Abstract
Cognitive dysfunction is a common feature of schizophrenia and deficits are present before the onset of psychosis, and are moderate to severe by the time of the first episode. Controversy exists over the course of cognitive dysfunction after the first episode. This study examined age-associated differences in performance on clinical neuropsychological (NP) and information processing tasks in a sample of geriatric community living schizophrenia patients (n=172). Compared to healthy control subjects (n=70), people with schizophrenia did not differ on NP tests across age groups but showed evidence for age-associated cognitive worsening on the more complex components of an information-processing test. Age-related changes in cognitive function in schizophrenia may be a function of both the course of illness and the processing demands of the cognitive measure of interest. Tests with fixed difficulty, such as clinical NP tests, may differ in their sensitivity from tests for which parametric difficulty manipulations can be performed.
Keywords: Schizophrenia, Neuropsychology, Cognition, Information Processing, Aging
1.1 Introduction
The question of whether cognitive impairments in schizophrenia are static or dynamic is fundamental to addressing the debate over neurodegenerative hypotheses regarding the illness. If the cognitive impairments detected at or prior to the first episode of illness remain stable throughout the lifetime course of illness relative to healthy comparison (HC) samples, including changes associated with healthy aging, then this finding would argue against such degenerative processes. However, if schizophrenia patients were to demonstrate a decline in cognitive functioning over the life-span in excess of that observed with normal aging, this type of course could be consistent with degenerative features such as Alzheimer’s Disease and Huntington’s Disease. Neurodevelopmental models need not be considered exclusive of neurodegenerative processes if both developmental and degenerative processes occur at different times in the life course. It is likely, for example, that adverse neurodevelopmental events make individuals more vulnerable to age-related cognitive decline.
To date, limited neuropsychological batteries and the failure to include truly older patients living outside of institutional settings have made studies regarding the course of cognitive functioning in schizophrenia have been difficult to compare. Two cross-sectional studies of ambulatory patients found no evidence for age-related cognitive decline in IQ (Mockler et al., 1997) or a global measure of dementia severity (Eyler Zorrila et al., 2000). In contrast, several cross-sectional studies of chronically institutionalized “poor-outcome” schizophrenia patients suggest worsening of cognitive impairment over the lifespan. For example, Davidson et al. (1995) found differences in scores on the Mini Mental State Examination across adjacent 10-year age-groups ranging from 25 to 95, with a resulting difference in performance of over 15 points when the 25–35 year olds were compared to those over 85. Similar findings were reported by Arnold et al. (1995). An additional cross-sectional analysis of a larger sample with an age range extending to also 95 years found evidence for age-related differences in cognitive performance across 22 items from standardized neuropsychological (NP) tests designed for use in the elderly (Harvey et al., 1995).
Using a comprehensive NP battery and longitudinal methodology, Heaton and colleagues (2001) found no evidence for cognitive decline regardless of age, illness variables, or length of follow-up. The outpatients in this study, however, were relatively young, with a mean age of 47.6 (SD=15.7). Using patients drawn from the same sample of institutionalized patients referenced above (Davidson et al, 1995; Harvey et al, 1995), longitudinal follow-up studies suggested an easily detected worsening in cognition over follow-up intervals of 2.5 (Harvey et al., 1999) and 6 (Friedman et al., 2001) years, with risk for worsening increasing only after the age of 65 (Friedman et al., 2001). These cognitive declines predicted subsequent functional declines (Harvey et al., 1999; Friedman et al., 2001) and patients could be classified at a baseline assessment into cognitive subtypes that predicted stability versus decline in cognitive and functional status over a 6-year follow-up (Bowie et al., 2004). Further, a 10-year epidemiological follow-up study of first admission patients who were living in the community found significant declines in visual spatial skills and visual memory over that period, with those cognitive declines also related to functional declines (Stirling et al., 2003). Results suggesting cognitive decline over the lifespan are consistent with the well-replicated finding of continuing ventricular enlargement over the lifespan in schizophrenia (see DeLisi et al., 2006 for a review).
One of the clear methodological concerns with most of these studies of truly geriatric patients is that most of them had an atypical course of illness characterized by long-term institutionalization, which might not generalize to the majority of patients who have a less chronic course (e.g., medication-responsive patients living in the community and receiving outpatient treatment). Additionally, due to the substantial cognitive impairments found in the majority of these chronic patients, the cognitive assessments were limited to less challenging tasks that would have less likelihood of a floor effect, precluding the use of more complex measures that would place high demands on information processing capacity or executive functions.
In a cross sectional study using a measure of complex information processing, the span of apprehension task, in ambulatory patients sampled from the same research site as Heaton et al. (2001), Granholm and colleagues (2000) found age-related declines in the ability to manage information processing loads. Similar cross-sectional differences in cognitive performance were reported in executive functioning and working memory, but not verbal skills, memory, attention, or perceptual motor functions (Fucetola et al., 2000) in a sample of older ambulatory patients. Taken together, these findings suggest that subtle changes in cognitive functions may occur at various points of the illness. One hypothesis that could be advanced is that traditional neuropsychological instruments, while sensitive to schizophrenia and to aging in healthy people, may not be sensitive to small, but still potentially detectable, declines in an already impaired population. The performance of schizophrenia patients may be in a range that is less sensitive to subsequent declines. The results of the Granholm et al. study suggest that tests that examine components of information processing that can be manipulated parametrically may be sensitive to changes not detected by traditional NP tests.
