Abstract
While typically classified as either ‘structural’ or ‘pigmentary’, bio-optical tissues of terrestrial animals are rarely homogeneous and typically contain both a structural material such as keratin or chitin and one or more pigments. These base materials interact physically and chemically to create colours. Combinations of structured base materials and embedded pigment molecules often interact optically to produce unique colours and optical properties. Therefore, to understand the mechanics and evolution of bio-optical tissues it is critical to understand their material properties, both in isolation and in combination. Here, we review the optics and evolution of coloured tissues with a focus on their base materials, using birds and butterflies as exemplar taxa owing to the strength of our current knowledge of colour production in these animals. We first review what is known of their base materials, and then discuss the consequences of these interactions from an optical perspective. Finally, we suggest directions for future research on colour optics and evolution that will be invaluable as we move towards a fuller understanding of colour in the natural world.
Keywords: structural colour, pigments, photonics, refractive index
1. Introduction
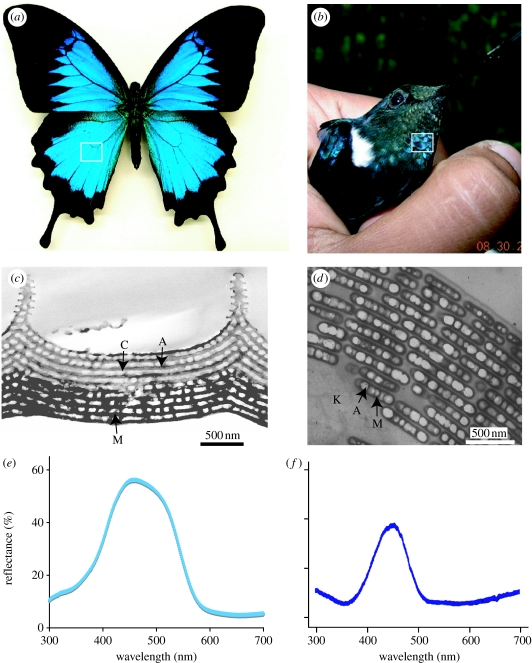
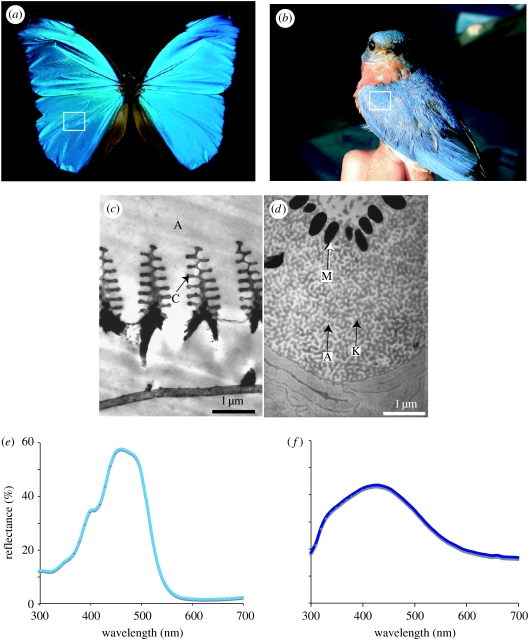
Coloration in living things can be created by selective absorption of light by pigments (pigmentary colour), by coherent or incoherent scattering of light from highly structured or unstructured tissues (structural colour) and by light scattering and absorption from combinations of these elements (combined colours) (Fox & Vevers 1960; Parker 2000; Shawkey & Hill 2005; Hill & McGraw 2006). These colours can also be categorized as either iridescent or non-iridescent. Broadly defined, iridescent colours change in appearance with the angle of observation or illumination, while non-iridescent colours remain similar in appearance regardless of angle of observation (Osorio & Ham 2002). The most well-studied animal colours are in butterflies (order Lepidoptera) and birds (class Aves). These two groups have an astonishing diversity of colours that range across the UV and visible spectrums (Nijhout 1991) from the near UV/purple colours of satin bowerbirds Ptilonorynchus violaceus (Doucet et al. 2006) and UV wing colours common in pierid butterflies (family Pieridae; e.g. Stavenga et al. 2006) to the fiery reds of hummingbirds (order Trochilidae) (Greenewalt et al. 1960) and memorable black-and-orange patterns of the monarch butterfly Danaus plexippus. This diversity of colour is matched by diversity in underlying optical mechanisms. For example, over 20 different ordered formations of melanin, keratin and air create iridescent colours in birds (Durrer 1986). In butterflies, the diversity of colour-producing, chitin-based structures is astounding, with new optical mechanisms described nearly every year (Kemp 2002). These arrangements are both convergent and divergent in form between these two groups. The layers of chitin and air creating colour in scales of the butterfly Papilio ulysses (Vukusic et al. 2004), for example, are strikingly similar to the layers of melanin and air seen in many hummingbird species such as Coeligena prunellei (figure 1; J. L. Parra & M.D. Shawkey 2007, unpublished data). However, structures such as the Christmas tree-like chitin and air structures seen in butterfly species such as Morpho didius (Vukusic et al. 1999) and the quasi-ordered channel-like ‘spongy layers’ of keratin and air in feathers of species such as the eastern bluebird Sialia sialis (Shawkey et al. 2003) have no known analogues in their avian or lepidopteran counterparts (figure 2).
Figure 1.
(a) The butterfly P. ulysses. (b) The hummingbird C. prunellei (photo by Juan Parra). In both cases, the box indicates the coloured region from which the transmission electron microscope (TEM) images and spectral data were taken. (c) TEM image of an iridescent blue scale from the butterfly P. ulysses, showing chitin (C) and air (A) arrays. The lower portion is much more darkly stained, suggesting the presence of diffuse melanin (M). (d) TEM image of an iridescent green barbule from the hummingbird Coeligena iris, showing highly ordered layers of air (A) filled melanin (M) platelets in a keratin (K) matrix. (e,f) Spectral data from P. ulysses and C. prunellei.
Figure 2.
(a) The butterfly M. didius. (b) The eastern bluebird S. sialis (photo by Mark Liu). In both cases, the box indicates the coloured region from which the TEM images and spectral data were taken. (c) TEM image of an iridescent scale from the butterfly M. didius showing ‘Christmas tree’-like formations of chitin (C) in air (A). (d) TEM image of a non-iridescent barb from blue feathers of the eastern bluebird S. sialis showing a solid keratin layer surrounding a spongy layer, or matrix of keratin (K) and air (A) above a layer of melanin granules (M) that absorb incoherently backscattered light. Note the granular packaging of melanin, contrasting with the diffuse distribution in figure 1c. (e,f) Spectral data from M. didius and S. sialis.
