Abstract
Cleaved high molecular weight kininogen (HKa), as well as its domain 5 (D5), inhibits migration and proliferation induced by angiogenic factors and induces apoptosis in vitro. To study its effect on tube formation we utilized a collagen-fibrinogen, three-dimensional gel, an in vitro model of angiogenesis. HKa, GST-D5 and D5 had a similar inhibitory effect of tube length by 90±4.5%, 86±5.5% and 77±12.9%, respectively. D5-derived synthetic peptides: G440-H455 H475-H485 and G486-K502 inhibited tube length by 51±3.7%, 54±3.8% and 77±1.7%, respectively. By a comparison of its inhibitory potency and its sequences, a functional sequence of HKa was defined to G486-G496. PP2, a Src family kinase inhibitor, prevented tube formation in a dose-dependent manner (100–400nM), but PP3 at 5 μM, an inactive analogue of PP2, did not. HKa and D5 inhibited Src 416 phosphorylation by 62±12.3% and 83±6.1%, respectively. The C-terminal Src kinase (Csk) inhibits Src kinase activity. Using a siRNA to Csk, expression of Csk was down-regulated by 86±7.0%, which significantly increased tube length by 27±5.8%. The addition of HKa and D5 completely blocked this effect. We further showed that HKa inhibited Src family kinase activity by disrupting the complex of uPAR, αvβ3 integrin and Src. Our results indicate that the anti-angiogenic effect of HKa and D5 is mediated at least in part through Src family kinases and identify a potential novel target for therapeutic inhibition of neovascularization in cancer and inflammatory arthritis.
Keywords: Angiogenesis, C-terminal Srk kinases, uPAR, αvβ3, Intergin, Src family kinase, kininogen
INTRODUCTION
Angiogenesis is the formation of new capillaries from preexisting blood vessels [1]. Extracellular matrix (ECM) proteins play an important role in physiological and pathological processes such as wound healing and tumor angiogenesis. Elevated tissue factor (TF) expression has been frequently observed in many types of tumor cells [2]. The TF-activated coagulation cascade leads to thrombin formation, which in turn promotes the pericellular deposition of fibrin in which thrombin is trapped [3, 4]. The resulting exposed collagen and fibrin/fibrinogen fragments in tissues form a provisional matrix suitable for attachment and invasion of tumor cells. This mixture also facilitates endothelial cells to invade the tumor in an attempt to provide the neoplastic mass with the necessary vasculature for growth [5]. Thus, anti-angiogenic therapy is an important goal for cancer therapy [1].
High molecular weight kininogen (HK) is a plasma protein that was initially identified as a precursor of bradykinin, a potent vasodilator that regulates many vascular functions. HK is recognized as a multifunctional protein that plays important roles in many pathophysiologic processes, such as fibrinolysis, thrombosis, inflammation and coagulation [6, 7]. HK can specifically bind to endothelial cells, where it can be cleaved by plasma kallikrein to release bradykinin [8]. The remaining portion of the molecule, cleaved HK or HKa, contains a heavy chain and light chain linked through a disulfide bond. Domain 5 (also known as kininostatin) is located in the light chain of HKa. We have shown that HKa as well as D5 inhibited migration, proliferation [9] and induced apoptosis in vitro [10, 11]. In ovo, these proteolytic derivatives of HK inhibit neovascularization [9, 10]. In this study, compared the inhibitory effect of HKa with D5 (its functional domain) and also used different synthetic peptides derived from D5 to narrow down the D5′s sub-domains which could exert the inhibitory effect of HKa on tube formation. Knowledge is incomplete regarding the HKa or D5-driven signal-transduction pathways leading to the inhibition of capillary morphogenesis. In order to delineate this mechanism, we focused on Src family kinases (SFKs). The role of SFKs in angiogenesis has been well appreciated. Recent studies from several other laboratories [12] demonstrated that SFKs promoted the formation of tubes and prevented their regression. SFKs suppressed regression by activating the ERK pathway that antagonized the Rho/ROCK pathway. In vivo, in a rat model of retinopathy of prematurity, a pronounced increase of retinal SFK Tyr-416 phosphorylation was observed that was specifically associated with pathologic angiogenesis [13]. Pyrazolo-pyrimidine-derived c-Src inhibitor reduces angiogenesis by suppressing vascular endothelial growth factor production and ERK activity [14]. Integrins have been shown to direct the activation of SFKs. The engagement of integrins by extracellular matrix ligands triggers “outside-in” signals that activate SFKs by direct interaction with the integrin β cytoplasmic domain [15]. An antibody to avβ3 can block human breast cancer growth and angiogenesis in human skin [16]. It has been well documented that uPAR associates with integrin avβ3 and a5β1 by its domain 2 and 3 and modulates the “outside-in” signal of integrins [17, 18]. Since uPAR is a major receptor of HKa and D5, we will now test this hypothesis that the inhibitory effect of HKa and D5 is by down-regulating the activity of Src family kinases (SFKs).
Materials and Methods
Reagents and antibodies
PP2, PP3, fibrinogen and vascular endothelial growth factor (VEGF) were purchased from Calbiochem (San Diego, CA). FGF-2 was purchased from Life Technologies (Grand Island, NY). Hiperfect transfect reagent, siRNA against Csk and scrambled siRNA were purchased from Qiagen (Valencia, CA). Phorbol myristate acetate (PMA) and monoclonal antibody against actin were purchased from Sigma Co. (St. Louis, MO). Collagen solution (purified type I collagen), monoclonal antibodies against caveolin-1 and caveolin-1 (pY14), and antibody against Csk were purchased from BD Biosciences (Bedford, MA). Anti-uPAR mAb was from American Diagnostica Inc (Stamford, CT). Polyclonal antibody against αvβ3 integrin (LM609), against α5β1, and monoclonal antibody against integrin β1 subunit were from Chemicon (Temecula, CA). Monoclonal antibody against integrin αv was from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-active Src (p416) antibody was from Cell Signaling (Beverly, MA). Monoclonal antibody against tubulin was from Oncogene (Boston, MA).
Cell culture
HUVECs and endothelial cell culture media were purchased from Cambrex (Walkersville, MD). HUVECs were maintained in endothelial cell growth medium (EGM) containing 10% fetal calf serum in cell culture dishes coated with 0.2% gelatin at 37°C in a humidified incubator (5% CO2, 95% air). Cells were split at a ratio of 1:3 every passage. Cells from three to eight passages were used in this study.
Coating of fibrin, fibrinogen or collagen for two dimension (2D) endothelial cell culture
Coating of fibrin: 12-well microplates were coated with fibrinogen mixed with 0.5U/ml (final concentration) of thrombin for 1h. The fibrin excess was aspirated. Plates were washed three times with Dulbecco’s Phosphate Buffered Saline (DPBS) and incubated with EGM for 1h in order to inactivate any residual thrombin. Coating of fibrinogen or collagen: 12-well microplates were coated with fibrinogen or collagen for 1h. The solution of fibrinogen or collagen was removed by aspiration. Plates washed three times with DPBS. HUVECs (0.12×106) were placed on each well previously coated with fibrin, fibrinogen or collagen and incubated for 3h. The medium was replaced with endothelial basal medium (EBM) plus 15 μM ZnCl2 and 1% fetal bovine serum and incubated for an additional hour. FGF-2 (20 ng/ml) and HKa or D5 (100 nM) were added into medium and incubated for 26h. HUVECs were fixed with 4% formaldehyde for 20 min and photographed with a digital camera.
Preparation of collagen gel matrices and 3 dimensional (3D) cell culture
Collagen and fibrinogen are two matrix proteins that have been used in 3D cell culture. Collagen is a major component of the basement membrane. Fibrin, the cleaved product of fibrinogen by thrombin, constitutes the predominant protein of the provisional matrix at wound sites where neovascularization is a prominent event. Therefore, collagen and fibrinogen/fibrin serve as scaffold proteins for vessel formation at angiogenic sites. In this study, 3D cell culture was performed according to the method initially described by Davis and Camarillo [19] with modifications as following [20]: Collagen gel solution was prepared on ice as: 3.5 mg/ml collagen solution, 7 mg/ml fibrinogen, 1×M199 medium, and 10 mM HEPES adjusted pH to 7.4 by 7.5% NaHCO2. HUVECs grown in EGM on 2D cell culture dishes (100% confluence) were detached with a trypsin solution (0.025% trypsin and 0.01% EDTA). After washing with EGM to inactivate trypsin, cells were resuspended in EBM containing 40 ng/ml FGF-2, 10 ng/ml VEGF and 25 nM PMA, referred to as angiogenic stimulators. The gel and cell solutions were mixed together in a ratio 2:1 (by volume). The resulted cell suspension contained a final concentration of 1.17 mg/ml of collagen, 2.34 mg/ml of fibrinogen and 1×106 cells/ml. Cells suspension (200μl) was transferred to a well of 48-well tissue culture plate and kept at 37°C for 20 minutes to allow gel formation. The gel matrices were then filled with EBM medium containing angiogenic stimulators. Cells embedded in gel matrices were incubated at 37°C in a humidified incubator (5% CO2, 95% air) for the time intervals indicated.