Deficiencies in information processing capacity resources are widely reported in the schizophrenia spectrum. Schizophrenia patients deteriorate more than healthy individuals when asked to divide their attentional focus (Granholm et al., 1991; Serper et al., 1990; Harvey et al., 2000) or ignore distracting information (Harvey et al., 1990). Similar results have also been reported in patients with schizotypal personality disorder (SPD; Moriarty et al., 2003; Harvey, et al., 2006). These impairments appear related to reductions in processing capacity resources, rather than strategic failures, as these studies have shown that patients with schizophrenia and SPD use similar strategies to those employed by healthy individuals. Further, psychophysiological indices of processing capacity reveal processing limitations in schizophrenia patients at load levels that are minimally demanding to healthy individuals (Granholm et al., 1997) and changes in the synchronization of cortical activation during working memory operations at load levels that HC can manage without deficit (Callicott et al., 1999; 2000).
1.2 Purpose
The present study is a cross-sectional examination of cognitive performance in a community-dwelling older sample of schizophrenia patients. This study has several methodological advantages, including the assessment of a large sample of truly older (age 50 to 85) patients with schizophrenia and a demographically similar group of healthy control subjects on a clinical NP assessment battery and on an information processing task that features varying levels of cognitive processing demands. Thus, in contrast to earlier studies, a generally representative sample of ambulatory schizophrenia patients, a wider age range, and a greater variability in the specific content of cognitive assessments were all employed. We hypothesized 1) that tasks that were sensitive to aging in general (processing speed and episodic memory) would be sensitive to the impairments seen in older people with schizophrenia, detecting age-related changes in the two samples even in the absence of group by aging interactions, and 2) that our information processing task would detect group by aging interactions (reflecting greater deficits on the part of older people with schizophrenia) in the condition with greater information processing demands. The basis for this second hypothesis comes from previous studies that suggest age-related differences in complex information processing tasks (Granholm et al, 2000; Fucetola et al, 2000).
2.1 Method
2.1.1 Subjects
Subjects were a community dwelling sample of schizophrenia patients (SZ; n=172) and demographically similar healthy comparison subjects (HC; n=70) selected from a longitudinal study of the course of cognitive and functional status in schizophrenia outpatients. All patients were in outpatient treatment at the time of recruitment at a VA, New York State, or academic clinical research site. Outpatient status was defined as living outside of an institutional setting (hospitals or nursing homes). Study subjects were also required to have evidence of continued illness at the time of recruitment, as evidenced by meeting at least one of three criteria: 1) an inpatient admission for psychosis in the past two years; 2) an emergency room visit for psychosis in the past two years; or 3) a score on the Positive and Negative Syndrome Scale items delusions, hallucinations, or conceptual disorganization of 4 (moderate) or more at the time of their baseline assessment.
Healthy comparison groups were recruited from flyers, newspaper advertisements, and referral from other research projects within the institution. It was required that they could not meet criteria for any DSM-IV Axis I disorder.
For both the patient and the healthy comparison group, exclusion criteria included active substance abuse, concurrent neurological conditions, medical conditions known to affect cognitive functions, or other Axis I psychiatric disorders. All participants were interviewed with the Comprehensive Assessment of History and Symptoms (Andreasen et al, 1992) and diagnoses were confirmed with a senior clinician. All participants were between the ages of 50 and 85, fluent in English, and had at least 8 years of education.
2.2.1 Measures
Subjects completed testing in a fixed order. A comprehensive NP battery was used. For this study, performance between the groups was compared on the clinical NP variables and by examining performance on the information processing measure across two levels of complexity. We also examined an estimate of premorbid intellectual functioning, the Wide Range Achievement Test Third Edition (WRAT-3; Wilkinson, 1993) Recognition Reading score. The clinical NP variables were total correct words over the five learning trials and delayed recall from the Rey Auditory Verbal Learning Test (RAVLT; Schmidt, 1996), number of categories attained from the 64-Card Computer version of the Wisconsin Card Sorting Test (WCST; Heaton et al., 1993), total correct responses on Phonological Fluency (FAS; Spreen and Strauss, 1998), total correct responses on Category Fluency (Animal Naming; Spreen and Strauss, 1998), total score on Letter Number Sequencing (LNS) from the Wechsler Adult Intelligence Scale (Psychological Corporation, 1998), total time to completion on parts A and B of the Trail-Making Test (TMT; Reitan and Wolfson, 1993) and the interference score from the Stroop Color and Word Test (Golden and Freshwater, 2002).
Assessments of two levels of processing demands were conducted with the Digit Span Distraction test (DSD; Oltmanns and Neale, 1975). This is a two-part test in which the subject must repeat lists of digits read by a female voice alone (Non-Distraction Condition) or with a male voice interspersed between digits (Distraction Condition). Seven trials per condition are presented, with 5 target digits for distraction trials and six for the nondistraction trials. The distraction condition is a more cognitively complex task as it requires selective attention to the targeted female voice while ignoring the male voice. Importantly, the two conditions of this test are matched a priori for factors affecting discriminating power in healthy individuals (most importantly true score variance). Thus, although the tasks differ in complexity, the two subtasks have been shown repeatedly to be equally difficult for healthy controls in several different studies (See Harvey et al., 1990 for a review), with performance deficits on the distraction condition as compared to the nondistraction condition consistently found in people with schizophrenia.
Patients were also administered the Positive and Negative Syndrome Scale (PANSS; Kay, 1991). To rate this scale, the examiner conducts a chart review, interviews a clinician, and conducts a structured interview, then rates on a 7-point behaviorally anchored scale (absent to severe) the presence and severity of 30-items. We examined the positive and negative factors derived from an empirically-based five-factor model (White et al., 1997).
