Abstract
Although other hantaviruses are associated with renal manifestations, hantavirus cardiopulmonary syndrome (HCPS) has not been associated with such sequelae. The HCPS survivors were prospectively evaluated for renal complications. Subjects underwent yearly evaluation, laboratory studies, and 24-hour urine collection. Thirty subjects were evaluated after recovery from HCPS with the first follow-up at a median of 7.4 months after discharge. Subjects were a wide age range (18–51) but had an equal gender composition. Eighteen of 30 (60%) returned for > 1 evaluation. Half (15/30) had a 24-hour urine collection with > 150 mg of total protein and 6 had > 300 mg. Seven had a Cockcroft-Gault creatinine clearance (CrClCG) < 90 mL/min/1.73 m2 and 2 were < 60. Fifty-three percent met the definition of chronic kidney disease. Those treated with extracorporeal membrane oxygenation had less renal sequelae (P = 0.035). Our data suggest that renal sequelae may occur in HCPS. Further study of renal complications of New World hantavirus infections are needed.
INTRODUCTION
Hantaviruses, enveloped RNA viruses of the family Bunyaviridae, are known to cause hemorrhagic fever with renal syndrome (HFRS) and hantavirus cardiopulmonary syndrome (HCPS) in humans. The HFRS is found primarily in Europe and Asia, and presents as an acute febrile illness associated with marked renal involvement.1–3 Hantavirus cardiopulmonary syndrome, found primarily in North and South America, is characterized by dramatic cardiopulmonary collapse. 4,5
In acute HFRS, proteinuria and elevated creatinine are common.6 Cases with more profound hemodynamic instability have more marked renal failure. 1,7,8 Renal pathology in HFRS classically shows tubulointerstitial nephritis with medullary hemorrhage.9 Data from long-term studies indicate that these diseases may be associated with chronic renal dysfunction, including reduced glomerular filtration rate (GFR), ongoing proteinuria, and hypertension. 10–12
In comparison, collected data on HCPS has focused on acute pulmonary and cardiac complications. Data on renal sequelae are unknown because of the infrequency of HCPS and lack of long term studies. To elucidate possible post-illness complications, we followed a cohort of HCPS patients prospectively for up to five years. This, the largest prospective cohort of survivors of HCPS, provided the opportunity to study potential renal complications associated with this emerging infection.
MATERIALS AND METHODS
Individuals in the United States with confirmed HCPS were eligible for study. Subjects were recruited from patients seen at or transferred to the University of New Mexico Health Science Center (UNM), patients referred by various state departments of health, and by a Hantavirus information coordinator at the Centers for Disease Control and Prevention (CDC). Confirmatory evidence of previous HCPS infection was considered to be a positive serology by western blot or modified western blot (RIBA Strip Immunoblot Assay, Chiron Corp., Inc., Emeryville, CA), 13,14 or by Sin Nombre virus (SNV)–specific ELISA. 15 Documentation of an appropriate clinical syndrome was confirmed using the CDC’s case definition for Hantavirus pulmonary syndrome. 16 All cases were reviewed for inclusion criteria by researchers with extensive experience in hantavirus disease. Subjects meeting entry criteria were admitted to the inpatient UNM General Clinical Research Center (GCRC) unit. Informed consent was obtained before enrollment. Patients signed a release of information so that records regarding their acute illness could be reviewed. The study was approved through the UNM Human Research Review Committee for study of human subjects.
Each subject’s previous history was reviewed in detail, with particular attention paid to previous medical history, history of acute hantavirus-related illness, and laboratory diagnosis of hantavirus infection. To consider severity of acute illness, each subject was graded using available historic information with a disease severity index (DSI) of 1 to 4 (Table 1). Because data were insufficient to categorize patients by standard disease severity indexes (e.g., Apache IV), we formulated, a priori of data analyses, a disease severity index based on other studies of hantavirus disease. Subjects had their acute HCPS illness categorized as mild disease (febrile illness, no oxygen requirement), moderate disease (requiring pulmonary support with oxygen ± mechanical ventilation), severe disease (mechanical ventilation and cardiac dysfunction requiring vasopressor or ionotropic support used), and catastrophic (extracorporeal membrane oxygenation [ECMO] used).
Table 1.
Disease severity index
| DSI | Description |
|---|---|
| 1 | Mild disease (febrile illness, no oxygen requirement) |
| 2 | Moderate disease (requiring pulmonary support with oxygen ± mechanical ventilation) |
| 3 | Severe disease (mechanical ventilation and cardiac dysfunction requiring vasopressors or ionotropic support) |
| 4 | Catastrophic dsease (ECMO* used) |
Extracorporeal membrane oxygenation.
Recruitment of subjects was limited to individuals at least 2 months out from acute HCPS to exclude the direct effects of the acute phase of illness. At each evaluation, patients were examined by a physician and had a complete history and physical examination. Follow-up visits were scheduled to occur at approximately yearly intervals over the course of the five-year study. New subjects were recruited as they were identified over time; therefore, follow-up was limited to remaining study years. Blood was drawn for chemistries, liver function, complete blood count, and sedimentation rate. Patients also underwent a 24-hour urine collection for total protein and creatinine. On the second hospital night, after termination of the 24-hour urine collection, an overnight 12-hour water deprivation was performed with pre- and post-urine specific gravity. Anthropomorphic measurements were also collected and used to calculate lean body mass and body mass index (BMI).
