Abstract
Recently, we [E.M.C. D’Agata, G.F. Webb, M.A. Horn, R.C. Moellering Jr., and S. Ruan, Modelling the invasion of community-acquired methicillin-resistant Staphylococcus aureus into the hospital setting, Clin. Infect. Dis. 48 (2009), pp. 274–284] proposed a deterministic mathematical model to characterize the factors contributing to the replacement of hospital-acquired methicillin-resistant Staphylococcus aureus (HA-MRSA) with the community-acquired MRSA (CA-MRSA) and to quantify the effectiveness of interventions aimed at limiting the spread of CA-MRSA in the hospital setting. Numerical simulations of the model strongly suggest that CA-MRSA will become the dominant MRSA strain in the hospital setting. In this companion paper, we provide steady-state analysis and more numerical simulations of the model. It is shown that when no colonized or infected patients enter the hospital, competitive exclusion of HA-MRSA by CA-MRSA will occur with increased severity of CA-MRSA infections resulting in longer hospitalizations and a larger in-hospital reservoir of CA-MRSA. Improving compliance with hand hygiene and decolonization of CA-MRSA carriers are effective control strategies.
Keywords: transmission dynamics, epidemic model, basic reproduction number, competition exclusion, disease-free and endemic steady states
1. Introduction
Staphylococcus aureus is a gram-positive bacterium that colonizes the skin and is present in the anterior nares in about a quarter of the population [5]. The bacterium acquires resistance against all classes of antibiotics by either mutation of an existing bacterial gene or horizontal transfer of a resistance gene from another bacterium [5]. Since it was first reported in the 1950s, methicillin-resistant Staphylococcus aureus (MRSA) has been regarded as a healthcare-associated pathogen affecting predominantly the elderly and debilitated [5,14], and MRSA infections are an important clinical and public health problem [6]. It was estimated that deaths in patients with MRSA in the USA in 2005 surpassed those caused by HIV/AIDS in the same year [1,12].
In 1998, a new strain of MRSA emerged in the community setting occurring among young healthy individuals with no exposure to the healthcare setting [9]. Since then, this community-acquired MRSA strain (CA-MRSA) has rapidly spread throughout the world [4, 11,13,23]. Outbreaks of CA-MRSA have been reported among children [9], athletes [16], nurseries [17] and obstetrical wards [20]. With the use of mathematical models, it has been shown that the presence of a community reservoir has a major impact on the control of MRSA in the hospital [2,15,19,20]. It has been suggested that CA-MRSA may be replacing the traditional hospital-acquired MRSA (HA-MRSA) [18].
Recently, we [3] developed a deterministic mathematical model to quantify the temporal patterns of CA-MRSA spread into the hospital setting and its competitive exclusion of HA-MRSA over time. The transmission dynamics of CA-MRSA within the hospital setting and the effectiveness of current infection control strategies were quantified to determine the optimal strategy or a combination of strategies, aimed at preventing the spread of CA-MRSA between patients. The deterministic model describes the transmission dynamics of CA-MRSA within a 400-bed tertiary-care hospital with approximately 25,000 admissions per year. The impact of an increasing influx of CA-MRSA into the hospital setting as a result of the persistent and rising dissemination of CA-MRSA within the community is quantified and the effect of different interventions aimed at limiting the spread of CA-MRSA is analysed and compared. Individuals within the hospital are in five mutually exclusive states: susceptible, colonized with either CA-MRSA or HA-MRSA and infected with either CA-MRSA or HA-MRSA. Individuals enter the hospital in one of these states and exit via death or discharge. Within the hospital, susceptible individuals can become colonized with either CA-MRSA or HA-MRSA and can subsequently become infected with the respective MRSA strain. Transmission of MRSA between individuals occurs through the hands of healthcare workers (HCWs), the main vectors of antimicrobial-resistant bacteria. Control strategies for preventing the spread of MRSA include improving compliance with hand hygiene and placing individuals who are infected with MRSA on contact precautions as per standard Centers for Disease Control and Prevention requirements.
