Abstract
Objective
IL-31 is a recently discovered helical cytokine. Its receptor consists of a ligand specific IL-31R subunit and a receptor chain that is shared with Oncostatin M (OSM), called OSM-Rβ. Because OSM-Rβ deficient animals have reduced hematopoietic progenitor cells (HPC) and OSM has effects on and is involved in homeostasis of HPC, we studied whether IL-31 and IL-31R play a role in hematopoiesis.
Methods
IL-31R-/- mice and their littermate WT controls were assessed for absolute numbers and cycling status of bone marrow and spleen HPC (CFU-GM, BFU-E, CFU-GEMM). Recombinant IL-31 was evaluated for stimulation, enhancement, or inhibition of colony formation by HPC, and for survival enhancing effects on HPC subjected to growth factor withdrawal and delayed addition of grown factors. Hematopoietic stem cells (HSC) from WT and IL-31R-/- mice were compared for competitive repopulating capacity in lethally irradiated congenic mice.
Results
IL-31R-/- mice demonstrated significantly decreased absolute numbers and cycling status of immature subsets of HPC in bone marrow bone and spleen compared to WT mice. There were no significant differences in absolute numbers of more mature subsets of WT and IL-31R-/- bone marrow CFU-GM. WT but not IL-31R-/- bone marrow CFU-GM responded to synergistic stimulation by combinations of cytokines. While IL-31 had neither colony stimulating, enhancing, or inhibiting activity for bone marrow HPC, it did enhance survival of these HPC in the context of delayed addition of growth factors. No significant differences were detected in competitive repopulating HSC activity between WT and IL-31R-/- bone marrow cells.
Conclusions
IL-31R is involved in positive regulation of absolute numbers and cycling status of immature subsets of HPC in vivo. While IL-31 in vitro does not modulate proliferation of HPC, it does enhance their survival, which may contribute to effects on cycling and numbers of HPC in vivo. Under steady state conditions, loss of IL-31R on HPC does not appear to influence the activity of competitive repopulating HSC. These results with HPC may be of future utility for manipulation of hematopoiesis in a pre-clinical setting.
Introduction
Helical cytokines and their receptors play important modulatory roles in the regulation of hematopoiesis in vivo and in vitro [1]. While factors such as erythropoietin (Epo), granulocyte colony stimulating factor (G-CSF) and thrombopoietin (Tpo) have been well characterized as the key physiological regulators of red blood cell (RBC), neutrophil and platelet (PLT) production, respectively, other factors that influence the growth and survival of hematopoietic stem cells (HSC) and early hematopoietic progenitor cells (HPC) remain an area of active investigation.
Interleukin (IL)-31 is a recently identified cytokine implicated in atopic dermatitis [2-4]. IL-31 is a four helix bundle cytokine and signals through a heterodimeric receptor complex composed of the IL-31 receptor (IL-31R) and the Oncostatin M receptor β chain (OSM-Rβ) [5-7]. The latter molecule also functions as a receptor for OSM, a cytokine with activities on hematopoietic cells [8-11]. An interesting symmetry in expression pattern of the two ligands exists, as OSM is made by CD4+ cells of the T-helper (TH)1 subtype [8], whereas IL-31 is preferentially expressed in TH2 cells [3].
Mice deficient in OSM-Rβ have been generated [12], and are predicted to be nonresponsive to both IL-31 and OSM. These mice have reduced levels of circulating erythrocytes and thrombocytes, and colony assays as well as transplantation experiments revealed that OSM has direct effects on hematopoietic progenitors as well as indirect effects mediated by the hematopoietic microenvironment [12]. Despite documented effects of OSM on hematopoietic progenitor cells [8], it remains to be determined how much of the phenotype in OSM-Rβ deficient mice can be attributed to loss of OSM signaling, and how much is due to loss of IL-31 function.
To address this question and further characterize the role of IL-31, we have generated mice deficient in IL-31R. These mice are predicted to be selectively deficient in IL-31 function but to have normal response to OSM. We found that IL-31R-/- mice have reduced levels of hematopoietic progenitors in the bone marrow and spleen but normal circulating blood cell counts. While IL-31 in vitro did not modulate proliferation of HPC, it supported their survival in the absence of growth factors. Our results illuminate for the first time an important function for IL-31 and IL-31R in HPC homeostasis.
Material and Methods
Mice
IL-31R-/- mice were generated using standard homologous recombination technique and C57BL/6 embryonic stem cells. The mutation was maintained in a congenic C57BL/6 background. Details of the targeting will be published elsewhere. Mice were housed in a specific pathogen free animal facility at Genentech Inc (South San Francisco, CA) and sent for analysis to the Indiana University School of Medicine. Studies reported in this paper were approved by the Indiana University Animal Care Committee.
Cytokines/reagents
Recombinant human and murine IL-31 were produced by overexpression in 293-T cells (Genentech). Activity of purified recombinant IL-31 was determined by proliferation of a responder cell line (BaF3) engineered to express combinations of human or murine IL-31Rα and OSM-Rβ. Recombinant human Stromal Cell Derived Factor (SDF)-1/CXCL12, and murine granulocyte macrophage colony stimulating factor (GM-CSF), interleukin (IL)-3, and stem cell factor (SCF) were purchased from R & D Systems (Minneapolis, MN, USA). Recombinant human Flt3-Ligand (FL) was a kind gift of Immunex/Amgen Corporation (Seattle, WA, USA). Recombinant human Erythropoietin (EPO) was purchased from Amgen Corporation (Thousand Oaks, CA, USA). Pokeweed mitogen mouse spleen cell conditioned medium (PWMSCM) was produced as described [13]. Hemin was purchased from Eastman Kodak Co. (Rochester, NY, USA). As shown in figure 5, IL-31 is species specific and murine IL-31 was used on primary mouse bone marrow cells. All other cytokines/reagents were active on mouse cells.