Evaluation of renal involvement
Total protein was evaluated by 24-hour urine collection and protein to creatinine ratio on the same specimen. Proteinuria was defined as greater than 150 mg of protein per 24-hour urine collection or 150 mg of protein per gram of creatinine. A protein/creatinine ratio based on the 24 hours sample was used to control for the possibility of inaccurately timed collections. Subjects were admitted to the GCRC and stayed overnight in a hospital bed to assure complete data collection and to exclude postural or orthostatic proteinuria.
Renal function was assessed with serum creatinine (Cr), creatinine clearance (CrCl) calculated from the 24-hour urine collection (CrCl24), and by the Cockcroft-Gault equation (CrClCG)17:
CrCl was then standardized per 1.73 m2 body surface area (BSA), using the Dubois method for calculation of BSA. 18 Subjects were assessed for chronic kidney disease (CKD) based on the National Kidney Foundation classification system (Table 2).19
Table 2.
National Kidney Foundation–stages of chronic kidney disease*
| Stage | Description | GFR (mL/min/1.73 m2) |
|---|---|---|
| 1 | Kidney damage with normal or ↑ GFR | ≥ 90 |
| 2 | Kidney damage with mild ↓ GFR | 60–89 |
| 3 | Moderate ↓ GFR | 30–59 |
| 4 | Severe ↓ GFR | 15–29 |
| 5 | Kidney failure | < 15 or dialysis |
From the National Kidney Foundation, 2002. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Kidney Disease Outcome Quality Initiative. Am J Kidney Dis 39 (Suppl 2): S1–S246.
GFR = glomerular filtration rate.
Statistical analysis
The Fisher’s exact, Wilcoxon rank-sum, and Kruskal-Wallis tests were used to determine whether baseline characteristics were significantly associated with either the highest level of proteinuria or lowest level of CrCl. Exact P values were computed for all tests. All statistical analyses were performed using SAS version 9.1 (Cary, NC).
RESULTS
Baseline subject characteristics
Thirty-five subjects were evaluated at the GCRC for follow-up after recovery from acute HCPS. Renal studies were incomplete in three subjects, and they were excluded from analysis. Two subjects under the age of 16 were not included in this analysis, leaving 30 total subjects. Study participants represented a diverse population (Table 3). Twenty-nine subjects had SNV. One subject had Bayou virus infection discriminated from SNV using a type–specific assay using Bayou virus and SNV nucleocapsid and G1 antibodies in a western blot format, and through reverse transcription-polymerase chain reaction (RT-PCR) of peripheral blood mononuclear cells with sequencing amplimers as previously described. 20,21 All subjects had evidence of virus–specific neutralizing antibodies to hantavirus.
Table 3.
Patient demographics
| N = 30 | ||
|---|---|---|
| Gender | Female | 15 (50) |
| Male | 15 (50) | |
| Ethnicity | Non-Hispanic White | 18 (60) |
| Hispanic | 6 (20) | |
| Native American | 5 (17) | |
| African American | 1 (3) | |
| DSI | 1 | 3 (10) |
| 2 | 3 (10) | |
| 3 | 17 (57) | |
| 4 | 6 (20) | |
| Unknown | 1 (3) | |
| Age | Median (range) | 39 (18–51) |
| BMI | Median (range) | 27.2 (17.2–36.2) |
Percentages may not equal 100 because of rounding. All values given as n (%) unless otherwise indicated.
DSI = disease severity index; BMI = body mass index.
Overall, subjects had a median age of 39 (range 18–51). Co-morbidity was low, with a single subject with known Type 2 diabetes mellitus and hypertension per history, and a second subject with an elevated hemoglobin A1C of 6.4. Neither of these subjects had proteinuria or reduced renal function. No other subjects had prior medical problems; therefore, no laboratory monitoring was available for comparison for most subjects. Those with medical records and laboratory studies for review had no evidence of renal disease before their acute HCPS admission. Furthermore, blood pressure monitoring during evaluation periods did not identify subjects with silent or undiagnosed hypertension, and no subject had an elevated erythrocyte sedimentation rate.
Other laboratory testing did not identify previously undetected medical problems that had not been specified in the patient’s clinical history or medical records. Two subjects had elevated gamma-glutamyl transpetidase (GGT) levels and one had transient elevation in total bilirubin; these returned to normal on subsequent visits. Two subjects had decreased serum albumin levels (3.0 and 3.2 g/dL, respectively), one of whom had an elevated aspartate aminotransferase (AST) level (127 U/L), but there was no repeat laboratory testing for these two subjects as they did not return for follow-up visits. No subject had abnormalities in their hemoglobin and only one patient had a mild transient elevation in their total white blood cell count (WBC). A total of 5/30 (17%) of subjects had platelets below 150,000/μL at least once during testing, with two persisting on follow-up. Platelets < 150,000/μL were not associated with an elevated urine protein, decreased creatinine clearance, or elevation in serum creatinine (data not shown).
All subjects’ first study visit was at a median of 7.4 months after their initial illness (interquartile range [IQR] 4–21). A total of 64 evaluations adequate for analysis were completed between January 1994 and December 2002. Twelve subjects had one evaluation, six subjects had two, eight subjects had three, and four subjects had three or more. In total, 18 subjects returned for repeat evaluations. Twelve subjects only made the initial visit because of late enrollment or other logistical reasons. Of the subjects with only one visit, two were undocumented foreign nationals who worked as migrant laborers and were lacking a permanent address.
