Abstract
While traumatic joint injuries are known to increase the risk of osteoarthritis (OA), the mechanism is not known. Models for injurious compression of cartilage may identify predictors of injury that suggest a clinical mechanism. We investigated the relationship between peak stress during compression and glycosaminoglycan (GAG) loss after injury for knee and ankle cartilages. Human cartilage explant disks were harvested post-mortem from the knee and ankle of three organ donors with no history of OA and subjected to injurious compression to 65% strain in uniaxial unconfined compression at 2 mm/s (400%/s). The GAG content of the conditioned medium was measured three days after injury. After injury of knee cartilage disks, damage was visible in 18 of 39 disks (46%). Three days after injury, the increase in GAG loss to the medium (GAG loss from injured disks minus GAG loss from location-matched uncompressed controls) was 1.5 ± 0.3 μg/disk (mean ± SEM). With final strain and compression velocity held constant, we observed that increasing peak stress during injury was associated with less GAG loss after injury (p<0.001). In contrast, ankle cartilage appeared damaged after injury in only one of 16 disks (6%), there was no increase in GAG loss (0.0 ± 0.3 μg/disk), and no relationship between peak stress and increase in GAG loss was detected (p=0.51). By itself, increasing peak stress did not appear to be an important cause of GAG loss from human cartilage in our injurious compression model. However, we observed further evidence for differences in the response of knee and ankle cartilages to injury.
Introduction
Osteoarthritis (OA) is a mechanical and functional failure of an articular joint that leads to pain and disability for a significant portion of the population. Degradation of the articular cartilage is one of the hallmark features of osteoarthritis. An interplay between mechanical forces and cellular responses that leads to excessive degradative activity is therefore thought to be crucial to understanding the pathogenesis of osteoarthritis (Radin et al. 1991; Felson et al. 2000; Aigner et al. 2002). In particular, the aggrecan molecules of the cartilage matrix, maintained by resident chondrocytes, provide much of the equilibrium compressive stiffness of the tissue due to electrostatic repulsion between the highly charged and closely packed aggrecan glycosaminoglycan (GAG) chains (Buschmann and Grodzinsky 1995). In addition, cartilage dynamic stiffness is primarily associated with interstitial fluid pressurization (Soltz and Ateshian 2000), due largely to the high resistance to fluid flow provided by aggrecan GAGs within the matrix (Maroudas 1979). Importantly, it is now well established that loss of aggrecan from the cartilage is a critical event in osteoarthritis (Sandy et al. 1992; Glasson et al. 2005; Stanton et al. 2005).
Along with risk factors such as age, obesity, and joint alignment, it has been observed that a traumatic joint injury leads to a higher risk for development of osteoarthritis in that joint (Roos et al. 1995; Felson et al. 2000; Gelber et al. 2000; Wilder et al. 2002). The increased risk was once thought to be primarily due to the mechanical joint instability resulting from the damage to the ligaments or meniscus during injury, but it now appears that even though joint instability is a risk factor for OA, joint repair surgery may not reduce the risk of post-traumatic OA (Feller 2004; Lohmander et al. 2004; von Porat et al. 2004). This suggests that early events after the injury have long-term effects on the cells and tissues of the joint. For example, within 24 hours after anterior cruciate ligament injury, a dramatic increase in the concentration of the inflammatory cytokines IL-1β and TNF-α has been observed in the synovial fluid of the injured knee (Irie et al. 2003), and inflammatory changes in the synovial fluid appear to be sustained above normal levels for months to years (Lohmander et al. 1993; Cameron et al. 1997).
To investigate these processes under defined conditions, in vitro models for injurious mechanical compression of the cartilage have been developed by a number of investigators [reviewed in (Patwari et al. 2001; Borrelli and Ricci 2004)]. These models may be useful for identifying the mechanical parameters of loading that are most responsible for damage to the cartilage matrix as well as for injury to the chondrocytes. This information could lead to a clinically useful characterization of the tolerances of the cartilage cells and matrix, and could also give insights into the mechanisms of mechanotransduction that are responsible for the effects of an injury.