These similarities and differences in form have ultimate evolutionary and proximate physical and chemical explanations. Variation and selection in both groups have moulded structures into their modern forms. However, some of the materials used in this moulding process differ. The most prominent difference is found in their primary structural materials: butterflies use chitin while birds use keratin (Srinivasarao 1999). Furthermore, both groups use selectively absorbing pigments such as carotenoids and pterins as well as broadly absorbing melanins, but the macroscale anatomical forms in which they are deposited (diffusely or packaged) differ (see §3 below). The physical and optical properties of these base materials have certainly affected the evolutionary trajectories of coloured tissues in both groups. This divergence, together with flexibility associated with the basic feather or wing-scale design templates, constrains or facilitates the evolution of novel colours and optical effects in integumentary tissues. Like a craftsman working with wood or metal, evolution tinkers within the limits of available materials.
These limits may vary both intrinsically and through combinations with other materials. Bio-optical tissues are rarely homogeneous and typically contain either keratin or chitin and one or more pigments (Srinivasarao 1999). The physical, chemical and optical interactions between these base materials create opportunities for colour production that would not be available otherwise. Thus, it is important to understand the properties of base materials both in isolation and in combination to understand the evolution of coloured tissues. Here, we take a bottom-up approach to reviewing coloured tissues, using birds and butterflies as exemplar taxa owing to the depth of current knowledge of colour production in these animals. We begin by describing what we know of their base materials and then discuss the various ways that they interact with one another and their optical consequences. Finally, we discuss aspects of future research on optics and evolution of colour that would be invaluable to the field and which would fill the significant gaps in our current knowledge.
2. Background
2.1 Refractive index
One of the central physical concepts of this field, and of particular importance to the optical role played by biomaterials and bio-tissues, relates to refractive index (RI). The RI of a material, or more precisely the contrast between the RI of the two or more neighbouring materials in direct contact with each other within a bio-optical system, dictates the magnitude of the reflective or transmissive scatter of light from the interfaces that separate them (Hecht & Zajac 1974). It is this that essentially underpins the system's optical properties.
More generally, however, the interaction between a material and the electromagnetic fields associated with light is affected by the nature of the material's permittivity value. This dielectric permittivity value determines the ability of the material to polarize in response to incident electric fields and therefore to enable the transmission of these fields (Pedrotti & Pedrotti 1993; such as a block of glass enabling the transmission of light through it). The response of normal dielectric materials, however, generally depends on the frequency of the field because their polarizability does not react instantaneously to an applied field, i.e. there is a phase lag in the polarizing response of a material to the applied field. For this reason, dielectric permittivity is often treated as a complex number function of the frequency of the applied field because complex numbers allow both magnitude and phase to be specified.
In non-specialist optical applications, it is much more common to consider the RI of a material rather than explicitly its permittivity. RI also describes how the material interacts with high-frequency electromagnetic fields such as those associated with light. In fact, the RI of a non-magnetic material is defined as the square root of its dielectric permittivity (Hecht & Zajac 1974). Since a material's permittivity is specified as a complex number, this implies that its RI will also be complex, comprising both a real and imaginary component.
It has become increasingly clear in the study of bio-optical materials, that the complex nature of their RI values is an important and often overlooked issue. In fact, it is especially important to consider for biomaterials comprising strongly absorbing materials or pigments. Complex RI is conventionally written as =n+iκ. The real component, n, indicates the phase velocity of light in the material relative to light in vacuum. The imaginary component κ is the extinction coefficient and indicates the loss to optical absorption of the material (Pedrotti & Pedrotti 1993).
Generally, both n and κ are dependent on wavelength, especially near regions of maximal absorption. However, in many instances, for transparent media or broadly absorbing pigments over suitably narrow wavelength ranges, their n and κ values may be treated as being approximately constant (Hummel 2001).
In a surprising number of systems and bio-optical materials, however, both the real and imaginary components of RI have not been measured directly. Most often, researchers have used values for the real component of RI for a generic substance (keratin, melanin) from the literature, and have ignored the imaginary component entirely (however, Vukusic et al. (1999) made an experimental measurement of the imaginary component of iridescent Morpho butterfly scales). While this may be sufficient for rough approximations of the underlying colour phenomena, more precise quantification is necessary because variation in either n or κ can have profound influences on the resultant colour. For example, changes in n can result in shifts in hue (by changing the optical path length of nanostructures and therefore the system's interference conditions). Changes in n and/or κ can result in wavelength-specific or wavelength-independent shifts in brightness by changing reflection and/or absorption. Consequently, more precise measurements of RI (for instance using methods such as those described in Vukusic et al. (2004)) are clearly needed for almost all coloured structures studied to date.
2.2 Light absorption by pigments
Pigments are molecules that selectively absorb certain wavelengths of light. When pigments are embedded in a material at low concentrations, absorption of incident light by the pigmented material is often represented by the Lambert–Beer law, A=εbc, where A is absorbance; ε is the wavelength-dependent molar extinction coefficient of the pigment (similar to the extinction coefficient κ in complex RI described above); b is the optical path length of the structure; and c is the concentration of the pigment (Hecht & Zajac 1974). It is important to note that this commonly referenced relationship only holds for low pigment concentrations where stray light and light scattering by pigments or nearby structures are trivial considerations (Hecht & Zajac 1974), conditions that are unlikely to hold for many if not most biological tissues.
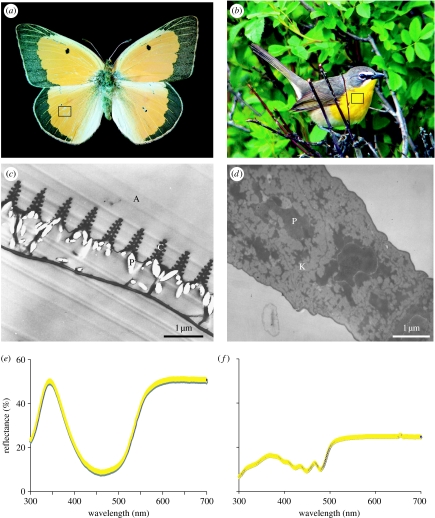
However, even when attempting to apply this simple model of pigmentary absorbance, scrutiny of the literature reveals several important empirical deficiencies. First, the absorption characteristics (i.e. molar extinction coefficients) of individual pigments are most often identified by suspending pigment molecules in extraction solutions, often of extreme (non-biological) pH. It is well known that the pH of the medium in which a pigment is suspended influences its absorptive properties (Fox 1976), and yet little work has been done to identify absorption profiles at biologically relevant pH values, resulting in a misalignment between published absorbance profiles and actual optical behaviours of pigments in vivo. Second, perhaps more empirically challenging, for most coloured tissues the concentration and localization of pigments within the tissues are at best coarsely known (but see Shawkey & Hill 2005; figure 3 of the current paper). This is particularly true in tissues where several pigments are known to play a role in coloration. Application of modern microscopy techniques such as confocal and atomic force microscopy may aid in quantifying the concentration and spatial arrangement of pigments. We encourage work to address these deficiencies, especially as they inform modelling efforts.
