Abstract
The interactions between predator diversity and primary consumer abundance can include direct effects and indirect, cascading effects. Understanding these effects on immature Anopheles mosquitoes is important in sub-Saharan Africa, where most cases of malaria occur. Aquatic predators and immature mosquitoes were collected from shallow pools of varying age previously excavated by brickmakers in the western highlands of Kenya. Path analysis showed an indirect negative effect of habitat age on An. gambiae (Giles, 1902) mediated by effects on predator diversity. Disturbance resets habitats to an earlier successional stage, diminishing predator diversity and increasing An. gambiae populations. The increase in vector abundance as a result of reduced predator diversity highlights the public health value in conserving native insect diversity.
Keywords: trophic cascades, diversity, Anopheles gambiae, malaria, Kenya
Diversity cascades are the indirect interactions between diversity and other community metrics across trophic levels. The effect of predator diversity on primary consumers (i.e., top-down diversity cascades, sensu Dyer 2008) varies depending on the food web being studied, but it is clear that biodiversity can have significant population-level effects on disease vectors (Ostfeld and Keesing 2000). For example, incidence of Lyme disease is positively correlated with species richness of good disease hosts, such as birds, but the densities of effective arthropod vectors of Lyme disease are negatively correlated with the species richness of small mammals, which vary widely in their ability to carry the disease. Increases in predator diversity can promote herbivore abundance by increasing intraguild predation and omnivory among predators (Hochberg 1996, Denoth et al. 2002), further showing that diversity at one trophic level can act to enhance or constrain populations at other levels. Alternately, if specialist predators are present, increased predator diversity may reduce the abundance of a particular prey species. In such a case, as predator diversity increases within a habitat, the probability of specialist predators being present also increases (Stireman et al. 2004). Several studies of top-down diversity cascades have been published by terrestrial ecologists (Dyer and Letourneau 2003, Dyer and Stireman 2003, Schmitz 2004, Pearson and Dyer 2006, Dyer 2008, Pearson et al. 2008), whereas the effect of predator diversity on the aquatic food webs that are dominated by immature mosquitoes has not been examined in detail, despite the large number of single-predator studies performed in such systems (Lacey and Orr 1994).
Enhancement or conservation of aquatic predator species richness may be an effective supplement to existing malaria control programs, particularly in Africa where a substantial majority of the world’s estimated 515 million malaria cases occur (Snow et al. 2005). Malaria epidemics in the African highlands have generated a great deal of political and scientific interest, and an ongoing effort to determine why malaria has spread to these previously malaria-free locations (Malakooti et al. 1998, Hay et al. 2002) and to establish methods for preventing these epidemics from recurring (Delacollette 2002, Abeku 2007). Surveys of aquatic habitats in the highlands of western Kenya have suggested that altered land use plays an important ecological role in determining the distribution of mosquito larvae (Carlson et al. 2004, Omlin et al. 2007, Howard and Omlin 2008); in particular, in many highland valleys, the excavation of clay for the large-scale production of bricks has generated hundreds of shallow pools that support high densities of immature Anopheles gambiae (Giles, 1902) but very few predator species (Carlson et al. 2004). In this paper, An. gambiae is sensu Giles, 1902, referring to a cryptic species complex, frequently denoted as An. gambiae s.l. for studies in which polymerase chain reaction (PCR) identification of the sibling species was not accomplished. Members of this complex are the primary malaria vectors in sub-Saharan Africa. Similarly, An. funestus is used sensu Giles, 1900; members of this cryptic species complex are secondary vectors of malaria in sub-Saharan Africa.
This study examined simple aquatic food webs in the same system described above: shallow brickmaking pools in the highlands of western Kenya. The following general hypotheses were tested:
Predator diversity and prey abundance will be positively associated with the length of time since a habitat was last disturbed (hereafter referred to as habitat age) caused by successional changes in habitat quality.
Habitat age affects An. gambiae abundance both directly, through discriminating oviposition behavior, and indirectly, through direct and indirect effects on predator diversity.