In this companion paper, we provide the steady-state analysis and more numerical simulations of the model. It is shown that when no colonized or infected patients enter the hospital, competitive exclusion of HA-MRSA by CA-MRSA will occur with increased severity of CA-MRSA infections, resulting in longer hospitalizations and a larger in-hospital reservoir of CA-MRSA. Numerical simulations also demonstrate that if some of the patients admitted to the hospital are colonized or infected with HA-MRSA and CA-MRSA strains, then both strains can persist in the hospital in terms of positive equilibria.
2. The model
The patients in the hospital are divided into five compartments:
S(t) = number of susceptible patients at time t.
CC(t) = number of patients colonized with the CA-MRSA strain at time t.
CH(t) = number of patients colonized with the HA-MRSA strain at time t.
IC(t) = number of patients infected with the CA-MRSA strain at time t.
IH(t) = number of patients infected with the HA-MRSA strain at time t.
Patients are admitted at a total rate of Λ per day with the fractions of CA-MRSA colonized, CA-MRSA infected, HA-MRSA colonized and HA-MRSA infected patient admissions = λCC, λIC, λCH, λIH, respectively. Susceptible patients have an average length of stay (LOS) = 1/γS, and colonized CA-MRSA and colonized HA-MRSA have average LOS = 1/γC and 1/γH, respectively. The colonization rates of susceptible patients to the colonized CA-MRSA compartment are (1 − η)βCC/N and (1 − η)βIC/N and to the colonized HA-MRSA compartment are (1 − η)βCH/N and (1 − η)βIH/N. Here η is the compliance with hand washing hygiene (with η = 0 corresponding to 0% compliance and η = 1 corresponding to 100% compliance), βCC, βIC, βCH, βIH the colonization transmission rates of patients from HCWs contaminated by colonized CA-MRSA, infected CA-MRSA, colonized HA-MRSA and infected HA-MRSA patients, respectively, and N the total number of patients in the hospital. The ratios βCC/βCH and βIC/βIH are approximately 4/3 because of the more rapid doubling time of CA-MRSA. In the simulations, the average LOS of susceptible patients (γS) is adjusted so that the number of patients in the hospital is maintained at N. The rates of infection of colonized CA-MRSA and colonized HA-MRSA patients are φC and φH, respectively. The cure rates of infected CA-MRSA and infected HA-MRSA patients are τC and τH, respectively. The death rates of infected CA-MRSA and infected HA-MRSA patients are δC and δH, respectively. The rates of decolonization of colonized CA-MRSA and colonized HA-MRSA patients are αCC and αCH, respectively. Parameter estimates were obtained from the Beth Israel Deaconess Medical Center’s computerized database system, which provides patient and infection control data and from the literature (Table 1).
Table 1.
Variables and parameters of the model [3].
| Symbol | Interpretation | Baseline value |
|---|---|---|
| N | Total number of patients in the hospital | 400 |
| Λ | Total admissions per day | 70 |
| λCC | Fraction of colonized CA-MRSA admissions | Varies |
| λCH | Fraction of colonized HA-MRSA admissions | Varies |
| λIC | Fraction of infected CA-MRSA admissions | Varies |
| λIH | Fraction of infected HA-MRSA admissions | Varies |
| 1/γS | Average LOS of susceptible patients | 5 days |
| 1/γCC | Average LOS of colonized CA-MRSA patients | 5 days |
| 1/γCH | Average LOS of colonized HA-MRSA patients | 7 days |
| 1/γIC | Average LOS of infected CA-MRSA patients | 10 days |
| 1/γIH | Average LOS of infected HA-MRSA patients | 18 days |
| η | Hand hygiene compliance fraction (0 to 1) | 0.6 (60%) |
| βCC | Colonized/colonized CA-MRSA transmission rate | 0.36 |
| βCH | colonized/colonized HA-MRSA transmission rate | 0.27 |
| βIC | Infected/colonized CA-MRSA transmission rate | 0.09 |
| βIH | Infected/colonized HA-MRSA transmission rate | 0.07 |
| δCγIC | Infected CA-MRSA patient death rate | 0.033/10 (3.3%) |
| δHγIC | Infected HA-MRSA patient death rate | 0.2/18 (20%) |
| φCγCC | Colonized CA-MRSA patient infection rate | 0.1/5 (10%) |
| φHγCH | Colonized HA-MRSA patient infection rate | 0.1/7 (10%) |
| τCγIC | Infected CA-MRSA patient cure rate | 0.967/10 (96.7%) |
| τHγIH | Infected HA-MRSA patient cure rate | 0.8/18 (80%) |
| αCγCC | Infected CA-MRSA patient cure rate | 0.0/5 (0%) |
| αHγCH | Infected HA-MRSA patient cure rate | 0.0/7 (0%) |
Note: LOS, length of stay.