Disease severity
Most subjects (17/30) had severe HCPS (DSI 3). Twenty percent (6/30) of subjects had catastrophic disease and received ECMO (DSI 4), indicating severe myocardial depression (cardiac index of < 2.5 L/min/m2).22 The remaining subjects had mild or moderate acute illness (DSI 1 and 2 respectively) with the exception of one subject who could not be assigned a disease severity score.
Renal outcomes
The principal outcomes evaluated were proteinuria and decreased creatinine clearance. Half of the subjects (15/30) had proteinuria > 150 mg by 24-hour urine collection, and another 21% (6/30) had > 300 mg/24 hours at some point during follow-up. In the 15 subjects who developed proteinuria, the median peak of proteinuria was 283 mg/24 hours (IQR 195–543), compared with a median of 88 mg/24 hours (IQR 42–140) in subjects without proteinuria (Table 4). Protein to creatinine ratios produced similar percentages of proteinuria. Nephrotic range proteinuria was not seen; the highest level of proteinuria was 537 mg/24 hours. There was a trend toward an association with DSI severity and proteinuria (P = 0.06), with subjects with severe disease (DSI 3) with the most significant proteinuria (median 230 mg/24 hours, IQR 140–322), and subjects with severe disease who received ECMO (DSI 4) with the lowest values (median 58 mg/24 hours, IQR 42–147) (Table 4). Compared with all levels of DSI, subjects with DSI 4 had a lower median of proteinuria then other DSI categories (230 mg/24 hours, IQR 115–322) (P = 0.035).
Table 4.
Proteinuria and creatinine clearance by selected patient characteristics*
| Proteinuria |
Creatinine clearance† |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| > 150‡ |
< 90§ |
||||||||
| n | Median (IQR)‡ | P | n (%) | P | Median (IQR)§ | P | n (%) | P | |
| Total | 30 | 171 (88–283) | – | 15 (50) | – | 141 (86–177) | – | 7 (25) | – |
| Gender | |||||||||
| Female | 15 | 142 (82–272) | 0.51 | 6 (40) | 0.47 | 111 (70–154) | 0.08 | 6 (40) | 0.08 |
| Male | 15 | 240 (114–289) | 9 (60) | 159 (119–179) | 1 (7) | ||||
| Race | |||||||||
| White | 18 | 205 (114–289) | 0.75¶ | 9 (50) | 0.76 | 119 (76–154) | 0.02¶ | 6 (35) | 0.34 |
| Latino | 6 | 222 (82–240) | 4 (66) | 152 (110–174) | 1 (20) | ||||
| Native American | 5 | 142 (74–195) | 2 (40) | 233 (179–234) | 0 (0) | ||||
| Age | |||||||||
| < 40 | 15 | 142 (82–289) | 0.63 | 7 (47) | 0.53 | 149 (92–185) | 0.35 | 3 (21) | 0.62 |
| ≥ 40 | 15 | 240 (114–283) | 8 (53) | 120 (79–159) | 4 (29) | ||||
| BMI | |||||||||
| < 28 | 16 | 142 (108–267) | 0.50 | 6 (38) | 0.27 | 111 (70–145) | 0.007 | 7 (47) | 0.007 |
| ≥ 28 | 13 | 230 (82–328) | 8 (62) | 174 (152–178) | 0 (0) | ||||
| DSI | |||||||||
| 1–2 | 6 | 191 (42–262) | 0.06¶ | 3 (50) | 0.14 | 115 (76–121) | 0.78¶ | 2 (40) | 0.70 |
| 3 | 17 | 230 (140–322) | 11 (65) | 145 (86–177) | 4 (25) | ||||
| 4 | 6 | 58 (42–147) | 1 (17) | 152 (110–233) | 1 (17) | ||||
All categorical values analyzed using Fisher’s exact test, and continuous variables with Wilcoxon rank-sum test unless otherwise indicated.
Calculated using Cockcroft-Gault methods.
Units mg/24 hours.
Units mL/min/1.73 m2.
Analyzed using Kruksal-Wallis test.
IQR = interquartile range; BMI = body mass index; DSI = disease severity index.
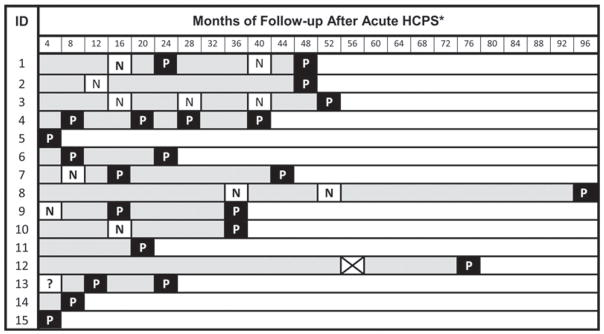
In subjects followed serially, prevalence of proteinuria appeared to increase over time. Proteinuria was found in 21% (6/29) of subjects at the first visit (one patient had 24-hour urine values only after his first visit), 56% (10/18) of subjects making a second visit, and 70% (7/10) making three or more visits (Figure 1). All subjects who developed proteinuria remained proteinuric on follow-up testing, except subject 1 who had a transient improvement. The number of months of lag between the initial illness and the detection of proteinuria varied, median 18.0 months (IQR 7.4–36.6).
Figure 1.