2.3.1 Data Analysis
Both subject groups (Healthy Control and Patient) were further classified into one of three age groups: 50–59, 60–69, 70–85.
Age- and group-associated NP performance was examined with Univariate Analysis of Covariance (ANOVA) tests with age group and subject group (Patients and Healthy Controls) as the between-subjects factors and estimated premorbid intellectual functioning (WRAT Reading) entered as the covariate the alpha level for the analyses of the 10 neuropsychological variables was adjusted using the Bonferroni correction to 0.005.
Repeated measures analysis of variance (rANOVA) tests were used to examine differences in information processing on the DSD with sample (Patients and Healthy Controls) and age group as the between subjects factors and test condition (Distraction and Nondistraction) as the within subject factor.
3.1.1 Results
172 Outpatient (SZ) and 70 Healthy Comparison (HC) subjects participated in this study. The HC group had significantly more years of education and higher estimated premorbid IQ (i.e., based on WRAT-III reading subtest scores), although there were no significant group by age-group interactions. Both years of education and estimated IQ were entered as covariates in the analyses. The SZ group had more male subjects overall, but not in the oldest age group. Therefore, regression analyses were performed for each of the NP and information processing variables to create standardized residual scores that were adjusted for gender. See Table 1 for demographic characteristics of the groups.
Table 1.
Demographic Data for Healthy Control and Schizophrenia Patients (Mean (SD))
| Schizophrenia Outpatients | Healthy Controls | Group | Age*Group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 50–59 | 60–69 | 70–85 | 50–59 | 60–69 | 70–85 | F | p-value | F | p-value | |
| N | 113 | 38 | 21 | 21 | 18 | 31 | --- | --- | --- | --- |
| Age | 54.4 (2.7) | 63.3 (2.7) | 76.6 (4.5) | 54.0 (2.7) | 64.1 (2.4) | 76.8 (5.1) | 2.3 | .12 | 2.0 | .14 |
| WRAT Reading | 44.5 (7.8) | 45.7 (9.3) | 44.1 (9.7) | 47.4 (5.9) | 51.1 (5.5) | 50.1 (4.1) | 15.7 | <.001 | .75 | .47 |
| Years of Education | 12.6 (2.3) | 12.1 (5.0) | 12.6 (1.8) | 14.2 (2.0) | 14.2 (2.5) | 14.5 (2.4) | 18.0 | <.001 | .06 | .94 |
| Gender*Group | Gender*Age | |||||||||
| χ2 | p-value | χ2 | p-value | |||||||
| % Male | 75.2 | 65.8 | 76.2 | 28.6 | 44.4 | 61.3 | 15.1 | <.001 | 1.4 | .48 |
3.1.2 Age × Group effects on Clinical Neuropsychological Variables
Test results and Univariate significance tests are presented in Table 2. No age-associated differences were found in the schizophrenia patients for any symptom variable. ANOVAs revealed significantly poorer performance by the SZ group across all of the NP measures (i.e., a statistically significant effect of diagnosis) except the Stroop Interference score, but no group by age-group interaction for any of the NP variables other than for Trail-Making Test Part A. Age-group differences were observed for all variables other than WCST categories, phonological fluency, and RAVLT delayed recall, but there were no group by age-group interactions on any of these variables.
Table 2.
Correlations with Age and Univariate Analysis of Covariance Tests between Age Groups on Traditional Neuropsychological Variables and Symptoms for Schizophrenia Outpatient and Healthy Control Subjects.
| Correlation with Age Pearson’s r (p-value) | Age Group Mean Raw Score(SD) | F (p-value)1 | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | 50–59 | 60–69 | 70–85 | Group | Age | Group × Age | ||