Materials and Methods
Mosquito larvae and aquatic predators were collected from shallow pools of varying ages, and path analysis was used to test hypothesized causal relationships between habitat age and predator diversity and mosquito abundance.
Study System
This study was conducted in Itumbe Valley, Kisii District, one of many similar valleys in the densely populated highlands of western Kenya. Itumbe Valley is the site of intensive brick production, for which clay is excavated and processed in shallow water-filled pits. These habitats were linked to proliferation of An. gambiae after a 2002 malaria epidemic in the region (Carlson et al. 2004).
Field Methods
One hundred brick-making pits were evaluated in April 2003. Abundance of immature mosquitoes was obtained by standard dipping method, averaging the yields of five dips per pit (Service 1993). Identification of immature mosquitoes was constrained by field conditions; larvae were identified as An. gambiae, An. funestus, or belonging to the culicine “competitor” mosquito genera. Macroscopic predators were qualitatively sampled by aquatic sweep net for a duration of ~5 min in each pit. Invertebrate predators were later separated into distinct morpho-species, and voucher specimens were preserved in ethanol for subsequent taxonomic identification. To establish habitat age, brick-makers were questioned regarding the date when each pit had last been used for clay extraction or processing.
Conceptual Model
Models incorporating Anopheles abundance began soon after the discovery was made that malaria was spread by mosquitoes (Ross 1911). As the complexity of the models has increased, there has been a tendency to examine the variables involved in producing adult mosquitoes separately from the interactions between the adult mosquitoes, humans, and Plasmodium parasites. The most comprehensive model available that describes adult Anopheles production hypothesizes that the effects of predation and competition are important variables affecting Anopheles larvae (Depinay et al. 2004).
In addition to the direct effects of predation and competition on Anopheles larvae, it is likely that there are important interactions within and between populations of predators and competitors. In this regard, the abundance of competitors may have an additional indirect effect on Anopheles larvae by altering the abundance, diversity, or feeding habits of predators (Chesson 1989). Like other dispersing insects, An. gambiae mosquitoes use various combinations of visual (Huang et al. 2005) and olfactory (Sumba et al. 2008) cues to select high-quality aquatic habitats for oviposition. Some of these cues are associated with biological succession of the habitat, such as the extent of microbial growth (Huang et al. 2006) and the presence of top predators (Munga et al. 2006). Thus, successional stage of a habitat is expected to play an important role in determining abundance of An. gambiae larvae directly by affecting oviposition behavior, and indirectly by affecting colonization rates of predators and competitor mosquitoes. The hypothesized relationships among these latent variables— habitat disturbance, predator diversity, competitor abundance, and vector abundance—and the ultimate production of adult vector mosquitoes were delineated in a conceptual model (Fig. 1), guided by previous structural equation models of diversity cascades in agricultural systems and grasslands (Dyer and Stireman 2003, Pearson and Dyer 2006).
Fig. 1.
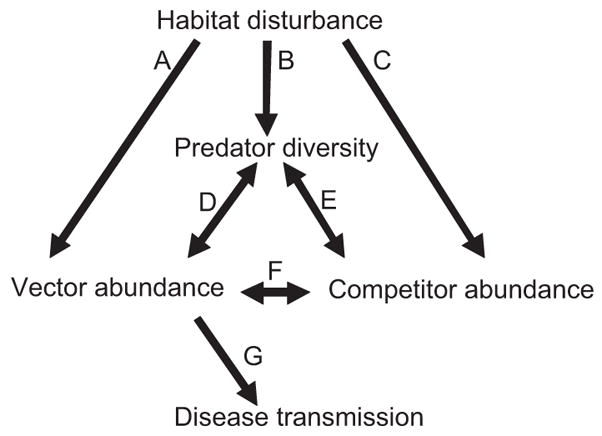
Conceptual model showing a hypothesized set of interactions in aquatic ecosystems that could combine to influence malaria transmission. Habitat condition affects immature vector abundance directly (A) by influencing oviposition behavior of gravid female Anopheles mosquitoes. Habitat similarly affects colonization by predators (B) and competitors (C), although the magnitude and sign of the effect may be inconsistent. The interactions between predator diversity and vector abundance (D) may be mitigated or enhanced by additional interactions between predators and competitors (E) and by density-dependent interactions between vectors and competitors (F). Direct and indirect effects on vector abundance ultimately influence the more well-established relationship between vector abundance and malaria transmission (G).