Timing of testing and results in subjects with proteinuria (> 150 mg/24 hours). □, White squares indicate when testing no proteinuria (N) detected (< 150 mg/dL); ■, black squares indicate when testing proteinuria (P) detected (> 150 mg/24 hours); ⊠, indicates a missed urine sample; ?, indicates start date of acute hantavirus cardiopulmonary syndrome (HCPS) unknown; and  indicates range of follow-up. * Equals first number of range of four months; ID = identification number.
indicates range of follow-up. * Equals first number of range of four months; ID = identification number.
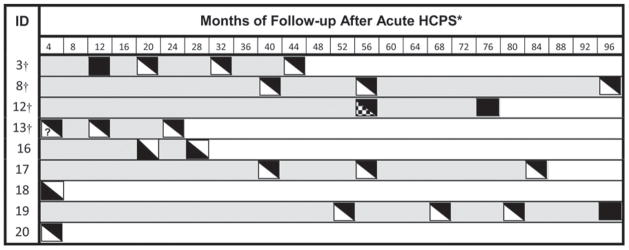
All subjects had serum Cr in the normal range for gender. A total of 29 subjects had at least a CrClCG and 29 had a CrCl24 at their first visit; one subject did not have urine collected on their first visit (but did have values on a subsequent visit), and one subject did not have a height and weight recorded, which prevented calculation of a CrClCG. When creatinine clearance was estimated by the Cockcroft-Gault equation, 25% (7/29) had a CrClCG < 90 mL/min/1.73 m2 and 7% (2/29) had a CrClCG < 60 mL/min/1.73 m2 (Table 4). When creatinine clearance was estimated from the 24-hour urine collection, similar percentages of subjects had reduced renal function, 17% (5/30) with < 90 mL/min/1.73 m2 and 7% (2/30) with CrCl24 < 60 mL/min/1.73 m2. Three subjects developed a decreased CrCl when calculated by both methods, and five had abnormalities on at least one (Figure 2). One patient (subject 3, Figure 2) who initially had abnormalities by 24-hour urine estimation improved over time. However, this subject’s CrClCG remained < 90 mL/min/1.73 m2 during all other follow-up visits and developed proteinuria (subject 3, Figure 1).
Figure 2.
Timing of testing and results in subjects with decreased creatinine clearance (< 90 mL/min/1.73 m2). ◣, indicates when 24-hour creatinine clearance < 90 mL/min/1.732 (black = yes, white = no); ◥, indicates when testing Cockcroft-Gault creatinine clearance < 90 mL/min/1.73 m2 (black = yes, white = no);  , indicates missed sample; ?, indicates start date of acute hantavirus cardiopulmonary syndrome (HCPS) unknown, and
, indicates missed sample; ?, indicates start date of acute hantavirus cardiopulmonary syndrome (HCPS) unknown, and  indicates range of follow-up. * Equals first number of range of four months. † Identification numbers (ID) are linked to specific subjects in Figure 1.
indicates range of follow-up. * Equals first number of range of four months. † Identification numbers (ID) are linked to specific subjects in Figure 1.
Subjects with a BMI ≥ 28 kg/m2 were less likely to have CrClCG < 90 mL/min/1.73 m2 (P = 0.007) and their median CrClCG (174 mL/min/1.73 m2, IQR 152–178) was also higher (P = 0.007) consistent with hyperfiltration (Table 4). Native Americans had similar findings with a median CrClCG (233 mL/min/1.73 m2, IQR 179–234), which was different from both Whites (119 mL/min/1.73 m2, IQR 76–154) and Latinos (152 mL/min/1.73 m2, IQR 110–174) (P = 0.02). On the basis of the estimates of creatinine clearance and/or the presence of proteinuria, 53% (16/30) of these long term survivors would qualify as having CKD: 23% (7/30) stage I, 17% (5/30) stage II, and 13% (4/30) stage III (Table 2).
To look for evidence of concentration deficits, we also completed provocative testing. After water deprivation, only one patient failed to concentrate urine normally. This patient had a urine specific gravity re-checked during an episode of gastroenteritis with vomiting, and again was noted to be unable to concentrate the urine appropriately. This patient also developed proteinuria year three post-illness.
On routine urinalysis (UA), seven subjects had a positive leukocyte esterase (LCE). The LCE had no relationship to proteinuria in any of the evaluated subjects, as those with proteinuria had repeat UAs that demonstrated either no LCE or no proteinuria. Two subjects had red blood cells in their urine on one sample, but not on repeat sampling. No other abnormalities were noted on microscopic analysis.
DISCUSSION
To our knowledge, this is the first study to assess survivors of HCPS for long-term renal sequelae. This prospective cohort study of HCPS survivors found an unexpected number of subjects with proteinuria and decreased CrCl in long-term follow-up; over half of the subjects met the standard clinical definition for CKD. Our data also suggest that subjects receiving ECMO treatment may be at lower risk.