The equations of the basic model are
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
with initial conditions S(0) = S0, CC(0) = CC0, CH(0) = CH0, IC(0) = IC0 and IH(0) = IH0 specified at time 0.
3. Steady-state analysis
In this section, we analyse the steady states of the model. We consider two cases. (i) No colonized or infected patients enter the hospital, that is, λCC = λCH = λIC = λIH = 0. In the absence of such admissions, there is a competitive exclusion effect as the two strains compete in the hospital. The strain that dominates has the higher basic reproduction number, which must also be higher than 1. (ii) There are patients colonized with CA-MRSA strain admitted to the hospitals, that is, λCC ≠ 0, λCH = λIC = λIH = 0. If colonized or infected patients with CA-MRSA strain are admitted each day, then both strains are more likely to co-exist in the hospital.
The CA-MRSA strain in the absence of the hospital strain has a basic reproduction number defined by
| (6) |
and the HA-MRSA strain in the absence of the community strain has a basic reproduction number defined by
| (7) |
3.1. No admission of MRSA colonized or infected patients
When λCC = λCH = λIC = λIH = 0, the model can have three types of steady states based on and .
3.1.1. : disease-free steady state
When , the disease-free steady state
| (8) |
exists. In fact, we have the following results.
Theorem 3.1
If S0, CC0, CH0, IC0, IH0 ≥ 0, then the solutions are non-negative and remain bounded in the positive cone of R5. If and , then the disease-free steady state E0 is locally asymptotically stable. If either or , then E0 is unstable.
Proof
It is easy to see that the solutions remain in the positive cone if the initial conditions are in the positive cone. Let T (t) = S(t) + CC(t) + CH(t) + IC(t) + IH(t). Then
Thus, the solutions remain bounded in the positive cone of R5 and the system induces a global semiflow in the positive cone of R5.
To determine the stability of the disease-free steady state E0, we use the results in van den Driessche and Watmough [4]. Re-order the components of E0 as CC = 0, IC = 0, CH = 0, IH =0, S = Λ/γS. Set
and
where
Then
Similarly,
Therefore,
which implies that the spectral radius of the matrix F V −1 is
If and , then ρ(F V −1) < 1. By Theorem 2 in van den Driessche and Watmough [4], we know that the disease-free steady state E0 is locally asymptotically stable. E0 is unstable if or .
Remark 1
The case when and corresponds to the situation that there are no MRSA strains prevailing in the hospital. By using the comparison method, one can show that the disease-free steady state E0 is indeed globally stable.
3.1.2. : steady state with only the HA-MRSA strain
When , there is a disease steady state with only the hospital MRSA strain
| (9) |
where
Theorem 3.2
If , then the HA-MRSA strain endemic steady state EH exists and is locally asymptotically stable.
Proof
Re-order the steady-state EH as (0, 0, CH, IH, SH). Similarly as in the proof of Theorem 3.1, we have
and
Thus, the spectral radius of F V −1 is given by
Since , we have ρ(FV−1) < 1, which implies that EH is locally asymptotically stable by Theorem 2 in van den Driessche and Watmough [4].
Remark 2
The case when corresponds to the situation that only the hospital MRSA strain is prevailing in the hospital.
3.1.3. :HA-MRSA strain wins
When , the community MRSA strain disease steady state
| (10) |
exists, where
Notice that the hospital MRSA strain disease steady state EH still exists in this case. However, the stability of EH and EC depends on the relationship between and . We first have the following result regarding the stability of EH and EC.