Figure 5.
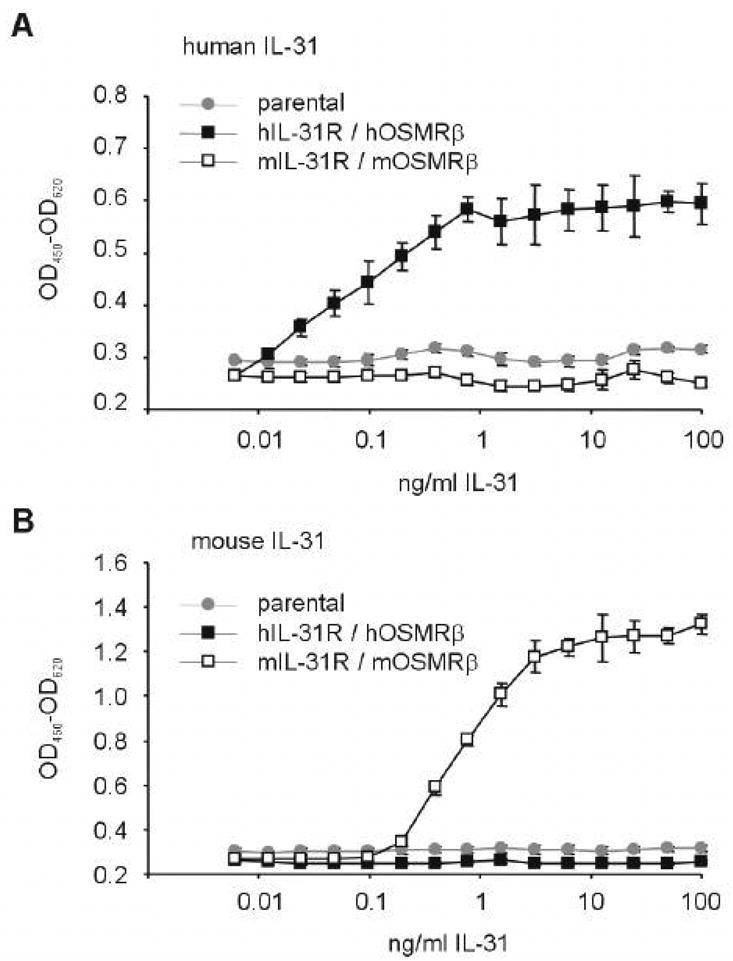
Titration of human (A) and murine (B) IL-31 with carboxyterminal His8-tag. Murine BaF3 cells transfected with hIL-31R/hOSM-Rβ and mIL-31R/mOSM-Rβ were incubated with increasing doses of human or murine IL-31. Proliferation was measured colorimetrically upon addition of XTT for the last 4 hours of the assay. Cross-species reactivity was not observed. EC50 for murine IL-31 was observed at 782 +/- 34 pg/ml, and EC50 for human IL-31 was 72 +/- 5 pg/ml.
Colony Assay
Unseparated femoral bone marrow or spleen cells from wild-type littermate (WT) or IL-31R-/- mice were plated at 5×104 and 5×105 cells/ml in the presence of the concentrations of cytokines denoted in the legends to the Figures or Tables. Colonies were scored 7 days after incubation of cells at 5% CO2 at lowered (5%) O2 tension in a humidified atmosphere [14]. Absolute numbers of marrow and spleen hematopoietic progenitor cells (HPC: colony forming unit-granulocyte, macrophage (CFU-GM), burst forming unit-erythroid (BFU-E), colony forming unit-erythroid, granulocyte, macrophage, megakaryocyte (CFU-GEMM)) were calculated by assessing the numbers of colonies formed per number of cells plated/ml, and multiplying this number by the total numbers of nucleated cells per femur or spleen. All experiments were repeated at least two times. Percent of HPC in S-phase of the cell cycle was estimated by the tritiated thymidine kill assay [14]. All mice were assessed individually. Cells from individual mice were not pooled.
Competitive repopulating HSC assay
IL-31R-/- (CD45.2+) or WT littermate control (CD45.2+) bone marrow cells were mixed at 5×105 cells/ml with 5×105 non-irradiated competitor B6.Boy J (CD45.1+) bone marrow cells (=1:1 ratio) and transplanted into lethally irradiated (950 cGy) B6.Boy J mice (purchased from Jackson Laboratories, Bar Harbor, ME, USA). The percentages of CD45.2+ Donor and CD45.1+ competitor cells, as determined by flow cytometry, were calculated at 1, 2, 4 and 6 months post-transplantation. The percentage of CD45.2+ plus CD45.1+ cells in the transplanted mice always equaled ∼100%. The assay was done as reported elsewhere for other donor cells [15,16].
Statistical Analysis
Statistical significance was determined by a two-tailed Students t-test, with p<0.05 considered to be statistically significant.