| PANSS Positive Symptom Factor2 |
SZ | −.12 (.13) | 3.8 (.65) | 3.8 (.80) | 3.8 (.69) | 1.1 (.32) | ||
| PANSS Negative Symptom Factor2 |
SZ | −.11 (.18) | 3.0 (.76) | 2.8 (.88) | 2.9 (.95) | .03 (.97) | ||
| WCST 64 Card Categories |
SZ | −.18 (.02) | 1.2 (1.4) | 1.0 (.99) | 0.62 (1.0) | 8.0 (.003) | 3.9 (.02) | .42 (.65) |
| HC | −.09 (.12) | 2.2 (1.6) | 2.1 (1.6) | 1.7 (1.4) | ||||
| Digit Symbol | SZ | −.42 (<.001) | 39.7 (15.4) | 35.8 (15.8) | 23.5 (16.7) | 47.2 (<.001) | 13.1 (<.001) | .62 (.54) |
| HC | −.38 (.001) | 60.5 (17.1) | 60.8 (17.2) | 47.6 (15.3) | ||||
| Letter Number Sequencing |
SZ | −.24 (.001) | 6.5 (2.7) | 6.8 (3.4) | 4.3 (3.5) | 26.1 (<.001) | 8.1 (<.001) | .15 (.85) |
| HC | −.36 (.002) | 10.3 (3.4) | 10.3 (3.7) | 8.2 (2.4) | ||||
| Phonological Fluency |
SZ | −.19 (.02) | 28.3 (10.9) | 29.1 (11.5) | 23.9 (12.1) | 30.3 (<001) | 3.6 (.69) | .86 (.42) |
| HC | −02 (.88) | 39.9 (13.4) | 46.6 (13.6) | 42.5 (13.3) | ||||
| Category Fluency | SZ | − 31(<.001) | 16.6 (5.3) | 15.5 (4.5) | 11.7 (5.5) | 5.9 (.01) | 7.2 (.001) | 1.2 (.29) |
| HC | −.20 (.10) | 18.6 (4.5) | 20.5 (7.1) | 16.9 (5.2) | ||||
| Stroop Interference |
SZ | −.07(.36) | −3.6 (7.5) | −4.0 (8.1) | 4.5 (9.1) | .07 (.78) | 5.9 (.003) | 1.6 (.20) |
| HC | −.40 (.001) | −77 (7.1) | −2.2 (6.6) | −7.9 (9.3) | ||||
| TMT part A Total Time3 |
SZ | .46 (<.001) | 64.2 (31.0) | 70.3 (34.8) | 110.6 (57.2) | 41.7 (<.001) | 18.9 (<.001) | 7.8 (<.001) |
| HC | .25 (.04) | 34.3 (12.4) | 46.8 (28.4) | 45.5 (15.2) | ||||
| TMT part B Total Time3 |
SZ | .32 (.001) | 149.1 (61.5) | 162.4 (52.9) | 199.8 (51.9) | 19.7 (<.001) | 7.3 (.001) | .54 (.58) |
| HC | .14 (.24) | 87.5 (46.7) | 100.1 (56.3) | 102.9 (48.1) | ||||
| RAVLT Learning | SZ | −.29 (<.001) | 34.2 (10.1) | 36.4 (11.5) | 23.9 (7.9) | 22.1 (<.001) | 6.5 (.002) | .96 (.38) |
| HC | −.18 (.12) | 44.5 (10.3) | 45.3 (11.3) | 40.9 (11.3) | ||||
| RAVLT Recall | SZ | −.19 (.01) | 6.0 (3.3) | 6.2 (3.3) | 4.1 (2.7) | 8.1 (.005) | 1.9 (.14) | .58 (.55) |
| HC | −.14 (.25) | 8.2 (2.4) | 8.6 (4.0) | 7.7 (2.9) | ||||
alpha level for the 10 neuropsychological tests was adjusted with the Bonferroni correction to 0.005. Significant effects surviving this correction are italicized. The ANCOVAs were performed with education-adjusted standardized residual scores with group (healthy control and participant) and age band as between-subject effects and estimated premorbid intellectual functioning (WRAT Reading score) as the covariate.
Positive and Negative Syndrome Scale positive and negative factors (White et al.,1997 ) expressed in terms of mean item scores
higher scores indicate poorer performance
3.1.3 Repeated Measures Effects on Information Processing
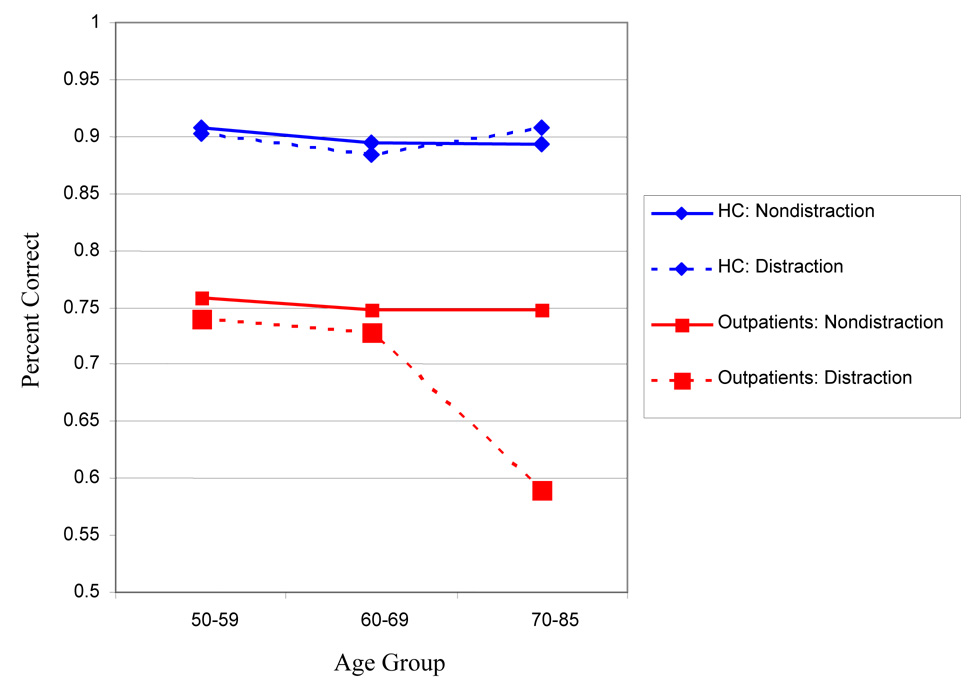
The rANOVA for the Digit Span Distraction Test resulted in a significant 2-way interaction of , for Test Condition by Age-Group, F(2,221)=5.2, p=.006. The test condition by Subject Group was not significant F(1,221)=0.52, p=.47. The three-way interaction of Test Condition by Subject Group by Age-Group was significant, F(2,221)=4.4, p=.01, with the SZ, but not HC, showing age related poorer performance on the distraction condition, and both groups showing no significant age-associated differences during the nondistraction condition. Simple effects tests showed that the schizophrenia patients showed a significant age effect on the distraction condition that was not seen in the healthy controls, which was driven by poorer performance in the patients over age 70. See Figure 1 for a graphical representation.
Figure 1.