Preparation of recombinant D5 of HK and synthetic peptides of D5
Glutathione-S-transferase (GST) and recombinant GST-D5 were prepared as previously described [9]. Briefly, GST was removed from GST-D5 by digestion with thrombin, which was neutralized with D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (PPACK). Free GST was removed with Glutathione Sepharose 4 Fast Flow column (Amersham Pharmicia Biotech, Piscataway, NJ). Residual thrombin and PPACK were removed with Amicon Centriprep YM-30 (Millipore Corp., Bedford, MA). Using YM-10, D5 solution was exchanged into 50 mM HEPES, 150 mM NaCl, pH 7.5 buffer. Endotoxin levels in the preparations were determined with the chromogenic limulus amebocyte lysate assay by use of an endotoxin testing kit (Cambrex, Walkersville, MD). Endotoxin level in D5 was below detectable limits (<0.1U/ml). D5 was visualized on 20% SDS PAGE and detected by Western blotting as a single band. Synthetic D5-peptides were produced as described previously [9].
Csk siRNA “knock down” experiment
The sequence 5′-CCCAACTGGTACAAAGCCAAA-3″, corresponding to 545–565 nucleotide of human Csk cDNA was the target region. A nonsilencing siRNA (Scrambled siRNA), 5′-AACCTGCGGGAAGAAGTGG-3′, was used as a control [17] Csk siRNA “knock down” experiments were performed according to the manufacturer’s instruction with the following modifications: HUVECs (0.80×106) were seeded into each dish (10 cm2), incubated 3h in 12 ml EGM at 60% confluence. Csk siRNA and transfection reagent “Hiperfect” with the Opti-MEM medium were mixed and incubated at room temperature about 20 min. The mixture was then added into dishes containing a final concentration of 10 nM Csk siRNA. Twenty-four hours later, the medium was replaced with fresh EGM and cells incubated with Csk siRNA again as the first day. On the third day, the medium was replaced with fresh EGM. In the fourth day, cells were split into two sets. Set one was subjected to Western blotting to evaluate Csk expression and quantified by densitometry. Set two was further split into several groups, which were used in 3D gel experiments to evaluate effects of HKa and D5 on tube formation and Src family kinases.
Immunoprecipitation (IP) and Immunoblotting (IB)
HUVECs in matrix gel were washed with ice-cold PBS containing 0.7 mM CaCl2, 0.5 mM MgCl2 and 1mM Na3VO4 prior to harvesting in extraction buffer A: 1% Triton X-100, 60 mM octyl glucoside, 10 mM Tris-HCl, pH 7.6, 50 mM NaCl, 30 mM Na4P207, 50 mM NaF, 1 mM Na3VO4, 2 mM CaCl2, plus mammalian protease inhibitor mixture (Sigma). After solubilization on ice for 15 min with intermittent vortexing, the extract was microcentrifuged for 10 min at 13,000×g and the supernatant recovered.
The complex formation of uPAR with other signaling molecules was determined by immunoprecipitation according to the methods described by Wei et al [18] with some modifications. Cell lysate was incubated with antibodies to αvβ3 or α5β1 followed by incubation of protein A/G beads. The immunoprecipitates were subjected to SDS-PAGE under non-reduced conditions, and immunoblot analysis was performed as described below.
Separately, the immunoprecipitated complex or the cell lysate containing equal amounts of protein (10–20 μg) were solubilized in Laemmli’s sample buffer, separated by SDS-PAGE, and transferred to polyvinylidene difluoride membrane (Millipore, Bedford, MA). The membranes were blocked with 5% milk, 0.05% Tween 20 in Tris-buffered saline, pH 7.5, and probed sequentially with primary and secondary antibodies diluted in the milk-containing buffer. Detection was by enhanced chemiluminescence (PerkinElmer Life Sciences, Shelton, CT) [21, 22]. Analysis of scanned images was performed using Quantity One software (BioRad, Hercules, CA).
Tube length analysis procedure
Endothelial cell tube length was quantified following the protocol published by Yang et al [23]. Experiments were done in triplicates; five digital images per well were taken. The images were analyzed using Image Pro-Plus 4.1 software (Media Cybernetics, Silver Springs, MD). A blinded observer measured the total length of each tube (defined as structures exceeding 100 micrometers in length) that was in clear focus in the image field. In those instances where several tube-like structures merged together or branched, the total length of the tube was calculated as the sum of the length of the individual branches. The results are reported as μm per square millimeter and standardized as percentages. Statistical analyses were performed by One Way Analysis Of Variance (ANOVA) and all pairwise multiple comparison procedures (Student-Newman-Keuls Method).
RESULTS
Effect of HKa, GST-D5 and D5 on tube formation of HUVECs in a collagen-fibrinogen 3D gel
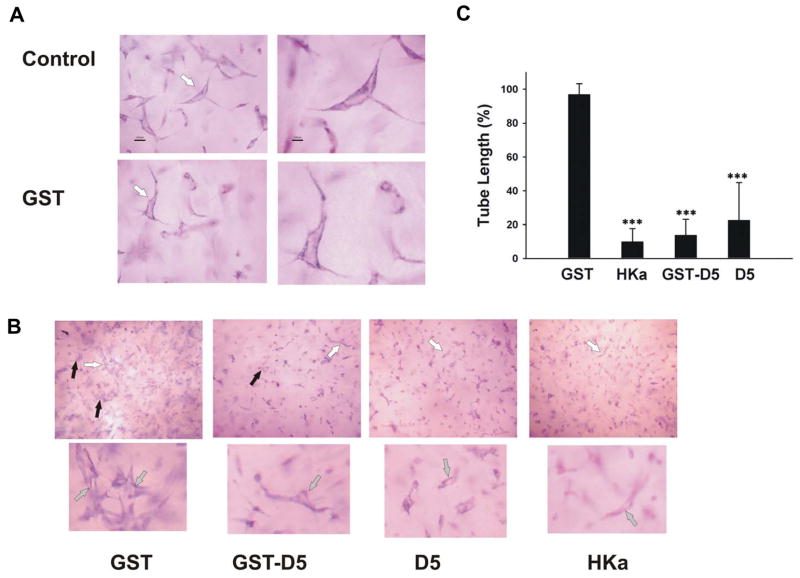
We have shown that HKa and GST-D5 inhibited endothelial cell proliferation and migration as well as induced apoptosis of endothelial cells by targeting uPAR [9]. The inhibitory effect of HKa and GST-D5 on angiogenesis was also shown in the chicken chorioallantoic membrane. However, there are several questions remaining to be answered: what is the potency of inhibitory effect of HKa and D5; who is the sub-domains of D5 which exert its inhibitory effect; what is the mechanism by which HKa and D5 inhibit angiogenesis. In this study, we utilized an in vitro model, a collagen-fibrinogen gel, to address these issues. In this 3D gel, HUVECs underwent a series of morphologic changes. At 6h, small vacuoles appeared in HUVECs (results not shown). These vacuoles coalesced to form tube-like structures containing lumens at 22 hours. This optimal time for tube formation was utilized to determine the effect of HKa and D5 on tube-like structure. The addition of HKa, GST-D5 as well as D5 inhibited the formation of tube-like structures at 22 hours as shown in figure 1A.
Figure 1. The effect of HKa, GST-D5 and D5 on tube formation in 3D gel.
A, HUVECs were cultured in 3D collagen-fibrinogen gel matrices for 22 hours at 37°C (Magnification of control and GST: 200X on left panels and 400X on right panels). White arrows point to lumens. B, HUVECs plus angiogenic stimulators with or without 300 nM GST, HKa, GST-D5 or D5, respectively. The image magnification of GST, HKa, GST-D5 and D5: top is 100X; bottom is 200X. The black arrows point to vacuoles. The lumens which white arrows point to were magnified. The addition of GST to the 3D gel matrices did not modify the appearance of endothelial cell tubes. C, tube formation in B was analyzed as described in Materials and Methods. Each bar represents the mean percentage of tube length ± SEM. (***p<0.005; HKa and D5 compared to control; GST-D5 compared to GST). n=3.
In order to determine the extent of inhibition of tube formation, quantification of tube length was carried out as indicated in Methods and Materials. Our data showed that HKa, GST-D5 and D5 significantly inhibited tube formation by 90±4.5%, 86±5.5% and 77±12.9%, respectively (figure 1B, 1C). No significant difference was found among HKa, GST-D5 and D5, suggesting that GST did not influence the results and HKa as well as D5 had similar effects on inhibition of tube formation.
