Abstract
Cholecystokinin (CCK) and its receptor CCK-2R have been shown to promote emotional responsivity and behavioral sensitization to psychostimulants in the rat. An animal model has been developed based on locomotor response to a novel inescapable environment. Animals exhibiting consistent differences in locomotor response to novelty have been termed as High and Low Responder rats (HR and LR respectively). This paradigm is deemed to model sensation-seeking, a personality trait closely associated with substance abuse. The present study provides genetic and pharmacological evidence that the CCK-ergic system modulates this behavior. Distinctive patterns of CCK-related gene expression in HR and LR animals occurred beyond the mesolimbic pathways. CCK gene expression was higher in hippocampus, amygdala, and prefrontal cortex, but lower in the ventral tegmental area of HR relative to LR rats. Levels of CCK-2R mRNA were more elevated in LR animals in some areas of the forebrain such as the prefrontal cortex, nucleus accumbens, and hippocampus. Additionally, CCK-2R blockade with the antagonist LY225.910 (0.5 mg/Kg) removed phenotype differences in sustained exploration of novel stimuli (i.e., a novel-object) in HR and LR rats exposed to an enriched open-field test series. Finally, CCK-2R blockade also altered M2 and 5-HT7 receptor gene expression in the mediodorsal thalamus (a strategic structure for corticothalamic trafficking) in a phenotype-dependent manner. Taken together, the findings reported here suggest that distinct CCK-ergic function may contribute to promoting individual differences in novelty-seeking behavior.
Keywords: Cholecystokinin, CCK-2R, LY225.910, novelty-seeking behavior, 5-HT7 receptor, M2 receptor
INTRODUCTION
Cholecystokinin (CCK), a gastrin-like peptide, is currently the most abundant neuropeptide found in the mammalian brain, (Crawley & Corwin, 1994; Dauge & Lena, 1999). There are two CCK receptor types in the brain. The CCKA receptor (recently termed CCK-1R) is expressed at relatively low levels, while the CCKB receptor (or CCK-2R) is densely distributed in the forebrain regions. There is general agreement about the influence of CCK-2R in generating anxiety-like behaviors and emotional responsiveness (Bradwejn et al., 1991; Van Megen, 1996; Farook et al., 2001; Chen et al., 2006). However, the anxiolytic profile of CCK-2R antagonists has been questioned (Griebel, 1999) thus raising serious concerns about the therapeutic potential of targeting the CCK system (Abelson, 1995). Recent evidence strongly suggests that CCK-ergic neurotransmission has a role in mood regulation after repeated stressful (social defeat) experience (Becker et al., 2007). Dopaminergic mesocorticolimbic system is a neural substrate through which CCK may mediate all these processes. CCK coexists with dopamine in the ventral tegmental area projection onto the nucleus accumbens (Crawley, 1994). CCK regulates mesolimbic function, strongly affecting dopamine-mediated behaviors such as behavioral responsivity to psychostimulants (Higgins et al., 1994) and stress-motivated behaviors (Rotzinger et al., 2002). In addition to serving other functions, dopamine-mediated transmission in the mesoaccumbal region has been implicated in the encoding of novel events (Horvitz, 2000), adaptative processing to cope with changing environmental demands (Le Moal and Simon; 1991), and novelty-seeking behavior (Piazza et al., 1991; Piazza & Le Moal, 1998). Although the grade of novelty or familiarity with context-dependent experimental conditions influences the manner CCK mediates dopamine-mediated behaviors (Ladurelle et al., 1995; Hökfelt et al., 2002), only one study gives support to a correlation between the expression of the CCK gene in the tegmental ventral area and novelty-seeking behavior (Lucas et al., 1998).
The term “novelty-seeking” portrays a behavior in the rat characterized by the vigor of the emotional response to a novel, inescapable environment. This trait has relevance in Psychiatry research as it is thought to model some aspects of sensation-seeking behavior in humans (Piazza et al., 1998; Dellu et al., 1996; Cain et al., 2005), a personality trait closely associated with drug abuse and related mental illnesses (Zuckerman & Neeb, 1979). Rats categorized into the high and low ranges of exploration when exposed to an inescapable novel environment (namely HR and LR animals respectively) differ in the rate at which they self-administer low doses of psychotropic drugs (HR rats higher self-administration than LR rats) (Piazza et al., 1989). Most importantly, the HR and LR model is of relevance in understanding the neurobiological bases of emotional responsiveness (Kabbaj, 2004). When compared to LR rats, HR exhibit higher behavioral activation following administration of psychostimulants, self-administer drugs of abuse at higher rates, and exhibit exaggerated emotional and stress responsivity after experiencing stressful, conflicting situations (Dellu et al., 1996; Kabbaj et al., 2001; Kabbaj, 2004). Prior work from our lab suggests the mediation of CCK in the capacity of emotional self-adjustment of HR and LR rats after repetitive anxiety-like training using unconditioned (novelty-based) tests (Ballaz et al., 2007a). These considerations led us to expand our knowledge of the involvement of CCK in novelty-seeking behavior by using a gene expression and behavioral approach. Additionally, we speculated about a putative interplay between the CCK-system and receptors like 5-HT7 and M2 as they contribute to novelty-seeking behavior and behavioral flexibility respectively (Ballaz et al., 2007b; Seeger et al., 2004).
EXPERIMENTAL PROCEDURES
Subjects
The present studies followed the European Communities Council Directive of 24 November 1986 (86/609/EEC) and the National Institute of Health guidelines on laboratory animal use and care (Publication No. 80-23). The University of Michigan Committee on Use and Care of Animals (UCUCA) approved the present study. Adequate measures were taken to minimize the number of animals used in the present study and their suffering. Male Sprague-Dawley rats from Charles River (Wilmington, MA), weighing 250–300 g at the beginning were used. Rats were acclimated in the housing unit for at least ten days before any experimental procedure. They were housed two per cage on a 12 hr light/dark cycle (lights on at 7 am) with access to food and water ad libitum. Stabulation remained unchanged until rats were sacrificed. Behavioral testing was conducted during the light cycle.