Theorem 3.3
If , then both the HA-MRSA strain steady state EH and the CA-MRSA strain steady state EC exist. Moreover, EH is locally asymptotically stable and EC is unstable.
Proof
The proof is similar to that of Theorem 3.4, so here we omit it.
Remark 3
In the case when , even the community MRSA strain is introduced into the hospital, it is not strong enough to spread, the hospital MRSA strain is dominant and is prevailing in the hospital.
3.1.4. : Co-existent steady state with both strains
When , there is a co-existent steady state
| (11) |
with both CA-MRSA and HA-MRSA strains, where
and CC* and CH* satisfy the relationship
Remark 4
In the very special case when , both the community and hospital MRSA strains co-exist and prevail in the hospital.
3.1.5. : CA-MRSA strain wins
Finally, if , then we have the following result regarding the stability of EH and EC.
Theorem 3.4
If , then both the HA-MRSA strain steady state EH and the CA-MRSA strain steady state EC exist. Moreover, EH is unstable and EC is locally asymptotically stable.
Proof
For the CA-MRSA strain steady state EC, following the proof of Theorem 3.2, we have since . Thus, EC is locally asymptotically stable.
The Jacobian matrix at the HA-MRSA strain steady state EH is
where
The Jacobian matrix has one eigenvalue , where
Since b > 0, and this eigenvalue must be positive. Thus, EH is unstable.
Remark 5
When increases to greater than 1 and the CA-MRSA strain invades the hospital and eventually overtakes the HA-MRSA strain.
3.1.6. Steady states chart and transcritical bifurcation
The above results on the existence and stability of equilibria are summarized in Table 2.
Table 2.
Steady states chart for the Model when λCC = λCH = λIC = λIH = 0.
| BRN | E0 | EH | EC | E* | |
|---|---|---|---|---|---|
|
|
Stable | Does not exist | Does not exist | Does not exist | |
|
|
Unstable | Stable | Does not exist | Does not exist | |
|
|
Unstable | Stable | Unstable | Does not exist | |
|
|
Unstable | Exists | Exists | Exists | |
|
|
Unstable | Unstable | Stable | Does not exist |
BRN, basic reproduction number.
From Table 2, we can observe the following scenarios. At the beginning when , there is no MRSA strains prevailing in the hospital. When , the HA-MRSA strain spreads out in the hospital. Now the CA-HRSA strain is introduced into the hospital. When increases to be greater than 1 but less than , that is, when , the CA-MRSA strain does not establish in the hospital and HA-MRSA is still dominant there. In the critical case when , the CA-MRSA strain and HA-MRSA strain co-exist in the hospital. Finally, when increases to pass so that , the CA-MRSA strain takes over the HA-MRSA strain and dominates the hospital.
From a dynamical system point of view, we can also see that the HA-MRSA strain steady state EH changes from stability to instability and the CA-MRSA strain steady-state EC changes from instability to stability when passes through . Therefore, there is a trans-critical bifurcation when .
3.2. With admission of CA-MRSA colonized patients
As the HA-MRSA strain is prevailing in many hospitals, the main concern now is if the CA-MRSA strain will invade the hospital and take over the HA-MRSA strain. In the previous section, we considered the equilibrium points of the model when there is no admission of MRSA colonized or infected patients into the hospital and discussed different possible outcomes. It will be interesting to discuss the model when there is admission of CA-MRSA colonized or/and infected patients into the hospital. For the sake of simplicity, in this section, we analyse the steady states of the model with admission of CA-MRSA colonized patients only. The case when there is admission of CA-MRSA infected patients or of both CA-MRSA colonized and infected patients can also be discussed.
When λCC > 0 and λCH = λIC = λIH = 0, there is a co-existent steady state
| (12) |
where
and and are given by
We still need to make sure that and . Lengthy and tedious calculations show that if
| (13) |
To have , we assume that
| (14) |
Remark 6
The existence of a positive steady state indicates that both the CA- and HA-MRSA strains can co-exist in the hospital if there is admission of CA-MRSA colonized patients into the hospital. However, to have such a positive equilibrium, condition (14) implies that the admission rate λCC of CA-MRSA colonized patients into the hospital must be greater than a threshold value, while condition (13) demonstrates that the basic reproduction number for the HA-MRSA strain must be greater than that for the CA-MRSA strain. This is reasonable and agrees with the observation in the previous section: since there are new cases of CA-MRSA colonized patients admitted into the hospital everyday, the HA-MRSA strain has to be stronger (i.e. ) in order to co-exist with the CA-MRSA strain in the hospital. Otherwise, it will be overtaken by the CA-MRSA strain.