Figure 3.
(a) The butterfly Colias eurytheme. (b) The yellow-breasted chat Icteria virens (photo by Denny Granstrand). In both cases, the box indicates the coloured region from which the TEM images and spectral data were taken. (c) TEM image of an iridescent scale of the butterfly C. eurytheme, showing ‘Christmas tree’-like formations of chitin above pterin pigments (P) packaged into discrete granules. (d) TEM image of a non-iridescent barb from yellow feather of the yellow-breasted chat I. virens, showing diffuse distribution of a carotenoid pigment (P) in an unordered keratin matrix. Note the diffuse packaging of pigments, contrasting with the granular distribution in (c). (e,f) Spectral data from C. eurytheme and I. virens.
3. Basic components of colour mechanisms
Keratin and chitin are, respectively, ubiquitous components of feathers and butterfly wing scales and hence form the central colour production materials in birds and butterflies.
3.1 Birds: keratin
Keratins are sulphur-rich, fibrous, non-crystalline proteins that make up the bulk of the integument in higher vertebrates. A first classification scheme groups them according to the mode of biosynthesis and tactile sensation as ‘soft’ (e.g. those in the stratum corneum and calluses) or ‘hard’ (e.g. those in hair, nails, claws and feathers) (Giroud & Leblond 1951), while a second classification scheme groups them according to X-ray diffraction patterns as amorphous, α, β and feather classes (Filshie & Rogers 1962; Stewart 1977; Brush 1978; Fraser & Parry 2008). Amorphous keratin, found for example in the cuticle extracted from animal hair, does not show any discrete reflections in X-ray patterns, indicating the absence of any ordered crystalline structure (Fraser et al. 1972). Hard mammalian tissues characteristically show an α-keratin diffraction pattern, while the diffraction patterns of hard feather keratins more closely resemble those of β-keratin (found in other avian and reptilian tissues such as scales, beaks and claws), but are considerably more complex (Astbury & Beighton 1961).
The remaining 10 per cent of the feathers not composed of keratin includes pigments such as melanins and carotenoids (discussed below), as well as amino acids, polysaccharides, ribose, nucleic acids and lipids (Gross 1956; Bollinger & Varga 1960). The non-pigmentary components are thought to be discarded by-products of the process of keratinization, and whether they play any significant role in feather structure or colour is not known.
3.1.1 Optical properties
Because all feathers contain it, keratin necessarily plays a critical role in feather optics. However, as far as we are aware, no direct measurements of RI of feather keratin have been made. Indeed, the approximate value used in most studies appears to have been taken from horn keratin early in the twentieth century (Bancroft et al. 1923). This value has ranged from 1.50 to 1.55 (Dyck 1971; Land 1972; Prum et al. 1999; Shawkey et al. 2003), although it was recently estimated to be closer to 1.58 (Brink & van der Berg 2004). Furthermore, keratin has in the past been treated as transparent, but recent work suggests that it absorbs light weakly (Brink & van der Berg 2004) and this absorption could affect colour production. Use of these estimates in optical models has provided good fits between predicted and observed spectra (Zi et al. 2003; Shawkey et al. 2006b), but more direct measurements are clearly needed. In all likelihood, RI (n and κ) varies within this range between different species, between different feather parts and even along the length of feather parts. Thus, future studies evaluating variation in n and κ of different feather parts and types (structurally coloured versus non-coloured) will be extremely valuable because they will allow for more accurate modelling.
3.2 Arthropods: chitin
Second only to cellulose in annual natural production (Roberts 1992), chitin is one of the most common biomaterials on the planet. As one of the major constituents of arthropod cuticle, it is also ubiquitously involved in the colour-producing structures found on the surfaces of insects, arachnids and other arthropods (Fox 1976). Much is known about the properties of chitin itself, especially in purified form. Chitin is a polymer composed of N-acetyl-d-glucosamine and d-glucosamine monomer subunits, linked by β-1,4-glycosidic bonds (Muzzarelli 1976). In both ‘native’ and purified forms, chitin has a highly ordered crystalline structure that has been found in three configurations, termed α-, β- and γ-chitin, referring to differences in the orientation of chitin chains (Rudall 1976). While surveys of arthropods are far from complete, all arthropod chitin described to date falls in to the highly ordered, more stable α configuration in which chitin chains are oriented anti-parallel to each other (Roberts 1992). This stands in contrast to the β-pleated sheets of feather keratin. However, with very few exceptions (Roberts 1992), chitin is not found in the animal kingdom in purified crystalline form, but rather as a complex matrix involving significant contributions of protein, minerals and other organic compounds (Muzzarelli 1976). For example, insect cuticle can be composed of anywhere from 40 to 76 per cent protein (Hackman 1976; Roberts 1992), and the cuticle of many crustaceans also includes significant quantities of calcium carbonate (Roberts 1992). This binding of proteins, minerals and pigments results in substantial changes to the physical and optical properties of the resulting chitin-based matrix and it is perhaps this protean capacity that has allowed chitin to be so pervasively used in the integument of the arthropod body. By contrast, keratin of feathers appears not to have the same degree of flexibility in binding, and is typically found in a relatively pure form (Gross 1956; Bollinger & Varga 1960).
When seeking to understand the optical properties of various components of colour-producing structures, the variations found in chitin matrices presents a significant challenge to deepen our theoretical and empirical understanding. Furthermore, it leaves important questions unanswered. How do different proteins contribute to absorbance and/or transmittance of a given cuticular structure? How does the melanization of cuticle influence RI? What is the most common cuticular composition in colour-producing structures on the wings of butterflies? The elytra of beetles? The coloured scales of jumping spiders?
While little work has been done to elucidate the specific biochemical properties of structurally coloured tissues in arthropods, results from other non-structurally coloured chitinaceous tissues are pertinent. Proteins are pervasively found in chitin matrices examined to date, and exhibit a wide range of bonding properties, from protein–chitin complexes held together with weak forces such as van der Waals' forces, to covalently bound proteins (Hackman 1976; Roberts 1992). The three-dimensional morphology of chitin–protein complexes is also variable, from regular hexagonally packed chitin fibrils embedded in a protein matrix to alternating sheets of chitin and protein (Muzzarelli 1976; Rudall 1976). Hackman (1976) suggested that chitin provides the primary structure upon which the associated protein is bound, but more research is needed to better understand this interaction.
In addition to protein, chitin matrices often include pigments such as ommochromes, melanins and carotenoids, compounds of direct importance to the optical properties of chitin-based structural colours (see §§3.3 and 3.4 below).