Locomotor activity test
Rats were briefly handled by the experimenter for the two days prior to testing to ensure habituation. On the test day, rats were transferred to an adjacent, unfamiliar room where locomotor activity was recorded for one hour. The animals were placed in Plexiglas® cages (40 × 20.5 × 20.5 cm) identical to the home cage (but with a novel grate floor) and were arranged in racks flanked by photo-beam cells to track horizontal displacement and rearing. Testing began when the rat was placed in the activity cage. All movements were processed by a central computer connected to the device using suitable software (Ratmove®, MBNI-University of Michigan, Ann Arbor). Because these measures lie upon a continuum of responses following a normal distribution, rats were assigned to the high responder (HR) group if the locomotor activity score was at least 1 SD above the mean locomotor activity score for each randomly selected subject sample or the low responder (LR) for 1 SD below the mean (Rosario & Abercrombie, 1999). As a result, the HR and LR group of rats were defined as those exhibiting locomotor scores in the upper and lower thirds of the sample, respectively.
In situ hybridization histochemistry (ISH)
To characterize phenotype differences in gene expression, brains were obtained from a cohort of rats tested only for HR-LR categorization and euthanatized two weeks after the locomotor activity test. In a second set of ISH experiments, brain samples were obtained from rats behaviorally tested for one week and sacrificed one day after the last test session. Brains were quickly frozen in n-methylbutane (−30°C) and stored at −80°C. Brains were sectioned on a cryostat at 10 μm (coronal sections), and thaw-mounted on poly-L-Lysine coated slides in 100 μm intervals. Tissue sections were fixed in 4% paraformaldehyde phosphate buffer for 1 hour, washed three times in 2 × SSC (300 mM sodium chloride, and 30 mM sodium citrate buffer pH 7.2), acetylated in 0.1 M triethanolamine buffer (0.1M pH 8.0) supplemented with acetic anhydride (0.25%) for 10 min, rinsed in distilled water, dehydrated in graded alcohol solutions (50–100%, 30s) and subsequently air-dried. Thereafter, tissues were hybridized with 35S-labeled cRNA probes. The following probes were cloned in our lab: CCK (Cck GenBank accession # M10353) a 260 bp sequence coding for the exon III sequence of preproCCK mRNA (nucleotides 210–470) which included the sequence for the translation of the CCK octapeptide c-terminal; and CCK-2R (Cckbr GenBank accession # NN013165) a 721 bp sequence in the coding region (nucleotides 1422–2143), M2 muscarinic acetylcholine receptor (Chrm2 GenBank accession # MN031016) an 835 bp sequence in the coding region (nucleotides 475–1310), 5-hydroxytryptamine (serotonin) receptor 7 (Htr7 GenBank accession # MN022938) was a fragment of 660 bp corresponding to a coding region of the same mRNA (nucleotides 650–1310). 35S-labeled cRNA probe was diluted in 50% hybridization buffer (50% formamide, 10% dextran sulphate, 2× SSC, 50 mM sodium phosphate buffer (pH 7.4), 1× Denhardt’s solution, 0.1 mg/ml yeast tRNA and 30 mM dithiothreitol), 70 μl of diluted probe placed on each slide (2× 106 dpm per four sections-slide) and cover slipped. Slides were placed in plastic trays moistened with 50% formamide and incubated at 55 °C overnight. Slides were rinsed three times in 2× SSC buffer the following day and then incubated in RNAase A solution (200 μg/ml) for 1 hour at 37 °C. Slides were then rinsed in increasingly stringent solutions (2× SSC, 1× SSC, 0.5× SSC) and placed in 0.1× SSC at 70 °C for 1 hour. After a final wash in distilled water, slides were dehydrated in graded alcohol and exposed to Kodak XAR film (Eastman Kodak, Rochester, NY) for a period ranging from 3 days for CCK to 3 weeks for 5-HT7 probes. The specificity of the hybridization signal was confirmed with sense probe controls for CCK, CCK-2R, M2, and 5-HT7.
Image analysis of autoradiograms
Analysis of digital images relied on optical densitometry in the linear range of the gray levels using NIH Scion Image™ (Scion Corporation, Frederick, MA). Digital images were captured from the X-ray Films using a CCD camera (TM-745; Pulnix, Sunnyvale, CA). Optical density measurements were multiplied by outlined area yielding integrated optical density (IOD) as the final unit of radioactive hybridized mRNA signal. Samples collected from each region of interest for each rat were used to obtain optical density measures. These data points were averaged to generate a single value for each rat. Even if a total of eight rats were used per each group, only brain samples located approximately in the same distance from bregma (a minimum of five) were used to estimate group averages.
Enriched Open Field (OF) test
The apparatus was an open field made of white Plexiglas® (base dimensions 1 m2; height 40 cm). Black lines drawn on the floor divided the open field into sixteen virtual quadrants. The room was illuminated with bright white light and the intensity at floor level was 75 lx. An array of different objects made of different materials was used. They were (A) a ceramic cup (6 cm base × 8 cm high), (B) a plastic bottle (4.5 cm base × 12 cm high), (C) a glass bottle (6 cm base × 14 cm high), and (D) a steel cylinder (5 cm base × 5 cm high). Rats received a single test session on every other day across one week. In the first trial (treatment day), HR and LR rats were administered either saline or drug, and 30 min later were exposed to a single object placed in the center of the arena. This first object (A) became the “old object” when paired with a “new” object (B, C, or D) in the following drug-free sessions (see Figure 1). In Trial 2 (D2 post-drug), the old object remained in the centre as a new object (B) occupied one of the corners 10 cm from the edges. In Trial 3 (D4 post-drug), both the old and new object (C) were arranged diagonally in opposite corners 80 cm apart. In the last trial (D6 post-drug), a new object (D) was positioned in the central arena while the old object remained in the corner. Objects and apparatus were thoroughly wiped with 70% ethanol between trials. The following measurements were monitored: interaction time with the new or the old objects, line crossings, and amount of entries into the center.
Figure 1. Enriched Open Field (OF) layout.