4. Numerical simulations and discussion
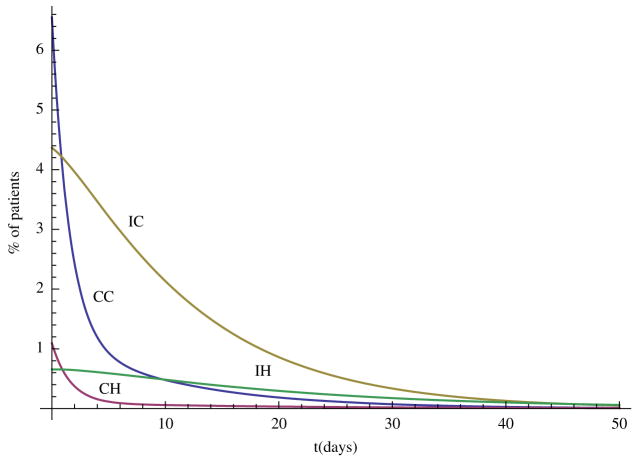
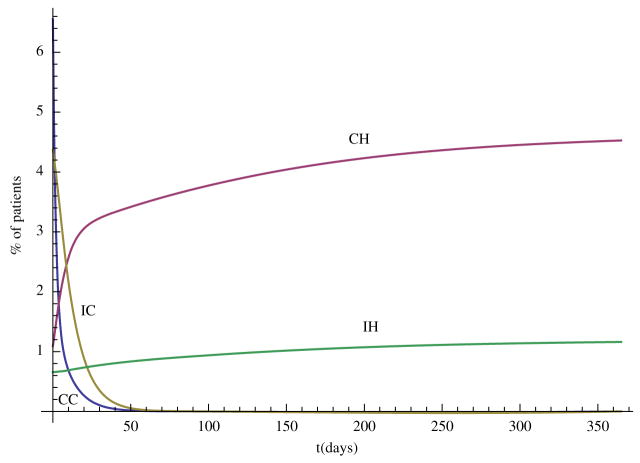
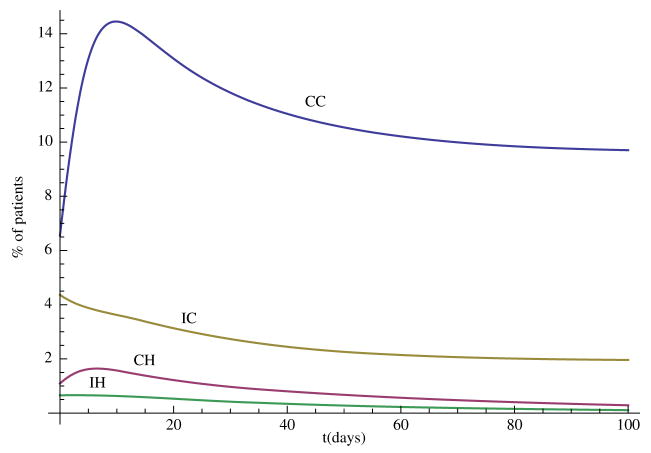
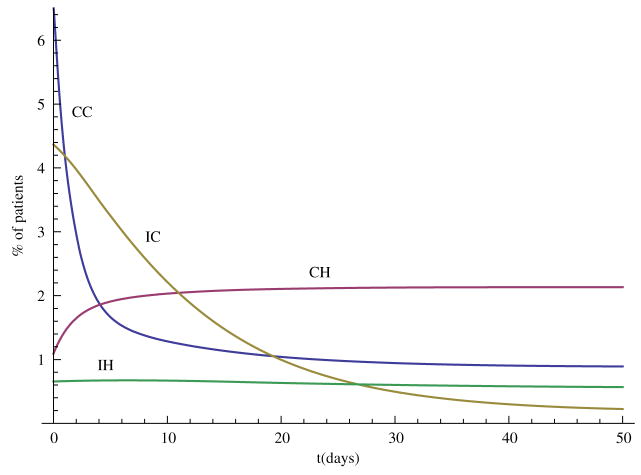
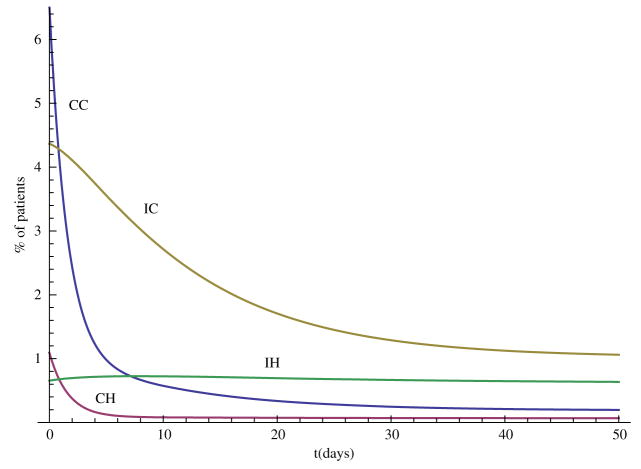
In this section, we carry out some numerical simulations to illustrate the results obtained in the previous section. Choose parameter as follows: Λ = 70, N = 400, η = 0.6, βCC = 0.36, βCH = 0.27, βIC = 0.09, βIH = 0.07, αC = 0.6, αH = 0.6, γS = 0.2, γC = 0.2, γH = 0.1429, τC = 0.0967, τH = 0.0444, φC = 0.02, φH = 0.0143, δC = 0.0033, δH = 0.0111. We can see that and , so the disease-free steady state E0 = (0.88, 0, 0, 0, 0) is stable (Figure 1). Now change some parameter values