Results
Hematopoiesis in vivo in IL-31R-/- mice
IL-31R deficient mice were generated by removing the four exons encoding the ligand binding domain of IL-31R from the genome, using standard homologous recombination technique in embryonic stem cells. These mice were phenotypically normal and did not show significant histological, morphological, or behavioral alterations (Data not shown).
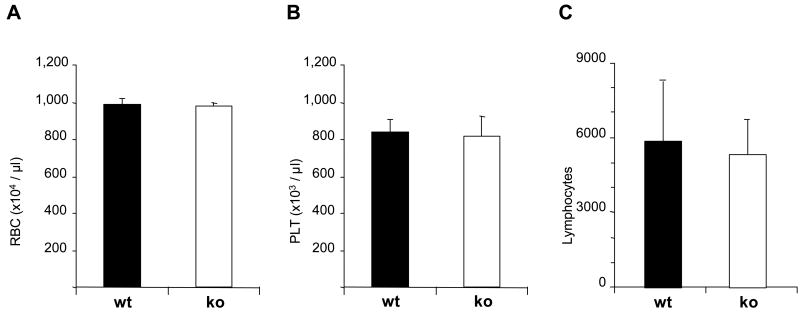
To investigate whether absence of IL-31R causes a hematopoietic phenotype, we first analyzed the number of circulating RBC, PLT, and lymphocytes. In contrast to mice with a disruption of OSM-Rβ [12], which show small but significant reduction of circulating RBC and PLT, IL-31R-/- mice had normal levels of these cells (Figure 1), and other blood cell types (not shown). There were also no significant differences in nucleated cellularity of femoral marrow (WT: 22±3 vs. IL-31R-/-: 22±3) and spleen (WT: 123±16 vs. IL-31R-/-: 138±19) (numbers represent mean ± SEM, in millions).
Figure 1.
Hematologic profile of IL-31R-/- mice. Blood was obtained by cardiac puncture from 8 animals per group and analyzed by automatic counter. Graphs represent mean counts and standard deviation of the number of circulating erythrocytes (A), platelets (B), and lymphocytes (C).
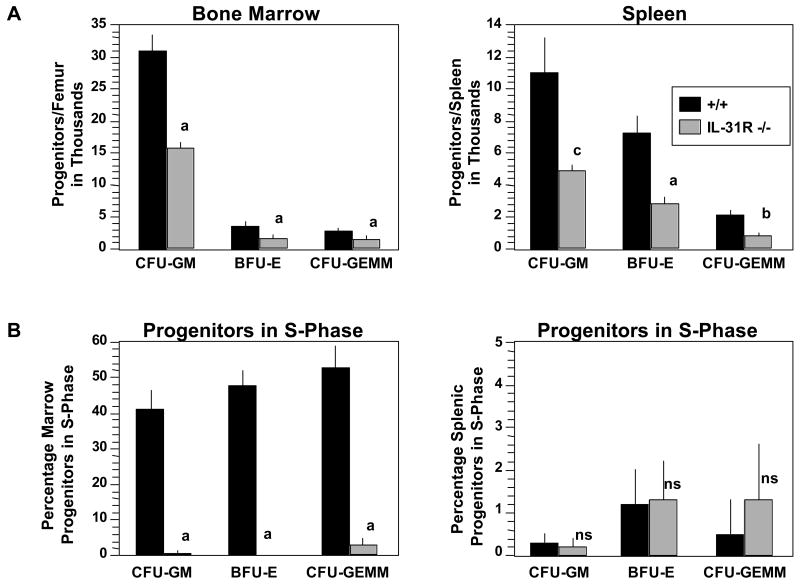
Normal levels of circulating blood cells in mice can be observed even if HPC numbers are compromised [17,18]. We therefore evaluated bone marrow and spleen of WT and IL-31R-/- mice for absolute numbers and cycling status (% in S-Phase) of CFU-GM, BFU-E, and CFU-GEMM. Unseparated bone marrow and spleen cells, treated with and without pulse-exposure to high specific activity tritiated thymidine, were plated in semi-solid methylcellulose culture medium with a combination of growth factors including Epo, PWMSCM, and SCF, plus hemin. Absolute numbers of progenitors per femur and spleen (Figure 2A), and the percent of progenitors in S-Phase (Figure 2B) were calculated. Significantly decreased absolute numbers and cycling status of bone marrow and absolute numbers of splenic CFU-GM, BFU-E, and CFU-GEMM were apparent in IL-31R-/- when compared to WT mice. HPC from both WT and IL-31R-/- mice were in a slow or non-cycling state.
Figure 2.
IL-31R-/- mice demonstrate decreased hematopoiesis in vivo of immature subsets of myeloid progenitor cells responsive to stimulation in vitro by combinations of cytokines. A) Absolute numbers and B) Cycling Status of progenitors in bone marrow, and spleen. Cells were stimulated in vitro with EPO (1U/ml), PWMSCM (5% vol/vol), SCF (50ng/ml), and hemin (0.1mM). Results shown are the averages of 7 individually assessed mice from each group from a total of 2 experiments, and are expressed as the mean ± ISEM. Significance: ap<0.004; bp<0.02; ns, not significant (p>0.05).