3.2.1 Optical properties
Optical investigations of basic materials involved in arthropod structural coloration have resulted in a wide range of estimates for RI, from 1.40 to 1.73 (Land 1972), with most estimates falling around the widely accepted value of 1.56. However, it is not clear whether a RI estimate of 1.40 (or 1.73) is the result of inaccurate measurement, or rather an indication of the influence of other compounds within the chitin matrix. For instance, Bernard & Miller (1968) asserted that a RI of 1.40 could be achieved in the corneal cuticle of Diptera by the inclusion of a significant amount of water, whereas, this estimate was criticized by Neville & Caveney (1969) who favoured the more common value of approximately 1.5. Conversely, does the recurrence of a RI of 1.56 indicate an underlying homogeneity to the chitin-based materials most commonly used in structural colours?
In addition to this uncertainty surrounding RI, there is limited knowledge about the variation in optical absorbance of chitin-containing optical structures. Currently, two studies have investigated the magnitude of this absorbance in chitin-based systems. In the first, using RI-matching experiments performed on single butterfly scales in conjunction with absolute reflectance measurements, the complex RI of the cuticular component of Morpho rhetenor wing scales was estimated to be n=1.56+0.06i (Vukusic et al. 1999). While this result may offer a guide to the magnitude of optical absorption (namely κ=0.06) inherent in butterfly scale systems of equivalent ultrastructure and melanin content, there is likely to be considerable variation across the full gamut of butterfly species' scales that exhibit structural colour. In the second study, a more optically absorbing system from another order was studied. The highly optically absorbing basal component of the iridescent wing membrane of the male damselfly, Neurobasis chinensis, was determined, using absolute reflectance measurements, to have an extinction coefficient of κ=0.13 (Vukusic et al. 2004). It is this high value of κ from the basal region that creates the necessary optical absorption of the unscattered light transmitted by the multilayer above it, thus significantly raising the saturation of the multilayer's coloured appearance. These examples illustrate the importance of determining optical properties of materials through direct measurements, as it is only through these measurements that precise determination of colour mechanisms was achieved.
Furthermore, it should be noted that the incorporation of different proteins as well as pigments such as melanins, pterins and ommochromes is likely to influence the absorption coefficient of chitinaceous structures, as well as their RI. Indeed, the presence of optically coloured pigments within structurally coloured systems creates a strongly wavelength-dependent absorption that should be very carefully represented when optically modelling such systems.
3.3 Melanins
While keratin and chitin are specific to birds and butterflies, respectively, melanins are found in both. Indeed, they are ubiquitous throughout the animal kingdom (Riley 1997). Melanins are indole biochromes, a family of polymer pigments (Fox & Vevers 1960; Fox 1976; Riley 1997; McGraw 2006b). The differences in light absorptive properties between the two major classes of melanins, eumelanins and phaeomelanins, appear to be caused by differences in the amount of indole quinones and carbonyl groups. Both types of melanins absorb a large amount of light (two to three times as much as carotenoids, for example; Sarna & Swartz 1998) and release it as heat, but eumelanins do so more strongly, particularly in the red wavelengths, than do phaeomelanins (Krishnaswamy & Baranoski 2004). The greater number of indole quinones and carbonyl groups in eumelanin causes this stronger absorption and makes them appear blacker than phaeomelanins that are light brown or red to yellow in colour (Riley 1997).
3.3.1 Optical properties
The RI of melanin is frequently cited as being close to 2.0 (e.g. Durrer 1962; Land 1972; Zi et al. 2003; Shawkey et al. 2006b) and good fits between predicted and measured spectra have been reported using this value. However, technical difficulties have made actually measuring the RI of melanin difficult, and the values obtained for human eumelanin range from 1.30 to 1.64 (Kurtz 1986). Similarly, extinction coefficients have been estimated for melanin (Brink & van der Berg 2004), but have never been directly measured. As is also true for keratin, explicit measurements of refractive indices and extinction coefficients will be critical to improve our understanding of their role in colour production. In birds and mammals, melanin is packaged in discrete organelles called melanosomes or granules (Fox 1976). By contrast, melanins in butterflies are most commonly found diffusely distributed throughout the chitin matrix of specific structures (figure 1c). However, while Hackman (1976) posited that pigments form conjugates with proteins embedded in the chitin matrix and are involved in stabilizing and controlling the configuration of associated protein chains, research in this area is needed.
3.4 Carotenoid and other pigments
Carotenoids, found throughout living organisms, are 40-carbon tetraterpenoid molecules that are lipid soluble and absorb light at particular wavelengths because of their conjugated double-bond system (McGraw 2006a). The degree of conjugation of the hydrocarbon chain and end rings determines the wavelengths of light absorbed (typically between 400 and 500 nm; McGraw 2006a) and thus the colour created by the pigment. The absorption profiles of many carotenoid pigments are well characterized (McGraw 2006a) but as far as we are aware their refractive indices and extinction coefficients are unstudied. Carotenoids in both birds and butterflies are diffusely distributed throughout coloured tissue and create colour solely through absorption (Olson 1970; Shawkey & Hill 2005). However, despite their pervasive use as plumage colourants in birds, carotenoids appear to be largely restricted in butterflies to coloured tissues in the larval and pupal stages with no known instances of adult wing coloration being produced using carotenoids (Nijhout 1991). Instead, red, orange and yellow colours in butterflies are produced by ommochromes or pterins. Ommochromes are kynurenine derivatives, whereas pterins are synthesized from guanosine diphosphate (Kayser 1985). For ommochromes, little work has been done to identify where and how these pigments are deposited within butterfly wing scales, but casual observation suggests that they are diffusely deposited within chitinaceous wing scale structures (N. I. Morehouse 2007, unpublished data). Pterins, on the other hand, exhibit a unique deposition pattern. Best known from work done on coloration in pierid butterflies, pterins are often deposited as large collections of pigment molecules that form oblong granules within coloured wing scales (Stavenga et al. 2004; Morehouse et al. 2007; figure 3c). The composition of these pigment granules is poorly understood, but may involve some contribution of chitin either throughout the granules or on the outer surface. However, the deposition of pterins in large, round or oblong structures (also found in bird irises and fish skin, for example; Oliphant 1987; Grether et al. 2001) may be dictated in part by the insolubility of pterin pigments at cellular pH values, making diffusion and transportation of these pigments through the integument more difficult.
These pigments differ from melanins in the strong wavelength dependence of their absorption (McGraw 2006a,c). While absorption by melanins varies relatively slightly and steadily across the spectrum (McGraw 2006a) absorption by carotenoids and other pigments varies wildly (McGraw 2006b). Thus, these pigments can potentially amplify or reduce reflectance patterns created by structural tissues, and may also add additional spectral patterns (Rutowski et al. 2005). The absorption profiles of many of these pigments are well characterized (McGraw 2006a) but continued work will doubtless uncover new pigments and clarify our understanding of existing pigments.