Statistical Analyses
To conform to the prerequisites for multiple regression analysis, all variables were tested and, with the exception of predator diversity, log transformed to meet assumptions of normality and homogeneity of variance. Pathway coefficients were generated using a series of multiple regressions using the CALIS procedure (SAS Institute 1989). These standardized coefficients, representing the relative magnitude of the relationships, are presented on a path diagram (Fig. 2).
Fig. 2.
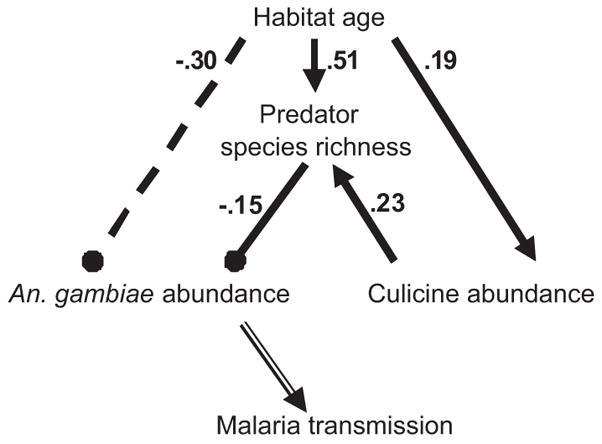
This causal pathway diagram is statistically supported by field data (i.e., no significant differences between the correlational matrix and that predicted by the causal model; χ2 = 0.88, df = 1, P = 0.35). Lines ending in arrowheads indicate a positive effect; lines ending in circles indicate a negative effect. Solid lines represent direct effects, whereas the dashed line shows an indirect effect. Only the relationships with statistically significant path coefficients (α = 0.05) are shown in the diagram, with the exception of the relationship between An. gambiae abundance and malaria transmission (indicated with a double lined arrow), but was not measured in this study. Habitat age had a direct positive effect on both predator diversity and abundance of culicine mosquito larvae but no detectable direct effect on Anopheles abundance. Habitat age also indirectly promoted predator diversity thorugh a direct enhancement of culicine larvae (alternative prey), which in turn acted to enhance predator colonization. The direct and indirect increases in predator diversity caused by habitat age resulted in an overall reduction of An. gambiae larvae, overwhelming any potential positive effect of habitat age on vector abundance.
Results
Mosquito dips from the sampled pits yielded an average of 8.0 ± 0.95 An. gambiae larvae and 5.7 ± 0.72 culicine larvae. A smaller number of An. funestus (1.8 ± 0.32) larvae were also collected. Across all pools, there were 28 predator morphospecies collected, representing 21 genera and 12 families (Table 1). The most common predators were Hemiptera (12 morpho spp.), including Hynesionella (Gerridae) and Anisops (Notonectidae), the only two genera found in >50% of the sampled pools. The next most common were Coleoptera, in particular the nine morphospecies of Dytiscidae, which were present in some combination in 52% of habitats. Mean species richness of predators for all pools was 5.0 ± 0.25. The average time since last disturbance of pools was 16.4 ± 2.8 mo, ranging from 0 (actively disturbed) to 13 yr since disturbance.
Table 1.