Several researchers have suggested that there may be a threshold level of peak stress that separates injurious from harmless loads to the cartilage (Repo and Finlay 1977; Newberry et al. 1998; Torzilli et al. 1999; Clements et al. 2001). At the same time, others have observed associations of cartilage injury with loading parameters such as the final strain (Loening et al. 2000; Ewers et al. 2001) and the rate of loading (Chen et al. 1999; Kurz et al. 2001; Quinn et al. 2001). Since these loading parameters (peak stress, peak strain, and rate of loading) are interdepedent, further conclusions about the significance of these loading parameters individually will require experiments to measure and consider all of the parameters simultaneously.
We report here the results of experiments subjecting normal articular cartilage from post-mortem human knee-ankle pairs to injurious compression. Since the pattern of osteoarthritis in the knee and ankle joints is different, with a lower incidence of OA in the ankle joint (Muehleman et al. 1997), investigation of the differences in biomechanical and biochemical differences between the two joint tissues may lead to insights into the pathogenesis of osteoarthritis. Previous studies have demonstrated that the ankle cartilage is stiffer, has a higher GAG content, and resists catabolic stimuli (reviewed in Kuettner and Cole 2005). In addition, an initial analysis of cartilage from a post-mortem knee-ankle pair suggested a major difference in the response of the two cartilages to injurious compression, and to the combination of injurius compression and exogenous cytokines (Patwari et al. 2003).
We therefore compared the results of injurious compression, which was defined here as unconfined compression to 65% strain at a velocity of 2 mm/s, between knee and ankle cartilage explants from multiple donor joints. Since this compression protocol applied a fixed final strain at a fixed compression velocity, we analyzed the data to determine whether the effect of peak stress applied to the cartilage during injurious compression was independently associated with the loss of proteoglycan from the cartilage matrix after injury.
Methods
Tissue Harvest
All research was approved by the Office of Research Affairs at Rush-Presbyterian-St. Luke's Medical Center and by the Committee on the Use of Humans as Experimental Subjects at the Massachusetts Institute of Technology. Tissue harvest, culture, and injurious compression were identical to the methods described in detail in our initial report (Patwari et al. 2003). Knee and ankle joints from the same limb were obtained post-mortem from two additional adult human organ donors with no history of OA and joint surfaces rated grade 2 or less on a modified Collins scale (Kuettner and Cole 2005) from the Gift of Hope Organ and Tissue Donor Network (Elmhurst, IL). All results presented here are for a cumulative analysis with three donors.
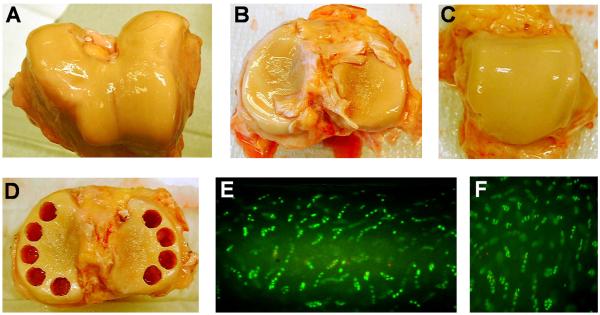
Bone-cartilage cylinders (9 mm in diameter) were harvested from knee (femoropatellar groove and tibiofemoral) and ankle (talotibial) joint surfaces [Figure 1, A-D and as shown previously (Treppo et al. 2000)]. To create slices of constant thickness, the cylinders were fixed in place and a microtome was used to first create a level surface (generally removing the superficial zone of the cartilage) and then cut either one or two 0.5-mm-thick slices, as allowed by the depth of the cartilage layer. The cut slices were measured by calipers to verify that the thickness was within 10% of 0.5 mm. From each slice, four smaller disks (3 mm in diameter) were then punched and equilibrated in culture for three days with DMEM plus 10% fetal calf serum, 1 mM HEPES, non-essential amino acids, proline, ascorbate, penicillin, streptomycin, and amphotericin B. In separate sections, cell viability was verified by incubation of slices with fluorescein diacetate and ethidium bromide (Figure 1E-F) (Kurz et al. 2001).
Fig. 1.
Donor knee and ankle joint harvest. Knee joint cartilage was harvested from the femoropatellar groove and femoral condyles (A) and from the tibial plateau (B). Ankle cartilage was harvested from the talar dome (C). The joints pictured here were from a 60-year-old male and were scored as modified Collins grade 1 (knee) and 0 (ankle). Bone-cartilage cylinders were then drilled, avoiding areas of fibrillated cartilage (D). To ensure live tissue explants after harvest, viable cells (cells stained green) and dead cells (nuclei stained red) were assessed in sections of ankle (E) and knee femoropatellar groove (F) cartilage.