Digit Span Distraction Test Performance by Group (Schizophrenia Outpatients and Healthy Control (HC)) and Age Group
3.1.4 Linearity Tests
Linearity effects were examined for the two variables that had significant group × age-group interactions. The SZ group, but not the HC group, demonstrated linear age-associated effects on the Trail-Making Test, Part A (SZ: F[1,168]=21.2, p<.001; HC: F[1,69]=2.8, p=.07) and the Distraction condition of the Digit Span Distraction Test (SZ: F[1,165]=5.5, p=.02; HC: F[2,69]=.06, p=.80).
3.1.5 Correlational Analyses
In order to ensure that designating patients into age groups was not biasing the results of the analyses by artificially trichotimizing groups by age, we examined age as a continuous variable. Pearson product-moment correlations were run on raw scores on age and test performance, separately in the two groups. As can be seen in Table 2, significant correlations between raw scores for performance and age were found for the schizophrenia patients on all variables other than Stroop interference and for the HC sample on digit symbol, letter number sequencing, and Stroop interference measures.
4.1.1 Discussion
There are several findings regarding age-associated differences in cognitive impairment from this study. Traditional NP tests were consistently sensitive to the diagnosis of schizophrenia and to age, but, other than Trail-Making Part A, they did not detect differential effects of aging in schizophrenic as compared to healthy individuals. The differential effects of aging in schizophrenia and HC subjects on trail-making part A is likely due to the combination of two factors: reduced sensitivity to age effects in the HC sample, as evidenced by a smaller correlation with age in the compared to other processing speed measures such as WAIS digit symbol and better performance on the part of the younger schizophrenia patients, leaving substantial room for age-associated differences. Thus, the interaction of age group × patient sample is not necessarily due to substantially greater change on the part of patients Similar to previous findings, a test designed to manipulate information processing capacity demands detected significant reductions in performance on the part of the oldest patients with schizophrenia in the cognitively complex condition, compared to the HC group. The test that detected these differences remained matched for difficulty across the two conditions and across the entire age range of the sample of HC subjects. Performance in the nondistraction condition also did not differ with age in the patients with schizophrenia. Thus, the poorer performance in the distraction condition with aging in schizophrenia meets criteria (Chapman and Chapman, 1973) for a differential deficit: not present in HC subjects and different across conditions only in the older members of the schizophrenia sample. In contrast to these age-associated differences in challenging conditions on the information processing task, differential age-associated differences in performance were not found in traditional measures of verbal fluency, reasoning and problem solving, verbal learning or memory, naming, inhibitory processing, or verbal working memory, when compared to age-related differences in a healthy comparison group. These cognitive differences also appear to be independent of clinical symptoms, as we found no evidence for age-associated changes in positive or negative symptoms. While we did not replicate previous reports of differential age-associated differences in performance on complex executive functioning tests (i.e., the WCST), the relatively low performance scores of the HC group may have contributed to our inability to find differences. In contrast to the 128-card version of the WCST employed by Fucetola et al. (2000), we used the 64-card version. While this version of the test is associated with high rates of completion by our elderly schizophrenia patients, it does not induce as much variance in performance, which may explain why it was not sensitive to age effects in either sample, in contrast to most of the other NP tests and other reports with the 128-card version.
4.1.2 Limitations
There are several limitations of this study. First, the present study is limited by its cross-sectional methodology, which introduces the possibility of cohort effects and precludes any definitive conclusions about true long term changes. The recruitment design of this research project, specifically selecting outpatients with an indication of active illness, raises the possibility that these findings might not generalize to other individuals with schizophrenia who were either recovered or institutionalized. Longitudinal analyses of geriatric patients will be needed to evaluate true decline in specific ability areas. Poor performance by the HC sample on the short version of the WCST limited our ability to replicate the Fucetola et al. (2000) results. Sampling biases may confound cross-sectional comparisons. One might expect more difficulty recruiting schizophrenia patients who are living in the community but still relatively low functioning. However, the present results suggest age-associated deficits can be detected in information processing in spite of this potential recruitment bias. Finally, our conclusions that age-associated differences in information processing might be detected in higher functioning schizophrenia patients as they age requires not only support from longitudinal follow-up, but the use of a broader range of tests that allow for parametric variation in processing capacity.
4.1.3 Implications
Despite these limitations, our results may provide insight into previous contradictory conclusions about the course of cognition in schizophrenia. Substantial research has shown that tasks that allow for parametric manipulations of difficulty levels, such as is possible with experimental neuroscience tests, such as the Digit Span Distraction Test and the N-back working memory test, can clarify patterns of differences between healthy controls and people with schizophrenia. For instance, changing processing demands on the n-back test lead to performance changes that could be interpreted as influencing the sensitivity of the test to diagnosis. Asking HC and schizophrenic patients to perform a 1-back version of the test leads to high levels of performance across both samples and low levels of diagnostic sensitivity, while 3 or 4-back performance leads to uniformly low performance across samples and equally poor diagnostic sensitivity. The 2-back condition is differentially challenging to HC and schizophrenia patients, leading to diagnostic differences in performance accompanied by interpretable patterns of brain activation (Calicott et al., 1999; 2000). In our study, the nondistraction condition of the test was not sensitive to age-related changes in performance in either sample (despite diagnostic differences in performance), but the distraction condition was particularly challenging for the oldest patients with schizophrenia. Thus, the parametric increase in processing load associated with adding selective attention demands apparently crossed a capacity threshold for a subset of the schizophrenia patients, much like engaging in minor manipulations of working memory load in healthy older individuals can lead to changes in performance that are greater than in younger subjects (Mitchell et al., 2000).
