb.204.12.2103. [DOI] [PubMed] [Google Scholar]
- Mäthger L.M., Hanlon R.T. Anatomical basis for camouflaged polarized light communication in squid. Biol. Lett. 2006;2:494–496. doi: 10.1098/rsbl.2006.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäthger L.M., Hanlon R.T. Malleable skin coloration in cephalopods: selective reflectance, transmission and absorbance of light by chromatophores and iridophores. Cell Tissue Res. 2007;329:179–186. doi: 10.1007/s00441-007-0384-8. [DOI] [PubMed] [Google Scholar]
- Mäthger L.M., Denton E.J., Marshall N.J., Hanlon R.T. Mechanisms and behavioral functions of structural colouration in cephalopods. J. R. Soc. 2009;6:S149–S163. doi: 10.1098/rsif.2008.0366.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw K.J. An update on the honesty of melanin-based color signals in birds. Pigment Cell Melanoma Res. 2008;21:133–138. doi: 10.1111/j.1755-148X.2008.00454.x. [DOI] [PubMed] [Google Scholar]
- McGraw K.J., Mackillop E.A., Dale J., Hauber M.E. Different colors reveal different information: how nutritional stress affects the expression of melanin- and structurally based ornamental plumage. J. Exp. Biol. 2002;205:3747–3755. doi: 10.1242/jeb.205.23.3747. [DOI] [PubMed] [Google Scholar]
- McNaught M.K., Owens I.P.F. Interspecific variation in plumage colour among birds: species recognition or light environment? J. Evol. Biol. 2002;15:505–514. doi: 10.1046/j.1420-9101.2002.00431.x. [DOI] [Google Scholar]
- Meadows M.G., Butler M.W., Morehouse N.I., Taylor L.A., Toomey M.B., McGraw K.J., Rutowski R.L. Iridescence: views from many angles. J. R. Soc. 2009;6:S203–S211. doi: 10.1098/rsif.2008.0460.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaoulis I.N., Heilman B.D. Butterfly thin films serve as solar collectors. Ann. Entomol. Soc. Am. 1998;91:122–127. [Google Scholar]
- Miller L.K., Brooks R. The effects of genotype, age, and social environment on male ornamentation, mating behavior, and attractiveness. Evolution. 2005;59:2414–2425. doi: 10.1554/05-160.1. [DOI] [PubMed] [Google Scholar]
- Møller A.P., Petrie M. Condition dependence, multiple sexual signals, and immunocompentence in peacocks. Behav. Ecol. 2002;13:248–253. doi: 10.1093/beheco/13.2.248. [DOI] [Google Scholar]
- Monroe E.A., Monroe S.E. Origin of iridescent colors on the indigo snake. Science. 1968;159:97–98. doi: 10.1126/science.159.3810.97-a. [DOI] [PubMed] [Google Scholar]
- Morrison R.L. A transmission electron microscopic (TEM) method for determining structural colors reflected by lizard iridophores. Pigment Cell Res. 1995;8:28–36. doi: 10.1111/j.1600-0749.1995.tb00771.x. [DOI] [PubMed] [Google Scholar]
- Morrison R.L., Rand M.S., Frost-Mason S.K. Cellular basis of color differences in three morphs of the lizard Sceloporus undulatus erythrocheilus. Copeia. 1995;1995:397–408. doi: 10.2307/1446903. [DOI] [Google Scholar]
- Morrison R.L., Sherbrooke W.C., Frost-Mason S.K. Temperature-sensitive, physiologically active iridophores in the lizard Urosaurus ornatus: an ultrastructural analysis of color change. Copeia. 1996;1996:804–812. doi: 10.2307/1447641. [DOI] [Google Scholar]
- Newton I.Opticks; or, a treatise of the reflections, refractions, inflections & colours of light. Based on the 4th ed., London, 17301952Dover Publications; New York, NY [Google Scholar]
- Omland K.E. Female mallard mating preferences for multiple male ornaments. I. Natural variation. Behav. Ecol. Sociobiol. 1996a;39:353–360. doi: 10.1007/s002650050300. [DOI] [Google Scholar]
- Omland K.E. Female mallard mating preferences for multiple male ornaments. II. Experimental variation. Behav. Ecol. Sociobiol. 1996b;39:361–366. doi: 10.1007/s002650050301. [DOI] [Google Scholar]