Insect predators collected from 100 brickmaking pits in Itumbe Valley, Kenya
| Order | Family | Genus | % habitats colonized |
|---|---|---|---|
| Hemiptera | Gerridae | Hynesionella | 66 |
| Limnogonus | 5 | ||
| Hydrometridae | Hydrometra | 12 | |
| Veliidae | Rhagovelia | 40 | |
| Notonectidae | Anisops | 68 | |
| Enithares | 5 | ||
| Pleidae | Plea | 12 | |
| Naucoridae | Laccocoris | 3 | |
| Nepidae | Ranatra | 8 | |
| Laccotrephes sp 1 | 23 | ||
| Laccotrephes sp 2 | 18 | ||
| Nepa | 2 | ||
| Odonata | Lestidae | Lestes | 32 |
| Coenagrionidae | Enallagma | 25 | |
| Libellulidae | Palpopleura | 20 | |
| Orthetrum | 16 | ||
| Coleoptera | Hydrophilidae | Hydrochara | 3 |
| Dytiscidae | Laccophilini sp. 1 | 32 | |
| Laccophilini sp. 2 | 12 | ||
| Laccophilini sp. 3 | 9 | ||
| Laccophilini sp. 4 | 2 | ||
| Laccophilini sp. 5 | 2 | ||
| Laccophilini sp. 6 | 1 | ||
| Copelatus | 5 | ||
| Cybister | 7 | ||
| Hydaticus | 3 |
Path analysis showed the following direct and indirect interactions: (1) strong positive effects of habitat age on predator diversity and on abundance of culicine competitors; (2) strong negative effects of predator diversity on An. gambiae abundance; and (3) a strong indirect negative effect of habitat age on An. gambiae abundance (Fig. 2). Our data support the hypothesis that habitat age both directly and indirectly increased predator diversity, which in turn depressed An. gambiae abundance; the model was supported by the data (χ2 = 0.88, df = 1, P = 0.35) and the path coefficients were significant (P < 0.05).
Discussion
Changes to predator diversity of any magnitude, even the loss of a single species, can have major impacts on ecosystems and on infectious disease risk (Keesing et al. 2006). This study showed the important role of aquatic predator diversity in suppressing local populations of the predominant malaria vector. Predator diversity was markedly diminished by habitat disturbance and recovered as biological succession of the habitat progressed over time. Part of this effect is attributable to the reduction of competitor mosquito populations, which may serve as an alternate food source for predators. In recently disturbed habitats, the reduction in predator diversity was associated with greater abundance of An. gambiae, the primary malaria vector in Africa. Ecological disturbance resulting from altered land use in highland swamps is further implicated as a possible cause for the puzzling increase in highland malaria (Minakawa et al. 2006).
In contrast to insecticidal control, biological control by a diverse predator assemblage is likely to persist over time (Ostfeld and Holt 2004). For example, Shililu et al. (2003) compared the efficacy against Anopheles larvae of the organophosphate temephos and two biolarvicides (Bacillus thuringiensis isrealensis and B. sphaericus) in a variety of aquatic habitats in Eritrea. For all three larvicides, mosquitoes were almost entirely eliminated from standing streambed pools in the days immediately after treatment, but within 14 d after treatment, mosquito densities were equivalent to untreated habitats (mean of 15.36 larvae per pool). Although vector control through use of larvicides is possible in aquatic habitats, it requires weekly evaluation and/or treatment (Fillinger and Lindsay 2006). Because competitor abundance has a positive effect on predator diversity, larvicidal elimination of non-target organisms (including other Dipteran larvae) may indirectly promote a resurgence of An. gambiae larval populations even when predators are not harmed by the treatment itself.
In this study, it is clear that diversity of aquatic predators is important for suppressing An. gambiae larval abundance and is associated with habitats that have remained undisturbed for an extended period of time. Although larval abundance is only one factor influencing subsequent vector-biting rate and malaria transmission, reductions in malaria cases have been observed after large-scale implementation of larval control (reviewed by Walker and Lynch 2007). This study found statistical support for a strong interaction between predator diversity and An. gambiae abundance, indicated by the magnitude of the path coefficient (−0.15, from Fig. 2); in the context of the study design, each additional predator species in a brick-making pit reduced the number of An. gambiae by approximately one larva per dip sample.
This study did not incorporate the rapidly diminishing areas of undisturbed wetlands in the Kenyan highlands, wherein the diversity of predators is expected to prohibit establishment of substantial An. gambiae populations. The benefits of ecosystem services have been cited as a compelling incentive for the preservation of biodiversity in tropical areas (Daily et al. 1997, Keesing et al. 2006). The local paucity of predator species after human disturbance is linked with the proliferation of malaria-vectoring mosquitoes in the mountains of western Kenya, implicitly showing the value of natural enemy diversity to human health.