Tasks requiring divided attention and dual-task processing have demonstrated relationships with the functioning of the prefrontal cortex (Barch, Sheline, Csernasky, & Snyder, 2003; Perlstein, Dixit, Carter, Noll, & Cohen, 2003; Schlosser et al., 2003; Stevens et al, 1998; Rypma, Berger, & Depositor, .2002). There is a potential neurobiological substrate of these information processing abnormalities in the frontal cortex. Evidence from neuroimaging studies shows progressive volume reductions in frontal lobes in people with schizophrenia (Gur et al, 1998; Siegel et al, 1994; Mathalon, Sullivan, Lim, & Pfefferbaum, 2001). Compared to healthy individuals, schizophrenia patients have a small overall reduction in whole brain white matter volume (Cannon et al, 1998) and in areas implicated in the cognitive dysfunction observed in schizophrenia, including the volume (Buchanan, Vladar, Barta, & Pearlson, 1998; Sigmudsson et al., 2001; Brier et al., 1992), integrity (Foong et al, 2001) and coherence of tracts (Buchsbaum et al., 1998) in the prefrontal cortex, and volume (Mitelman et al., 2003) , integrity (Foong et al., 2000; 2001) and coherence of tracts (Lim et al., 1999) of the temporal lobes. Further, longitudinal investigations have found reductions in frontal white matter volume after the first episode of illness to be correlated with executive functioning deficits (Ho et al., 2003). Consequences of white matter abnormalities might manifest in complex information processing tasks but not in other measures, such as declarative memory or simple attention span. White matter diseases such as multiple sclerosis (MS) are associated with deficient performance on complex cognitive tasks, particularly those that assess processing speed (Beatty, Goodkin, Monson, Beatty, Hertsgaard, 1988). Similar to the present age-associated findings with schizophrenia patients, MS patients demonstrate impairment on complex measures of attentional processing despite normal performance on simple immediate span (Rao, Leo, Bernardin, & Unverzagt, 1991).
Age-associated declines can be difficult to demonstrate in illnesses such as schizophrenia, where cognitive impairment is already substantial at early ages on many measures. Tracking changes over time therefore requires not simply repeating tests, but examining performance on tests of varying levels of difficulty and manipulating levels of difficulty within qualitatively similar task demands. Thus, a more feasible approach for future studies aimed at detection of cognitive impairments, even in younger patients than those in this study, might be to use tests with multiple levels of complexity, such as the N-Back or tests with dynamic titration that adjust the difficulty level to within-session performance or within-subject over time.
Acknowledgement
The authors thank Hannah Anderson, Kushik Jaga, and Brooke Halpern for assistance with data collection and technical aspects of the report preparation.
Role of Funding Source.
This research was supported by NIMH Grant Number MH 63116 to Dr. Harvey, the Mt. Sinai Silvio Conte Neuroscience Center (NIMH MH 36692; KL Davis PI) and the VA VISN 3 MIRECC. These sponsors had no role in study design, collection of data, data analysis, interpretation of results, or writing of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest.
None.
References
- Andreasen N, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH): An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Gur RE, Shapiro RM, Fisher KR, Moberg PJ, Gibney MR, Gur RC, Blackwell P, Trojanowski JQ. Prospective clinicopathologic studies of schizophrenia: accrual and assessment of patients. Am. J. Psychiatry. 1995;152:731–737. doi: 10.1176/ajp.152.5.731. [DOI] [PubMed] [Google Scholar]
- Barch DM, Sheline YI, Csernansky JG, Snyder AZ. Working memory and prefrontal cortex dysfunction: specificity to schizophrenia compared with major depression. Biol. Psychiatry. 2003;53:376–384. doi: 10.1016/s0006-3223(02)01674-8. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Goodkin DE, Monson N, Beatty PA, Hertsgaard D. Anterograde and retrograde amnesia in patients with chronic progressive multiple sclerosis. Arch Neurol. 1988;45:611–619. doi: 10.1001/archneur.1988.00520300029013. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Reichenberg A, Rieckmann N, Parrella M, White L, Harvey PD. Stability and functional correlates of memory-based classification in older schizophrenia patients. Am. J. Ger. Psychiatry. 2004;12:376–386. doi: 10.1176/appi.ajgp.12.4.376. [DOI] [PubMed] [Google Scholar]
- Breier A, Buchanan RW, Elkashef A, Munson RC, Kirkpatrick B, Gellad F. Brain morphology and schizophrenia: a magnetic resonance imaging study of limbic, prefrontal cortex, and caudate structures. Arch. Gen. Psychiatry. 1992;49:921–926. doi: 10.1001/archpsyc.1992.01820120009003. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Vladar K, Barta PE, Pearlson GD. Structural evaluation of the prefrontal cortex in schizophrenia. Am. J. Psychiatry. 1998;155:1049–1055. doi: 10.1176/ajp.155.8.1049. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Tang CY, Peled S, Gudbjartsson H, Lu D, Hazlett EA, Downhill J, Haznedar M, Fallon JH, Atlas SW. MRI white matter diffusion anisotropy and PET metabolic rate in schizophrenia. Neuroreport. 1998;9:425–430. doi: 10.1097/00001756-199802160-00013. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, Goldberg TE, Weinberger DR. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cerebral Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cerebral Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Cannon TD, van Erp TGM, Huttunen M, Lonnqvist J, Salonen O, Valanne L, Poutanen V, Standertskjold-Nordenstam CG, Gur RE, Yan M. Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Arch. Gen. Psychiatry. 1998;55:1084–1091. doi: 10.1001/archpsyc.55.12.1084. [DOI] [PubMed] [Google Scholar]