as follows: βCH = 0.71, βIH = 0.17, η = 0.3, αH = 0.3, then and the hospital MRSA strain steady-state EH is stable (Figure 2). Finally, choose βCC = 0.87 and βIC = 0.19, we have , and the community MRSA strain drives out the hospital MRSA strain (Figure 3).
Figure 1.
When and , the disease-free steady state E0 is stable.
Figure 2.
When , the hospital MRSA strain steady state EH is stable.
Figure 3.
When , the community MRSA strain takes over.
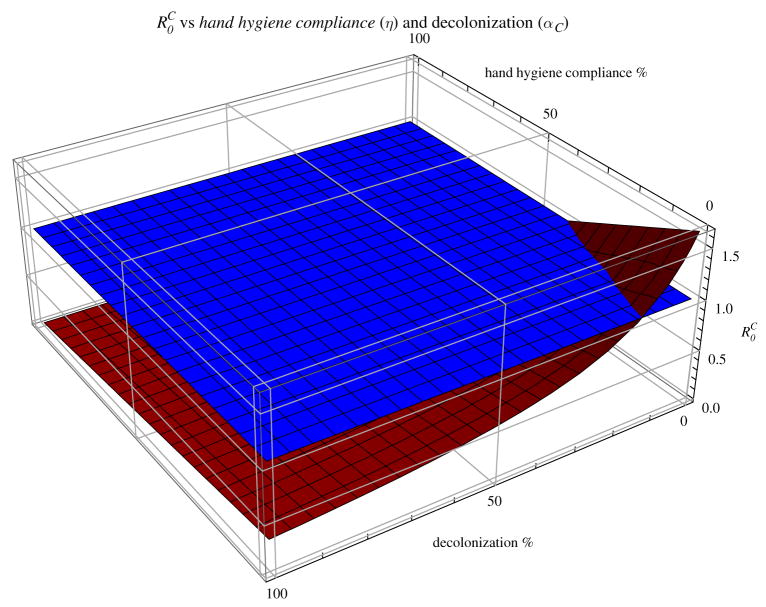
Hand hygiene and decolonization are both efficient interventions. Figure 4 shows that, when there is no entry of new cases, combining hand hygiene and decolonization can reduce the basic reproduction number for CA-MRSA to less than 1.