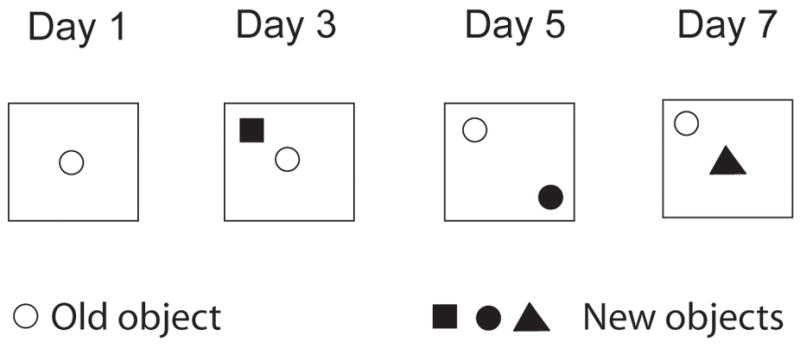
Notice that the empty circle representing the “new object” on Day 1 (D0 post-drug) becomes the “old object” on days 3 (D2 post-drug), 5 (D4 post-drug), and 7 (D6 post-drug), when it is combined with the “new object” (filled in shapes). Injections were given 30 min before the first test session.
Drug administration
The selective CCK-2R antagonist LY225.910 (Suman-Chauhan et al., 1996) was purchased from Tocris (Ellisville, Missouri). Dosage was based on the effective range removing phenotype differences in novelty-seeking behavior in the rat (Ballaz et al., 2007a). It was prepared fresh at 0.5 mg/ml in 0.9% NaCl plus DMSO (5%) and administered 1 ml/Kg via intraperitoneal (IP). A cohort of 32 rats (16 HR and 16 LR) was used in in the enriched OF test. Eight HR and eight LR animals received 0.5 mg/Kg of LY225.910 while eight HR and eight LR rats received vehicle thirty minutes before the first OF trial.
Statistical analysis
One-way or two-way (Treatment × Phenotype) ANOVAs were applied to gene expression data. Exploration in the enriched OF was analyzed using a three-way (Treatment × Phenotype × Trial) ANOVA. Planned contrasts were made using the Fisher’s probable least-squares difference (PLSD) post hoc analyses. An alpha value of 0.05 was used to declare statistical significance. All the analyses were carried out using SPSS® 14 for Windows.
RESULTS
Phenotype differences in CCK-related gene expression (Table 1 and Figure 2)
Table 1.
Individual difference in mRNA levels coding for CCK and CCK-2R.
| GENE | Brain region a | HR, IOD (× 103) | LR, IOD (× 103) | Percent of LR value |
|---|---|---|---|---|
| CCK | PFC | 53.3 ± 3.7 | 42.3 ± 2.4 | + 26 * |
| mPFC | 65.2 ± 4.9 | 47.3 ± 4.8 | + 38 * | |
| RtC | 8.7 ± 1.5 | 4.4 ± 1.0 | + 45 * | |
| Hipp CA1-field | 28.8 ± 4.5 | 16.1 ± 3.3 | + 79 * | |
| Hipp CA3-field | 17.8 ± 2.2 | 11.8 ± 1.4 | + 51 * | |
| DG | 5.3 ± 1.1 | 2.5 ± 0.5 | + 53 * | |
| Bla | 32.9 ± 4.6 | 20.9 ± 3.4 | + 57 * | |
| VTA | 20.6 ± 1.7 | 33.2 ± 3.2 | − 38 ** | |
| SN | 37.1 ± 5.6 | 50.8 ± 5.2 | − 27 | |
| MDTh | 37.1 ± 2.6 | 34.0 ± 2.8 | + 9 | |
|
|
||||
| CCK-2R | PFC | 44.2 ± 3.1 | 56.0 ± 5.3 | − 21 * |
| CCant | 45.7 ± 2.2 | 55.3 ± 2.3 | − 17 * | |
| Pir | 21.3 ± 1.9 | 28.2 ± 2.2 | − 24 * | |
| Hipp CA1-field | 4.5 ± 0.7 | 7.0 ± 1.0 | − 36 | |
| Hipp CA2-field | 1.9 ± 0.3 | 3.4 ± 0.5 | − 44 * | |
| Hipp CA3-field | 14.4 ± 1.7 | 22.0 ± 1.8 | −34 * | |
| DG | 6.9 ± 1.3 | 10.9 ± 1.0 | − 40 * | |
| OT | 11.0 ± 2.1 | 9.7 ± 1.7 | + 13 | |
| NAcc RP | 28.6 ± 3.9 | 44.4 ± 1.1 | − 36 * | |
| NAcc Core | 14.7 ± 8.4 | 19.2 ± 1.7 | −23 * | |
Abbreviations: Bla basolateral amygdala, CCant anterior cingulated cortex, DG denate gyrus, Hipp hippocampus, MDTh mediodorsal thalamus, mPFC medial prefrontal cortex, Nacc core nucleus accumbens core, Nacc RP nucleus accumbens rostral pole, OT olfactory tubercle, Pir piriform cortex, PFC prefrontal cortex, Rtc retrosplenial cortex, SN substantia nigra, and VTA ventral tegmental area. Results are in mean ± S.E.M;
p < 0.05,
p < 0.01; N = 5 – 8.
Figure 2. Phenotype differences in CCK and CCK-2R 35S-mRNA hybridization signal.