Injurious compression
Each cartilage disk was paired with another disk punched from the same 9-mm-slice, and thus from the same joint location and depth. The thickness of each disk was measured again just prior to injury. From each pair one disk was subjected to injurious compression and one remained uncompressed as a control (Fig. 2A). Injurious compression consisted of uniaxial compression, in a uniaxial radially unconfined geometry, to 65% strain at a velocity of 2 mm/s, in an incubator-housed loading instrument (Fig 2B-C), as previously described in detail (Patwari et al. 2003). Displacement was continuously measured by a linear variable differential transformer, and the load during compression was acquired at a rate of 200 samples per second from a load cell (Frank et al. 2000). Strain was calculated based on a measurement of thickness just prior to injury and stress based on the area of the disk before compression. After injury, cartilage damage was graded by visual inspection as unchanged (0) (Fig 2D), deformed to a non-circular or non-cylindrical shape (1) (Fig 2E-F), or grossly fractured (2). Injured and control disks were returned to culture in fresh medium for 3 more days, after which the conditioned medium was collected and analyzed for sulfated GAG content by the dimethylmethylene blue assay.
Fig. 2.
Schematic of injurious compression methods and results. From each 9-mm-diameter cartilage slice, we obtained four 3-mm-diameter cartilage disks. Two were assigned to receive injury and two to remain unloaded (A). For injury, a single disk was placed in a loading chamber (B) and was subjected to uniaxial unconfined compression in an incubator-housed loading apparatus (C). Compared to unloaded controls, which retained a normal round and cylindrical shape (D), injured disks displayed a range of damage including bulges (E, lower right and F, upper left) and elliptical shapes (F).
Statistical Analysis
The primary measure of effect for this study was the difference of GAG loss from injured disks minus GAG loss from the location-matched control disks. The difference in GAG loss was defined such that positive values indicate an increase in GAG loss after injury compared to control cartilage. The main independent variable was peak stress. Generalized linear mixed-effects regression models for correlated data (Diggle et al. 1994) were used to assess the relationship between peak stress and difference in GAG loss after injury. These regression models account for the correlation in the data due to multiple measures taken from the same subject.
We considered original anatomical location of the cartilage disks and the score for damage (dichotomized as 0 vs. >0) observed after injury as potential confounders in the analyses. Location of the cartilage disks was measured using the following four factors: 1) medial vs. lateral aspect of the articular surface, 2) position in the anterior-posterior plane (from proximal to distal along the femur and from anterior to posterior on the tibia), 3) the depth of the slice from which the cartilage disk was punched (top vs bottom), 4) femoral surface vs. tibial plateau (knee analyses only). Separate regression models were fit for the knee and ankle. In knee analyses, adjusted models were fit that included all potential covariates. In addition, interaction terms were included to assess whether damage score modified the relationship between peak stress and difference in GAG loss. Ankle analyses were adjusted for medial vs. lateral aspect and depth but not damage due to the lack of variation in damage score. All analyses were conducted using 2-sided tests and a significance level of 0.05.
Results
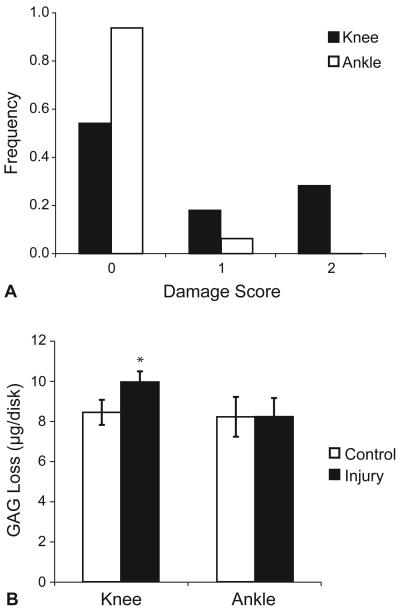
Injurious compression experiments were performed on knee and ankle cartilage tissue from three organ donors (two male and one female) whose ages ranged from 60 to 72 years. A total of 78 disks were harvested from the knee joint articular surfaces, which were rated Collins grade 1 to 2. During equilibration in culture, the change in thickness, calculated as final minus initial thickness normalized to initial thickness, was minimal (−3.2 ± 7.1 %, mean ± SD). Injury of knee cartilage disks produced peak stresses of 13.5 ± 4.6 MPa (mean ± SD) and damage (damage score of 1 or 2) was observed in 18 of 39 disks (46%) (Fig. 3A). Injured and control disks were replaced in fresh medium for three more days, after which the GAG content of the conditioned medium was measured. Uncompressed control cartilage released 8.4 ± 0.6 μg GAG per disk, while injured cartilage released 9.9 ± 0.5 μg/disk (mean ± SEM) (Figure 3B). GAG loss was higher from injured cartilage compared to location-matched controls (mean increase, 1.5 μg/disk; SEM = 0.3; p < 0.001 by paired t-test, N = 39). For reference, the total GAG content of these specimens is typically 125 μg per disk.