Figure 4.
Basic reproduction number for the community MRSA strain with baseline parameter values as in Table 1, except that the hand hygiene % and decolonization % vary from 0 to 100.
The above simulations were carried out with the assumption that no colonized or infected cases enter the hospital, that is, λCC = λCH = λIC = λIH = 0. If there are colonized or infected cases entering the hospital, then the outcomes are completely different. For example, if λCC = 0.03, λCH = 0.07, that is, some patients admitted to the hospital are colonized with HA-MRSA and CA-MRSA strain, respectively, then both strains will co-exist in the hospital (Figure 5). Similarly, if λIC = 0.005, λIH = 0.0017, that is, some patients admitted to the hospital are infected with HA-MRSA and CA-MRSA strain, respectively, then once again both strains will co-exist in the hospital (Figure 5).
Figure 5.
When λCC = 0.03, λCH = 0.07 and λIC = λIH = 0, both HA-MRSA and CA-MRSA strains establish in the hospital.
Entry of new cases into the hospital is crucial for the spread and control of both HA-MRSA and CA-MRSA strains. The simulations in Figures 5 and 6 strongly support the suggestion of screening for MRSA at hospital admission for colonized and infected cases [7,8]. However, screening requires action and compliance with infection control precautions. If HCWs do not comply with hand hygiene and other contact precautions when a patient is identified with MRSA through screening, then this intervention would not prevent the spread of MRSA. It also indicates that, when λCC, λCH, λIC and λIH are not all zero, the modal can have positive steady states with the endemicity of both HA-MRSA and CA-MRSA strains.
Figure 6.
When λCC = λCH = 0, λIC = 0.005 and λIH = 0.0017, there is a stable positive endemic steady state with both HA-MRSA and CA-MRSA strains in the hospital.
Acknowledgments
The authors would like to thank the two anonymous referees for their careful reading and valuable comments. This work was supported in part by the joint DMS/NIGMS Initiative through the NIH grant R01GM083607 (E.M.C.D., G.F.W., S.R.). This work was also partially supported by the NSF (M.A.H., S.R.). Any opinion, findings, conclusions or recommendations expressed are those of the authors and do not necessarily reflect the views of the NSF.
Contributor Information
Glenn F. Webb, Email: glenn.f.webb@vanderbilt.edu.
Mary Ann Horn, Email: mhorn@nsf.gov.
Erika M. C. D’Agata, Email: edagata@bidmc.harvard.edu.
Robert C. Moellering, Jr, Email: rmoeller@bidmc.harvard.edu.
References
- 1.Bancroft EA. Antimicrobial resistance: It’s not just for hospitals. JAMA. 2007;298:803–804. doi: 10.1001/jama.298.15.1803. [DOI] [PubMed] [Google Scholar]
- 2.Cooper BS, et al. Methicillin-resistant Staphylococcus aureus in hospitals and the community: Stealth dynamics and control catastrophes. Proc Natl Acad Sci USA. 2004;101:10223–10228. doi: 10.1073/pnas.0401324101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Agata EMC, Webb GF, Horn MA, Moellering RC, Jr, Ruan S. Modeling the invasion of community-acquired methicillin-resistant Staphylococcus aureus into the hospital setting. Clin Infect Dis. 2009;48:274–284. doi: 10.1086/595844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Driessche P, Watmough J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease tranmission. Math Biosci. 2002;180:29–48. doi: 10.1016/s0025-5564(02)00108-6. [DOI] [PubMed] [Google Scholar]
- 5.Grundmann H, Aires-de-Sousa M, Boyce J, Tiemersma E. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet. 2006;368:874–885. doi: 10.1016/S0140-6736(06)68853-3. [DOI] [PubMed] [Google Scholar]
- 6.Harbarth S. Control of endemic methicillin-resistant Staphylococcus aureus – recent advances and future challenges. Clin Microbiol Infect. 2006;12:1154–1162. doi: 10.1111/j.1469-0691.2006.01572.x. [DOI] [PubMed] [Google Scholar]