Effect of synthetic D5-peptides on tube formation
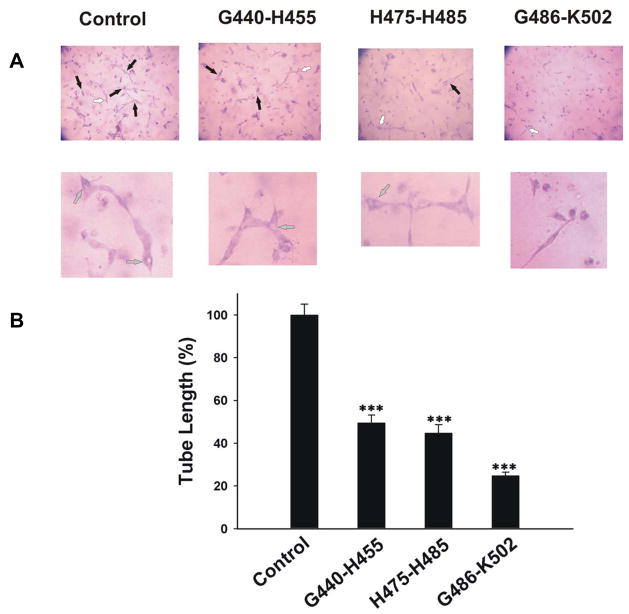
In a previous study [9], we showed that synthetic D5-peptides, such as G486-K502, H475-H485 and G440-H455, had different potency on either migration or proliferation, both of which are critical steps in angiogenesis. The percentages of endothelial cell migration inhibition induced by G486-K502, G440-H455 and H475-H485 were 51, 16 and 12 respectively at 0.2 μM concentration. In contrast, the concentration of G486-K502, G440-H455 and H475-H485 to yield 50% inhibition of endothelial cell proliferation was 55± 15μM, 0.11± 0.08μM and 1.1± 0.5μM, respectively [9]. The same peptides were evaluated in 3D collagen-fibrinogen gel for their effect on tube formation. In figure 2, G440-H455, H475-H485 and G486-K502 significantly inhibited tube formation by 51±3.7%, 54±3.8% and 77±1.7%, respectively. There were significant differences when comparing G486-K502 to either G440-H455 or H475-H485. No significant difference was found between G440-H455 and H475-H485.
Figure 2. The effect of D5 peptides G440-H455, G486-K502 and H475-H485 on tube formation in 3D gel.
A, HUVECs were cultured in 3D collagen-fibrinogen gel matrices in the presence of angiogenic stimulators plus 300 nM D5 peptides. The magnification of images: top is 100X; bottom is 200X. The black arrows point to vacuoles. The lumens which white arrows point to were magnified. B, the analysis of tube formation was similar to figure 1C. Each bar represents the mean percentage of tube length ± SEM. (***p<0.005 compared to control). n=3.
Role of Src family kinases in 3D gel and effect of HKa and D5 on Src family kinases
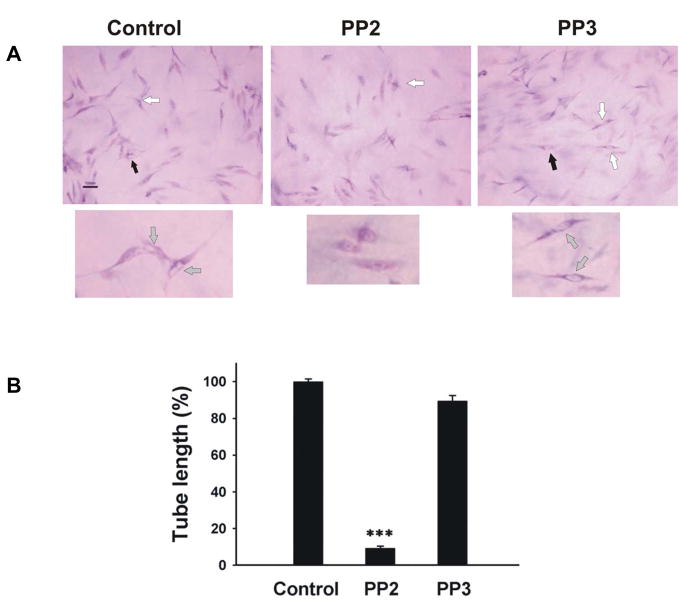
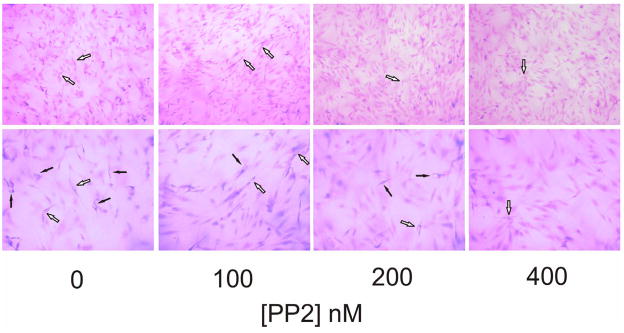
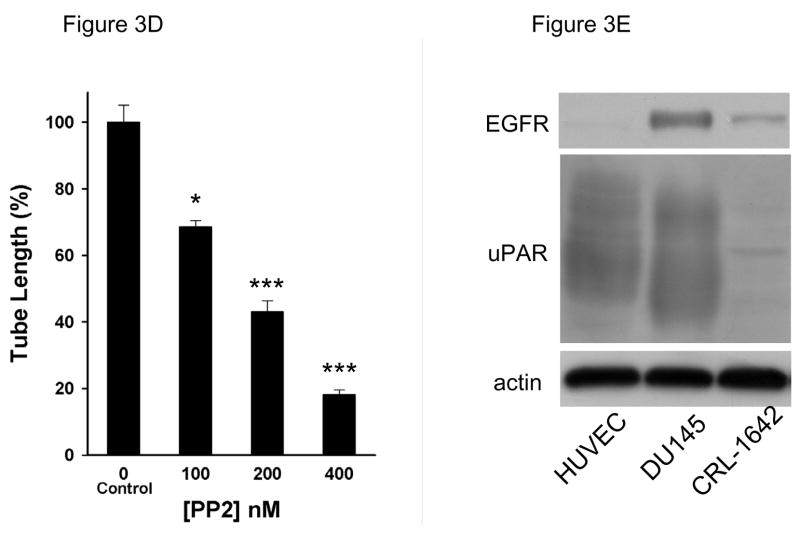
Liu et al demonstrated that collagen initiates capillary morphogenesis, which coincides with activation of Src family kinases [24]. A novel Src kinase inhibitor AZM475271 inhibit metastasis and tumor angiogenesis in human pancreatic cancer by its inhibition of migration and proliferation of HUVECs [25]. In agreement with this finding, PP2 (5 μM), a potent and selective Src family kinase inhibitor, almost completely inhibit tube formation (figure 3A). The tube length was significantly decreased to 9.1±1.1% by PP2 but not by PP3 (to 91±2.9%, figure 3B). Besides inhibiting Src family kinases, PP2 may inhibit other tyrosine kinases (JAK, ZAP-70 and EGF-R). At the concentration used in our studies (5μM) PP2 does not inhibit JAK or ZAP-70. Much higher concentration of PP2 is required to inhibit JAK or ZAP-70 since the IC50 for JAK is 50μM and for ZAP-70 is 100μM. However PP2 could potentially inhibit epidermal growth factor receptor (EGF-R; IC50=480nM), which is expressed by tumor cells and contributes to tumor progression. The IC50 of PP3 needed to inhibit EGF-R kinase is 2.7μM. PP3 at 5μM in our study had no significant effect on tube formation, so, EGF-R did not contribute appreciably to tube formation [26]. This result is expected because normal endothelial cells do not express EGFR and are not responsive to EGF [27]. To further address this issue, we performed a PP2 dose experiment shown as in figure 3C. PP2 dose-dependently inhibited tube formation in a very low concentration at 100, 200 and 400 nM by 69±1.9%, 43±3.2% and 18±1.3%, respectively. As shown as in figure 3E, HUVEC express very high level of uPAR but very low level of EGFR compared to DU145, indicating that the activity of SFK is specifically required in tube formation.
Figure 3. Inhibition of Src family kinase inhibitor PP2 on tube formation in 3D gel.
A, to test the effect of PP2 on tube formation, HUVECs were cultured in 3D collagen-fibrinogen gel matrices in the presence of angiogenic stimulators with or without PP2 and PP3 at 5μM. The images were described as seen in figure 2A. Bar=100 μm. Experiments were repeated three times independently with similar results. B, the analysis of tube formation was performed as described in figure 1C. Each bar represents the mean percentage of tube length ± SEM. (***p<0.005 compared to control). C, The PP2 dose dependent experiments were performed in 0, 100, 200, 400 nM concentration. Black arrows point to vacuoles. White arrows point to lumens. The magnification of upper panel is 100×, bottom is 200×, bar=100 μm. D, The analysis of tube formation was performed as described in figure 1C (n=3). E, Western blotting was performed as described as in “Materials and Methods” The expression of EGFR and uPAR was detected by antibodies against EGFR (Santa Cruz, CA)and uPAR. DU145 (prostate cancer cell) and CRL-1642 (lung carcinoma cell) are from ATCC (Manassas, VA ). The data was represented experiments of 2.