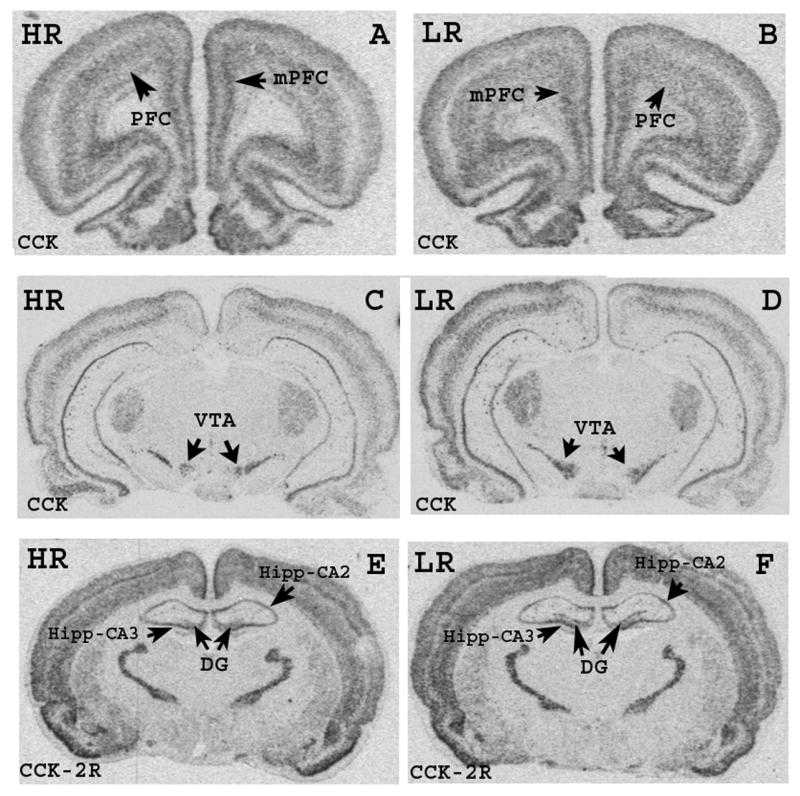
Autoradiograms illustrate 35S-mRNA hybridization signal for CCK (A, B, C and D) and for CCK-2R (E, F) in selected coronal sections of the brain. Abbreviations: DG, dentate gyrus; Hipp, hippocampus; mPFC, medial prefrontal cortex; PFC, prefrontal cortex; VTA, ventral tegmental area.
In HR animals, CCK expression was significantly higher in prefrontal cortex (PFC: F(1,12) = 6.356, p < 0.05), medial prefrontal cortex (mPFC: F(1,13) = 6.665, p < 0.05), retrosplenial cortex (RtC: F(1,11) = 4.881, p < 0.05), hippocampus CA1 subfield (Hipp-CA1: F(1,10) = 5.329, p < 0.05), hippocampus CA3 subfield (Hipp-CA3: F(1,11) = 5.518, p < 0.05), dentate gyrus (DG: F(1,13) = 5.363, p < 0.05), basolateral amygdala (Bla: F(1,10) = 4.687, p < 0.05). In contrast, in the ventral tegmental area (VTA) CCK mRNA signal was enhanced in LR relative to HR rats (F(1,9) = 9.665, p < 0.05). CCK-2R transcript levels were elevated in LR relative to HR in the PFC (F(1,12) = 5.377, p < 0.05), anterior cingulate cortex (CC: (F(1,12) = 8.566, p < 0.05), Hipp-CA2 (F(1,10) = 7.588, p < 0.05), Hipp-CA3 (F(1,10) = 9.023, p < 0.05), DG (F(1,10) = 5.827, p < 0.05), piriform cortex (Pir: F(1,11) = 5.057, p < 0.05), anterior pole of the nucleus accumbens (NAcc: F(1,12) = 8.220, p < 0.05), and NAcc core (F(1,12) = 5.647, p < 0.05).
Exploration in the enriched OF (Figure 3 and Table 2)
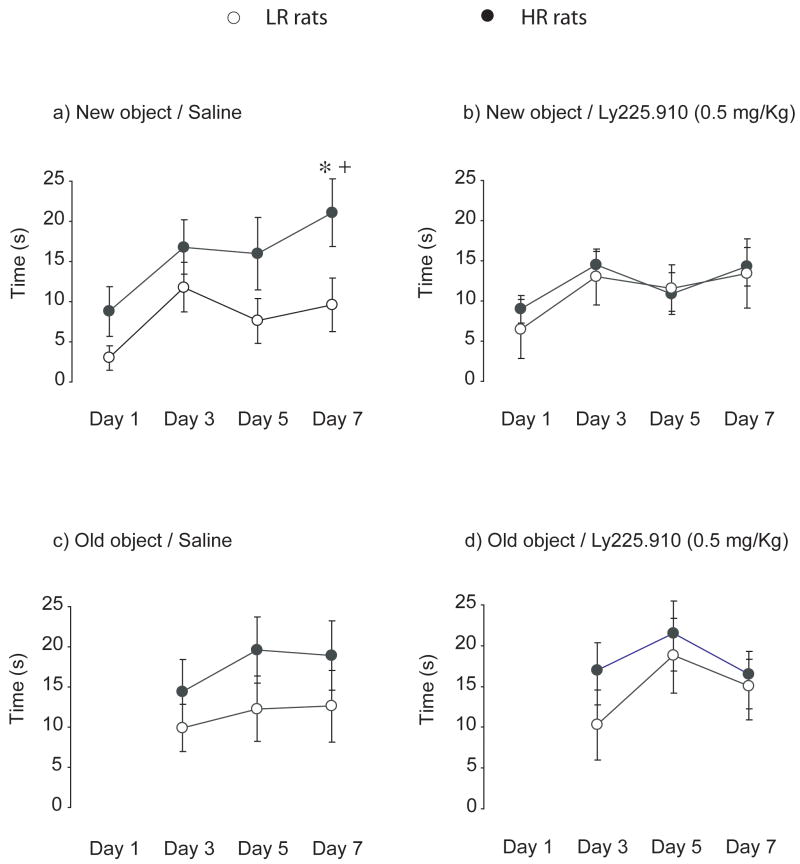
Figure 3. Effects of acute administration of LY225.910 (0.5 mg/Kg) on the sustained object exploration in HR and LR rats exposed to the enriched OF.
Line graphs represent interaction time with each object across the enriched OF sequence in HR (filled in circles) and LR (empty circles) rats. Single administration of LY225.910 was given 30 min before the first trial on day 1. Drug-free test sessions were conducted on day 3 (D2 post-drug), 5 (D4 post-drug), and 7 (D6 post-drug). Results are in mean ± S.E.M; * p < 0.05 compared to LR rats; + p < 0.05 compared to the performance of the same group in Trial 1; N = 8.
Table 2. Effects of LY225.910 (0.5 mg/Kg) on general activity in the OF sequence.