Acknowledgments
Funding for this research was provided by the Government of Finland through Grant 24811201 and BioVision Switzerland. Travel expenses for J.C.C. were provided through NIH ICIDR Grant U19 AI45511 and financial support was provided through CDC fellowship training Grant CCT 622308-02.
References Cited
- Abeku TA. Response to malaria epidemics in Africa. Emerg Infect Dis. 2007;13:681–686. doi: 10.3201/eid1305.061333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JC, Byrd BD, Omlin FX. Field assessments in western Kenya link malaria vectors to environmentally disturbed habitats during the dry season. BMC Public Health. 2004;4:33. doi: 10.1186/1471-2458-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesson J. The effect of alternate prey on the functional response of Notonecta hoffmani. J Vector Ecol. 1989;29:277–284. [Google Scholar]
- Daily GC, Alexander S, Ehrlich PR, Goulder L, Lubchenco J, Matson PA, Mooney HA, Postel S, Schneider SH, Tilman D, Woodwell GM. Ecosystem services: benefis supplied to human societies by natural ecosytems. Issues Ecol. 1997;2:1–16. [Google Scholar]
- Delacollette C, editor. Prevention and control of malaria epidemics. World Health Organization/Roll Back Malaria; Geneva, Switzerland: 2002. [Google Scholar]
- Denoth M, Frid L, Myers JH. Multiple agents in biological control: improving the odds? Biol Control. 2002;24:20–30. [Google Scholar]
- Depinay JM, Mbogo CM, Killeen G, Knols B, Beier J, Carlson J, Dushoff J, Billingsley P, Mwambi H, Githure J, Toure AM, McKenzie FE. A simulation model of African Anopheles ecology and population dynamics for the analysis of malaria transmission. Malar J. 2004;3:29. doi: 10.1186/1475-2875-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer LA. The ecology of tri-trophic interactions in the tropics. In: Carson WP, Schnitzer SA, editors. Tropical forest community ecology. Blackwell; Oxford, United Kingdom: 2008. pp. 275–293. [Google Scholar]
- Dyer LA, Letourneau D. Top-down and bottom-up diversity cascades in detrital vs. living food webs. Ecol Lett. 2003;6:60–68. [Google Scholar]
- Dyer LA, Stireman JO. Community-wide trophic cascades and other indirect interactions in an agricultural community. Basic Appl Ecol. 2003;4:423–432. [Google Scholar]
- Fillinger U, Lindsay SW. Suppression of exposure to malaria vectors by an order of magnitude using microbial larvicides in rural Kenya. Trop Med Int Health. 2006;11:1629–1642. doi: 10.1111/j.1365-3156.2006.01733.x. [DOI] [PubMed] [Google Scholar]
- Hay SI, Cox J, Rogers DJ, Randolph SE, Stern DI, Shanks DG. Climate change and the resurgence of malaria in the East African highlands. Nature (Lond) 2002;415:905–909. doi: 10.1038/415905a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg ME. Consequences for host population levels of increasing natural enemy species richness in classical biological control. Am Nat. 1996;147:307–318. [Google Scholar]
- Howard AF, Omlin FX. Abandoning small-scale fish farming in western Kenya leads to higher malaria vector abundance. Acta Trop. 2008;105:67–73. doi: 10.1016/j.actatropica.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Huang J, Walker ED, Giroux PY, Vulule J, Miller JR. Ovipositional site selection by Anopheles gambiae: influences of substrate moisture and texture. Med Vet Entomol. 2005;19:442–450. doi: 10.1111/j.1365-2915.2005.00588.x. [DOI] [PubMed] [Google Scholar]