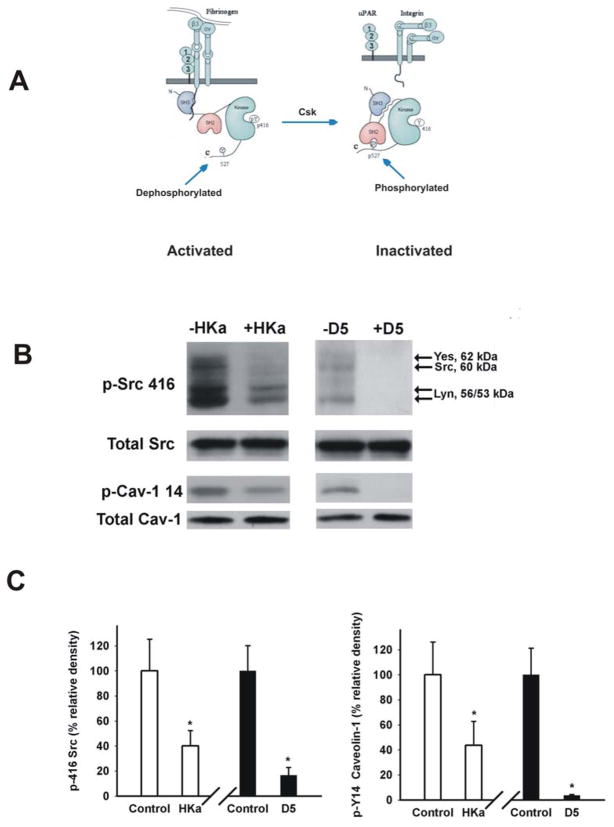
The Src family has nine members including Src, Lck, Hck, Fyn, Blk, Lyn, Fgr, Yes and Yrk. All family members share a conserved domain structure consisting of consecutive SH3, SH2 and tyrosine kinase (SH1) domains [28]. Src family members require phosphorylation within a segment of the kinase domain termed the activation loop (at Tyrosine residue 416) for full catalytic activity [28]. Recent data have demonstrated that Src can be immunoprecipitated by an antibody to the β3 integrin. Furthermore, upon adhesion, the Src co-immunoprecipitating with the β3 integrin is activated. The Src-integrin interaction is mediated by the SH3 domain of Src [15]. Considering that uPAR clusters with integrins [17, 18], we thought that HKa and D5 might interfere with the Src kinase activity by binding to uPAR (figure 4A). As shown in figure 4B, HKa and D5 each significantly decreased the activity of Src family kinases by 62±12.3% and 83±6.1% (figure 4C), respectively as measured by the decrease in phosphorylation of tyrosine 416. The downstream target of Src family kinases, caveolin tyrosine-14 reflected more precisely the inhibition of Src family kinases by HKa and D5. HKa and D5 (figure 4B) decreased caveolin-1 phosphorylation to 44±19.0% and 14±0.7%, respectively (figure 4C). Antibody against Tyr 416 recognized a number of Src family members. Yes (62KD), Src (60KD) and Lyn (56/53 KD) are indicated in figure 4B based on the molecular weight of each member of Src family kinases [29, 30].
Figure 4. HKa and D5 inhibited Tyr 416 phosphorylation of Src family kinases.
A, fibrinogen binds to the integrin αvβ3 or α5β1, resulting the conformational change of integrins. The outside-in signal of integrin αvβ3 induces the association of SH3 domain of Src with the intracellular tail of integrin β3. This association results from Src activation by dephosphorylation of Tyr 527 and auto-phosphorylation of Tyr416. In contrast, Csk inactivates Src by phosphorylating Tyr 527. B, HUVECs were cultured in 3D collagen-fibrinogen gel matrices for 22 hours at 37°C in the presence of angiogenic stimulators plus 300 nM HKa or D5. The gel containing cells was washed once with ice-cold Dulbecco’s PBS and then the cells were lyzed by adding extraction buffer. The gel was removed by centrifugation at 5000×g for 5min at 4°C. The protein concentration was measured by Coomassie Blue Plus (PIERCE). Proteins were separated on SDS PAGE and membranes were probed with an antibody to Src phospho-tyrosine 416 as well as an antibody to caveolin-1 phospho-tyrosine 14. Total Src and caveolin-1 showed equal loading. C, Src phospho-tyrosine 416 and caveolin-1 phospho-tyrosine 14 were quantified by densitometry. The data were collected from three independent experiments, represented as mean ± SEM (*p<0.05 compared to control).
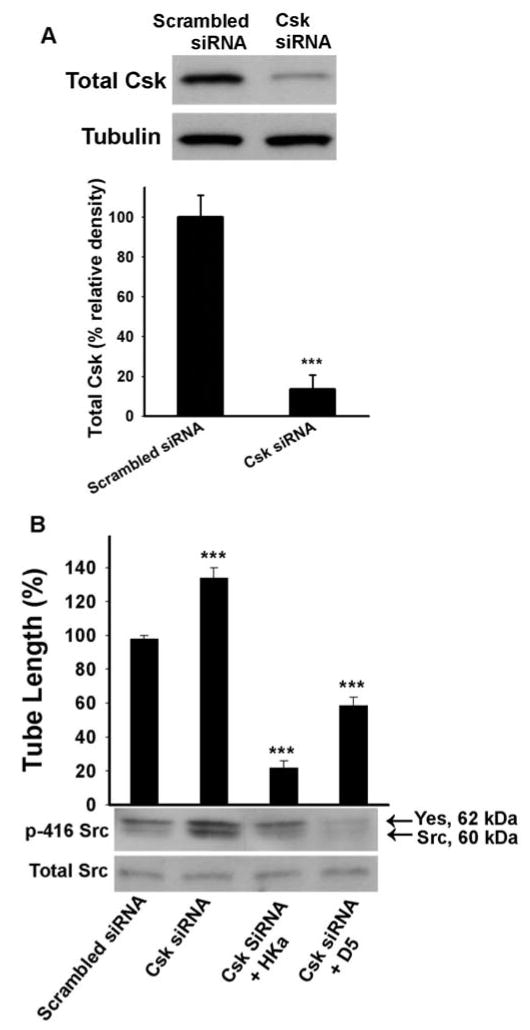
The inactivating phosphorylation on Tyr 527 of Src is carried out by the Src C-terminal kinase (Csk), or its homology kinase (Chk). However, only Csk exists in endothelial cells [31]. The phosphorylation of tyrosine residue 527 at the Src C-terminal tail leads to its intramolecular interaction with the SH2 domain of Src, and promotes an intramolecular SH3 domain-mediated interaction, which inhibits catalytic activity. Mouse knockout studies revealed that complete deficiency of Csk caused embryonic death by day 9.5. Csk −/− embryos display branching defects during vascular development. Remarkable, Csk −/− yolk sacs have fewer but larger blood vessels than the healthy control, suggesting that enhanced activity of Src family kinases will increase the size of vessels [32]. These results suggest that if we can decrease Csk expression in 3D gel, it would increase tube length. We carried out in vitro Csk “knock down” experiments by using a specific siRNA (figure 5A). Csk siRNA decreased Csk expression by 86±7.0% (figure 5A). Consistent with the in vivo studies of others, down regulation of Csk expression significantly enhanced tube length by 27±5.8% (figure 5B). The addition of HKa and D5 not only completely reversed this effect, but also decreased the basal tube length by 78±3.9% and 42±4.8% (respectively) compared with the control group (figure 5B). Genetic targeting Csk results in enhanced Src kinase activity [33]. As shown in figure 5B bottom panel, down-regulating Csk expression would increase the Tyr 416 phosphorylation of Src family kinase by Csk siRNA while HKa and D5 inhibited it.
Figure 5. Effect of HKa and D5 on Csk “knockdown” endothelial cells.
Csk siRNA “knock down” experiments were performed according to the method described in Materials and Methods. A, after 48 hours, one half of the cells were subjected to Western Blotting for total Csk expression evaluation (Top) and densitometry quantification (Bottom). B, the remaining cells were used for 3D experiments with or without 300 nM HKa or D5 (***p<0.005 compared to control). Tube formation was measured as described in figure 1C. Src 416 phosphorylation was probed by an antibody as described in figure 4. Data represents three independent experiments (Mean ± SEM).