Fig. 3.
Damage score and GAG loss following injurious compression. Immediately following injury, injured ankle and knee cartilage disks from three donors were scored for visible damage (A). Injured and uncompressed control disks were replaced in fresh culture medium, and three days later the sGAG content of the conditioned medium was measured (B). Injury of knee cartilage resulted in an increase in GAG loss to the medium after injury compared to location-matched controls (p<0.001 by paired t-test), whereas there was no observed difference after injury of ankle cartilage (p=0.97).
Cartilage from the ankle joint (a total of 32 disks) was harvested from surfaces all rated Collins grade 0. During equilibration in culture, the change in thickness was minimal (−3.2 ± 6.5 %, mean ± SD). Injury produced peak stresses of 13.9 ± 4.6 MPa (mean ± SD) and damage was observed in only 1 of 16 disks (6%) (Fig. 3A). Three days after injury, the control cartilage had released 8.2 ± 1.0 μg GAG/disk and injured cartilage had released 8.2 ± 0.9 μg/disk (mean ± SEM) (Figure 3B). No difference was observed in GAG loss from injured cartilage compared to location-matched controls (mean difference, 0.0 μg/disk; SEM = 0.3; p = 0.97 by paired t-test, N = 16).
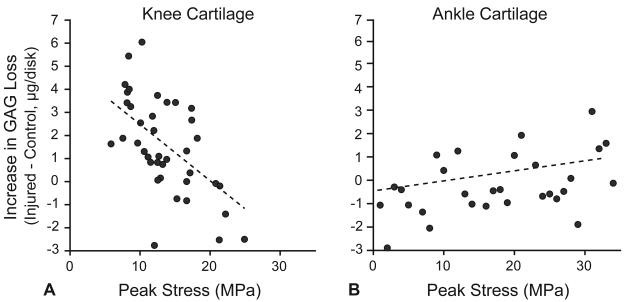
We first examined the unadjusted linear relationship between the peak stress during injury and the increase in GAG loss after injury (Fig. 4). In knee cartilage GAG loss significantly decreased with increasing peak stress (coefficient: −0.25 ± 0.06 μg/MPa, p < 0.001) (Table 1A). In the adjusted analysis, peak stress remained a significant predictor of GAG loss (coefficient: −0.22 ± 0.06 μg/MPa, p = 0.001). No significant interaction was observed between peak stress and damage score. In contrast, in ankle cartilage, we did not detect an association between peak stress and GAG loss in either the unadjusted or the adjusted analysis (Table 1B).
Fig. 4.
Relationship of peak stress to the increase in GAG loss after injury. The increase in GAG loss after injury was defined as the GAG loss from an injured disk minus the GAG loss from a location-matched uncompressed control disk. In knee cartilage (A) there was a statistically significant negative relationship between peak stress and increase in GAG loss (p < 0.001, 39 observations from 3 donors). In ankle cartilage (B), there was a positive association between peak stress and difference in GAG loss after injury that was not statistically significant (p = 0.51, 16 observations from 3 donors).
Table 1.