4. Involvement of pigments in structurally coloured tissues
4.1 Pigments and incoherently scattering tissues
Interactions between pigment molecules and light-scattering tissue matrices are common in animal coloration, but are rarely explicitly analysed, as only one component is typically considered at a time. However, recent work has highlighted the importance of understanding both the scattering and absorptive properties of coloured traits (Grether et al. 2004; Vukusic et al. 2004; Rutowski et al. 2005; Shawkey & Hill 2005; Morehouse et al. 2007) because variations in either scattering efficiency and/or concentration of pigment molecules can result in distinct optical effects. In the simplest and perhaps most common cases, pigment molecules are deposited diffusely in unordered tissues that randomly scatter incident light. This is the case in many bird feathers, where pigments such as carotenoids are often found within the unordered keratin matrix of feather barbs, with coloration resulting from combined structural scattering and pigment-based wavelength-selective light absorption (Shawkey & Hill 2005; figure 3). More complex interactions between diffusely scattering tissues and pigments have also been identified. For example, Vukusic et al. (2004) investigated the highly absorbing black coloured scale region that forms the border around the central saturated blue wing colour of the butterfly P. ulysses. In these extremely absorbing scales, highly disordered filaments of melanin-packed cuticle are responsible for creating a high absorption cross section that results in increased light absorbance and the scale's optically black appearance. In the butterfly species Pontia protodice, Morehouse et al. (2007) demonstrated that the arrangement of pigment granules simultaneously contributed to increased light scattering in long wavelengths and light absorption in short wavelengths, resulting in the highly chromatic wing colours characteristic of pierid butterflies. This latter case is unusual because the pigments themselves participate directly in both optical effects.
4.2 Pigments and coherently scattering tissues
Pigments are also ubiquitously found in coherently scattering tissues, where they can serve a variety of functions. The most common pigments found in structural coloration of birds and butterflies are melanins that are often found deposited below or within structural arrays, where they function to absorb diffusely scattered light, thus contributing to increases in saturation and iridescence of the resulting colour pattern (Land 1972; Fox 1976; Prum & Torres 2003; Shawkey & Hill 2006; Yoshioka & Kinoshita 2006). However, in some cases, melanin is deposited above structurally coloured tissues, and used to modulate the brightness of the underlying coloration (Doucet et al. 2004; Shawkey et al. 2006a). In addition to serving an absorptive function, melanin may serve a role in influencing the RI of optical structures due to its high RI (but see §3.4 above). For example, melanin may be responsible for the modulation of RI found in many multilayer thin films in insects and other arthropods, especially colours found on the body integument or thick chitinaceous structures such as beetle elytra (Land 1972; Fox 1976; Parker et al. 1998). In some butterfly wing scales, melanin appears in discrete regions of colour-producing nanostructures (figure 1c), but its role in the optical properties of these wing scales remains virtually unexplored.
In avian coloration, however, the role of melanin is much better understood. The discrete granular packaging of melanin in bird feathers allows for melanin to play a role in producing a diverse range of structural colours. All known iridescent colours in birds are created by laminar or crystalline arrays of melanin granules embedded in keratin (Durrer 1986; Prum 2006). Although they are all produced by the same underlying processes of coherent light scattering, considerable variation exists in the structure and arrangement of the alternating layers of keratin, melanin and air, and consequently in the appearance of these different colours. The melanin granules can be rod- or disc-shaped, solid or hollow and can be arranged in single or multiple layers (Prum 2006). For example, ‘thick films’ have a single layer of melanin granules below a single superficial keratin layer (Brink & van der Berg 2004; Doucet et al. 2006; Prum 2006; Shawkey et al. 2006b), while ‘thin films’ have multiple layers of melanin granules and keratin (Durrer 1986; Zi et al. 2003; Prum 2006). The brilliantly coloured iridescent gorgets of many species of hummingbirds (family Trochiledae), for example, are produced by coherent light scattering from multiple, alternating layers of keratin and air-filled, disc-shaped melanin platelets (Greenewalt et al. 1960). These hollow granules further increase the opportunities for diverse colour production by introducing a sharp interface between two materials of highly divergent refractive indices (1.0 for air and approx. 2.0 for melanin). This interface should produce large amounts of scattering, and indeed birds with hollow melanin granules such as hummingbirds typically produce much brighter displays than those with solid melanin granules such as grackles (Shawkey et al. 2006b; J. L. Parra & M. D. Shawkey 2007, unpublished data).
Other pigments with more complex wavelength-dependent absorption profiles, such as pterins, carotenoids and ommochromes, are also found in structural colour patterns (Schmidt & Paulus 1970; Fox 1976; Steinbrecht et al. 1985; Rutowski et al. 2005; Wickham et al. 2006). The contributions of these pigments to the optical properties of colour patterns are various. In some instances, they shape the colour of light reflected from broadband reflectors, such as the chirped multilayers in butterfly pupae (Steinbrecht et al. 1985), the reflective scales of fish (Denton & Land 1971; Land 1972) and the skin of cephalopods (Mathger & Hanlon 2007). In other cases, they influence the scattering of light wavelengths not reflected by nearby optical structures, resulting in novel and complex colour phenotypes (Mason 1923, 1926; Schmidt & Paulus 1970; Prum & Torres 2003; Stavenga et al. 2006; Wickham et al. 2006). In even more specific instances, pigments may absorb extraneous light in wavelengths reflected by optical nanostructures while transmitting light in other wavelengths, thus creating a complex colour element that simultaneously showcases both the structural and pigment-based components of the colour trait (Rutowski et al. 2005).
5. Directions for future research
In reviewing the available information regarding basic materials in animal colours, it is clear that our knowledge of single components such as chitin and keratin, while not complete, is in some ways fairly sophisticated (e.g. chemical and conformational understandings). However, barring a few exceptional cases, little work has been done to look at the complex interplay between structured tissues and associated pigments in colour arrays. We argue that further work in this area should be profitable for a number of reasons.