Injections were given 30 min before the first test session on day 1 (D0 post-drug). Drug-free test sessions were conducted on day 3 (D2 post-drug), 5 (D4 post-drug), and 7 (D6 post-drug).Data are in mean ± S.E.M, N = 8.
| Line crossings | Day 1 | Day 3 | Day 5 | Day 7 |
|---|---|---|---|---|
| Vehicle-HR | 54 ± 11 | 67 ± 11 | 53 ± 9 | 66 ± 10 |
| LY225.910-HR | 65 ± 8 | 75 ± 10 | 64 ± 12 | 69 ± 13 |
| Vehicle-LR | 35 ± 9 | 49 ± 11 | 31 ± 9 | 46 ± 11 |
| LY225.910-LR | 36 ± 9 | 41 ± 10 | 44 ± 11 | 54 ± 14 |
| Centre entries | ||||
| Vehicle-HR | 4 ± 2 | 6 ± 1 | 4 ± 1 | 7 ± 2 |
| LY225.910-HR | 4 ± 1 | 7 ± 2 | 4 ± 1 | 8 ± 2 |
| Vehicle-LR | 1 ± 1 | 4 ± 1 | 3 ± 1 | 5 ± 2 |
| LY225.910-LR | 2 ± 1 | 3 ± 1 | 2 ± 1 | 5 ± 2 |
Figure 3 illustrates the time interacting with each object across the enriched OF series. The three-way (Phenotype × Treatment × Trial) ANOVA conducted on the time spent exploring the novel object revealed a significant Phenotype effect (F(1,112) = 7.46, p < 0.01), Trial effect (F(3,112) = 5.00, p < 0.01), and Phenotype × Treatment interaction (F(1,112) = 4.33, p < 0.05). In the last trial in which a new object was positioned in the center of the arena, non-drugged HR rats significantly explored the new object more than non-drugged LR rats (p < 0.05, Figure 3a). In addition, non-drugged HR rats explored the new object in Trial 4 more than they did in Trial 1 (p < 0.01). Prior exposure to the LY225.910 (0.5 mg/Kg) removed all differences (Figure 3b). Main effects and significant interactions were not significant for the interaction time with of the old object (Figure 3c and 3d) in either HR or LR rats. Table 2 illustrates the additional indices of exploration across the OF series. There was an overall Phenotype effect for the amount of line crossings (F(1,112) = 18.31, p < 0.001), as well as an overall Phenotype (F(1,112) = 12.94, p < 0.001) and Trial effect (F(3,112) = 7.02, p < 0.001) for entries into the center. The Phenotype x Treatment interaction did not result in significance for either the number of line crossings (p = 0.6349) or entries into the centre (p = 0.7284).
Differences in 5-HT7 and M2 mRNA in OF-trained HR and LR rats (Figure 4)
Figure 4. Differences in mRNA coding for the 5-HT7 and M2 receptor in the mediodorsal thalamic nuclei of OF-trained HR and LR rats.
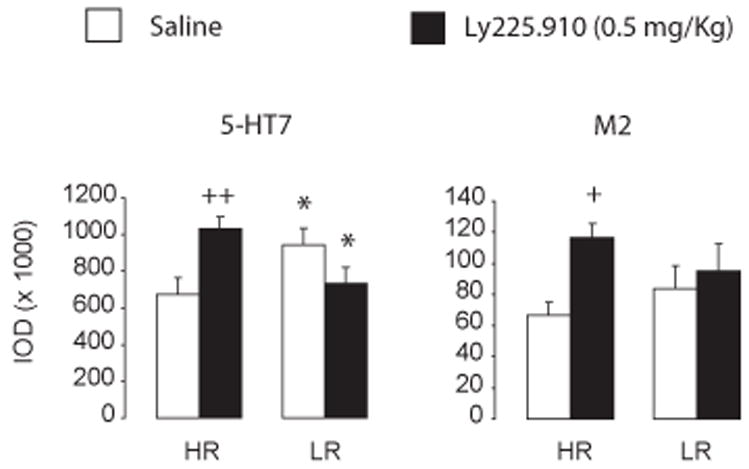
Charts represent mRNA levels as integrated optical density (IOD) units one day after the last OF test session (D7 post-drug). Results are in mean ± S.E.M; * p < 0.05, relative to same-treatment HR group; + p < 0.05; ++ p < 0.01, relative to drug-naïve HR group; N = 5–8.
Figure 4 displays gene expression in the mediodorsal thalamic nuclei one day after the last OF task (D7 post-drug). The sampled area was located approximately in a −1.80 mm distance from bregma and was comprised of the anteroventral, anteromedial, and central medial thalamic nuclei, as well as the anterior paraventricular thalamic nucleus. At this level, the Phenotype × Treatment interaction was significant for the 5-HT7 35S-mRNA hybridization signal (F(1,25) = 10.78, p < 0.01; Fig. 4a). Compared to drug-naïve HR rats, 5-HT7 gene expression was higher in drug-naïve LR rats. In LY225.910-(0.5 mg/Kg)-exposed HR rats, however, 5-HT7 gene expression was significantly higher than LY225.910-(0.5 mg/Kg)-exposed LR rats (p < 0.05) and drug-naïve HR rats (p < 0.01). A Treatment effect was significant for levels of M2 receptor transcript (F(1,28) = 5.23, p < 0.05; Figure 4b). The levels of mRNA coding for M2 in LY225.910-(0.5 mg/Kg)-exposed HR rats were higher than in drug-naïve HR rats (p < 0.05). Intensity of the hybridization signal between drug-naïve HR and LR rats did not differ significantly (p = 0.3906).
DISCUSSION
The main findings of the present study are: (1) CCK gene expression was higher in HR when compared to LR rats in the hippocampus, amygdala, and subregions of the prefrontal cortex (although the inverse was found in the ventral tegmental area) while CCK-2R expression was lower in HR versus LR rats in rostral regions of the forebrain; (2) When compared to LR animals, HR animals exhibited a sustained response to novel visual stimuli after being repeatedly tested in the Open Field across one week. Interestingly, phenotype differences were not observed after acute exposure to the CCK-2R antagonist LY225.910; and (3) HR and LR rats reacted to the antagonist challenge by expressing different levels of M2 and 5-HT7 receptor mRNA in the dorsomedial thalamic nuclei (a region exhibiting important functional interactions with the prefrontal cortex and ventral tegmental area) in a phenotype-dependent manner. These observations suggest that CCK may mediate different capacities of self-adjustment in HR and LR rats as repeatedly exposed to novel visual stimuli.