Analysis of the relationship between peak stress and the increase in GAG loss after injury by a generalized linear mixed-effect regression model.
| A. Human Knee Cartilage | |||||
|---|---|---|---|---|---|
| Unadjusted Estimate | Adjusted Estimate | ||||
| Coefficient | p-value | Coefficient | p-value | ||
| Peak Stress | −0.25 ± 0.06 | <0.001 | −0.22 ± 0.06 | 0.001 | |
| B. Human Ankle Cartilage | |||||
| Unadjusted Estimate | Adjusted Estimate | ||||
| Peak Stress | Coefficient | p-value | Coefficient | p-value | |
| 0.043 ± 0.064 | 0.51 | 0.015 ± 0.065 | 0.82 | ||
Discussion
In adult human knee cartilage from normal post-mortem donors, increasing peak stress during injury, with final (peak) strain and velocity held constant, was associated with less loss of proteoglycan from the tissue. This unexpected result may reflect a type of tissue yielding due to microstructural damage during loading, and emphasizes the importance of considering which compression parameters are held constant when interpreting this type of analysis. Nevertheless, peak stress, by itself, does not appear to be the critical determinant of proteoglycan loss in this injurious compression model. Secondly, in striking contrast to the results in knee cartilage, we observed that in ankle cartilage the same level of injurious compression caused little visible damage and had no significant effect on proteoglycan loss. These results significantly add to the evidence that knee and ankle cartilages, whether by design or adaptation, respond differently to mechanical and biological stimuli.
The concept of a threshold for cartilage injury is interesting for its implications both in understanding chondrocyte mechanotransduction and in clinical practice (Newberry et al. 1998; Torzilli et al. 1999; Loening et al. 2000). In one of the first in vitro investigations of this phenomenon, Torzilli et al. (1999) showed evidence for a threshold level of injury in terms of cell death and collagen damage in their injury model using adult bovine occipital joint cartilage. However, since the experiments involved loading that varied both peak stress and strain, these data were consistent with either a threshold in peak stress or in peak strain. Subsequently, investigators have also demonstrated the importance of other injury parameters such as compression velocity (Chen et al. 1999; Kurz et al. 2001).
It is therefore critical to consider that the observed effect of any one loading parameter is dependent on how the other loading parameters are varied in the experiment. For example, Ewers et al. (2001) have reported the counter-intuitive result that higher peak stress during injury was associated with less damage and cell death, and explained the seeming paradox by focusing on the rate of loading. Since compression was applied under load control, the specimens were not all loaded at the same velocities. The cartilage disks loaded to higher peak stress were loaded at higher velocities and, therefore, generated higher intratissue pressures more quickly. As a result, the higher peak stress was generated by compression to a lower strain than the cartilage loaded to the lower peak stress. Consideration of these results, therefore, suggests that the peak strain of injury can be more important than the peak stress for causing cartilage damage and chondrocyte death. Similarly, over a full range of velocities, Morel and Quinn (2004) showed quite clearly that loading cartilage to a fixed peak stress can cause much higher cell death at slower loading rates due to the very high final strain and accompanying water loss that results.
In the experiment reported here, cartilage was compressed to one fixed strain (65%) at one fixed velocity (2 mm/s, corresponding to a strain rate of approximately 400%/s), allowing us to examine the effects of variation in the peak stress generated by the cartilage during injury. We did not observe a threshold level of peak stress for injury, and instead observed a statistically significant negative association of peak stress with GAG loss. These observations are supported by a recent analysis of data from injurious compression of newborn bovine articular cartilage by several of the current authors (DiMicco et al. 2004), in which the peak stress during injurious compression was not associated with GAG loss after injury. This lack of a positive association in data from both adult human and immature bovine tissue supports the hypothesis that the peak stress is not directly responsible for matrix damage under these loading conditions.
We hypothesize that the negative association between peak stress and GAG loss observed in knee cartilage here reflects the generation of unvisualized microstructural damage in the cartilage matrix during the injury and the association of GAG loss with such damage. Since the tissue was loaded to a fixed strain and velocity, the variations in peak stress observed in this experiment reflect variations among cartilage samples in mechanical properties such as the stiffness and permeability. Thus, damage to matrix mechanical properties during the injury would be expected to cause a decrease in the peak stress generated by the tissue.