First, theoretical modelling of the optical properties of coloured animal tissues has approximated a number of systems with success (reviewed in Grether et al. 2004; Prum 2006), suggesting that current theory adequately describes many basic optical mechanisms responsible for colour production. However, in many cases, explicit incorporation of the pigmentary components of coloured tissues (by inclusion of changes in absorbance and RI associated with pigmentation) should lead to better model fitting. In addition, most modelling work seeks to understand the basic mechanisms responsible for the ‘average’ case, rather than the span of variation found within a colour pattern or colour element. From both an evolutionary and functional standpoint, however, the variation ubiquitously present in coloured tissues is of central importance and modelling that begins to tease out the underlying reasons for such variation should be extremely valuable. Some work has already looked at variation due to changes in the morphology of colour producing arrays (Shawkey et al. 2003; Kemp et al. 2006). However, variation in colour patterns due to changes in pigment deposition and associated shifts in RI and absorbance remain mostly unexplored (Grether et al. 2004; Shawkey & Hill 2005; Morehouse et al. 2007). Further work in this area promises to inform both empirical and theoretical understandings of the optical parameters important to variation in colour traits.
Second, more complete knowledge of the biochemical demands of coloured tissues is necessary for evaluating the costs associated with their development and production. Contemporary understandings of the evolution of colour traits are placing increasing importance on quantifying such costs and their role in driving responses to natural and sexual selection. From this perspective, colour traits that involve contributions of both structural and pigmentary colour mechanisms are of considerable interest, because a single colour may draw from distinct biochemical pools (e.g. diet-derived carotenoids and synthesized keratins). In such situations, these colour traits could potentially be used to communicate multiple pieces of information about the bearer (Møller & Pomiankowski 1993; Grether et al. 2004), increasing their usage as indicator traits during social interactions. Once again, theoretical treatments of such complex colour phenotypes should be useful in identifying the underlying components most important to observable variation in colour patterns. These insights can in turn be used to better understand life-history tradeoffs associated with the evolution of such colour traits.
Third, colour traits that involve both pigmentary and structural components offer the opportunity to evaluate developmental mechanisms responsible for both pigment deposition and formation of nanostructures and the degree to which such underlying developmental pathways are coupled or under independent control. For example, work by Yoshioka & Kinoshita (2006) on the butterfly Morpho cypris revealed that this species produces the bold white patterning on its largely iridescent blue wings by removing melanin from the white wing scales and their underlying wing substrate without simultaneously removing the blue-reflecting multilayer structures in the wing scales. This suggests that melanin deposition in this butterfly is under developmental control independent of mechanisms responsible for scale ultrastructure formation. By contrast, in other butterflies developmental mechanisms involved in pigmentation and ultrastructure appear more tightly coupled (Gilbert et al. 1988; Janssen et al. 2001). Similar work has begun in bird coloration, using insights from amelanic individuals (Shawkey & Hill 2006), but more work is needed.
Last, efforts to elucidate phylogenetic patterns of colour trait evolution have largely treated structural and pigmentary colour production mechanisms independently (Badyaev & Hill 2000; Shawkey et al. 2006b). However, as we have laid out above, these mechanisms are often combined in colour elements, making evolutionary histories potentially more complex than currently appreciated. We suggest that attention to the interplay between pigments and structural coloration should deepen our understanding of the evolution of coloration across phylogenetic scales.
Acknowledgments
We thank the organizers and participants of the Iridescence: More than Meets the Eye Conference for providing the inspiration for this work.
Footnotes
One contribution of 13 to a Theme Supplement ‘Iridescence: more than meets the eye’.
References
- Astbury W.T., Beighton E. Structure of feather keratin. Nature. 1961;191:171–173. doi: 10.1038/191171b0. [DOI] [Google Scholar]
- Badyaev A.V., Hill G.E. Evolution of sexual dichromatism: contribution of carotenoid- versus melanin-based coloration. Biol. J. Linn. Soc. 2000;69:153–172. doi: 10.1111/j.1095-8312.2000.tb01196.x. [DOI] [Google Scholar]
- Bancroft W.D., Chamot E.M., Merritt E., Mason C.L. Blue feathers. Auk. 1923;40:275–300. [Google Scholar]
- Bernard G.D., Miller W.H. Interference filters in the corneas of Diptera. Invest. Ophtholmol. 1968;7:416–434. [PubMed] [Google Scholar]
- Bollinger A., Varga D. Cholestanol in avian plumage. Aust. J. Exp. Biol. 1960;38:265–270. doi: 10.1038/icb.1960.28. [DOI] [Google Scholar]
- Brink D.J., van der Berg N.G. Structural colours from feathers of the bird Bostrychia hagedash. J. Phys. D: Appl. Phys. 2004;37:813–818. doi: 10.1088/0022-3727/37/5/025. [DOI] [Google Scholar]
- Brush A.H. Feather keratins. In: Brush A.H., editor. Chemical zoology. Aves. vol. X. Academic Press; New York, NY: 1978. pp. 117–139. [Google Scholar]
- Denton E.J., Land M.F. Mechanism of reflexion in silvery layers of fish and cephalopods. Proc. R. Soc. B. 1971;178:43–61. doi: 10.1098/rspb.1971.0051. [DOI] [PubMed] [Google Scholar]
- Doucet S.M., Shawkey M.D., Rathburn M.D., Mays H.L., Jr, Montgomerie R. Concordant evolution of plumage colour, feather microstructure, and a melanocortin receptor gene between mainland and island populations of a fairy-wren. Proc. R. Soc. B. 2004;271:1663–1670. doi: 10.1098/rspb.2004.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet S.M., Shawkey M.D., Hill G.E., Montgomerie R. Iridescent structural plumage coloration in satin bowerbirds: structure, mechanisms and individual variation. J. Exp. Biol. 2006;209:380–390. doi: 10.1242/jeb.01988. [DOI] [PubMed] [Google Scholar]