Relative to LR animals, HR rats showed higher levels of CCK mRNA in specific areas of the cortex. These areas included the hippocampus, basolateral amygdala, retrosplenial cortex, dorsal prefrontal cortex, and anterior cingulate cortex (including mPFC). The ventral tegmental area of the mesencephalon (VTA) was a region where the intensity of the CCK mRNA signal showed a negative correlation with environmentally induced exploration (i.e., LR higher than HR animals). This finding was in agreement with that reported elsewhere (Lucas et al., 1998). Gene expression of CCK-2R in the nucleus accumbens of HR animals was lower than that of LR animals. The combination of low levels of expression of CCK-2R gene expression in the nucleus accumbens and reduced CCK transcript levels in the VTA likely contributes to down-regulation of the mesoaccumbal CCK-afferents in HR relative to LR rats. Because adjustments of dopamine neurotransmission are essential to appropriately interact with the environment (Le Moal & Simon, 1991) and CCK modulates them depending on the grade of novelty (Ladurelle et al., 1995), it is suggested that this specific pattern of gene expression of CCK-related molecules relates to salience of environmental novelty. Expression of the CCK-2R gene in the cortex, hippocampus, and dentate gyrus were negatively related to environmentally-induced novel exploration. This agreed with other studies demonstrating that general exploration is negatively associated with brain levels of this receptor (Harro & Vasar, 1991; Matto et al., 1997; Dauge et al., 2001). The hippocampus is one of the areas where over-expression of CCK-2R contributes to the onset of anxiety (Wang et al., 2003). Activation of CCK-2R in the hippocampus causes excitation of pyramidal neurons by releasing excitatory amino acids (Breukel et al., 1997), an effect that is antagonized by benzodiazepines (Bradwejn & de Montigny, 1984). Interestingly, deletion of the CCK-2R gene produces specific adaptive disturbances (Noble & Roques, 2002) reminiscent of the exploratory pattern exhibited by HR rats.
Considering the experimental approach used in the study, we cannot assert that mRNA expression denotes levels of endogenous ligand and all functional receptors, but a differential function of the CCK-ergic system. In order to establish a causative link of the CCK-related gene expression with novelty-seeking behavior, effects of acute CCK-2R receptor antagonism on sustained exploration of various new and old objects were investigated in HR and LR rats submitted to a sequence of enriched OF tests. Routine exposure to assorted new and old objects revealed subtle but significant phenotype differences, as drug-naïve HR rats showed sustained exploration of novel objects more consistently than drug-naïve LR rats. These differences were not observed in rats exposed to LY225.910. There is evidence that CCK-2R antagonists administered to naïve un-habituated rats decreases exploration, suggesting that CCK under these circumstances is released endogenously to increase exploration (Hokfelt et al., 2002). This is comparable to the overall decrease in novel-object exploration observed with LY225.910 in our HR sample given that the period of time rats were tested (5 min every other day) was not enough for rats to adapt to the experimental setting and that object localizations (i.e., spatial components of the task) were not constant across all test sessions. In light of the inverse pattern of differences in the expression of CCK between the VTA and the forebrain in HR and LR rats, responses to novel visual stimuli may be related to a distinct CCK-ergic function. Indeed, baselines of novel-object exploration in drug-exposed rats changed in opposite directions following acute blockade of the CCK-2R receptor. Given the pattern of CCK-2R gene expression in HR and LR rats, synthesis of CCK-2R receptor (as a compensatory mechanism of the antagonist effects) may occur in drug-exposed HR rats at a higher rate than in drug-exposed LR rats. This may account for HR rats not showing increased interest for the novel-object across the OF sequence. Drug-induced change in CCK-2R receptors deserves further investigation. Carry-over effects of acute LY225.910 likely denoted impaired learning (Dauge and Lena, 1998; Sebret et al., 1999). In this case, drug would alter the course of gene expression long after administration as learning brings about changes in gene expression.
The mediodorsal thalamic nuclei was of interest as it interacts with the VTA in a cooperative manner to modulate the activity of prefrontal cortex neurons (Floresco and Grace, 2003). The mediodorsal thalamic nuclei also acts as a critical link between basal forebrain and the prefrontal cortex (Krettek and Price, 1977). Whereas this cortex is relevant to novelty detection (Dias et al., 2002), exposure of rats to unfamiliar environments results in metabolic activation of mediodorsal thalamic nuclei (Sarter et al., 1989). Additionally, some evidence suggests that rats with mediodorsal thalamic damage show impaired learning (Beracochea et al., 1989, Harrigan et al., 1991). Among many others participating in learning processes, M2 (Seeger et al., 2004) and 5-HT7 (Meneses, 2004) receptors were selected because their mRNA is densely expressed in the mediodorsal thalamic nuclei (Ballaz et al., 2007b; Vilaro et al., 1992). No other attempts to examine further differences in gene expression were made since it was beyond the scope of the present study. Given the complex experimental design, gene expression data reported here should be considered as illustrative examples of the phenotype-dependent effects of the antagonist rather than as a mechanistic insight into them.
Levels of 5-HT7 transcript in drug-naïve HR rats were significantly lower than drug-naïve LR rats thus replicating our prior findings (Ballaz et al., 2007b). Most importantly, this pattern was the opposite to that of drug-exposed animals. Given the influence of 5-HT7 in emotion (Hedlund et al., 2005) and learning (Meneses, 2004), the collapse of differences in the behavioral baseline of HR and LR rats after experience with the drug could be attributable to the inverse pattern of 5-HT7 gene expression. These findings warrant further investigation about a putative interaction between the 5-HT7 receptor and CCK-ergic system. Prior experience with the drug increased M2 receptor gene expression specifically in HR rats. This finding is not surprising as there exists evidence of the mediation of acetylcholine muscarinic receptors in the down-stream mechanisms activated by CCK when triggering anxiety (Biró et al., 1997). An increased expression of M2 may be related to the habituation to novel visual stimuli observed in HR rats, since lack of its function produces impaired behavioral flexibility (Seeger et al., 2004). As a result of the exposure to LY225.920, gene expression was altered as a function of phenotype, thereby leading to a collapse of the phenotype differences once the drug was cleared. Yet puzzling, carry-over effects of LY225.910 give support to the notion that a differential CCK-ergic function may be critical for keeping the pattern of novelty-seeking behavior in HR and LR rats.