Although renal complications have not been commonly reported in HCPS, renal disease is a hallmark of acute clinical HFRS. There are a few published reports regarding long-term renal outcomes after HFRS in highly endemic areas. A small number of survivors of Korean hemorrhagic fever years after their illness were found to have deficits in tubular concentration without evidence of decreased GFR or proteinuria. 11 Renal function was also found to be maintained in post-Puumula virus patients, but proteinuria and hypertension compared with healthy age matched controls were more common 10; however, at ten years these findings appeared to be attenuated. 12 Furthermore, although some claim there is epidemiologic data linking CKD to seropositivity for HFRS viruses, 23,24 others have disputed this finding. 25,26
Renal involvement in acute HCPS resulting from SNV is not well defined, but elevated creatinine and proteinuria can be seen in acute disease. 27,28 In the initial case series describing the syndrome, abnormalities of renal function were common, but serum creatinine levels did not rise above 2.5 mg/dL (220 μmol/L) in any patient.5 Examination of autopsy specimens did not find evidence of histopathologic renal damage, however, immunohistochemistry demonstrated SNV antigen in the interstitial capillaries of the medulla, the cortex, and glomerular endothelial cells. 27,29 Limited experience with HCPS caused by Bayou and Black Creek Canal viruses indicate evidence of clinical renal involvement, 21,30,31 and at least one case report has described a presentation similar to HFRS secondary to SNV. 32
Because of a lack of understanding of renal disease in acute HCPS, we can only speculate on the cause for these late renal abnormalities. Most authorities feel the renal impairment seen acutely in HCPS is secondary to the profound hemodynamic collapse, i.e., ischemic acute tubular necrosis. This type of renal injury has been linked to long-term renal sequelae. 33–35 One hypothesis would be that permanent loss of nephrons during the acute phase of illness could lead to chronic hyperfiltration in the remaining nephrons. Hyperfiltration, initially a homeostatic mechanism that maintains GFR through increased efficiency at individual nephron units, is widely felt to be a possible mechanism for progression of CKD. 36 Recent research into the long-term effects of ischemic acute tubular necrosis suggests an alternate hypothesis where irreversible injury to peritubular capillaries leads to chronic tubulo-interstitial injury and kidney disease. 33,37
Experimental evidence also indicates hantavirus infections have a predilection for endothelium. 38,39 Pathogenic hantaviruses have been shown to increase vascular permeability by disruption of tight junctions during acute infection. 40 In HFRS, this involvement is most prominent in the kidney, whereas in HCPS the pulmonary vascular bed is principally involved. 27 However, cytotoxic T-lymphocytes (CTL) along hantavirus–infected vascular endothelial cells in the lung in HCPS patients 27 have also been shown near tubuli in the kidney in HFRS. 41 Because these endothelial cells infected with SNV have also been shown to be recognized and lased by an experimental CD8+ hantavirus–specific CTL line, 42 it is possible that the virus affects both target organs independently. These experimental data coupled with clinical cases demonstrating HCPS associated renal disease 28,32 and HFRS associated pulmonary disease 43 suggest potential overlap in pathogenesis between HFRS and HCPS. Although there is no current data that glomerular capillary permeability is increased in HCPS, these data suggest this may need further study.
The relative lack of renal disease in our most severely ill subjects who required ECMO also suggests that renal disease may be more complex. The apparent protective effect of ECMO is counterintuitive, because patients who receive ECMO support are uniformly hemodynamically unstable and renal sequelae have been described in children and adults on ECMO for other reasons. 44–46 The ECMO itself is also felt to be somewhat nephrotoxic, producing significant vascular trauma through placement of large bore arterial access and by producing pigment nephropathy from hemolysis in the extra-corporeal circuit. 44–46
ECMO may have long-term benefits for several other reasons. The pathophysiology of hantavirus infection is most likely a result of the host immune response. Although there is a lack of hantavirus specific cytopathologic effects, cytotoxic T cells are found in tissues during disease. 27,41 In both SNV and Andes viruses, a higher viral load at time of presentation is associated with a more severe clinical course and increased mortality, whereas rapid production of neutralizing antibody is associated with improved survival. 47 Furthermore, both live and UV inactivated hantavirus particles are capable of inducing interferon stimulating genes, suggesting that viral replication itself is not needed to stimulate an appropriate host immune response. 48 Because ECMO requires large volumes of blood and blood product, it may serve to dilute virus and therefore decrease host response and immune mediated tissue injury. The ECMO process may also remove or filtrate pro-inflammatory cytokines and chemokines induced by the virus. Finally, from a physiologic perspective, ECMO may improve blood flow and protect the kidneys during cardiovascular collapse. Because ECMO has been shown to improve overall survival in patients with severe HCPS, 49 future studies comparing CD8 + CTL responses and cytokine production in patients treated with ECMO and those treated with more conventional methods may explain ECMO’s potential role in protection.
We are limited in our conclusions by the small size of this cohort. Given the low incidence and high mortality of HCPS this limitation is understandable and unavoidable. Follow-up and monitoring were difficult because of the geographic diversity of this cohort, and also limited the study to intermittent clinical monitoring. The proteinuria described here was generally mild and cannot be specifically linked to the prior Hantavirus infection or acute illness. Orthostatic proteinuria, a relatively benign process generally described in children and young adults, 50 cannot be ruled out. However, given the fact that timed urine specimens were collected in an inpatient setting where activity was kept to a minimum, we feel this is an unlikely confounder. Intermittent proteinuria or “functional proteinuria” are also possibilities given the limited number of urine specimens collected in many patients, but these processes tend to be self-limited. 51
Another limitation is the assertion of chronicity on these renal abnormalities given the limited follow-up in some individuals. A more definitive answer with regard to the nature of this renal involvement could be better ascertained through biopsy and histopathologic evaluation. Because no patient met criteria for biopsy, pathologic specimens were not available to determine potential causes for renal sequelae. Future studies are planned, but the relative infrequency of this disease makes prospective studies difficult.