- Durrer H. Schillerfarben beim Pfau. Vehr. Naturforsch. Ges. Besel. 1962;73:204–224. [Google Scholar]
- Durrer H. The skin of birds: colouration. In: Bereiter-Hahn J., Matoltsy A.G., Richards K.S., editors. Biology of the integument. Vertebrates. vol. 2. Springer; Berlin, Germany: 1986. pp. 239–247. [Google Scholar]
- Dyck J. Structure and spectral reflectance of green and blue feathers of the Lovebird (Agapornis roseicollis) Biol. Skr. 1971;18:1–67. [Google Scholar]
- Filshie B.K., Rogers G.E. An electron microscope study of the fine structure of feather keratin. J. Cell Biol. 1962;13:1–12. doi: 10.1083/jcb.13.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox D.L. University of California Press; Berkeley, CA: 1976. Animal biochromes and structural colours. [Google Scholar]
- Fox H.M., Vevers G. Macmillan; New York, NY: 1960. The nature of animal colors. [Google Scholar]
- Fraser R.D.B., Parry D.A.D. Molecular packing in the feather keratin filament. J. Struct. Biol. 2008;162:1–13. doi: 10.1016/j.jsb.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Fraser R.D.B., MacRae T.P., Rogers G.E. C. C. Thomas; Springfield, IL: 1972. Keratins. Their composition, structure and biosynthesis. [Google Scholar]
- Gilbert L.E., Forrest H.S., Schultz T.D., Harvey D.J. Correlations of ultrastructure and pigmentation suggest how genes control development of wing scales of Heliconius butterflies. J. Res. Lepidoptera. 1988;26:141–160. [Google Scholar]
- Giroud A., Leblond C.P. The keratinization of epidermis and its derivatives, especially the hair, as shown by X-ray diffraction and histochemical studies. Ann. N. Y. Acad. Sci. 1951;53:613–626. doi: 10.1111/j.1749-6632.1951.tb31963.x. [DOI] [PubMed] [Google Scholar]
- Greenewalt C.H., Brandt W., Friel D. The iridescent colors of hummingbird feathers. J. Opt. Soc. Am. 1960;50:1005–1013. doi: 10.1364/JOSA.50.001005. [DOI] [Google Scholar]
- Grether G.F., Hudon J., Endler J.A. Carotenoid scarcity, synthetic pteridine pigments and the evolution of sexual coloration in guppies (Poecilia reticulata) Proc. R. Soc. B. 2001;268:1245–1253. doi: 10.1098/rspb.2001.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grether G.F., Kolluru G.R., Nersissian K. Individual colour patches as multicomponent signals. Biol. Rev. 2004;79:583–610. doi: 10.1017/S1464793103006390. [DOI] [PubMed] [Google Scholar]
- Gross R. Water soluble compounds associated with the plumage of the pigeon (Columba livia) Aust. J. Exp. Biol. 1956;34:65–70. doi: 10.1038/icb.1956.9. [DOI] [PubMed] [Google Scholar]
- Hackman R.H. The interactions of cuticular proteins and some comments on their adaptation to function. In: Hepburn H.R., editor. The insect integument. Elsevier; New York, NY: 1976. pp. 107–120. [Google Scholar]
- Hecht E., Zajac A. Addison-Wesley; New York, NY: 1974. Optics. [Google Scholar]
- Hill G.E., McGraw K.J., editors. Bird coloration. Mechanisms and measurements. vol. I. Havard University Press; Boston, MA: 2006. [Google Scholar]
- Hummel R.E. Springer; Berlin, Germany: 2001. Electronic properties of materials. An introduction for engineers. [Google Scholar]
- Janssen J.M., Monteiro A., Brakefield P.M. Correlations between scale structure and pigmentation in butterfly wings. Evol. Dev. 2001;3:415–423. doi: 10.1046/j.1525-142X.2001.01046.x. [DOI] [PubMed] [Google Scholar]
- Kayser, H. 1985 Pigments. In Comprehensive insect physiology, biochemistry and pharmacology, vol. 10 (ed. G. A. Kerkut & L. I. Gilbert), pp. 367–415. New York, NY: Pergamon.
- Kemp D.J. Shedding new light on nature's brightest signals. Trends Ecol. Evol. 2002;17:298–300. doi: 10.1016/S0169-5347(02)02516-8. [DOI] [Google Scholar]
- Kemp D.J., Vukusic P., Rutowski R.L. Stress-mediated covariance between nano-structural architecture and ultraviolet butterfly coloration. Funct. Ecol. 2006;20:282–289. doi: 10.1111/j.1365-2435.2006.01100.x. [DOI] [Google Scholar]
- Krishnaswamy A., Baranoski G.V.G. A biophysically-based spectral model of light interaction with human skin. Eurographics. 2004;23:331–340. doi: 10.1111/j.1467.8659.2004.00764.x. [DOI] [Google Scholar]
- Kurtz S.K. Light scattering calculations for melanin pigments from the Rayleigh to the Mie regime. J. Invest. Dermatol. 1986;87:400–401. [Google Scholar]
- Land M.F. The physics and biology of animal reflectors. Prog. Biophys. Mol. Biol. 1972;24:77–106. doi: 10.1016/0079-6107(72)90004-1. [DOI] [PubMed] [Google Scholar]
- Mason C.W. Structural colors in feathers. II. J. Phys. Chem. 1923;27:401–448. doi: 10.1021/j150230a001. [DOI] [Google Scholar]
- Mason C.W. Structural colors in insects. I. J. Phys. Chem. 1926;30:383–395. doi: 10.1021/j150261a009. [DOI] [Google Scholar]
- Mathger L.M., Hanlon R.T. Malleable skin coloration in cephalopods: selective reflectance, transmission and absorbance of light by chromatophores and iridophores. Cell Tiss. Res. 2007;329:179–186. doi: 10.1007/s00441-007-0384-8. [DOI] [PubMed] [Google Scholar]
- McGraw K.J. Mechanics of carotenoid-based coloration. In: Hill G.E., McGraw K.J., editors. Bird coloration. Mechanisms and measurements. vol. I. Harvard University Press; Cambridge, MA: 2006a. pp. 177–242. [Google Scholar]
- McGraw K.J. Mechanics of melanin-based coloration. In: Hill G.E., McGraw K.J., editors. Bird coloration. Mechanisms and measurements. vol. I. Harvard University Press; Cambridge, MA: 2006b. pp. 243–294. [Google Scholar]
- Møller A.P., Pomiankowski A. Why have birds got multiple sexual ornaments? Behav. Ecol. Sociobiol. 1993;32:167–176. doi: 10.1007/BF00173774. [DOI] [Google Scholar]
- Morehouse N.I., Vukusic P., Rutowski R.L. Pterin pigment granules are responsible for both broadband light scattering and wavelength selective absorption in the wing scales of pierid butterflies. Proc. R. Soc. B. 2007;274:359–366. doi: 10.1098/rspb.2006.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzarelli R.A.A. Biochemical modifications of chitin. In: Hepburn H.R., editor. The insect integument. Elsevier; New York, NY: 1976. pp. 63–87. [Google Scholar]
- Neville A.C., Caveney S. Scarabeid beetle exocuticle as an optical analogue of cholesteric liquid crystals. Biol. Rev. 1969;44:531–562. doi: 10.1111/j.1469-185X.1969.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Nijhout H.F. Smithsonian Institution Press; Washington, DC: 1991. The development and evolution of butterfly wing patterns. [Google Scholar]