Effect of HKa and D5 on endothelial cell adhesion to extracellular matrix
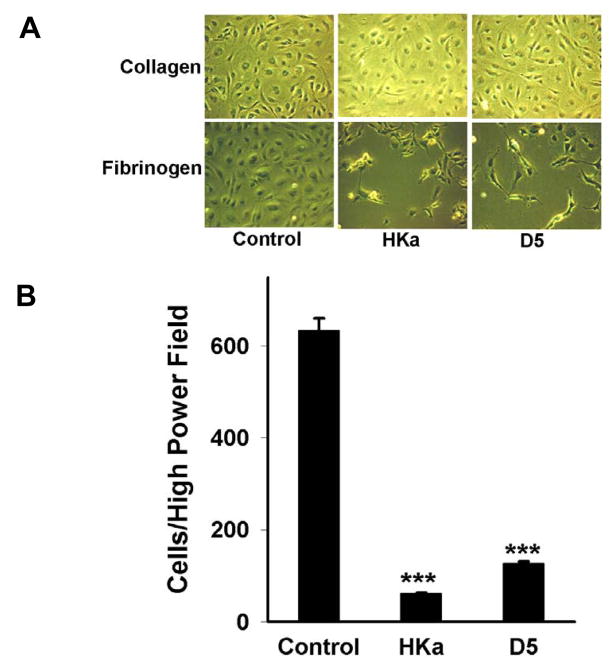
Fibrin/fibrinogen is not a component of the normal constitutive extravascular matrix, while collagen is a constituent of interstitial matrix. Fibrin/fibrinogen is usually present in the provisional matrix found in wound healing and tumor angiogenesis [2]. In the early stage of wound healing, cytokines, growth factors and bradykinin stimulate endothelial cells to form new capillaries, which are surrounded by fibrin, fibrinogen and collagen matrix. In the later stage of wound healing, those capillaries attached to fibrin and fibrinogen, but not collagen, would be expected to regress and disappear. Thus, the HKa inhibitory effect would first start by detach endothelial cells from fibrinogen therefore inducing apoptosis of the detached cells and inhibiting cell migration and spreading. In view of that, we performed cell detachment experiments to confirm our hypothesis (figure 6). HUVECs spread and coalesced well when cultured on fibrin, collagen or fibrinogen. When HUVECs were cultured on fibrinogen (control) and treated with HKa or D5, cell spreading was inhibited and the cells detached from the culture dish matrix (the lower panel of figure 6A). Similar results were found when HUVECs were cultured on fibrin (data not shown). In contrast, neither HKa nor D5 inhibited spreading of HUVECs or caused cell detachment when HUVECs were cultured on collagen alone (the upper panel of figure 6A). HKa decreased the number of cells per high power field by 91±2.4% and D5 by 80±4.2% (figure 6B). Our results suggest that the receptors required by HKa and D5 to exhibit an anti-adhesive effect are expressed on fibrin/fibrinogen but not on collagen, allowing the selective detachment of endothelial cells from fibrin/fibrinogen by HKa and D5.
Figure 6. The antiadhesive effect of HKa and D5 on plates coated with fibrin and fibrinogen.
A series of 12-well microplates were coated with fibrinogen or collagen as described in materials and methods. A, HUVECs 0.12×106 were placed on each well and incubated for 3h. Then, the medium was replaced with EBM plus 15μM ZnCl2 and 1% fetal bovine serum and incubated for an additional 1h. FGF-2 (20 ng/ml) and HKa or D5 (100 nM) were added into medium and incubated for 26h. HUVECs were fixed with 4% formaldehyde for 20min and photographed with a digital camera. Control coated fibrinogen/collagen wells were treated identically except that they received neither D5 nor HKa. Experiments were repeated three times independently. B, three different 200X fields from each well were photographed and the attached cells counted. Results are plotted as mean cell number± SEM (***p<0.005 compared to control).
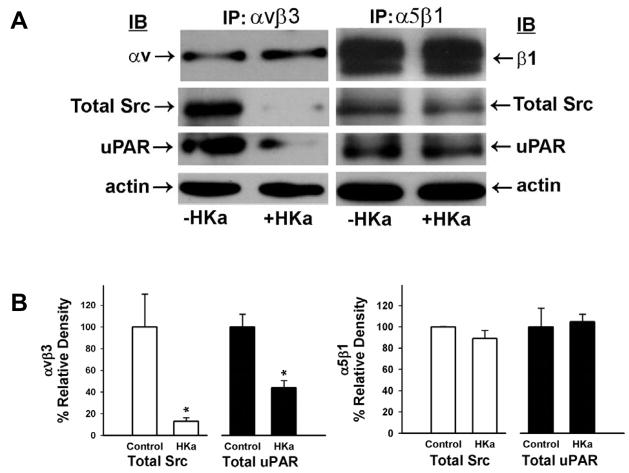
Several receptors have been implicated in mediating fibrinogen binding to endothelial cells. These include αvβ3 and α5β1 integrins [34]. Receptors for collagen are the β1 containing integrins α1β1, α2β1, α10β1 and α11β1 [35]. None of them has been shown to associate with uPAR. However, several papers reported that uPAR associated with αvβ3, α3β1 and α5β1 integrins [36]. Since HKa and D5 selectively detached endothelial cells from fibrinogen but not from collagen, we wondered whether αvβ3 or α5β1 integrin plays a role in cell detachment and tube formation. As shown in figure 7A, cell lysates from 3D gels were precipitated by an antibody to either αvβ3 or α5β1, indicating that uPAR, αvβ3 or α5β1, and Src form a complex. However, HKa prevents the antibody to αvβ3 from precipitating Src by 87±3.3% and uPAR by 56±6.4% but has not effect on immunoprecipitation by the antibody to α5β1 (Figure 7B). The presence of integrin αvβ3 and α5β1 was confirmed by probing the immunoprecipitates with anti-integrin αv or β1 subunit, respectively.
Figure 7. The complex disruption of uPAR-integrin-Src complex by HKa.
A, HUVECs were cultured in 3D collagen-fibrinogen gel matrices for 22 hours at 37°C in the presence of angiogenic stimulators with or without 300 nM HKa. The gel containing cells was washed once with ice-cold Dulbecco’s PBS and then the cells were lyzed by adding extraction buffer. The gel was removed by centrifugation at 5000×g for 5min at 4°C. The protein concentration was measured by Coomassie Blue Plus. Immunoprecipitation procedures were performed as described in Materials and Methods using antibodies to αvβ3 or α5β1. β-actin showed equal protein loading. B, Src and uPAR to αvβ3 (n=3) and α5β1 (n=4) were quantified by densitometry. The data were represented as mean ± SEM (*p<0.05 compared to control).
Because uPAR can form complexes with several integrins, including α3β1, α5β1 and αvβ3 [36], it is possible that HKa or D5 disrupt integrins “outside-in” signaling pathways by dissociating those complexes.
DISCUSSION
In previous studies, we showed that HKa can disrupt the uPAR-integrin complex [37], but no evidence has been provided to illustrate the downstream signaling events that were modified by the HKa-uPAR-integrin complex interaction. For the first time, we demonstrate that HKa and its D5 domain inhibit Src family kinase 416 phosphorylation, which reflects the Src family kinase activity, as well as caveolin-1 14 phosphorylation, which is a downstream effector of Src Kinase. Down-regulating Csk expression increases the Src family kinase activity as shown by increase in Src 416 phosphorylation and vessel size as reflected by increase in tube length. HKa and D5 completely reversed this effect. We further demonstrate that HKa disrupts the uPAR-αvβ3-Src complex, but not the uPAR-α5β1-Src complex, to modulate the Src kinase activity.
Smaller functional peptides can be easily synthesized and used in in vivo angiogenesis experiments. In order to find out the functional sub-domains of HKa. We uitilized HKa, GST-D5, D5 and synthetic D5-peptides to test its inhibitory effect in a collagen-fibrinogen three dimensional gel. As shown as figure 1 and 2, HKa, D5 and peptide G486-K502 has a similar inhibitory effect. Mahdi F, et al. [38] identified a higher affinity site in the light chain (His477-Gly496) and a lower affinity site in the heavy chain (Cys333-Lys345) for HKa binding to suPAR. Domain 5 (K420-S513) overlaps the entire high affinity uPAR-binding site, while the peptides G486-K502 and H475-H485 only contain a portion of that sequence. However, G486-K502, the most potent peptide in inhibiting migration, has a similar inhibitory effect (77%) on tube formation with D5, which could narrow down the most important uPAR-binding and migration-inhibiting sequence to G486-G496, not H477-H485. In contrast, the G440-H455 peptide, a Zn binding site for D5 [39], exerts most of the inhibitory effect of D5 on proliferation, which only has a modest inhibition of tube formation. Thus, it is indicated that the functional subdomain of D5 for tube formation is G486-G496, not G440-H455. But G440-H455 is still an important area for D5 binding to endothelial cells.