These results provide intriguing information about long-term renal outcomes following HCPS, and to the potential of overlap between Old World and New World hantavirus disease and pathogenesis. We feel that these data support careful screening of patients with HCPS for long-term renal involvement. Referral to a nephrologist should be considered in all patients with evidence of renal involvement, particularly patients with GFR < 60 and proteinuria greater than one gram. Studies evaluating renal involvement during acute infection and renal sequelae caused by New World hantavirus are needed.
Acknowledgments
We acknowledge the significant contributions of the following individuals and groups: Cindy Wootton, the nurses and support staff of the University of New Mexico GCRC, the New Mexico State Department of Health, and the members of the Hantavirus Long-term Survivors Study Group.
Financial support: This study was supported by DHHS/NIH/NCRR/GCRC Grant #5M01RR00997 (DG) and the Dedicated Research Funds of the University of New Mexico Health Science Center. Dr. Pergam is supported by NIAID Grant # T32 AI007044 and the Joel Meyers Foundation.
References
- 1.Bruno P, Hassell LH, Brown J, Tanner W, Lau A. The protean manifestations of hemorrhagic fever with renal syndrome. A retrospective review of 26 cases from Korea. Ann Intern Med. 1990;113:385–391. doi: 10.7326/0003-4819-113-5-385. [DOI] [PubMed] [Google Scholar]
- 2.Vapalahti O, Mustonen J, Lundkvist A, Henttonen H, Plyusnin A, Vaheri A. Hantavirus infections in Europe. Lancet Infect Dis. 2003;3:653–661. doi: 10.1016/s1473-3099(03)00774-6. [DOI] [PubMed] [Google Scholar]
- 3.Mckee K, LeDuc J, Peters CJ. Hantaviruses. In: Belshe RB, editor. Textbook of Human Virology. St. Louis: Mosby Year Book; 1991. pp. 615–632. [Google Scholar]
- 4.Khan A, Khan AS. Hantaviruses: a tale of two hemispheres. Panminerva Med. 2003;45:43–51. [PubMed] [Google Scholar]
- 5.Duchin JS, Koster FT, Peters CJ, Simpson GL, Tempest B, Zaki SR, Ksiazek TG, Rollin PE, Nichol S, Umland ET, Moolenaar RL, Reef SE, Nolte KB, Gallaher MM, Butler JC, Breiman RF for the Hantavirus Study Group. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. The Hantavirus Study Group. N Engl J Med. 1994;330:949–955. doi: 10.1056/NEJM199404073301401. [DOI] [PubMed] [Google Scholar]
- 6.Settergren B. Clinical aspects of nephropathia epidemica (Puumala virus infection) in Europe: a review. Scand J Infect Dis. 2000;32:125–132. doi: 10.1080/003655400750045204. [DOI] [PubMed] [Google Scholar]
- 7.Faulde M, Sobe D, Kimmig P, Scharninghausen J. Renal failure and hantavirus infection in Europe. Nephrol Dial Transplant. 2000;15:751–753. doi: 10.1093/ndt/15.6.751. [DOI] [PubMed] [Google Scholar]
- 8.Cosgriff TM. Mechanisms of disease in Hantavirus infection: pathophysiology of hemorrhagic fever with renal syndrome. Rev Infect Dis. 1991;13:97–107. doi: 10.1093/clinids/13.1.97. [DOI] [PubMed] [Google Scholar]
- 9.Mustonen J, Helin H, Pietila K, Brummer-Korvenkontio M, Hedman K, Vaheri A, Pasternack A. Renal biopsy findings and clinicopathologic correlations in nephropathia epidemica. Clin Nephrol. 1994;41:121–126. [PubMed] [Google Scholar]
- 10.Makela S, Ala-Houhala I, Mustonen J, Koivisto AM, Kouri T, Turjanmaa V, Vapalahti O, Vaheri A, Pasternack A. Renal function and blood pressure five years after puumala virus-induced nephropathy. Kidney Int. 2000;58:1711–1718. doi: 10.1046/j.1523-1755.2000.00332.x. [DOI] [PubMed] [Google Scholar]
- 11.Rubini ME, Jablon S, Mc DM. Renal residuals of acute epidemic hemorrhagic fever. Arch Intern Med. 1960;106:378–387. doi: 10.1001/archinte.1960.03820030066011. [DOI] [PubMed] [Google Scholar]
- 12.Miettinen MH, Makela SM, Ala-Houhala IO, Huhtala HS, Koobi T, Vaheri AI, Pasternack AI, Porsti IH, Mustonen JT. Ten-year prognosis of Puumala hantavirus-induced acute interstitial nephritis. Kidney Int. 2006;69:2043–2048. doi: 10.1038/sj.ki.5000334. [DOI] [PubMed] [Google Scholar]
- 13.Bharadwaj M, Nofchissey R, Goade D, Koster F, Hjelle B. Humoral immune responses in the hantavirus cardiopulmonary syndrome. J Infect Dis. 2000;182:43–48. doi: 10.1086/315657. [DOI] [PubMed] [Google Scholar]
- 14.Hjelle B, Jenison S, Torrez-Martinez N, Herring B, Quan S, Polito A, Pichuantes S, Yamada T, Morris C, Elgh F, Lee HW, Artsob H, Dinello R. Rapid and specific detection of Sin Nombre virus antibodies in patients with hantavirus pulmonary syndrome by a strip immunoblot assay suitable for field diagnosis. J Clin Microbiol. 1997;35:600–608. doi: 10.1128/jcm.35.3.600-608.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. [Accessed June 14, 2008];Hantavirus Pulmonary Syndrome Labratory-confirmed Diagnosis. Available at: http://www.cdc.gov/ncidod/diseases/hanta/hps/noframes/hpsslideset/hpsslides13-24.htm.