- Chapman JP, Chapman LJ. Problems in the measurement of cognitive deficit. Psychol. Bull. 1973;79:380–385. doi: 10.1037/h0034541. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Szulc KU, Bertisch HC, Majcher M, Brown K. Understanding structural brain changes in schizophrenia. Dialog. Clin. Neurosci. 2006;8:71–78. doi: 10.31887/DCNS.2006.8.1/ldelisi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M, Harvey PD, Powchik P, Parrella M, White L, Knobler HY, Losonczy MF, Keefe RS, Katz S, Frecska E. Severity of symptoms in chronically institutionalized geriatric schizophrenic patients. Am. J. Psychiatry. 1995;152:197–207. doi: 10.1176/ajp.152.2.197. [DOI] [PubMed] [Google Scholar]
- Eyler Zorrilla LT, Heaton RK, McAdamsm LA, Zisookm S, Harrism MJ, Jestem DV. Cross-sectional study of older outpatients with schizophrenia and healthy comparison subjects: no differences in age-related cognitive decline. Am J Psychiatry. 2000;157:1324–1326. doi: 10.1176/appi.ajp.157.8.1324. [DOI] [PubMed] [Google Scholar]
- Foong J, Maier M, Barker GJ, Brocklehurst S, Miller DH, Ron MA. In vivo investigation of white matter pathology in schizophrenia with magnetisation transfer imaging. J Neurol Neurosurg Psychiatry. 2000;68:70–74. doi: 10.1136/jnnp.68.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foong J, Symms MR, Barker GJ, Maier M, Woermann FG, Miller DH, Ron MA. Neuropathological abnormalities in schizophrenia: evidence from magnetization transfer imaging. Brain. 2001;124:882–892. doi: 10.1093/brain/124.5.882. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Harvey PD, Coleman T, Moriarty PJ, Bowie C, Parrella M, White L, Adler D, Davis KL. Six-year follow-up study of cognitive and functional status across the lifespan in schizophrenia: a comparison with Alzheimer's disease and normal aging. Am. J. Psychiatry. 2001;158:1441–1448. doi: 10.1176/appi.ajp.158.9.1441. [DOI] [PubMed] [Google Scholar]
- Fucetola R, Seidman LJ, Kremen WS, Faraone SV, Goldstein JM, Tsuang MT. Age and neuropsychologic function in schizophrenia: a decline in executive abilities beyond that observed in healthy volunteers. Biol. Psychiatry. 2000;48:137–146. doi: 10.1016/s0006-3223(00)00240-7. [DOI] [PubMed] [Google Scholar]
- Golden CJ, Freshwater SM. The Stroop Color and Word Test. A Manual for Clinical and Experimental Users. Wood Dale, IL: Stoelting; 2002. [Google Scholar]
- Granholm E, Morris S, Asarnow RF, Chock D, Jeste DV. Accelerated age-related decline in processing resources in schizophrenia: evidence from pupillary responses recorded during the span of apprehension task. J. Int. Neuropsychological. Soc. 2000;6:30–43. doi: 10.1017/s1355617700611049. [DOI] [PubMed] [Google Scholar]
- Granholm E, Asarnow RF, Marder SR. Controlled information processing resources and the development of automatic detection responses in schizophrenia. J. Abnorm Psychology. 1991;100:22–30. doi: 10.1037//0021-843x.100.1.22. [DOI] [PubMed] [Google Scholar]
- Granholm E, Morris SK, Sarkin AJ, Asarnow RF, Jeste DV. Pupillary responses index overload of working memory resources in schizophrenia. J. Abnorm. Psychology. 1997;106:458–467. doi: 10.1037//0021-843x.106.3.458. [DOI] [PubMed] [Google Scholar]
- Gur RE, Cowell P, Turetsky BI, Gallacher F, Cannon T, Bilker W. A follow-up magnetic resonance imaging study of schizophrenia: Relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch. Gen. Psychiatry. 1998;55:145–152. doi: 10.1001/archpsyc.55.2.145. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Docherty N, Serper MR, Rasmussen M. Cognitive deficits and thought disorder: II. An eight-month follow-up study. Schizophr Bull. 1990;16:147–156. doi: 10.1093/schbul/16.1.147. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Lombardi J, Kincaid MM, Parrella M, White L, Powchik P, Davidson M. Cognitive functioning in chronically hospitalized schizophrenic patients: age-related changes and age disorientation as a predictor of impairment. Schiz. Res. 1995;17:15–24. doi: 10.1016/0920-9964(95)00026-i. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Silverman JM, Mohs RC, Parrella M, White L, Powchik P, Davidson M, Davis KL. Cognitive decline in late-life schizophrenia: a longitudinal study of geriatric chronically hospitalized patients. Biol. Psychiatry. 1999;45:32–40. doi: 10.1016/s0006-3223(98)00273-x. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Moriarty PJ, Serper MR, Schnur E, Lieber D. Practice-related improvement in information processing with novel antipsychotic treatment. Schiz. Res. 2000;46:139–148. doi: 10.1016/s0920-9964(00)00033-5. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Reichenberg A, Romero M, Granholm E, Siever LJ. Dual-task information processing in schizotypal personality disorder: evidence of impaired processing capacity. Neuropsychology. 2006;20:453–460. doi: 10.1037/0894-4105.20.4.453. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Gladsjo JA, Palmer BW, Kuck J, Marcotte TD, Jeste DV. Stability and course of neuropsychological deficits in schizophrenia. Arch. Gen. Psychiatry. 2001;58:24–32. doi: 10.1001/archpsyc.58.1.24. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chellune CJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test Manual-Revised and expanded. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- Ho BC, Andreasen NC, Nopoulosm P, Arndtm S, Magnottam V, Flaumm M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch. Gen. Psychiatry. 2003;60:585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- Kay SR. Positive and negative syndromes in schizophrenia. New York: Brunner/Mazel; 1991. [Google Scholar]