In 2D cell culture, HKa and D5 detach cells from fibrin/fibrinogen but not collagen, suggesting that HKa and D5 recognize cells attached to fibrin/fibrinogen. Therefore, in the later stage of wound healing, HKa and D5 would only induce the regression of blood vessels attached to fibrin/fibrinogen in a (provisional) extracellular matrix and not of the resting vessels surrounded by collagen. HKa and D5 detach endothelial cells from fibrin/fibrinogen in a 2D system and inhibit tube formation in 3D gels. These results suggest that HKa and D5 exert an inhibitory effect on tumor cell growth by preventing endothelial cells from invading the fibrin/fibrinogen matrix around tumor cells. Thereby, these HK fragments arrest the formation of new blood vessels which would offer nutrients and oxygen for tumor cell growth.
Src family kinases have been implicated in angiogenesis, especially in regulating vascular permeability, cell motility and endothelial cell differentiation [24, 40]. We demonstrated that Src family kinase selective inhibitor PP2, but not, PP3 (an inactive analogue) inhibited tube formation. Although PP2 also can inhibit EGFR activation, most of studies suggested that HUVECs do not express EGFR. Di Fulvio et al [41] recently showed that HUVECs do response to EGF. By using Western blot techniques, we found that HUVECs expressed a very low level of EGFR compared with DU145 tumor cells (Figure 3E). Since PP2 significantly inhibited tube formation in a very low concertion (100–400nM), indicating SFKs play a critical role in angiogenesis. The fact that HKa and D5 significantly decreased the phosphorylation of Src family kinases 416 and caveolin-1 14 provides a mechanistic explanation of the inhibitory effect of HKa and D5 in tube formation. Caveolin-1 was first identified as a major tyrosine-phosphorylated protein in v-Src-transformed embryo fibroblast. In endothelial cells, phosphorylation of caveolin-1 on tyrosine 14 is required for caveolin-1 accumulation in the leading extension of transmigrating endothelial cells, a process essential for the initiation of angiogenesis [42].
Other investigators have indicated that Src, Yes, and Lyn are expressed in endothelial cells [29, 43, 44] We confirmed that an antibody to phospho-tyrosine 416 in Src recognized several bands around 50–60 KD in figure 4B. Based on the molecular weight of each member in the Src family kinases, we suggest that the top band is Yes (62KD), the band below Yes is Src (or Fyn, 60KD) and the bottom two bands are Lyn (56/53KD).
In Figure 5, both D5 and HKa inhibited the phosphorylation of Src 416 and the increase of tube length seen in Csk −/− endothelial cells. However, D5 shows a more potent effect on phosphorylation of Src, while HKa is a more potent inhibitor of tube length. In this case, the deficiency of Csk enhanced the proliferation of endothelial cells, which would increase the size of vessels, not only by increased phosphorylation of Src family kinases, but also by disassociating the complex with VE-cadherin. Csk suppress cell growth by a binding via its SH2 domain to the phosphorylated tyrosine 685 of VE-cadherin [45]. Cadherin and intermediate filament (such as cytokeratin) form cell-cell junction known as desmosome. HK binds to cytokeratin 1 in a zinc-dependent manner via its domain 3 (D3) in the heavy chain of HK, which is lacking in D5. An antibody against domain 3 blocks the binding of HK to endothelial cells about 30% [46]. Two D3 derived peptides potently inhibited endothelial cell proliferation with IC50 values of 5–10 μM [47]. Colocalization of cytokeratin 1 and uPAR on endothelial cells [48] indicate HKa can bridge cytokeratin 1 and uPAR together via its domain 3 and domain 5. The additional domain 3 effect of HKa might inhibit the secondary effect of deficiency of Csk, which D5 might have less effect. Thus, HKa can completely reverse the effect of Csk deficiency. The mechanism via cytokeratin-cadherin-contact inhibition of cell growth by which domain 3 inhibits cell proliferation is not completely understood at this moment. It would be nice to further address this issue in the future.
The inhibitory effect of HKa on Src family kinase activity might be through an interaction of HKa with its receptors: cytokeratin 1, gC1qR, tropomyosin and uPAR. [48–50]. The association of uPAR with α3β1, αvβ3 and α5β1 integrins has been well appreciated for many years [36]. Although uPAR can cluster with either β3 or β1[17], β3 integrin can associate with almost all Src kinases such as Src, Fyn, Lyn and Yes while β1 integrin only can associate with Lyn and Yes [15]. The antibody against total Src also detects other Src family members. The data in figure 7A can be explained as integrin β3 associated with Yes, Fyn and Src (figure 7A, left panel) while integrin β1 associates only with Yes in figure 7A right panel. Src has been shown to bind constitutively and selectively to β3 integrin through an interaction involving the Src SH3 domain and carboxyl-terminal region of the β3 cytoplasmic tail. Clustering of β3 integrin activates Src and induces phosphorylation of Tyr-416 in the Src activation loop. Thus, the interaction of β3 integrin and Src plays an essential role in angiogenesis. Colman R, et al. [49] showed that HKa binds to Domain 2 and 3 of the uPAR, indicating that HKa can either dissociate uPAR-integrin complex directly or interfere with the integrin conformational change needed to associate with uPAR, and may thus dissociate integrins and Src, which would inactivate Src kinase. In contrast, αvβ3 integrin is not a receptor for HKa as evidenced by the observation that the binding of HKa to endothelial cells was not inhibited by either a known αvβ3 ligand, fibrinogen, or a specific anti-β3 integrin antibody. We now present a working model of how HKa disrupts signaling, based on the data presented in this study (figure 8). uPA can interact with αvβ3, but not α5β1 [51]. We speculate that endogenous uPA can be a bridge between αvβ3 and uPAR, but not α5β1 and uPAR. In this case quantification demonstrates that HKa disrupts uPAR-αvβ3-Src (figure 7B left panel), but not uPAR-α5β1-Src complex (figure 7B right panel) by removing endogenous uPA.
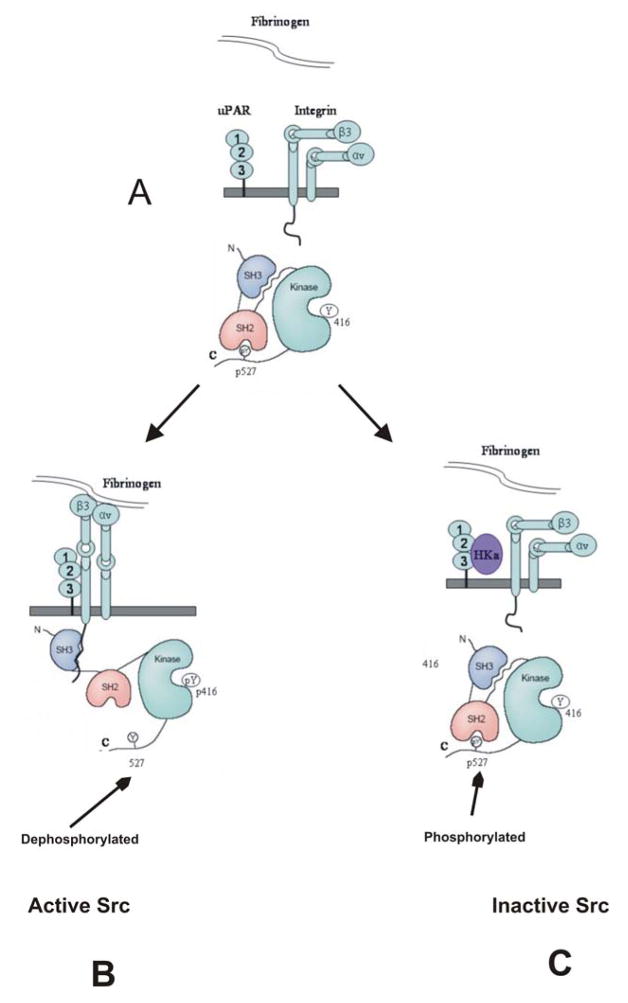
Figure 8. Illustration of Src activity modulated by HKa in response to outside-in signaling of αvβ3 integrin.
A, Src remains an inactive form where SH2 domain is engaged with phosphorylated Tyr 527, SH3 domain is engaged with the SH2-kinase-linker and Tyr416 (SH1) is unphosphorylated. B, the binding of fibrinogen to the αvβ3 integrin on the cell membrane results the conformational change of integrin αvβ3 and initiates the outside-in signaling, which induces the association of the intracellular tail of the β3 integrin with SH3 domain of Src. This association facilitates de-phosphorylation of pTyr 527 and auto-phosphorylation of Tyr 416 in Src kinase, which results in Src activation. The clustering of uPAR to αvβ3 integrin in response to integrin activation modulates the bidirectional signaling of αvβ3 integrin. C, addition of HKa decreases Src kinase activity by targeting uPAR.