- 16.Centers for Disease Control and Prevention. Case definitions for infectious conditions under public health surveillance. MMWR Recomm Rep. 1997;46:1–55. [PubMed] [Google Scholar]
- 17.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 18.Silkenson J, Kasiske B. Laboratory assessment of kidney disease. In: Brenner B, editor. Brenner and Rector’s Textbook of Nephrology. Phildelphia: Saunders; 2004. pp. 1107–1128. [Google Scholar]
- 19.Korevaar JC, Jansen MA, Dekker FW, Boeschoten EW, Bossuyt PM, Krediet RT. Evaluation of DOQI guidelines: early start of dialysis treatment is not associated with better health-related quality of life. National Kidney Foundation-Dialysis Outcomes Quality Initiative. Am J Kidney Dis. 2002;39:108–115. doi: 10.1053/ajkd.2002.29896. [DOI] [PubMed] [Google Scholar]
- 20.Torrez-Martinez N, Bharadwaj M, Goade D, Delury J, Moran P, Hicks B, Nix B, Davis JL, Hjelle B. Bayou virus-associated hantavirus pulmonary syndrome in Eastern Texas: identification of the rice rat, Oryzomys palustris, as reservoir host. Emerg Infect Dis. 1998;4:105–111. doi: 10.3201/eid0401.980115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hjelle B, Goade D, Torrez-Martinez N, Lang-Williams M, Kim J, Harris RL, Rawlings JA. Hantavirus pulmonary syndrome, renal insufficiency, and myositis associated with infection by Bayou hantavirus. Clin Infect Dis. 1996;23:495–500. doi: 10.1093/clinids/23.3.495. [DOI] [PubMed] [Google Scholar]
- 22.Crowley MR, Katz RW, Kessler R, Simpson SQ, Levy H, Hallin GW, Cappon J, Krahling JB, Wernly J. Successful treatment of adults with severe Hantavirus pulmonary syndrome with extracorporeal membrane oxygenation. Crit Care Med. 1998;26:409–414. doi: 10.1097/00003246-199802000-00047. [DOI] [PubMed] [Google Scholar]
- 23.Glass GE, Watson AJ, LeDuc JW, Kelen GD, Quinn TC, Childs JE. Infection with a ratborne hantavirus in US residents is consistently associated with hypertensive renal disease. J Infect Dis. 1993;167:614–620. doi: 10.1093/infdis/167.3.614. [DOI] [PubMed] [Google Scholar]
- 24.Glass G, Childs JE, Watson AJ, LeDuc JW. Association of chronic renal disease, hypertension, and infection with a ratborne hantavirus. Arch Virol Suppl. 1990;1:69–80. [Google Scholar]
- 25.Botros BA, Sobh M, Wierzba T, Arthur RR, Mohareb EW, Frenck R, El Refaie A, Mahmoud I, Chapman GD, Graham RR. Prevalence of hantavirus antibody in patients with chronic renal disease in Egypt. Trans R Soc Trop Med Hyg. 2004;98:331–336. doi: 10.1016/S0035-9203(03)00063-4. [DOI] [PubMed] [Google Scholar]
- 26.Settergren B, Stegmayr BG, Elgh F. Lack of serologic evidence for an association between Hantavirus infection and end-stage renal disease. Nephron. 1998;80:93. doi: 10.1159/000045139. [DOI] [PubMed] [Google Scholar]
- 27.Zaki SR, Greer PW, Coffield LM, Goldsmith CS, Nolte KB, Foucar K, Feddersen RM, Zumwalt RE, Miller GL, Khan AS, Rollin PE, Ksiazek TG, Nichol ST, Mahy BWJ, Peters CJ. Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am J Pathol. 1995;146:552–579. [PMC free article] [PubMed] [Google Scholar]
- 28.Dara SI, Albright RC, Peters SG. Acute sin nombre hantavirus infection complicated by renal failure requiring hemodialysis. Mayo Clin Proc. 2005;80:703–704. doi: 10.4065/80.5.703. [DOI] [PubMed] [Google Scholar]
- 29.Nolte KB, Feddersen RM, Foucar K, Zaki SR, Koster FT, Madar D, Merlin TL, McFeeley PJ, Umland ET, Zumwalt RE. Hantavirus pulmonary syndrome in the United States: a pathological description of a disease caused by a new agent. Hum Pathol. 1995;26:110–120. doi: 10.1016/0046-8177(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 30.Khan AS, Gaviria M, Rollin PE, Hlady WG, Ksiazek TG, Armstrong LR, Greenman R, Ravkov E, Kolber M, Anapol H, Sfakianaki ED, Nichol ST, Peters CJ, Khabbaz RF. Hantavirus pulmonary syndrome in Florida: association with the newly identified Black Creek Canal virus. Am J Med. 1996;100:46–48. doi: 10.1016/s0002-9343(96)90010-8. [DOI] [PubMed] [Google Scholar]
- 31.Khan AS, Spiropoulou CF, Morzunov S, Zaki SR, Kohn MA, Nawas SR, McFarland L, Nichol ST. Fatal illness associated with a new hantavirus in Louisiana. J Med Virol. 1995;46:281–286. doi: 10.1002/jmv.1890460320. [DOI] [PubMed] [Google Scholar]
- 32.Passaro DJ, Shieh WJ, Hacker JK, Fritz CL, Hogan SR, Fischer M, Hendry RM, Vugia DJ. Predominant kidney involvement in a fatal case of hantavirus pulmonary syndrome caused by Sin Nombre virus. Clin Infect Dis. 2001;33:263–264. doi: 10.1086/321832. [DOI] [PubMed] [Google Scholar]