- Oliphant L.W. Pteridines and purines as major pigments of the avian iris. Pigm. Cell Res. 1987;1:129–131. doi: 10.1111/j.1600-0749.1987.tb00401.x. [DOI] [PubMed] [Google Scholar]
- Olson S.L. Specializations of some carotenoid-bearing feathers. Condor. 1970;72:424–430. doi: 10.2307/1366389. [DOI] [Google Scholar]
- Osorio D., Ham A.D. Spectral reflectance and directional properties of structural coloration in bird plumage. J. Exp. Biol. 2002;205:2017–2027. doi: 10.1242/jeb.205.14.2017. [DOI] [PubMed] [Google Scholar]
- Parker A.R. 515 million years of structural colour. J. Opt. A Pure Appl. Opt. 2000;2:R15–R28. doi: 10.1088/1464-4258/2/6/201. [DOI] [Google Scholar]
- Parker A.R., McKenzie D.R., Large M.C.J. Multilayer reflectors in animals using green and gold beetles as contrasting examples. J. Exp. Biol. 1998;201:1307–1313. doi: 10.1242/jeb.201.9.1307. [DOI] [PubMed] [Google Scholar]
- Pedrotti F., Pedrotti L. Prentice-Hall; New York, NY: 1993. Introduction to optics. [Google Scholar]
- Prum R.O. Anatomy, physics and evolution of avian structural colors. In: Hill G.E., McGraw K.J., editors. Bird coloration: mechanisms. Mechanisms and measurements. vol. I. Harvard University Press; Boston, MA: 2006. pp. 295–353. [Google Scholar]
- Prum R.O., Torres R. Structural colouration of avian skin: convergent evolution of coherently scattering dermal collagen arrays. J. Exp. Biol. 2003;206:2409–2429. doi: 10.1242/jeb.00431. [DOI] [PubMed] [Google Scholar]
- Prum R.O., Torres R., Williamson S., Dyck J. Two-dimensional Fourier analysis of the spongy medullary keratin of structurally coloured feather barbs. Proc. R. Soc. B. 1999;266:13–22. doi: 10.1098/rspb.1999.0598. [DOI] [Google Scholar]
- Riley P.A. Melanin. Int. J. Biochem. Cell Biol. 1997;29:1235–1239. doi: 10.1016/S1357-2725(97)00013-7. [DOI] [PubMed] [Google Scholar]
- Roberts G.A.F. MacMillan Press; London, UK: 1992. Chitin chemistry. [Google Scholar]
- Rudall K.M. Molecular structure in arthropod cuticles. In: Hepburn H.R., editor. The insect integument. Elsevier; New York, NY: 1976. pp. 21–41. [Google Scholar]
- Rutowski R.L., Macedonia J.M., Morehouse N.I., Taylor-Taft L. Pterin pigments amplify iridescent ultraviolet signal in males of the orange sulphur butterfly, Colias eurytheme. Proc. R. Soc. B. 2005;272:2329–2335. doi: 10.1098/rspb.2005.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarna T., Swartz H.M. The physical properties of melanins. In: Nordlund J.J., Boissy R.E., Hearing V.J., King R.A., Ortonne J.P., editors. The pigmentary system: physiology and pathophysiology. Oxford University Press; New York, NY: 1998. pp. 333–358. [Google Scholar]
- Schmidt K., Paulus H. Die feinstruktur der flugelschuppen einiger Lycaeniden (Insecta, Lepidoptera) Z. Morph. Tiere. 1970;66:224–241. doi: 10.1007/BF00280735. [DOI] [Google Scholar]
- Shawkey M.D., Hill G.E. Carotenoids need structural colors to shine. Biol. Lett. 2005;1:121–124. doi: 10.1098/rsbl.2004.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawkey M.D., Hill G.E. Significance of a basal melanin layer to production of non-iridescent structural plumage colour: evidence from an amelanotic Steller's jay (Cyanocitta stelleri) J. Exp. Biol. 2006;209:1245–1250. doi: 10.1242/jeb.02115. [DOI] [PubMed] [Google Scholar]
- Shawkey M.D., Estes A.M., Siefferman L.M., Hill G.E. Nanostructure predicts intraspecific variation in ultraviolet-blue plumage colours. Proc. R. Soc. B. 2003;270:1455–1460. doi: 10.1098/rspb.2003.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawkey M.D., Balenger S.L., Hill G.E., Siefferman L. Mechanisms of evolutionary change in structural plumage coloration among bluebirds. J. R. Soc. Interface. 2006a;3:527–532. doi: 10.1098/rsif.2006.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawkey M.D., Hauber M.E., Estep L.K., Hill G.E. Evolutionary transitions and structural mechanisms of avian plumage coloration in grackles and allies (Icteridae) J. R. Soc. Interface. 2006b;3:777–783. doi: 10.1098/rsif.2006.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasarao M. Nano-optics in the biological world: beetles, butterflies, birds and moths. Chem. Rev. 1999;99:1935–1961. doi: 10.1021/cr970080y. [DOI] [PubMed] [Google Scholar]
- Stavenga D.G., Stowe S., Siebke K., Zeil J., Arikawa K. Butterfly wing colours: scale beads make white pierid wings brighter. Proc. R. Soc. B. 2004;271:1577–1584. doi: 10.1098/rspb.2004.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavenga D.G., Giraldo M.A., Hoenders B.J. Reflectance and transmittance of light scattering scales stacked on the wings of pierid butterflies. Opt. Expr. 2006;14:4880–4890. doi: 10.1364/OE.14.004880. [DOI] [PubMed] [Google Scholar]
- Steinbrecht R.A., Mohren W., Pulker H.K., Schneider D. Cuticular interference reflectors in the golden pupae of Danaine butterflies. Proc. R. Soc. B. 1985;226:367–390. doi: 10.1098/rspb.1985.0100. [DOI] [Google Scholar]
- Stewart M. The structure of chicken scale keratin. J. Ultrastruct. Res. 1977;60:27–33. doi: 10.1016/S0022-5320(77)80038-5. [DOI] [PubMed] [Google Scholar]
- Vukusic P., Sambles J.R., Lawrence C.R., Wootton R.J. Quantified interference and diffraction in single Morpho butterfly scales. Proc. R. Soc. B. 1999;266:1403–1411. doi: 10.1098/rspb.1999.0794. [DOI] [Google Scholar]
- Vukusic P., Sambles J.R., Lawrence C.R. Structurally assisted blackness in butterfly scales. Proc. R. Soc. B. 2004;271:S237–S239. doi: 10.1098/rsbl.2003.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham S., Large M.C.J., Poladian L., Jermiin L.S. Exaggeration and suppression of iridescence: the evolution of two-dimensional butterfly structural colours. J. R. Soc. Interface. 2006;3:99–108. doi: 10.1098/rsif.2005.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka S., Kinoshita S. Structural or pigmentary? Origin of the distinctive white stripe on the blue wing of a Morpho butterfly. Proc. R. Soc. B. 2006;273:129–134. doi: 10.1098/rspb.2005.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zi J., Yu X., Li Y., Hu S., Xu C., Wang X., Liu X., Fu R. Coloration strategies in peacock feathers. Proc. Natl Acad. Sci. USA. 2003;100:12 576–12 578. doi: 10.1073/pnas.2133313100. [DOI] [PMC free article] [PubMed] [Google Scholar]