- Huang J, Miller JR, Chen SC, Vulule JM, Walker ED. Anopheles gambiae (Diptera: Culicidae) oviposition in response to agarose media and cultured bacterial volatiles. J Med Entomol. 2006;43:498–504. doi: 10.1603/0022-2585(2006)43[498:agdcoi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Keesing F, Holt RD, Ostfeld RS. Effects of species diversity on disease risk. Ecol Lett. 2006;9:485–498. doi: 10.1111/j.1461-0248.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- Lacey LA, Orr BK. The role of biological control of mosquitoes in integrated vector control. Am J Trop Med Hyg. 1994;50:97–115. doi: 10.4269/ajtmh.1994.50.97. [DOI] [PubMed] [Google Scholar]
- Malakooti MA, Biomndo K, Shanks GD. Re-emergence of epidemic malaria in the highlands of western Kenya. Emerg Infect Dis. 1998;4:671–676. doi: 10.3201/eid0404.980422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakawa N, Omukunda E, Zhou G, Githeko A, Yan G. Malaria vector productivity in relation to the highland environment in Kenya. Am J Trop Med Hyg. 2006;75:448–453. [PubMed] [Google Scholar]
- Munga S, Minakawa N, Zhou G, Barrack OO, Githeko AK, Yan G. Effects of larval competitors and predators on oviposition site selection of Anopheles gambiae sensu stricto. J Med Entomol. 2006;43:221–224. doi: 10.1603/0022-2585(2006)043[0221:eolcap]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Omlin FX, Carlson JC, Ogbunugafor CB, Hassanali A. Anopheles gambiae exploits the treehole ecosystem in western Kenya: a new urban malaria risk? Am J Trop Med Hyg. 2007;77:264–269. [PubMed] [Google Scholar]
- Ostfeld RS, Keesing F. Biodiversity and disease risk: the case of Lyme disease. Conserv Biol. 2000;14:722–728. [Google Scholar]
- Ostfeld RS, Holt RD. Are predators good for your health? Evaluating evidence for top-down regulation of zoonotic disease reservoirs. Front Ecol Environ. 2004;2:13–20. [Google Scholar]
- Pearson CV, Dyer LA. Trophic diversity in two grassland ecosystems. J Insect Sci. 2006;6:23. doi: 10.1673/2006_06_25.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson CV, Massad TJ, Dyer LA. Diversity cascades in alfalfa fields: from plant quality to agroecosystem diversity. Environ Entomol. 2008;37:947–955. doi: 10.1603/0046-225x(2008)37[947:dciaff]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ross R. The prevention of malaria. John Murray; London, United Kingdom: 1911. [Google Scholar]
- SAS Institute. SAS user’s guide: statistics. SAS Institute; Cary, NC: 1989. [Google Scholar]
- Schmitz OJ. Perturbation and abrupt shift in trophic control of biodiversity and productivity. Ecol Lett. 2004;7:403–409. [Google Scholar]
- Service MW. Mosquito ecology: field sampling methods. 2. Elsevier Applied Science; New York: 1993. [Google Scholar]
- Shililu JI, Tewolde GM, Brantly E, Githure JI, Mbogo CM, Beier JC, Fusco R, Novak RJ. Efficacy of Bacillus thuringiensis israelensis, Bacillus sphaericus, and temephos for managing Anopheles larvae in Eritrea. J Am Mosq Cont Assoc. 2003;19:251–258. [PubMed] [Google Scholar]
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature (Lond) 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stireman JO, Dyer LA, Matlock RB. Top-down forces in managed versus unmanaged habitats. In: Barbosa P, Castellanos I, editors. Ecology of predator-prey interactions. Oxford University Press; Oxford, United Kingdom: 2004. pp. 303–323. [Google Scholar]
- Sumba LA, Ogbunugafor CB, Deng AL, Hassanali A. Regulation of oviposition in Anopheles gambiae s.s.: role of inter- and intra-specific signals. J Chem Ecol. 2008;34:1430–1436. doi: 10.1007/s10886-008-9549-5. [DOI] [PubMed] [Google Scholar]
- Walker K, Lynch M. Contributions of Anopheles larval control to malaria suppression in tropical Africa: review of achievements and potential. Med Vet Entomol. 2007;21:2–21. doi: 10.1111/j.1365-2915.2007.00674.x. [DOI] [PubMed] [Google Scholar]