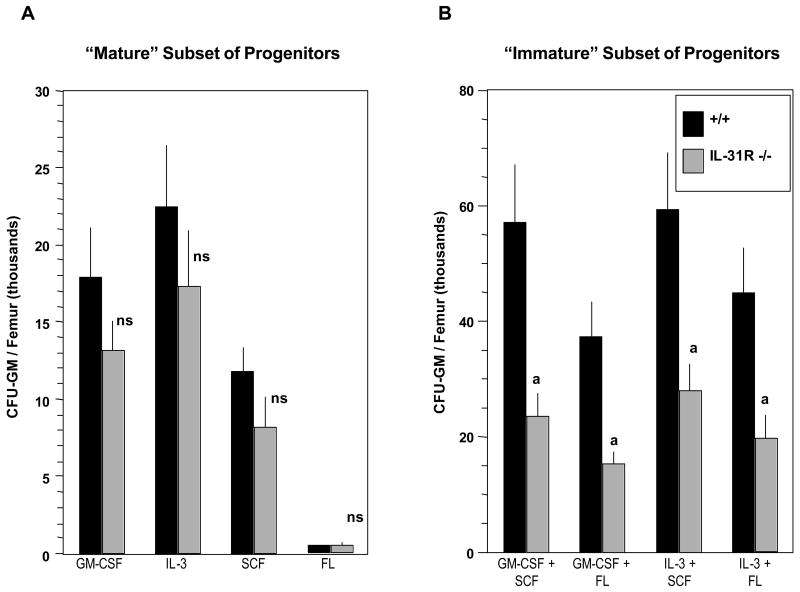
Stimulation of progenitor cells in vitro with combinations of cytokines allows detection of earlier, more immature subsets of progenitors, whereas stimulation with single cytokines allows detection of mature subset of progenitor cells [1,14]. To determine if deletion of IL-31R also influenced the later, more mature subset of progenitors, bone marrow cells from WT and IL-31R-/- mice were plated in vitro in semi-solid agar culture medium in the presence of either GM-CSF, IL-3, SCF, FL alone (Figure 3A) or a combination of GM-CSF or IL-3 with either SCF or FL (Figure 3B). As seen in Figure 3A, no significant differences were detected between the absolute numbers of CFU-GM in vivo in WT and IL-31R-/- mice when these cells were stimulated in vitro by individual cytokines. In contrast, and consistent with the results presented in Figure 2A, absolute numbers of CFU-GM per femur responsive to stimulation by combinations of cytokines were significantly decreased in IL-31R-/- when compared to WT animals [Figure 3B]. These results suggest that the IL-31R has positive regulatory effects in vivo on immature, compared to more mature, subsets of myeloid progenitor cells.
Figure 3.
IL-31R-/- mice demonstrate normal hematopoiesis in vivo of mature CFU-GM, but decreased hematopoiesis of immature subsets of CFU-GM. The following concentrations of growth factors were used: GM-CSF (10ng/ml), IL-3 (10ng/ml), SCF (50ng/ml), FL (100ng/ml). Results are the average of 7 WT and 8 IL-31R-/- mice, each individually analyzed, from a total of 2 complete experiments, and are expressed as mean +/- 1 SEM. Significance: a p<0.001; ns, not significant (p>0.05).
Assessment of the Responsiveness of WT and IL-31R-/- progenitor cells in vitro to synergistic stimulation
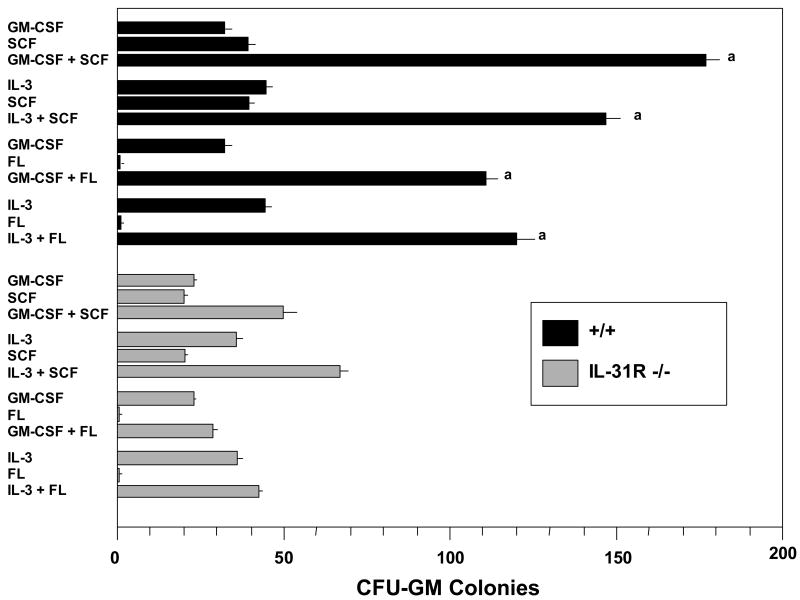
In order to better understand the role of the IL-31R in the regulation of hematopoiesis, we evaluated the responsiveness of CFU-GM from WT and IL-31R-/- bone marrow cells to synergistic combinations of growth factors. As seen in Figure 4, CFU-GM from bone marrow of WT mice responded synergistically to GM-CSF or IL-3 in combination with either SCF or IL-3. In sharp contrast, the effects of GM-CSF or IL-3 with SCF or FL on IL-31R-/- CFU-GM were essentially additive. These results suggest that the IL-31R either directly or indirectly influences the responsiveness of CFU-GM to synergistic stimulation by the combinations of cytokines evaluated in Figure 4.