The role of integrin αvβ3 and α5β1 in tumor angiogenesis has been argued for a decade. Early data showed that the disruption of αvβ3 integrin ligation by either blocking antibodies or cyclic peptide antagonists prevented blood vessel formation in mouse retina, rabbit cornea, and chick chorioallantoic membrane [52]. However, the tumors in these mice lacking integrin β3 or both β3 and β5 integrins display enhanced angiogenesis and tumor growth, strongly suggesting that neither β3 nor β5 integrins are essential for neovascularization [53]. To clarify this issue, Mahabelshwar et al. [54] recently generated knock-in mice that express a mutant β3 integrin unable to undergo tyrosine phosphorylation, demonstrating defective tyrosine phosphorylation in mutant β3 knock-in cells resulted in impaired adhesion, spreading, and migration of endothelial cells, indicating integrin β3 signaling is critical for pathologic angiogenesis. At the initial experiment, we thought that HKa might affect integrin α5β1 function since volociximab, a chimeric integrin alpha5beta1 antibody, inhibits the growth of VX2 tumors in rabbits [55], but actually not. Instead, HKa disrupted uPAR-αvβ3-Src complex. We think that is reasonable because c-Src via its SH3 domain binds to the cytoplasmic tail of integrin β3.
In this study, we first narrowed down the functional sequence of HKa to G486-G496. Such small peptide can be easily used in in vivo study for the therapeutic purpose. Then, we showed that HKa and D5 can inhibit SFKs phosphorylation, which is required in angiogenesis. Scrambled siRNA did not inhibit Csk expression, which means that SFKs is still a key in prompting tube formation in the control. However, Csk siRNA decreased Csk expression resulting two effects. In the one hand, it increased SFKs phosphorylation. In the other hand, it loosed cell-cell junction, which Csk normally binds to VE-cadherin and tightens cell-cell junction. Both events increased cell proliferation prompting tube formation. Our experiment indicated that HKa and D5 could reverse both effects with different potency. Integrin αvβ3 and α5β1 mediate “outside-in” signal which is critical in tumor angiogenesis. Our data suggested that HKa affected Integrin αvβ3 function resulting disruption of integrin “outside-in” signal which is required for c-Src activation. Although the function of the integrin α5β1 is important in angiogenesis, HKa seems not to affect it. Taken together, we demonstrated that HKa suppress angiogenesis by affecting the uPAR-intergrin αvβ3-Src signaling axis.
Acknowledgments
We would like to thank Drs. Baohua Yang, Su Hung and Harlan Bradford for valuable assistance. We also thank Ms. Princess D. Graham for her help in editing as well as manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. [Review] [72 refs] Nature Medicine. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez PM, Patierno SR, Rickles FR. Tissue factor and fibrin in tumor angiogenesis. Semin Thromb Hemost. 2004;30:31–44. doi: 10.1055/s-2004-822969. [DOI] [PubMed] [Google Scholar]
- 3.Ruf W, Mueller BM. Thrombin generation and the pathogenesis of cancer. Semin Thromb Hemost. 2006;32(Suppl 1):61–8. doi: 10.1055/s-2006-939555. [DOI] [PubMed] [Google Scholar]
- 4.Nagy JA, Brown LF, Senger DR, Lanir N, Van de Water L, Dvorak AM, Dvorak HF. Pathogenesis of tumor stroma generation: a critical role for leaky blood vessels and fibrin deposition. Biochim Biophys Acta. 1989;948:305–26. doi: 10.1016/0304-419x(89)90004-8. [DOI] [PubMed] [Google Scholar]
- 5.Tsopanoglou NE, Maragoudakis ME. Role of thrombin in angiogenesis and tumor progression. Semin Thromb Hemost. 2004;30:63–9. doi: 10.1055/s-2004-822971. [DOI] [PubMed] [Google Scholar]
- 6.Colman RW. Biologic activities of the contact factors in vivo--potentiation of hypotension, inflammation, and fibrinolysis, and inhibition of cell adhesion, angiogenesis and thrombosis. Thromb Haemost. 1999;82:1568–77. [PubMed] [Google Scholar]
- 7.Colman RW, Schmaier AH. Contact system: A vascular biology modulator with anticoagulant, profibrinolytic, antiadhesive, and proinflammatory attributes. Blood. 1997;90:3819–3843. [PubMed] [Google Scholar]
- 8.Mori K, Nagasawa S. Studies on human high molecular weight (HMW) kininogen. II. Structural change of HMW kininogen by the action of human plasma kallikrein. J Biochem (Tokyo) 1981;89:1465–1473. doi: 10.1093/oxfordjournals.jbchem.a133339. [DOI] [PubMed] [Google Scholar]
- 9.Colman RW, Jameson BA, Lin Y, Johnson D, Mousa SA. Domain 5 of high molecular weight kininogen (kininostatin) down- regulates endothelial cell proliferation and migration and inhibits angiogenesis. Blood. 2000;95:543–50. [PubMed] [Google Scholar]
- 10.Guo YL, Wang S, Colman RW. Kininostatin, an angiogenic inhibitor, inhibits proliferation and induces apoptosis of human endothelial cells. Arterioscler Thromb Vasc Biol. 2001;21:1427–33. doi: 10.1161/hq0901.095277. [DOI] [PubMed] [Google Scholar]
- 11.Guo YL, Wang S, Cao DJ, Colman RW. Apoptotic effect of cleaved high molecular weight kininogen is regulated by extracellular matrix proteins. J Cell Biochem. 2003;89:622–32. doi: 10.1002/jcb.10536. [DOI] [PubMed] [Google Scholar]
- 12.Im E, Kazlauskas A. Src Family Kinases Promote Vessel Stability by Antagonizing the Rho/ROCK Pathway. J Biol Chem. 2007;282:29122–9. doi: 10.1074/jbc.M702637200. [DOI] [PubMed] [Google Scholar]
- 13.Werdich XQ, Penn JS. Specific involvement of SRC family kinase activation in the pathogenesis of retinal neovascularization. Invest Ophthalmol Vis Sci. 2006;47:5047–56. doi: 10.1167/iovs.05-1343. [DOI] [PubMed] [Google Scholar]
- 14.Donnini S, Monti M, Castagnini C, Solito R, Botta M, Schenone S, Giachetti A, Ziche M. Pyrazolo-pyrimidine-derived c-Src inhibitor reduces angiogenesis and survival of squamous carcinoma cells by suppressing vascular endothelial growth factor production and signaling. Int J Cancer. 2007;120:995–1004. doi: 10.1002/ijc.22410. [DOI] [PubMed] [Google Scholar]
- 15.Arias-Salgado EG, Lizano S, Sarkar S, Brugge JS, Ginsberg MH, Shattil SJ. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc Natl Acad Sci U S A. 2003;100:13298–302. doi: 10.1073/pnas.2336149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks PC, Stromblad S, Klemke R, Visscher D, Sarkar FH, Cheresh DA. Antiintegrin alpha v beta 3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest. 1995;96:1815–1822. doi: 10.1172/JCI118227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei Y, Czekay RP, Robillard L, Kugler MC, Zhang F, Kim KK, Xiong JP, Humphries MJ, Chapman HA. Regulation of {alpha}5{beta}1 integrin conformation and function by urokinase receptor binding. J Cell Biol. 2005;168:501–11. doi: 10.1083/jcb.200404112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei Y, Eble JA, Wang Z, Kreidberg JA, Chapman HA. Urokinase receptors promote beta1 integrin function through interactions with integrin alpha3beta1. Mol Biol Cell. 2001;12:2975–86. doi: 10.1091/mbc.12.10.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis GE, Camarillo CW. An alpha 2 beta 1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp Cell Res. 1996;224:39–51. doi: 10.1006/excr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 20.Yang B, Cao DJ, Sainz I, Colman RW, Guo YL. Different roles of ERK and p38 MAP kinases during tube formation from endothelial cells cultured in 3-dimensional collagen matrices. J Cell Physiol. 2004;200:360–9. doi: 10.1002/jcp.20025. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Pelekanakis K, Woolkalis MJ. Thrombin and tumor necrosis factor alpha synergistically stimulate tissue factor expression in human endothelial cells: regulation through c-Fos and c-Jun. J Biol Chem. 2004;279:36142–7. doi: 10.1074/jbc.M405039200. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Zalewski A, Liu Y, Mazurek T, Cowan S, Martin JL, Hofmann SM, Vlassara H, Shi Y. Diabetes-induced oxidative stress and low-grade inflammation in porcine coronary arteries. Circulation. 2003;108:472–8. doi: 10.1161/01.CIR.0000080378.96063.23. [DOI] [PubMed] [Google Scholar]