- Osorio D., Ham A.D. Spectral reflectance and directional properties of structural coloration in bird plumage. J. Exp. Biol. 2002;205:2017–2027. doi: 10.1242/jeb.205.14.2017. [DOI] [PubMed] [Google Scholar]
- Owens I.P.F., Hartley I.R. Sexual dimorphism in birds: why are there so many different forms of dimorphism? Proc. R. Soc. B. 1998;265:397–407. doi: 10.1098/rspb.1998.0308. [DOI] [Google Scholar]
- Papke R.S., Kemp D.J., Rutowski R.L. Multimodal signalling: structural ultraviolet reflectance predicts male mating success better than pheromones in the butterfly Colias eurytheme L. (Pieridae) Anim. Behav. 2007;73:47–54. doi: 10.1016/j.anbehav.2006.07.004. [DOI] [Google Scholar]
- Parker A.R. Discovery of functional iridescence and its coevolution with eyes in the phylogeny of Ostracoda (Crustacea) Proc. R. Soc. B. 1995;262:349–355. doi: 10.1098/rspb.1995.0216. [DOI] [Google Scholar]
- Parker A.R. Colour in Burgess Shale animals and the effect of light on evolution in the Cambrian. Proc. R. Soc. B. 1998;265:967–972. doi: 10.1098/rspb.1998.0385. [DOI] [Google Scholar]
- Parker A.R. 515 million years of structural colour. J. Opt. A: Pure Appl. Opt. 2000;2:R15–R28. doi: 10.1088/1464-4258/2/6/201. [DOI] [Google Scholar]
- Parker A.R., McKenzie D.R. The cause of 50 million-year-old colour. Proc. R. Soc. B. 2003;270:S151–S153. doi: 10.1098/rsbl.2003.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A.R., Townley H.E. Biomimetics of photonic nanostructures. Nat. Nanotech. 2007;2:347–353. doi: 10.1038/nnano.2007.152. [DOI] [PubMed] [Google Scholar]
- Parker A.R., McKenzie D.R., Ahyong S.T. A unique form of light reflector and the evolution of signalling in Ovalipes (Crustacea: Decapoda: Portunidae) Proc. R. Soc. B. 1998;265:861–867. doi: 10.1098/rspb.1998.0385. [DOI] [Google Scholar]
- Parker A.R., McPhedran R.C., McKenzie D.R., Botten L.C., Nicorovici N.-A. P. Aphrodite's iridescence. Nature. 2001;409:36–37. doi: 10.1038/35051168. [DOI] [PubMed] [Google Scholar]
- Pearson D.L., Blum M.S., Jones T.H., Fales H.M., Gonda E., Witte B.R. Historical perspective and the interpretation of ecological patterns: defensive compounds of tiger beetles (Coleoptera: Cicindelidae) Am. Nat. 1988;132:404–416. doi: 10.1086/284860. [DOI] [Google Scholar]
- Penz C.M., DeVries P.J. Phylogenetic analysis of Morpho butterflies (Nymphalidae, Morphinae): implications for classification and natural history. Am. Mus. Novit. 2002;3374:1–33. doi: 10.1206/0003-0082(2002)374%3C0001:PAOMBN%3E2.0.CO;2. [DOI] [Google Scholar]
- Perrier C., de Lope F., Møller A.P., Ninni P. Structural coloration and sexual selection in the barn swallow Hirundo rustica. Behav. Ecol. 2002;13:728–736. doi: 10.1093/beheco/13.6.728. [DOI] [Google Scholar]
- Pitelka F.A. Territoriality and related problems in North American hummingbirds. Condor. 1942;44:189–204. doi: 10.2307/1364129. [DOI] [Google Scholar]
- Plattner L. Optical properties of the scales of Morpho rhetenor butterflies: theoretical and experimental investigation of the back-scattering of light in the visible spectrum. J. R. Soc. Interface. 2004;1:49–59. doi: 10.1098/rsif.2004.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poladian L., Wickham S., Lee K., Large M.C.J. Iridescence from photonic crystals and its suppression in butterfly scales. J. R. Soc. 2009;6:S203–S211. doi: 10.1098/rsif.2008.0460.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potyrailo R.A., Ghiradella H., Vertiatchikh A., Dovidenko K., Cournoyer J.R., Olson E. Morpho butterfly wing scales demonstrate highly selective vapour response. Nat. Photonics. 2007;1:123–128. doi: 10.1038/nphoton.2007.2. [DOI] [Google Scholar]