Figure 4.
Wild type littermate control (WT), but not IL-31 R -/- CFU-GM are responsive in vitro to the synergistic proliferative effects of GM-CSF or Il-3 with the combinations of either SCF or FL. Concentrations of Cytokine used: GM-CSF or IL-3 (10ng/ml), SCF (50ng/ml), FL (100ng/ml). Results are shown as mean +/- 1 SEM for CFU-GM derived colonies (expressed per 5×104 bone marrow cells plated) for 7 different mice in each group from a total of 2 separate experiments. Significance: a p<0.001 for colonies formed in the presence of GM-CSF or IL-3 plus SCF or FL compared to that of the additive colonies formed in plates of GM-CSF alone plus SCF or FL alone, and of IL-3 alone plus SCF or FL alone.
Influence of IL-31 in vitro on proliferation and survival of progenitor cells
To test whether IL-31 had colony stimulating activity or could modulate proliferation of HPC in the context of colony stimulating and/or co-stimulating factors, we first produced recombinant IL-31 with a carboxyterminal His8 tag by transient overexpression in 293T cells, followed by purification over a Nickel-NTA column. To determine the EC50 of this material, we assessed proliferation of BaF3 cells stably transfected with either human IL-31R / human OSM-Rβ, or murine IL-31R / murine OSM-Rβ, in response to IL-31. As shown in Figure 5, IL-31 displayed strict species specificity. Half-maximal activity (EC50) of human and murine IL-31 were determined be 72 +/- 5 pg/ml and 782 +/- 34 pg/ml, respectively.
Colony formation in response to recombinant murine IL-31 alone and in combination with other colony stimulating and/or co-stimulating factors was assessed in semi-solid methylcellulose culture medium. As shown in Table 1, IL-31 did not manifest direct colony stimulating capacity for CFU-GM BFU-E or CFU-GEMM, nor did it enhance or inhibit colony formation of CFU-GM, BFU-E, or CFU-GEMM stimulated by the cytokines or cytokine combinations shown in Table 1.
Table 1. IL-31 has no stimulating effects on CFU-GM, nor does it influence effects of GM-CSF, SCF, or FL, alone or in combination.
| Colonies/5×104 +/+Bone Marrow Cells | ||||
|---|---|---|---|---|
| Control Medium | IL-31 | |||
| 1ng/ml | 10ng/ml | 100ng/ml | ||
| CFU-GM | ||||
| Control Medium | 0 | 0 | 0 | 0 |
| GM-CSF | 31±3 | 31±2 | 30± | 32±1 |
| SCF | 25±2 | 24±1 | 25± | 27±2 |
| FL | 3±1 | 2±1 | 3± | 3±1 |
| GM-CSF + SCF | 74±3 | 74±2 | 74± | 78±3 |
| GM-CSF + FL | 50±4 | 50±2 | 50± | 52±2 |
| BFU-E | ||||
| Control Medium | 0 | 0 | 0 | 0 |
| Epo | 3±0.5 | 3±1 | 4±1 | 3±0.5 |
| Epo+SCF | 7±1 | 6±1 | 8±2 | 12±1* |
| CFU-GEMM | ||||
| Control Medium | 0 | 0 | 0 | 0 |
| Epo+SCF | 5±1 | 4±1 | 4±1 | 5±1 |
Growth factors were used at the following concentrations: GM-CSF or IL-3 (10ng/ml), SCF (50ng/ml), FL (100ng/ml)
This value was a significant increase compared to control medium, but was not reproducible in two other experiments
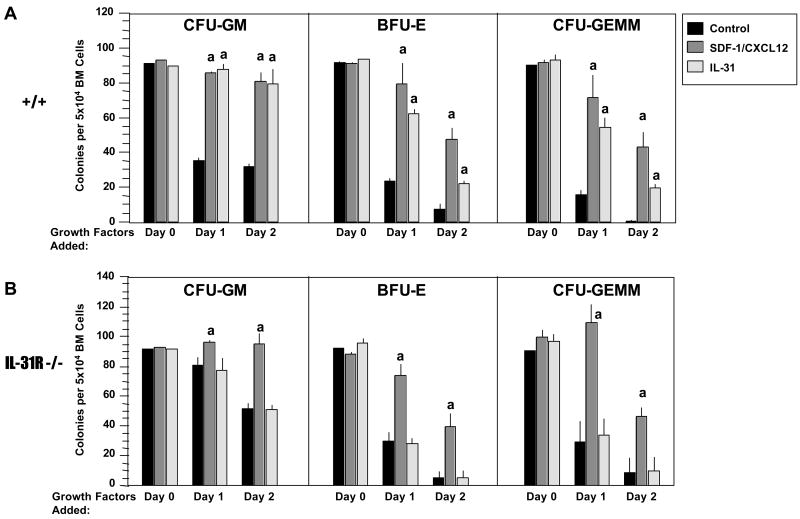
Myeloid progenitor cells require cytokines for survival, and undergo apoptosis upon growth factor withdrawal [1]. Thus, delayed addition of growth factors allowed us to evaluate whether IL-31 has survival enhancing activity for bone marrow CFU-GM, BFU-E, and/or CFU-GEMM. Bone marrow cells from WT and IL-31R-/- mice were plated in semi-solid methylcellulose culture medium with hemin in the presence of either control diluent, SDF-1/CXCL12, a known survival enhancing cytokine for myeloid progenitor cells [19], or IL-31. The combination of Epo, PWMSCM, and SCF was added to the culture plates either at time 0 (= Day 0), 24 hr (= Day 1) or 48 hr (= Day 2) of initiation of the cultures. As seen in Figure 6, IL-31 had significant survival enhancing activity for CFU-GM, BFU-E and CFU-GEMM in bone marrow from WT (Figure 6A), but not from IL-31R-/- animals (Figure 6B). By comparison, SDF-1/CXCL12 significantly increased survival of bone marrow progenitor cells from both WT and IL-31R-/- mice (Figure 6A and B), indicating that absence of IL-31R did not cause a general survival defect. While the survival enhancing activity of IL-31 on bone marrow CFU-GM was comparable to that of SDF-1/CXCL12, it was somewhat less active on BFU-E and CFU-GEMM (Figure 6A). These results demonstrate a positive influence of IL-31 on in vitro survival of myeloid progenitor cells, an effect directly mediated through the IL-31R. Consistent with the data in Table 1, an assessment of the day 0 counts in Figure 6 demonstrates that IL-31 neither enhances nor suppresses colony formation of myeloid progenitor cells stimulated by combinations of cytokines.