- 23.Yang S, Graham J, Kahn JW, Schwartz EA, Gerritsen ME. Functional roles for PECAM-1 (CD31) and VE-cadherin (CD144) in tube assembly and lumen formation in three-dimensional collagen gels. Am J Pathol. 1999;155:887–95. doi: 10.1016/S0002-9440(10)65188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Senger DR. Matrix-specific activation of Src and Rho initiates capillary morphogenesis of endothelial cells. Faseb J. 2004;18:457–68. doi: 10.1096/fj.03-0948com. [DOI] [PubMed] [Google Scholar]
- 25.Ischenko I, Guba M, Yezhelyev M, Papyan A, Schmid G, Green T, Fennell M, Jauch KW, Bruns CJ. Effect of Src kinase inhibition on metastasis and tumor angiogenesis in human pancreatic cancer. Angiogenesis. 2007;10:167–82. doi: 10.1007/s10456-007-9071-3. [DOI] [PubMed] [Google Scholar]
- 26.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 27.Amin DN, Hida K, Bielenberg DR, Klagsbrun M. Tumor endothelial cells express epidermal growth factor receptor (EGFR) but not ErbB3 and are responsive to EGF and to EGFR kinase inhibitors. Cancer Res. 2006;66:2173–80. doi: 10.1158/0008-5472.CAN-05-3387. [DOI] [PubMed] [Google Scholar]
- 28.Boggon TJ, Eck MJ. Structure and regulation of Src family kinases. Oncogene. 2004;23:7918–7927. doi: 10.1038/sj.onc.1208081. [DOI] [PubMed] [Google Scholar]
- 29.Schieven GL, Kallestad JC, Brown TJ, Ledbetter JA, Linsley PS. Oncostatin M induces tyrosine phosphorylation in endothelial cells and activation of p62yes tyrosine kinase. J Immunol. 1992;149:1676–82. [PubMed] [Google Scholar]
- 30.Kannan S, Audet A, Knittel J, Mullegama S, Gao GF, Wu M. Src kinase Lyn is crucial for Pseudomonas aeruginosa internalization into lung cells. Eur J Immunol. 2006;36:1739–52. doi: 10.1002/eji.200635973. [DOI] [PubMed] [Google Scholar]
- 31.Roskoski R., Jr Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 2005;331:1–14. doi: 10.1016/j.bbrc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Duan LJ, Imamoto A, Fong GH. Dual roles of the C-terminal Src kinase (Csk) during developmental vascularization. Blood. 2004;103:1370–2. doi: 10.1182/blood-2003-05-1701. [DOI] [PubMed] [Google Scholar]
- 33.Nada S, Yagi T, Takeda H, Tokunaga T, Nakagawa H, Ikawa Y, Okada M, Aizawa S. Constitutive activation of Src family kinases in mouse embryos that lack Csk. Cell. 1993;73:1125–35. doi: 10.1016/0092-8674(93)90642-4. [DOI] [PubMed] [Google Scholar]
- 34.Herrick S, Blanc-Brude O, Gray A, Laurent G. Fibrinogen. Int J Biochem Cell Biol. 1999;31:741–6. doi: 10.1016/s1357-2725(99)00032-1. [DOI] [PubMed] [Google Scholar]
- 35.White DJ, Puranen S, Johnson MS, Heino J. The collagen receptor subfamily of the integrins. Int J Biochem Cell Biol. 2004;36:1405–10. doi: 10.1016/j.biocel.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Wei Y, Tang CH, Kim Y, Robillard L, Zhang F, Kugler MC, Chapman HA. Urokinase Receptors Are Required for {alpha}5beta1 Integrin-mediated Signaling in Tumor Cells. J Biol Chem. 2007;282:3929–39. doi: 10.1074/jbc.M607989200. [DOI] [PubMed] [Google Scholar]
- 37.Cao DJ, Guo YL, Colman RW. Urokinase-type plasminogen activator receptor is involved in mediating the apoptotic effect of cleaved high molecular weight kininogen in human endothelial cells. Circ Res. 2004;94:1227–34. doi: 10.1161/01.RES.0000126567.75232.46. [DOI] [PubMed] [Google Scholar]
- 38.Mahdi F, Shariat-Madar Z, Kuo A, Carinato M, Cines DB, Schmaier AH. Mapping the interaction between high molecular weight kininogen and the urokinase plasminogen activator receptor. J Biol Chem. 2004;279:16621–16628. doi: 10.1074/jbc.M313850200. [DOI] [PubMed] [Google Scholar]
- 39.Hasan AAK, Cines DB, Herwald H, Schmaier AH, Muller-Esterl W. Mapping the cell binding site on high molecular weight kininogen domain 5. J Biol Chem. 1995;270:19256–19261. doi: 10.1074/jbc.270.33.19256. [DOI] [PubMed] [Google Scholar]
- 40.Klint P, Kanda S, Kloog Y, Claesson-Welsh L. Contribution of Src and Ras pathways in FGF-2 induced endothelial cell differentiation. Oncogene. 1999;18:3354–64. doi: 10.1038/sj.onc.1202680. [DOI] [PubMed] [Google Scholar]
- 41.Di Fulvio M, Frondorf K, Henkels KM, Lehman N, Gomez-Cambronero J. The Grb2/PLD2 interaction is essential for lipase activity, intracellular localization and signaling in response to EGF. J Mol Biol. 2007;367:814–24. doi: 10.1016/j.jmb.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Labrecque L, Nyalendo C, Langlois S, Durocher Y, Roghi C, Murphy G, Gingras D, Beliveau R. Src-mediated tyrosine phosphorylation of caveolin-1 induces its association with membrane type 1 matrix metalloproteinase. J Biol Chem. 2004;279:52132–40. doi: 10.1074/jbc.M409617200. [DOI] [PubMed] [Google Scholar]
- 43.Kanda S, Kanetake H, Miyata Y. HGF-induced capillary morphogenesis of endothelial cells is regulated by Src. Biochem Biophys Res Commun. 2006;344:617–22. doi: 10.1016/j.bbrc.2006.03.183. [DOI] [PubMed] [Google Scholar]
- 44.Werdich XQ, Penn JS. Src, Fyn and Yes play differential roles in VEGF-mediated endothelial cell events. Angiogenesis. 2005;8:315–26. doi: 10.1007/s10456-005-9021-x. [DOI] [PubMed] [Google Scholar]
- 45.Baumeister U, Funke R, Ebnet K, Vorschmitt H, Koch S, Vestweber D. Association of Csk to VE-cadherin and inhibition of cell proliferation. Embo J. 2005;24:1686–95. doi: 10.1038/sj.emboj.7600647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joseph K, Ghebrehiwet B, Kaplan AP. Cytokeratin 1 and gC1qR mediate high molecular weight kininogen binding to endothelial cells. Clin Immunol. 1999;92:246–55. doi: 10.1006/clim.1999.4753. [DOI] [PubMed] [Google Scholar]
- 47.Zhang JC, Qi X, Juarez J, Plunkett M, Donate F, Sakthivel R, Mazar AP, McCrae KR. Inhibition of angiogenesis by two-chain high molecular weight kininogen (HKa) and kininogen-derived polypeptides. Can J Physiol Pharmacol. 2002;80:85–90. doi: 10.1139/y02-011. [DOI] [PubMed] [Google Scholar]
- 48.Mahdi F, Shariat-Madar Z, Todd RF, 3rd, Figueroa CD, Schmaier AH. Expression and colocalization of cytokeratin 1 and urokinase plasminogen activator receptor on endothelial cells. Blood. 2001;97:2342–50. doi: 10.1182/blood.v97.8.2342. [DOI] [PubMed] [Google Scholar]
- 49.Colman RW, Pixley RA, Najamunnisa S, Yan W, Wang J, Mazar A, McCrae KR. Binding of high molecular weight kininogen to human endothelial cells is mediated via a site within domains 2 and 3 of the urokinase receptor. J Clin Invest. 1997;100:1481–7. doi: 10.1172/JCI119669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan MM, Bradford HN, Isordia-Salas I, Liu Y, Wu Y, Espinola RG, Ghebrehiwet B, Colman RW. High-Molecular-Weight Kininogen Fragments Stimulate the Secretion of Cytokines and Chemokines Through uPAR, Mac-1, and gC1qR in Monocytes. Arterioscler Thromb Vasc Biol. 2006 doi: 10.1161/01.ATV.0000240290.70852.c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tarui T, Akakura N, Majumdar M, Andronicos N, Takagi J, Mazar AP, Bdeir K, Kuo A, Yarovoi SV, Cines DB, Takada Y. Direct interaction of the kringle domain of urokinase-type plasminogen activator (uPA) and integrin alpha v beta 3 induces signal transduction and enhances plasminogen activation. Thromb Haemost. 2006;95:524–34. doi: 10.1160/TH05-06-0457. [DOI] [PubMed] [Google Scholar]
- 52.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–71. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 53.Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke KM. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8:27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- 54.Mahabeleshwar GH, Feng W, Phillips DR, Byzova TV. Integrin signaling is critical for pathological angiogenesis. J Exp Med. 2006 doi: 10.1084/jem.20060807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhaskar V, Fox M, Breinberg D, Wong MH, Wales PE, Rhodes S, Dubridge RB, Ramakrishnan V. Volociximab, a chimeric integrin alpha5beta1 antibody, inhibits the growth of VX2 tumors in rabbits. Invest New Drugs. 2007 doi: 10.1007/s10637-007-9078-z. [DOI] [PubMed] [Google Scholar]