- Lim KO, Hedehus M, Moseley M, de Crespigny A, Sullivan EV, Pfefferbaum A. Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Arch. Gen. Psychiatry. 1999;56:367–374. doi: 10.1001/archpsyc.56.4.367. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch. Gen. Psychiatry. 2001;58:148–157. doi: 10.1001/archpsyc.58.2.148. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, Mather M, D'Esposito M. Aging and reflective processes of working memory: binding and test load deficits. Psychol. Aging. 2000;15(3):527–541. doi: 10.1037//0882-7974.15.3.527. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS. MRI assessment of gray and white matter distribution in Brodmann's areas of the cortex in patients with schizophrenia with good and poor outcomes. Am. J. Psychiatry. 2003;160:2154–2168. doi: 10.1176/appi.ajp.160.12.2154. [DOI] [PubMed] [Google Scholar]
- Mockler D, Riordan J, Sharma T. Memory and intellectual deficits do not decline with age in schizophrenia. Schiz. Res. 1997;26:1–7. doi: 10.1016/S0920-9964(97)00031-5. [DOI] [PubMed] [Google Scholar]
- Moriarty PJ, Harvey PD, Mitropoulou V, Granholm E, Silverman JM, Siever LJ. Reduced processing resource availability in schizotypal personality disorder: evidence from a dual-task CPT study. J. Clin. Exp. Neuropsychology. 2003;25:335–347. doi: 10.1076/jcen.25.3.335.13808. [DOI] [PubMed] [Google Scholar]
- Oltmanns TF, Neale JM. Schizophrenic performance when distractors are present: attentional deficit or differential task difficulty? J. Abnorm. Psychology. 1975;84:205–209. doi: 10.1037/h0076721. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Dixit NK, Carter CS, Noll DC, Cohen JD. Prefrontal cortex dysfunction mediates deficits in working memory and prepotent responding in schizophrenia. Biol. Psychiatry. 2003;53:25–38. doi: 10.1016/s0006-3223(02)01675-x. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation. WAIS-III and WMS-III Technical Manual. San Antonio, TX: Author; 1998. [Google Scholar]
- Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology. 1991;41:685–691. doi: 10.1212/wnl.41.5.685. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. 2nd edition. Tucson, AZ: Neuropsychology Press; 1993. [Google Scholar]
- Rypma B, Berger JS, D'Esposito M. The influence of working-memory demand and subject performance on prefrontal cortical activity. J. Cog. Neurosci. 2002;14:721–731. doi: 10.1162/08989290260138627. [DOI] [PubMed] [Google Scholar]
- Schlosser R, Gesierich T, Kaufmann B, Vucurevic G, Hunsche S, Gawehn J, Stoeter P. Altered effective connectivity during working memory performance in schizophrenia: a study with fMRI and structural equation modeling. Neuroimage. 2003;19:751–763. doi: 10.1016/s1053-8119(03)00106-x. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Rey Auditory and Verbal Learning Test. Los Angeles: Western Psychological Services; 1996. [Google Scholar]
- Serper MR, Bergman RL, Harvey PD. Medication may be required for the development of automatic information processing in schizophrenia. Psychiatry Res. 1990;32:281–288. doi: 10.1016/0165-1781(90)90033-2. [DOI] [PubMed] [Google Scholar]
- Siegel BV, Reynolds C, Lohr JB, Nuechterlein KH, Bracha HS, Potkin SG. Changes in regional cerebral metabolic rate with age in schizophrenics and normal adults. Dev. Brain Dysf. 1994;7:132–146. [Google Scholar]
- Sigmundsson T, Suckling J, Maier M, Williams S, Bullmore E, Greenwood K, Fukuda R, Ron M, Toone B. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am. J. Psychiatry. 2001;158:234–243. doi: 10.1176/appi.ajp.158.2.234. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests and norms. 2ed. New York: Oxford University Press; 1998. [Google Scholar]
- Stevens AA, Goldman-Rakic PS, Gore JC, Fulbright RK, Wexler BE. Cortical dysfunction in schizophrenia during auditory word and tone working memory demonstrated by functional magnetic resonance imaging. Arch. Gen. Psychiatry. 1998;55:1097–1103. doi: 10.1001/archpsyc.55.12.1097. [DOI] [PubMed] [Google Scholar]
- Stirling J, White C, Lewis S, Hopkins R, Tantam D, Huddy A, Montague L. Neurocognitive function and outcome in first-episode schizophrenia: a 10-year follow-up of an epidemiological cohort. Schiz. Res. 2003;65:75–86. doi: 10.1016/s0920-9964(03)00014-8. [DOI] [PubMed] [Google Scholar]
- White L, Harvey PD, Opler L, Lindenmayer JP. Empirical assessment of the factorial structure of clinical symptoms in schizophrenia. A multisite, multimodel evaluation of the factorial structure of the Positive and Negative Syndrome Scale. The PANSS Study Group. Psychopathology. 1997;30:263–274. doi: 10.1159/000285058. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. The Wide Range Achievement Test. Wilmington, DE: Wide Range, Inc.; 1993. [Google Scholar]