Figure 6.
Wild-type littermate control (WT) but not IL-31R-/- myeloid progenitor cells are responsive in vitro the survival enhancing effects of IL-31 in the context of delayed growth factor addition. Bone marrow cells were plated at 5×104/ml in the presence of control diluent, SDF-1/CXCL12 (100ng/ml) or Il-31 (100ng/ml) in the presence of hemin. A combination of EPO (1U/ml) PWMSCM (5% vol/vol) and SCF (50ng/ml) were added to plates at either day 0, 1 or 2.
Competitive Stem Cell Repopulating Capacity of WT and IL-31R-/- bone marrow cells
The data shown in Figure 2 demonstrated a positive effect of the IL-31R on HPC growth. To determine whether expression of IL-31R on HSC and HPC was required for efficient repopulation of irradiated recipients, we compared the competitive repopulating activity of stem cells from the bone marrow of WT and IL-31R-/- mice (CD45.2+) using congenic B6.Boy J bone marrow competitor cells (CD45.1+) and lethally irradiated B6.Boy recipient mice. As seen in Table 2 for 1, 2, 4 and 6 month recipient mouse chimerism data, there was no significant difference in the repopulating capabilities of WT and IL-31R-/- bone marrow HSC. Thus, IL-31R does not appear, under steady-state conditions to influence the competitive repopulating capacity of HSC in lethally irradiated primary mouse recipients.
Table 2. Competitive Repopulating Capacity of IL-31 R -/- and Control (+/+) Bone Marrow Hematopoietic Stem Cells in Lethally Irradiated Recipients.
| Percent Chimerism | ||||
|---|---|---|---|---|
| 1 Month | 2 Months | 4 Months | 6 Months | |
| +/+ | 60±8 | 67±9 | 70±10 | 70±12 |
| IL-31 R -/- | 59±9 | 61±14 | 61±20 | 60±24 |
Mononuclear bone marrow cells from +/+ and IL-31 R -/- mice (both C45.2+) were competed against an equal number of congenic B6/BoyJ (CD45.1+) mononuclear bone marrow cells and injected into lethally irradiated B6.BoyJ mice. Percent CD45.2 chimerism was assessed at 1, 2, 4, and 6 months post transplantation. Results, shown as mean ± 1SD, are based on cells from 3 donors in each group, with each donor collection transplanted into 5 lethally irradiated recipients.
Discussion
IL-31 is a T-cell derived cytokine, which signals through a heterodimeric receptor composed of IL-31R and OSM-Rβ. The role of IL-31 in hematopoiesis had not been previously studied. However, mice deficient in OSM-Rβ, which is also utilized by the hematopoietically active cytokine OSM, have decreased numbers of hematopoietic progenitors. Since these mice are predicted to be nonresponsive to both OSM and IL-31, it is unclear whether that phenotype reflects loss of OSM or loss of IL-31 signals, or a combination of both. We therefore tested the role of IL-31 in hematopoiesis, using IL-31R-/- mice, which are nonresponsive to IL-31 but fully responsive to OSM.
IL-31R-/- mice had normal levels of circulating RBC, PLT, lymphocytes (Figure 1), other blood cell types (Data not shown), and mature HPC capable of responding to single cytokine stimuli (Figure 3A). However, these mice manifested significantly decreased numbers and cycling status of the more immature subsets of HPC, those CFU-GM, BFU-E, and CFU-GEMM that are responsive to the synergistic stimulating effects of combinations of cytokines for induction of optimal proliferation (Figures 2, 3B, and 4). Thus, genetic deletion of IL-31R affects HPC development at a very early stage, but under normal conditions does not manifest in abnormalities of the mature morphologically recognizable hematopoietic cell compartment, nor does it influence the competitive repopulating HSC compartment (Table 2). Our studies do not rule out additional effects of IL-31R deletion on early stages of lymphopoiesis and assessment of this possibility is warranted.