- Prum R.O. Anatomy, physics, and evolution of avian structural colors. In: Hill G.E., McGraw K.J., editors. Bird coloration. Mechanisms and measurements. vol. 1. Harvard University Press; Cambridge, MA: 2006. pp. 295–355. [Google Scholar]
- Prum R.O., Quinn T., Torres R. Anatomically diverse butterfly scales all produce structural colours by coherent scattering. J. Exp. Biol. 2006;209:748–765. doi: 10.1242/jeb.02051. [DOI] [PubMed] [Google Scholar]
- Rassart M., Colomer J.F., Tabarrant T., Vigneron J.P. Diffractive hygrochromic effect in the cuticle of the Hercules beetle Dynastes hercules. New J. Phys. 2008;10:033014. doi: 10.1088/1367-2630/10/3/033014. [DOI] [Google Scholar]
- Robinson M.H. Defenses against visually hunting predators. Evol. Biol. 1969;3:225–259. [Google Scholar]
- Rohrlich S.T., Porter K.R. Fine structural observations relating to the production of color by the iridophores of a lizard, Anolis carolinensis. J. Cell Biol. 1972;53:38–52. doi: 10.1083/jcb.53.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohwer S., Fretwell S.D., Niles D.M. Delayed maturation in passerine plumages and the deceptive acquisition of resources. Am. Nat. 1980;115:400–437. doi: 10.1086/283569. [DOI] [Google Scholar]
- Rutowski R.L. The use of visual cues in sexual and species discrimination by males of the small sulphur butterfly Eurema lisa (Lepidoptera, Pieridae) J. Comp. Physiol. 1977;115:61–74. doi: 10.1007/BF00667785. [DOI] [Google Scholar]
- Rutowski R.L. Mate-locating behavior in the common eggfly, Hypolimnas bolina (Nymphalidae) J. Lep. Soc. 1992;46:24–38. [Google Scholar]
- Rutowski R.L., Macedonia J.M., Merry J.W., Morehouse N.I., Yturralde K., Taylor-Taft L., Gaalema D., Kemp D.J., Papke R.S. Iridescent ultraviolet signal in the orange sulphur butterfly (Colias eurytheme): spatial, temporal and spectral properties. Biol. J. Linn. Soc. 2007;90:349–364. doi: 10.1111/j.1095-8312.2007.00749.x. [DOI] [Google Scholar]
- Sargent, T. D. 1990 Startle as an anti-predator mechanism, with special reference to the underwing moths (Catocala). In Insect defenses: adaptive mechanisms and strategies of prey and predators, pp. 229–249. Albany, NY: SUNY Press.
- Sarrazin, M., Vigneron, J. P., Welch, V. & Rassart, M. 2008 Nanomorphology of the blue iridescent wings of a giant tropical wasp, Megascolia procer javanenesis (Hymenoptera). (http://arxiv.org/abs/0710.2692v1) [DOI] [PubMed]
- Schultz T.D. Role of structural colors in predator avoidance by tiger beetles of the genus Cicindela (Coleoptera: Cicindelidae) Bull. Entomol. Soc. Am. 1986;32:142–146. [Google Scholar]
- Schultz T.D. Tiger beetle defenses revisited: alternative defense strategies and colorations of two neotropical tiger beetles, Odontocheila nicaraguensis Bates and Pseudoxycheila tasalis Bates (Carabidae: Cicindelinae) Coleopt. Bull. 2001;55:153–163. doi: 10.1649/0010-065X(2001)055%5B0153:TBDRAD%5D2.0.CO;2. [DOI] [Google Scholar]
- Schultz T.D., Bernard G.D. Pointillistic mixing of interference colours in cryptic tiger beetles. Nature. 1989;337:72–73. doi: 10.1038/337072a0. [DOI] [Google Scholar]
- Schultz T.D., Hadley N.F. Structural colours of tiger beetles and their role in heat transfer through the integument. Physiol. Zool. 1987;60:737–745. [Google Scholar]
- Seago A.E., Brady P., Vigneron J.-P., Schultz T.D. Gold bugs and beyond: a review of iridescence and structural colour mechanisms in beetles (Coleoptera) J. R. Soc. Interface. 2009;6:S165–S184. doi: 10.1098/rsif.2008.0354.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shand J. Corneal iridescence in fishes: light-induced colour changes in relation to structure. J. Fish Biol. 1988;32:625–632. doi: 10.1111/j.1095-8649.1988.tb05400.x. [DOI] [Google Scholar]