In summary, basal expression of CCK-related genes and long-term effects of the CCK-2R receptor antagonist on sustained exploration of novel stimuli denote a contribution of CCK to the way in which environmental experience biases emotional responsiveness in HR and LR rats. The knowledge gained from this study gives support to the notion that CCK-ergic function may predispose organisms to readily react and adapt in specific ways to arousing changes in their environment. It also may in the future help to explain the controversies in the CCK-hypothesis of anxiety.
Acknowledgments
Grants source disclosure: This work was supported by NIMH (PO1 MH42251 to Stanley Watson), NIDA (5RO1 DA013386 to Huda Akil), Office of Naval Research (ONR) N00014-02-1-0879 to HA and SJW, and the Pritzker Neuropsychiatry Disorders Research Consortium
We thank Tracy Simmons for revising this manuscript. We would also like to acknowledge the technical assistance of Sharon Burke.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abelson JL. Cholecystokinin in psychiatric research: a time for cautious excitement. J Psychiat Res. 1995;29:389–96. doi: 10.1016/0022-3956(95)00027-3. [DOI] [PubMed] [Google Scholar]
- Ballaz S, Akil H, Watson SJ. The CCK-system mediates adaptation to novelty-induced stress in the rat: A pharmacological evidence. Neurosci Lett. 2007a;428:27–32. doi: 10.1016/j.neulet.2007.09.035. [DOI] [PubMed] [Google Scholar]
- Ballaz S, Akil H, Watson SJ. Analysis of 5-HT6 and 5-HT7 receptor gene expression in rats showing differences in novelty-seeking behavior. Neuroscience. 2007b;147:428–38. doi: 10.1016/j.neuroscience.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Becker C, Zeau B, Rivat C, Blugeot A, Hamon M, Benoliel JJ. Repeat social defeat-induced depression-like behavioral and biological alterations in rats: involvement of cholecystokynin. Mol Psychiat. 2007 doi: 10.1016/sj.mp.4002097. [DOI] [PubMed] [Google Scholar]
- Beracochea DJ, Jaffard R, Jarrad LE. Effects of anterior or dorsomedial thalamic ibotenic lesions on learning and memory in rats. Behav Neural Biol. 1989;51:364–376. doi: 10.1016/s0163-1047(89)91000-5. [DOI] [PubMed] [Google Scholar]
- Biró E, Penke B, Teledgy G. Role of different neurotransmitter systems in the cholecystokinin octapeptide-induced anxiogenic response in rats. Neuropeptides. 1997;31:281–285. doi: 10.1016/s0143-4179(97)90060-3. [DOI] [PubMed] [Google Scholar]
- Bradwejn J, de Montigny C. Benzodiazepines antagonize cholecystokinin-induced activation of rat hippocampal neurones. Nature. 1984;312:363–364. doi: 10.1038/312363a0. [DOI] [PubMed] [Google Scholar]
- Bradwejn J, Koszycki D, Shriqui C. Enhanced sensitivity to cholecystokinin tetrapeptide in panic disorder. Clinical and behavioral findings Arch Gen Psychiat. 1991;48:603–610. doi: 10.1001/archpsyc.1991.01810310021005. [DOI] [PubMed] [Google Scholar]
- Breukel AI, Lopes da Silva FH, Ghijsen WE. Cholecystokinin (CCK-8) modulates vesicular release of excitatory amino acids in rat hippocampal nerve endings. Neurosci Lett. 1997;234:67–70. doi: 10.1016/s0304-3940(97)00678-2. [DOI] [PubMed] [Google Scholar]
- Cain ME, Saucier DA, Bardo MT. Novelty seeking and drug use: contribution of an animal model. Exp Clin Psychopharmacol. 2005;13:367–375. doi: 10.1037/1064-1297.13.4.367. [DOI] [PubMed] [Google Scholar]
- Chen Q, Nakajima A, Meacham C, Tang YP. Elevated cholecystokininergic tone constitutes an important molecular/neuronal mechanism for the expression of anxiety in the mouse. Proc Natl Acad Sci U S A. 2006;103:3881–3886. doi: 10.1073/pnas.0505407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Cholecystokinin modulates dopamine-mediated behaviors. Differential actions in medial posterior versus anterior nucleus accumbens Ann N Y Acad Sci. 1994;713:138–142. doi: 10.1111/j.1749-6632.1994.tb44060.x. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Corwin RL. Biological actions of cholecystokinin. Peptides. 1994;15:731–755. doi: 10.1016/0196-9781(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Dauge V, Lena I. CCK in anxiety and cognitive processes. Neurosci Biobehav Rev. 1998;22:815–825. doi: 10.1016/s0149-7634(98)00011-6. [DOI] [PubMed] [Google Scholar]
- Dauge V, Sebret A, Beslot F, Matsui T, Roques BP. Behavioral profile of CCK2 receptor-deficient mice. Neuropsychopharmacology. 2001;25:690–698. doi: 10.1016/S0893-133X(01)00291-3. [DOI] [PubMed] [Google Scholar]
- Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. Novelty-seeking in rats--biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34:136–145. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- Dias R, Honey RC. Involvement of the rat medial prefrontal cortex in novelty detection. Behav Neurosci. 2002;116:498–503. doi: 10.1037//0735-7044.116.3.498. [DOI] [PubMed] [Google Scholar]
- Farook JM, Zhu YZ, Wang H, Moochhala S, Lee L, Wong PT. Strain differences in freezing behavior of PVG hooded and Sprague-Dawley rats: differential cortical expression of cholecystokinin2 receptors. Neuroreport. 2001;12:2717–2720. doi: 10.1097/00001756-200108280-00025. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Grace AA. Gating of hippocampal-evoked activity in prefrontal cortical neurons by inputs from the mediodorsal thalamus and ventral tegmental area. J Neurosci. 2003;23:3930–3943. doi: 10.1523/JNEUROSCI.23-09-03930.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G. Is there a future for neuropeptide receptor ligands in the treatment of anxiety disorders? Pharmacol Ther. 1999;82:1–61. doi: 10.1016/s0163-7258(98)00041-2. [DOI] [PubMed] [Google Scholar]
- Harrigan T, Predery O, Persinger M. Radial Maze learning deficits and mediodorsal thalamic damage in context of multifocal seizure-induced brain lesions. Behav Neurosci. 1991;105:482–486. doi: 10.1037//0735-7044.105.3.482. [DOI] [PubMed] [Google Scholar]