The precise mechanism by which IL-31 selectively affects the subset of immature HPC is presently unclear. That IL-31R -/- mice have decreased numbers of immature HPC may relate, at least in part, to the possibility that wildtype mice manifest IL-31R-associated up-regulation of IL-3, SCF and/or GM-CSF receptors at the cell surface or that IL-31R induces amplification of signaling cascades activated by IL-3, SCF and/or GM-CSF. This would not occur in IL-31R -/- mice. Alternatively, a characteristic feature of many cytokine receptors is their ability to interact with more than one cytokine [1,20], especially when common subunits of receptors are involved. However, IL-31R is not known to be activated by OSM, and presently there is no evidence that the IL-31R might act as a signal transducer for IL-3, GM-CSF or SCF. Also, there is no evidence to suggest that alternate receptors exist for IL-3, GM-CSF or SCF. We don't believe it is likely that IL-31 uses the IL-3-, GM-CSF- or SCF-receptor. Future studies will be needed to definitively address actions of IL-3, GM-CSF, and/or SCF in the context of the IL-31R, and IL-31 actions in the context of other receptors. Differences may also relate to differential quantitative or qualitative expression of IL-31R on immature vs. mature MPC, mature HPC expressing splice variants of IL-31R subunits incapable of transducing the IL-31 signal, or alternatively, indirect effects mediated on accessory cells that produce cytokines that differentially effect immature vs. mature HPC. To evaluate the first two possibilities requires the ability to phenotypically identify and perhaps isolate the immature vs. mature subsets of HPC. To our knowledge, this is not yet possible, as markers for these differentiation stage-HPC subsets are not available. Future studies will be necessary to define mechanisms.
Recombinant IL-31 in vitro did not influence proliferation of HPC, neither alone nor in combination with other growth factors (Table 1). WT BM cells transplanted into OSM-Rβ deficient recipients failed to rescue the recipient's defect in peripheral RBC and PLT, and engrafted mice continued to show defects at the progenitor level [12]. This result suggests that absence of OSM-Rβ, and hence nonresponsiveness to OSM and IL-31, in the host derived stroma contributes to the hematologic abnormalities observed in OSM-Rβ-/- mice. To determine conclusively whether IL-31 acts through stromal cells, studies addressing the effects of selective IL-31R deficiency in stromal cells are warranted.
We did find that IL-31 had survival enhancing effects on CFU-GM, BFU-E, and CFU-GEMM. WT HPC, but not IL-31R-/- HPC, subjected in vitro to growth factor-withdrawal and subsequent delayed addition of growth factors showed enhanced survival in response to IL-31. This could be a direct effect on HPC survival, or alternatively an indirect effect mediated by IL-31 responsive non-HPC BM cells present in the culture. We found in a preliminary experiment that conditioned medium by unseparated bone marrow cells treated with or without IL-31 did not contain activity for stimulation, enhancement or inhibition of HPC proliferation (data not shown). Thus, we have no evidence at this time for an indirect effect mediated through IL-31 action on accessory cells. More rigorous experiments will be needed to test this further. Regardless of whether the effect of IL-31 on HPC survival is direct or indirect, IL-31/IL-31R survival effects may at least partially explain the decreased absolute numbers and cycling of immature subsets of HPC in IL-31R-/- mice. In agreement with the role of a survival factor, IL-31 has been shown to activate the PI3 Kinase/Akt pathway in certain cell lines [21]. Finally, OSM-Rβ-/- donor cells transplanted the defects observed in the OSM-Rβ-/- mice to WT recipients [12], indicating that IL-31 and/or OSM responsiveness is not only required on stromal cells, as discussed above, but also on HPC l cells for normal hematopoiesis.
A positive effect of IL-31 on survival of HPC is also consistent with the failure of IL-31R-/- HPC to respond efficiently to synergistic stimulation (Figure 4). The ability to respond to synergistic stimulation is a hallmark feature of immature progenitors, and therefore a lower abundance of these cells due to compromised survival would result in a diminished synergistic response to combinations of cytokines like that observed in IL-31R-/- HPC.
Why should two cytokines that are produced by specialized subsets of T-helper cells promote hematopoiesis? OSM is produced by TH1 cells [8], which are activated in response to infection with bacteria, whereas IL-31 is produced by TH2 cells [3], which mediate defense against helminths and are critical for humoral responses. Either immune response will occur in reaction to an immunological insult, which generally causes destruction of myeloid cells. Excessive TH2 response in the absence of IFN-γ resulted in strongly increased HPC activity [22]. IL-31 is now a possible candidate to mediate this response. Thus, the function of cytokines like IL-31 and OSM in this context may ensure increased production of myeloid immune cells in the face of various immunological challenges.
In summary, this is the first report implicating the IL-31/IL-31R axis in regulation of hematopoiesis. Further studies in the hematopoietic area of IL-31 and its receptor are now warranted in order to determine their precise cellular and intracellular mechanisms of action. Such studies may yield information of practical clinical utility for ex-vivo and in vivo manipulation of hematopoiesis.