- 7.Harbarth S, et al. Evaluating the probability of previously unknown carriage of MRSA at hospital admission. Am J Med. 2006;119:275.e15–275.e23. doi: 10.1016/j.amjmed.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 8.Harbarth S, et al. Universal screening for methicillin-resistant Staphylococcus aureus at hospital admission and nosocomial infection in surgical patients. JAMA. 2008;299:1149–1157. doi: 10.1001/jama.299.10.1149. [DOI] [PubMed] [Google Scholar]
- 9.Herold BC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593–598. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 10.Kajita E, Okano JT, Bodine EN, Layne SP, Blower S. Modelling an outbreak of an emerging pathogen. Nat Rev Microbiol. 2007;5:700–709. doi: 10.1038/nrmicro1660. [DOI] [PubMed] [Google Scholar]
- 11.Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillinresistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis. 2007;12:1840–1846. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klevens RM, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 13.Kluytmans-VandenBergh MFQ, Kluytmans JAJW. Community-acquired methicillin-resistant Staphylococcus aureus: Current perspectives. Clin Microbiol Infect. 2006;12(Suppl 1):9–15. doi: 10.1111/j.1469-0691.2006.01341.x. [DOI] [PubMed] [Google Scholar]
- 14.Kuehnert MJ, Hill HA, Kupronis BA, Tokars JI, Solomon SL, Jernigan DB. Methicillin-resistant Staphylococcus aureus hospitalizations, United States. Emerg Infect Dis. 2005;11:868–872. doi: 10.3201/eid1106.040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin BR. Minimizing potential resistance: A population dynamics view. Clin Infect Dis. 2001;33:S161–S169. doi: 10.1086/321843. [DOI] [PubMed] [Google Scholar]
- 16.MMWR. Methicillin-resistant Staphylococcus aureus infections among competitive sports participants-Colorado, Indiana, Pennsylvania, and Los Angeles county, 2000–2003. Morb Mortal Wkly Rep. 2003;52:793–795. [PubMed] [Google Scholar]
- 17.Otter JA, French GL. Nosocomial transmission of community-associated methicillin-resistant Staphylococcus aureus: An emerging threat. Lancet Infect Dis. 2006;6:753–755. doi: 10.1016/S1473-3099(06)70636-3. [DOI] [PubMed] [Google Scholar]
- 18.Popovich KJ, Weinstein RA, Hota B. Are community-associated methicillin-resistant Staphylococcus aureus (MRSA) strains replacing traditional nosocomial MRSA strains? Cl in. Infect Dis. 2008;46:795–798. doi: 10.1086/528716. [DOI] [PubMed] [Google Scholar]
- 19.Robotham JV, Scarff CA, Jenkins DR, Medley GF. Methicillin-resistant Staphylococcus aureus (MRSA) in hospitals and the community: Model predictions based on the UK situation. J Hosp Infect. 2007;65(S2):93–99. doi: 10.1016/S0195-6701(07)60023-1. [DOI] [PubMed] [Google Scholar]
- 20.Saiman L, et al. Hospital transmission of community-acquired methicillin-resistant Staphylococcus aureus among postpartum women. Clin Infect Dis. 2003;37:1313–1319. doi: 10.1086/379022. [DOI] [PubMed] [Google Scholar]
- 21.Smith DL, Dushoff J, Perencevich EN, Harris AD, Levin SA. Persistent colonization and the spread of antibiotic resistance in nosocomial pathogens: Resistance is a regional problem. Proc Natl Acad Sci USA. 2004;101:3709–3714. doi: 10.1073/pnas.0400456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tristan A, et al. Global distribution of panton-valentine leukocidin-positive methicillin-resistant Staphylococcus aureus. Emerg Infect Dis. 2006;13:594–600. doi: 10.3201/eid1304.061316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zetola N, Francis JS, Nuermberger EL, Bishai WR. Community-acquired meticillin-resistant Staphylococcus aureus: An emerging threat. Lancet Infect Dis. 2005;5:275–286. doi: 10.1016/S1473-3099(05)70112-2. [DOI] [PubMed] [Google Scholar]