- Shashar N., Rutledge P.S., Cronin T.W. Polarization vision in cuttlefish—a concealed communication channel? J. Exp. Biol. 1996;199:2077–2084. doi: 10.1242/jeb.199.9.2077. [DOI] [PubMed] [Google Scholar]
- Shawkey M.D., Hauber M.E., Estep L.K., Hill G.E. Evolutionary transitions and mechanisms of matte and iridescent plumage coloration in grackles and allies (Icteridae) J. R. Soc. Interface. 2006;3:777–786. doi: 10.1098/rsif.2006.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawkey M.D., Morehouse N.I., Vukusic P. A protean palette: colour materials and mixing in birds and butterflies. J. R. Soc. 2009;6:S221–S231. doi: 10.1098/rsif.2008.0459.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebeck U.E., Collin S.P., Ghoddusi M., Marshall N.J. Occlusable corneas in toadfishes: light transmission, movement and ultrastruture of pigment during light- and dark-adaptation. J. Exp. Biol. 2003;206:2177–2190. doi: 10.1242/jeb.00401. [DOI] [PubMed] [Google Scholar]
- Silberglied R.E. Communication in the ultraviolet. Annu. Rev. Ecol. Syst. 1979;10:373–398. doi: 10.1146/annurev.es.10.110179.002105. [DOI] [Google Scholar]
- Silberglied R.E., Taylor O.R. Ultraviolet differences between the sulphur butterflies, Colias eurytheme and C. philodice, and a possible isolating mechanism. Nature. 1973;241:406–408. doi: 10.1038/241406a0. [DOI] [PubMed] [Google Scholar]
- Silberglied R.E., Taylor O.R. Ultraviolet reflection and its behavioral role in courtship of sulfur butterflies Colias eurytheme and Colias philodice (Lepidoptera, Pieridae) Behav. Ecol. Sociobiol. 1978;3:203–243. doi: 10.1007/BF00296311. [DOI] [Google Scholar]
- Smith B.L., et al. Molecular mechanistic origin of the toughness of natural adhesives, fibres and composites. Nature. 1999;399:761–763. doi: 10.1038/21607. [DOI] [Google Scholar]
- Srinivasarao M. Nano-optics in the biological world: beetles, butterflies, birds, and moths. Chem. Rev. 1999;99:1935–1961. doi: 10.1021/cr970080y. [DOI] [PubMed] [Google Scholar]
- Stavenga D.G. Colour in the eyes of insects. J. Comp. Phys. A. 2002;188:337–348. doi: 10.1007/s00359-002-0307-9. [DOI] [PubMed] [Google Scholar]
- Stavenga D.G., Stowe S., Siebke K., Zeil J., Arikawa K. Butterfly wing colours: scale beads make white pierid wings brighter. Proc. R. Soc. B. 2004;271:1577–1584. doi: 10.1098/rspb.2004.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrecht R.A., Mohren W., Pulker H.K., Schneider D. Cuticular interference reflectors in the golden pupae of danaine butterflies. Proc. R. Soc. B. 1985;226:367–390. doi: 10.1098/rspb.1985.0100. [DOI] [Google Scholar]
- Stiles F.G. Aggressive and courtship displays of the male Anna's hummingbird. Condor. 1982;84:208–225. doi: 10.2307/1367674. [DOI] [Google Scholar]
- Stride G.O. Further studies on the courtship behaviour of African mimetic butterflies. Anim. Behav. 1958;6:224–230. doi: 10.1016/0003-3472(58)90055-1. [DOI] [Google Scholar]
- Stutchbury B.J. The adaptive significance of male subadult plumage in purple martins—plumage dyeing experiments. Behav. Ecol. Sociobiol. 1991;29:297–306. doi: 10.1007/BF00163988. [DOI] [Google Scholar]
- Summers K. Convergent evolution of bright coloration and toxicity in frogs. Proc. Natl Acad. Sci. USA. 2003;100:12 533–12 534. doi: 10.1073/pnas.2335928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpter D.J.T. The principles of collective animal behaviour. Phil. Trans. R. Soc. B. 2006;361:5–22. doi: 10.1098/rstb.2005.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney A., Jiggins C., Johnsen S. Insect communication: polarized light as a butterfly mating signal. Nature. 2003;423:31–32. doi: 10.1038/423031a. [DOI] [PubMed] [Google Scholar]