Acknowledgments
These studies were supported by US Public Health Service Grants RO1 HL56416, RO1 HL67384, and a Project in PO1 HL53586 from the NIH to HEB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shaheen M, Broxmeyer HE. Hematology : basic principles and practice. 4. Philadelphia, Pa: Elsevier Churchill Livingstone; 2005. The humoral regulation of hematopoiesis; pp. 233–265. [Google Scholar]
- 2.Bilsborough J, Leung DY, Maurer M, et al. IL-31 is associated with cutaneous lymphocyte antigen-positive skin homing T cells in patients with atopic dermatitis. J Allergy Clin Immunol. 2006;117:418–425. doi: 10.1016/j.jaci.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 3.Dillon SR, Sprecher C, Hammond A, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–760. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 4.Sonkoly E, Muller A, Lauerma AI, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117:411–417. doi: 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 5.Diveu C, Lelievre E, Perret D, et al. GPL, a novel cytokine receptor related to GP130 and leukemia inhibitory factor receptor. J Biol Chem. 2003;278:49850–49859. doi: 10.1074/jbc.M307286200. [DOI] [PubMed] [Google Scholar]
- 6.Dreuw A, Radtke S, Pflanz S, Lippok BE, Heinrich PC, Hermanns HM. Characterization of the signaling capacities of the novel gp130-like cytokine receptor. J Biol Chem. 2004;279:36112–36120. doi: 10.1074/jbc.M401122200. [DOI] [PubMed] [Google Scholar]
- 7.Ghilardi N, Li J, Hongo JA, Yi S, Gurney A, de Sauvage FJ. A novel type I cytokine receptor is expressed on monocytes, signals proliferation, and activates STAT-3 and STAT-5. J Biol Chem. 2002;277:16831–16836. doi: 10.1074/jbc.M201140200. [DOI] [PubMed] [Google Scholar]
- 8.Broxmeyer HE, Bruns HA, Zhang S, et al. Th1 cells regulate hematopoietic progenitor cell homeostasis by production of oncostatin M. Immunity. 2002;16:815–825. doi: 10.1016/s1074-7613(02)00319-9. [DOI] [PubMed] [Google Scholar]
- 9.Miyajima A, Kinoshita T, Tanaka M, Kamiya A, Mukouyama Y, Hara T. Role of Oncostatin M in hematopoiesis and liver development. Cytokine Growth Factor Rev. 2000;11:177–183. doi: 10.1016/s1359-6101(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 10.Mukouyama Y, Hara T, Xu M, et al. In vitro expansion of murine multipotential hematopoietic progenitors from the embryonic aorta-gonad-mesonephros region. Immunity. 1998;8:105–114. doi: 10.1016/s1074-7613(00)80463-x. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimura A, Ichihara M, Kinjyo I, et al. Mouse oncostatin M: an immediate early gene induced by multiple cytokines through the JAK-STAT5 pathway. Embo J. 1996;15:1055–1063. [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka M, Hirabayashi Y, Sekiguchi T, Inoue T, Katsuki M, Miyajima A. Targeted disruption of oncostatin M receptor results in altered hematopoiesis. Blood. 2003;102:3154–3162. doi: 10.1182/blood-2003-02-0367. [DOI] [PubMed] [Google Scholar]
- 13.Cooper S, Broxmeyer HE. Clonogenic methods in vitro for the enumeration of granulocyte-macrophage progenitor cells (CFU-GM) in human bone marrow and mouse bone marrow and spleen. Methods in Cell Science. 1991;13:77–81. [Google Scholar]
- 14.Cooper S, Broxmeyer HE. Current Protocols in Immunology. New York: John Wiley&Sons; 1996. Measurement of interleukin-3 and other hematopoietic growth factors, such as GM-CSF, G-CSF, M-CSF, erythropoietin, and the potent co-stimulating cytokines steel factor and Flt-3 ligand; pp. 6.4.1–6.4.12. [DOI] [PubMed] [Google Scholar]
- 15.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christopherson KW, 2nd, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 17.Broxmeyer HE, Cooper S, Lasky LA, De Sauvage F. Identification of a massive reserve of hematopoietic progenitors in mice. Stem Cells Dev. 2005;14:105–110. doi: 10.1089/scd.2005.14.105. [DOI] [PubMed] [Google Scholar]
- 18.Cheng J, Baumhueter S, Cacalano G, et al. Hematopoietic defects in mice lacking the sialomucin CD34. Blood. 1996;87:479–490. [PubMed] [Google Scholar]
- 19.Lee Y, Gotoh A, Kwon HJ, et al. Enhancement of intracellular signaling associated with hematopoietic progenitor cell survival in response to SDF-1/CXCL12 in synergy with other cytokines. Blood. 2002;99:4307–4317. doi: 10.1182/blood.v99.12.4307. [DOI] [PubMed] [Google Scholar]
- 20.Ozaki K, Leonard WJ. Cytokine and Cytokine Receptor Pleiotropy and Redundancy. J Biol Chem. 2002;277:29355–29358. doi: 10.1074/jbc.R200003200. [DOI] [PubMed] [Google Scholar]
- 21.Diveu C, Lak-Hal AH, Froger J, et al. Predominant expression of the long isoform of GP130-like (GPL) receptor is required for interleukin-31 signaling. Eur Cytokine Netw. 2004;15:291–302. [PubMed] [Google Scholar]
- 22.Murray PJ, Young RA, Daley GQ. Hematopoietic remodeling in interferon-gamma-deficient mice infected with mycobacteria. Blood. 1998;91:2914–2924. [PubMed] [Google Scholar]