- 33.Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens. 2004;13:1–7. doi: 10.1097/00041552-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Block CA, Schoolwerth AC. The epidemiology and outcome of acute renal failure and the impact on chronic kidney disease. Semin Dial. 2006;19:450–454. doi: 10.1111/j.1525-139X.2006.00206.x. [DOI] [PubMed] [Google Scholar]
- 35.Finn WF. Recovery for acute renal failure. In: Molitoris BA, Finn WF, editors. Acute Renal Failure: A Companion to Brenners and Rector’s the Kidney. 1. Philadelphia: WB Saunders; 2001. pp. 425–450. [Google Scholar]
- 36.Taal M, Luyck V, Brenner B. Adaption to nephron losss. In: Brenner B, editor. Brenner and Rector’s Textbook of Nephrology. Philadelphia: Saunders; 2004. pp. 1954–1997. [Google Scholar]
- 37.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281:F887–F899. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 38.Pensiero MN, Sharefkin JB, Dieffenbach CW, Hay J. Hantaan virus infection of human endothelial cells. J Virol. 1992;66:5929–5936. doi: 10.1128/jvi.66.10.5929-5936.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yanagihara R, Silverman DJ. Experimental infection of human vascular endothelial cells by pathogenic and nonpathogenic hantaviruses. Arch Virol. 1990;111:281–286. doi: 10.1007/BF01311063. [DOI] [PubMed] [Google Scholar]
- 40.Gavrilovskaya IN, Peresleni T, Geimonen E, Mackow ER. Pathogenic hantaviruses selectively inhibit beta3 integrin directed endothelial cell migration. Arch Virol. 2002;147:1913–1931. doi: 10.1007/s00705-002-0852-0. [DOI] [PubMed] [Google Scholar]
- 41.Temonen M, Mustonen J, Helin H, Pasternack A, Vaheri A, Holthofer H. Cytokines, adhesion molecules, and cellular infiltration in nephropathia epidemica kidneys: an immunohistochemical study. Clin Immunol Immunopathol. 1996;78:47–55. doi: 10.1006/clin.1996.0007. [DOI] [PubMed] [Google Scholar]
- 42.Hayasaka D, Maeda K, Ennis FA, Terajima M. Increased permeability of human endothelial cell line EA.hy926 induced by hantavirus-specific cytotoxic T lymphocytes. Virus Res. 2007;123:120–127. doi: 10.1016/j.virusres.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Schutt M, Meisel H, Kruger DH, Ulrich R, Dalhoff K, Dodt C. Life-threatening Dobrava hantavirus infection with unusually extended pulmonary involvement. Clin Nephrol. 2004;62:54–57. doi: 10.5414/cnp62054. [DOI] [PubMed] [Google Scholar]
- 44.Sell LL, Cullen ML, Whittlesey GC, Lerner GR, Klein MD. Experience with renal failure during extracorporeal membrane oxygenation: treatment with continuous hemofiltration. J Pediatr Surg. 1987;22:600–602. doi: 10.1016/s0022-3468(87)80107-0. [DOI] [PubMed] [Google Scholar]
- 45.Meyer DM, Jessen ME. Results of extracorporeal membrane oxygenation in neonates with sepsis. The Extracorporeal Life Support Organization experience. J Thorac Cardiovasc Surg. 1995;109:419–425. doi: 10.1016/S0022-5223(95)70272-5. discussion 425–427. [DOI] [PubMed] [Google Scholar]
- 46.Kolla S, Awad SS, Rich PB, Schreiner RJ, Hirschl RB, Bartlett RH. Extracorporeal life support for 100 adult patients with severe respiratory failure. Ann Surg. 1997;226:544–564. doi: 10.1097/00000658-199710000-00015. discussion 565–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao R, Yang S, Koster F, Ye C, Stidley C, Hjelle B. Sin Nombre viral RNA load in patients with hantavirus cardiopulmonary syndrome. J Infect Dis. 2006;194:1403–1409. doi: 10.1086/508494. [DOI] [PubMed] [Google Scholar]
- 48.Prescott J, Ye C, Sen G, Hjelle B. Induction of innate immune response genes by Sin Nombre hantavirus does not require viral replication. J Virol. 2005;79:15007–15015. doi: 10.1128/JVI.79.24.15007-15015.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dietl CA, Wernly JA, Pett SB, Yassin SF, Sterling JP, Dragan R, Milligan K, Crowley MR. Extracorporeal membrane oxygenation support improves survival of patients with severe hantavirus cardiopulmonary syndrome. J Thorac Cardiovasc Surg. 2008;135:579–584. doi: 10.1016/j.jtcvs.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 50.Hogg RJ. Adolescents with proteinuria and/or the nephrotic syndrome. Adolesc Med Clin. 2005;16:163–172. doi: 10.1016/j.admecli.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 51.Wingo CS, Clapp WL. Proteinuria: potential causes and approach to evaluation. Am J Med Sci. 2000;320:188–194. doi: 10.1097/00000441-200009000-00010. [DOI] [PubMed] [Google Scholar]