- Tada H., Mann S.E., Miaoulis I.N., Wong P.Y. Effects of a butterfly scale microstructure on the iridescent color observed at different angles. Appl. Opt. 1998;37:1579–1584. doi: 10.1364/AO.37.001579. [DOI] [PubMed] [Google Scholar]
- Taylor L.A., McGraw K.J. Animal coloration: sexy spider scales. Curr. Biol. 2007;17:R592–R593. doi: 10.1016/j.cub.2007.05.064. [DOI] [PubMed] [Google Scholar]
- Théry M., Vehrencamp S.L. Light patterns as cues for mate choice in the lekking white-throated manakin (Corapipo gutturalis) Auk. 1995;112:133–145. [Google Scholar]
- Théry M., Pincebourde S., Feer F. Dusk light environment optimizes visual perception of conspecifics in a crepuscular horned beetle. Behav. Ecol. 2008;19:627–634. doi: 10.1093/beheco/arn024. [DOI] [Google Scholar]
- Vigneron J.P., et al. Switchable reflector in the Panamanian tortoise beetle Charidotella egregia (Chrysomelidae: Cassidinae) Phys. Rev. E. 2007;76:031907. doi: 10.1103/PhysRevE.76.031907. [DOI] [PubMed] [Google Scholar]
- Vukusic P. Natural photonics. Phys. World. 2004;17:35–39. [Google Scholar]
- Vukusic P., Sambles J.R. Photonic structures in biology. Nature. 2003;424:852–855. doi: 10.1038/nature01941. [DOI] [PubMed] [Google Scholar]
- Vukusic P., Stavenga D. Physical methods for investigating structural colours in biological systems. J. R. Soc. 2009;6:S133–S148. doi: 10.1098/rsif.2008.0386.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukusic P., Sambles J.R., Lawrence C.R., Wootton R.J. Quantified interference and diffraction in single Morpho butterfly scales. Proc. R. Soc. B. 1999;266:1403–1411. doi: 10.1098/rspb.1999.0794. [DOI] [Google Scholar]
- Vukusic P., Wootton R.J., Sambles J.R. Remarkable iridescence in the hindwings of the damselfly Neurobasis chinensis chinensis (Linnaeus) (Zygoptera: Calopterygidae) Proc. R. Soc. B. 2004;271:595–601. doi: 10.1098/rspb.2003.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukusic P., Kelly R., Hooper I. A biological sub-micron thickness optical broadband reflector characterised using both light and microwaves. J. R. Soc. 2009;6:S193–S201. doi: 10.1098/rsif.2008.0345.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulinec K. Iridescent dung beetles: a different angle. Fla. Entomol. 1997;80:132–141. doi: 10.2307/3495550. [DOI] [Google Scholar]
- Wagner T., Neinhuis C., Barthlott W. Wettability and contaminability of insect wings as a function of their surface sculptures. Acta Zool. 1996;77:213–225. [Google Scholar]
- Wallace A.R. Macmillan; London, UK: 1889. Darwinism. [Google Scholar]
- Wasserthal L.T. The role of butterfly wings in the regulation of body temperature. J. Insect Physiol. 1975;21:1921–1930. doi: 10.1016/0022-1910(75)90224-3. [DOI] [Google Scholar]
- Welch V.L., Vigneron J.P., Parker A.R. The cause of colouration in the ctenophore Beroë cucumis. Curr. Biol. 2005;15:R985–R986. doi: 10.1016/j.cub.2005.11.060. [DOI] [PubMed] [Google Scholar]
- Wijnen B., Leertouwer H.L., Stavenga D.G. Colors and pterin pigmentation of pierid butterfly wings. J. Insect Phys. 2007;53:1206–1217. doi: 10.1016/j.jinsphys.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Wolf L.L., Stiles F.G. Evolution of pair cooperation in a tropical hummingbird. Evolution. 1970;24:759–773. doi: 10.2307/2406556. [DOI] [PubMed] [Google Scholar]
- Zahavi A. Mate selection—a selection for a handicap. J. Theor. Biol. 1975;53:205–214. doi: 10.1016/0022-5193(75)90111-3. [DOI] [PubMed] [Google Scholar]
- Zi J., Yu X., Li Y., Hu X., Xu C., Wang X., Liu W., Fu R. Coloration strategies in peacock feathers. Proc. Natl Acad. Sci. USA. 2003;100:12 576–12 578. doi: 10.1073/pnas.2133313100. [DOI] [PMC free article] [PubMed] [Google Scholar]