This hypothesis therefore proposes that rather than peak stress, the cause of proteoglycan release under these loading conditions is simply macro or micro-structural damage to the cartilage matrix. In support of this hypothesis, we have previously reported in newborn bovine tissue that GAG loss in the first three days after injury is not affected by inhibition of cell biosynthesis and is not strongly affected by inhibition of MMP and aggrecanase proteolysis (Patwari et al. 2003; DiMicco et al. 2004), suggesting that the mechanical forces directly cause most of the initial GAG loss after injury. For the current study, this suggested that the relation between peak stress and GAG loss would change depending on whether the matrix sustained damage during injury, so we tested an interaction between peak stress and damage score. The interaction was not found to be significant and therefore not included in the final model. However, since the matrix could be significantly damaged at the molecular level even if gross fractures and alterations in shape are not observed after injury, the ability of the current analysis to detect an interaction between peak stress and matrix damage may be limited. On the other hand, the lack of a relationship between peak stress and GAG loss in the ankle cartilage, where there was little observed damage, is consistent with this hypothesis. Finally, we would note that the relationships among peak stress, GAG loss, and damage are likely to change under different loading regimes and conditions that would be expected to produce different mechanisms of damage (Quinn et al. 2001; Milentijevic and Torzilli 2005).
The second major observation of this study was the striking difference between injury to the knee cartilage and injury to the ankle cartilage with the same compression protocol and similar peak stresses. In the ankle cartilage, despite compression to 65% strain, injury damaged only 6% of the cartilage disks and the mean increase in GAG loss after injury was less than 0.1 ug per disk, suggesting that the matrix of the ankle cartilage is substantially more resistant to mechanical injury than the matrix of the knee cartilages. There was in addition no observed relation between peak stress and GAG loss in the ankle, probably due to the limited range of GAG loss and/or damage to the ankle disks. This analysis of joint cartilages from three donors is still limited in power by the small sample size. However, these cumulative results provide important evidence to confirm and extend our initial report of similar results from the first donor tissues (Patwari et al. 2003).
Since the pattern of OA is different in these two joints, with OA affecting the ankle joint less often, we have previously hypothesized that there may be aspects of the ankle cartilage matrix and cells that provide it with resistance to OA. Prior studies have documented differences in the ankle compared to the knee cartilage in both the properties of the matrix and the responses of the cells, including higher compressive stiffness and proteoglycan density (Treppo et al. 2000), lower matrix degradation (Aurich et al. 2005), and less response to catabolic stimuli such as IL-1 and fibronectin fragments (Eger et al. 2002; Dang et al. 2003). Therefore, the relative resistance of ankle cartilage to damage in our in vitro model for traumatic joint injury is not only consistent with these prior reports but also suggests an additional explanation for the resistance of the ankle matrix itself to OA beyond the differences in overall joint mechanics.
Acknowledgments
The authors would like to express their appreciation to the Gift of Hope Organ and Tissue Donor Network and to the donors and their families for their permission to conduct research with the donor tissue. This work was funded in part by grants AR45779 and AR2P50-39239 from the National Institutes of Health and a fellowship from the Whitaker Foundation.
References
- Aigner T, Kurz B, Fukui N, Sandell L. Roles of chondrocytes in the pathogenesis of osteoarthritis. Curr Opin Rheumatol. 2002;14:578–584. doi: 10.1097/00002281-200209000-00018. [DOI] [PubMed] [Google Scholar]
- Aurich M, Squires GR, Reiner A, Mollenhauer JA, Kuettner KE, Poole AR, Cole AA. Differential matrix degradation and turnover in early cartilage lesions of human knee and ankle joints. Arthritis Rheum. 2005;52:112–119. doi: 10.1002/art.20740. [DOI] [PubMed] [Google Scholar]