- Harro J, Vasar E. Evidence that CCKB receptors mediate the regulation of exploratory behaviour in the rat. Eur J Pharmacol. 1991;193:379–81. doi: 10.1016/0014-2999(91)90156-k. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Huitron-Resendiz S, Henriksen SJ, Sutcliffe JG. 5-HT7 receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biol Psychiat. 2005;58:831–837. doi: 10.1016/j.biopsych.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Sills TL, Tomkins DM, Sellers EM, Vaccarino FJ. Evidence for the contribution of CCKB receptor mechanisms to individual differences in amphetamine-iduced locomotion. Pharmacol Biochem Behav. 1994;48:1019–1024. doi: 10.1016/0091-3057(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Blacker D, Broberger C, Herrera-Marschitz M, Snyder G, Fisone G, Cortes R, Morino P, You ZB, Ogren SO. Some aspects on the anatomy and function of central cholecystokinin systems. Pharmacol Toxicol. 2002;91:382–386. doi: 10.1034/j.1600-0773.2002.910617.x. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20:6983–6988. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbaj M. Neurobiological basis of individual differences in emotional and stress responsiveness. Arch Neurol. 2004;61:1009–1012. doi: 10.1001/archneur.61.7.1009. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol. 1977;171:157–192. doi: 10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- Ladurelle N, Roques BP, Dauge V. The transfer of rats from a familiar to a novel environment prolongs the increase of extracellular dopamine efflux induced by CCK8 in the posterior nucleus accumbens. J Neurosci. 1995;15:3118–3127. doi: 10.1523/JNEUROSCI.15-04-03118.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moal M, Simon H. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol Rev. 1991;71:155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- Lucas LR, Angulo JA, Le Moal M, McEwen BS, Piazza PV. Neurochemical characterization of individual vulnerability to addictive drugs in rats. Eur J Neurosci. 1998;10:3153–3163. doi: 10.1046/j.1460-9568.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- Matto V, Harro J, Allikmets L. The effects of cholecystokinin A and B receptor antagonists on exploratory behaviour in the elevated zero-maze in rat. Neuropharmacology. 1997;36:389–396. doi: 10.1016/s0028-3908(97)00011-7. [DOI] [PubMed] [Google Scholar]
- Meneses A. Effects of the 5-HT7 receptor antagonists SB-269970 and DR 4004 in autoshaping Pavlovian/instrumental learning task. Behav Brain Res. 2004;155:275–282. doi: 10.1016/j.bbr.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Noble F, Roques BP. Phenotypes of mice with invalidation of cholecystokinin (CCK(1) or CCK(2)) receptors. Neuropeptides. 2002;36:157–170. doi: 10.1054/npep.2002.0904. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Rouge-Pont F, Deminiere JM, Kharoubi M, Le Moal M, Simon H. Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Res. 1991;567:169–174. doi: 10.1016/0006-8993(91)91452-7. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Rosario LA, Abercrombie ED. Individual differences in behavioral reactivity: correlation with stress-induced norepinephrine efflux in the hippocampus of Sprague-Dawley rats. Brain Res Bull. 1999;48:595–602. doi: 10.1016/s0361-9230(99)00040-4. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bodewitz G, Steckler T. 2-[3H]deoxyglucose uptake patterns in rats exploring a six-arm radial tunnel maze: differenecs between experienced and nonexperienced rats. Behav Neurosci. 1989;103:1217–25. doi: 10.1037//0735-7044.103.6.1217. [DOI] [PubMed] [Google Scholar]
- Sebret A, Lena I, Crete D, Matsui T, Roques BP, Dauge V. Rat hippocampal neurons are critically involved in physiological improvement of memory processes induced by cholecystokinin-B receptor stimulation. J Neurosci. 1999;19:7230–7237. doi: 10.1523/JNEUROSCI.19-16-07230.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger T, Fedorova I, Zheng F, Miyakawa T, Koustova E, Gomeza J, Basile AS, Alzheimer C, Wess J. M2 muscarinic acetylcholine receptor knock-out mice show deficits in behavioral flexibility, working memory, and hippocampal plasticity. J Neurosci. 2004;24:10117–10127. doi: 10.1523/JNEUROSCI.3581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suman-Chauhan N, Meecham KG, Webdale L, Hunter JC, Pritchard MC, Woodruff GN, Hill DR. The influence of guanyl nucleotide on agonist and antagonist affinity at guinea-pig CCK-B/gastrin receptors: binding studies using [3H]PD140376. Regul Pept. 1996;65:37–43. doi: 10.1016/0167-0115(96)00070-5. [DOI] [PubMed] [Google Scholar]
- van Megen HJ, Westenberg HG, den Boer JA, Kahn RS. Cholecystokinin in anxiety. Eur Neuropsychopharmacol. 1996;6:263–280. doi: 10.1016/s0924-977x(96)00038-7. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhu YZ, Farook JM, Moochhala S, Teo AL, Lee LK, Wong PT. Genetic variations in CCK2 receptor in PVG hooded and Sprague-Dawley rats and its mRNA expression on cat exposure. Behav Neurosci. 2003;117:385–390. doi: 10.1037/0735-7044.117.2.385. [DOI] [PubMed] [Google Scholar]
- Vilaro MT, Wiederhold KH, Palacios JM, Mengod G. Muscarinic M2 receptor mRNA expression and receptor binding in cholinergic and non-cholinergic cells in the rat brain: a correlative study using in situ hybridization histochemistry and receptor autoradiography. Neuroscience. 1992;47:367–693. doi: 10.1016/0306-4522(92)90253-x. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Neeb M. Sensation seeking and psychopathology. Psychiatry Res. 1979;1:255–264. doi: 10.1016/0165-1781(79)90007-6. [DOI] [PubMed] [Google Scholar]