- Borrelli J, Jr., Ricci WM. Acute effects of cartilage impact. Clin Orthop Relat Res. 2004:33–39. doi: 10.1097/01.blo.0000132627.13539.02. [DOI] [PubMed] [Google Scholar]
- Buschmann MD, Grodzinsky AJ. A molecular model of proteoglycan-associated electrostatic forces in cartilage mechanics. J Biomech Eng. 1995;117:179–192. doi: 10.1115/1.2796000. [DOI] [PubMed] [Google Scholar]
- Cameron M, Buchgraber A, Passler H, Vogt M, Thonar E, Fu F, Evans CH. The natural history of the anterior cruciate ligament-deficient knee. Changes in synovial fluid cytokine and keratan sulfate concentrations. Am J Sports Med. 1997;25:751–754. doi: 10.1177/036354659702500605. [DOI] [PubMed] [Google Scholar]
- Chen CT, Burton-Wurster N, Lust G, Bank RA, Tekoppele JM. Compositional and metabolic changes in damaged cartilage are peak-stress, stress-rate, and loading-duration dependent. J Orthop Res. 1999;17:870–879. doi: 10.1002/jor.1100170612. [DOI] [PubMed] [Google Scholar]
- Clements KM, Bee ZC, Crossingham GV, Adams MA, Sharif M. How severe must repetitive loading be to kill chondrocytes in articular cartilage? Osteoarthritis Cartilage. 2001;9:499–507. doi: 10.1053/joca.2000.0417. [DOI] [PubMed] [Google Scholar]
- Dang Y, Cole AA, Homandberg GA. Comparison of the catabolic effects of fibronectin fragments in human knee and ankle cartilages. Osteoarthritis Cartilage. 2003;11:538–547. doi: 10.1016/s1063-4584(03)00085-2. [DOI] [PubMed] [Google Scholar]
- Diggle P, Liang K-Y, Zeger SL. Analysis of longitudinal data. Clarendon Press; New York: 1994. [Google Scholar]
- DiMicco MA, Patwari P, Siparsky PN, Kumar S, Pratta MA, Lark MW, Kim YJ, Grodzinsky AJ. Mechanisms and kinetics of glycosaminoglycan release following in vitro cartilage injury. Arthritis Rheum. 2004;50:840–848. doi: 10.1002/art.20101. [DOI] [PubMed] [Google Scholar]
- Eger W, Schumacher BL, Mollenhauer J, Kuettner KE, Cole AA. Human knee and ankle cartilage explants: catabolic differences. J Orthop Res. 2002;20:526–534. doi: 10.1016/S0736-0266(01)00125-5. [DOI] [PubMed] [Google Scholar]
- Ewers BJ, Dvoracek-Driksna D, Orth MW, Haut RC. The extent of matrix damage and chondrocyte death in mechanically traumatized articular cartilage explants depends on rate of loading. J Orthop Res. 2001;19:779–784. doi: 10.1016/S0736-0266(01)00006-7. [DOI] [PubMed] [Google Scholar]
- Feller J. Anterior cruciate ligament rupture: is osteoarthritis inevitable? Br J Sports Med. 2004;38:383–384. doi: 10.1136/bjsm.2004.013300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, Kington RS, Lane NE, Nevitt MC, Zhang Y, Sowers M, McAlindon T, Spector TD, Poole AR, Yanovski SZ, Ateshian G, Sharma L, Buckwalter JA, Brandt KD, Fries JF. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- Frank EH, Jin M, Loening AM, Levenston ME, Grodzinsky AJ. A versatile shear and compression apparatus for mechanical stimulation of tissue culture explants. J Biomech. 2000;33:1523–1527. doi: 10.1016/s0021-9290(00)00100-7. [DOI] [PubMed] [Google Scholar]
- Gelber AC, Hochberg MC, Mead LA, Wang NY, Wigley FM, Klag MJ. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133:321–328. doi: 10.7326/0003-4819-133-5-200009050-00007. [DOI] [PubMed] [Google Scholar]
- Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, Majumdar MK, Morris EA. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- Irie K, Uchiyama E, Iwaso H. Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. Knee. 2003;10:93–96. doi: 10.1016/s0968-0160(02)00083-2. [DOI] [PubMed] [Google Scholar]
- Kuettner KE, Cole AA. Cartilage degeneration in different human joints. Osteoarthritis Cartilage. 2005;13:93–103. doi: 10.1016/j.joca.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Kurz B, Jin M, Patwari P, Cheng DM, Lark MW, Grodzinsky AJ. Biosynthetic response and mechanical properties of articular cartilage after injurious compression. J Orthop Res. 2001;19:1140–1146. doi: 10.1016/S0736-0266(01)00033-X. [DOI] [PubMed] [Google Scholar]
- Loening AM, James IE, Levenston ME, Badger AM, Frank EH, Kurz B, Nuttall ME, Hung HH, Blake SM, Grodzinsky AJ, Lark MW. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys. 2000;381:205–212. doi: 10.1006/abbi.2000.1988. [DOI] [PubMed] [Google Scholar]
- Lohmander LS, Hoerrner LA, Dahlberg L, Roos H, Bjornsson S, Lark MW. Stromelysin, tissue inhibitor of metalloproteinases and proteoglycan fragments in human knee joint fluid after injury. J Rheumatol. 1993;20:1362–1368. [PubMed] [Google Scholar]
- Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50:3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- Maroudas A, Freeman MAR. Adult Articular Cartilage. Pittman Medical Publishing Corp. Lmtd.; Great Britain: 1979. Physicochemical Properties of Articular Cartilage; pp. 215–290. [Google Scholar]
- Milentijevic D, Torzilli PA. Influence of stress rate on water loss, matrix deformation and chondrocyte viability in impacted articular cartilage. J Biomech. 2005;38:493–502. doi: 10.1016/j.jbiomech.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Muehleman C, Bareither D, Huch K, Cole AA, Kuettner KE. Prevalence of degenerative morphological changes in the joints of the lower extremity. Osteoarthritis Cartilage. 1997;5:23–37. doi: 10.1016/s1063-4584(97)80029-5. [DOI] [PubMed] [Google Scholar]
- Newberry WN, Garcia JJ, Mackenzie CD, Decamp CE, Haut RC. Analysis of acute mechanical insult in an animal model of post-traumatic osteoarthrosis. J Biomech Eng. 1998;120:704–709. doi: 10.1115/1.2834882. [DOI] [PubMed] [Google Scholar]
- Patwari P, Cook MN, DiMicco MA, Blake SM, James IE, Kumar S, Cole AA, Lark MW, Grodzinsky AJ. Proteoglycan degradation after injurious compression of bovine and human articular cartilage in vitro: interaction with exogenous cytokines. Arthritis Rheum. 2003;48:1292–1301. doi: 10.1002/art.10892. [DOI] [PubMed] [Google Scholar]
- Patwari P, Fay J, Cook MN, Badger AM, Kerin AJ, Lark MW, Grodzinsky AJ. In vitro models for investigation of the effects of acute mechanical injury on cartilage. Clin Orthop. 2001:S61–71. doi: 10.1097/00003086-200110001-00007. [DOI] [PubMed] [Google Scholar]
- Quinn TM, Allen RG, Schalet BJ, Perumbuli P, Hunziker EB. Matrix and cell injury due to sub-impact loading of adult bovine articular cartilage explants: effects of strain rate and peak stress. J Orthop Res. 2001;19:242–249. doi: 10.1016/S0736-0266(00)00025-5. [DOI] [PubMed] [Google Scholar]
- Radin EL, Burr DB, Caterson B, Fyhrie D, Brown TD, Boyd RD. Mechanical determinants of osteoarthrosis. Semin Arthritis Rheum. 1991;21:12–21. doi: 10.1016/0049-0172(91)90036-y. [DOI] [PubMed] [Google Scholar]
- Repo RU, Finlay JB. Survival of articular cartilage after controlled impact. Journal of Bone and Joint Surgery. 1977;59A:1068–1076. [PubMed] [Google Scholar]
- Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage. 1995;3:261–267. doi: 10.1016/s1063-4584(05)80017-2. [DOI] [PubMed] [Google Scholar]
- Sandy JD, Flannery CR, Neame PJ, Lohmander LS. The structure of aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthritis of a novel proteinase which cleaves the Glu 373-Ala 374 bond of the interglobular domain. J Clin Invest. 1992;89:1512–1516. doi: 10.1172/JCI115742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltz MA, Ateshian GA. Interstitial fluid pressurization during confined compression cyclical loading of articular cartilage. Ann Biomed Eng. 2000;28:150–159. doi: 10.1114/1.239. [DOI] [PubMed] [Google Scholar]
- Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, Little CB, Last K, Farmer PJ, Campbell IK, Fourie AM, Fosang AJ. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- Torzilli PA, Grigiene R, Borrelli J, Jr., Helfet DL. Effect of impact load on articular cartilage: cell metabolism and viability, and matrix water content. J Biomech Eng. 1999;121:433–441. doi: 10.1115/1.2835070. [DOI] [PubMed] [Google Scholar]
- Treppo S, Koepp H, Quan EC, Cole AA, Kuettner KE, Grodzinsky AJ. Comparison of biomechanical and biochemical properties of cartilage from human knee and ankle pairs. J Orthop Res. 2000;18:739–748. doi: 10.1002/jor.1100180510. [DOI] [PubMed] [Google Scholar]
- von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63:269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder FV, Hall BJ, Barrett JP, Jr., Lemrow NB. History of acute knee injury and osteoarthritis of the knee: a prospective epidemiological assessment. The Clearwater Osteoarthritis Study. Osteoarthritis Cartilage. 2002;10:611–616. doi: 10.1053/joca.2002.0795. [DOI] [PubMed] [Google Scholar]