Abstract
Objective
Determine if antiretroviral (ARV) regimens with good central nervous system (CNS) penetration control HIV in cerebrospinal fluid (CSF) and improve cognition.
Design
Multi-site longitudinal observational study.
Setting
Research clinics.
Subjects
101 individuals with advanced HIV beginning or changing a new potent ARV regimen. Data for 79 subjects were analyzed. Participants underwent structured history and neurological examination, venipuncture, lumbar puncture, neuropsychological tests at entry, 24 and 52 weeks.
Intervention
ARV regimens were categorized as CNS penetration effectiveness (CPE) rank ≥ 2 or < 2. Generalized estimating equations were used to examine associations over the course of the study.
Main Outcome Measures
Concentration of HIV RNA in CSF and blood, neuropsychological test scores.
Results
Odds of suppression of CSF HIV RNA were higher when CPE rank ≥ 2 compared to < 2. Odds of suppression of plasma HIV RNA were not associated with CPE rank. Among subjects with impaired neuropsychological performance at entry, those prescribed regimens with a CPE rank ≥ 2 or more ARVs had lower NPZ4 over the course of the study.
Conclusions
ARV regimens with good CNS penetration, as assessed by CPE rank, are more effective in controlling CSF (and presumably CNS) viral replication than regimens with poorer penetration. In this study, ARVs with good CNS penetration were associated with poorer neurocognitive performance. A larger, controlled trial is required before any conclusions regarding the influence of specific ARVs on neurocognitive performance should be made.
Keywords: Cerebrospinal fluid, HIV, cognition, neuropsychological tests, antiretroviral therapy
Introduction
In the era before combination antiretroviral (cART) therapy, several studies showed that cerebrospinal fluid (CSF) HIV RNA levels correlated with severity of HIV-associated dementia [1, 2]. In subjects with advanced HIV, CSF HIV RNA concentration correlated with neuropsychological test performance [3, 4], and elevated HIV RNA levels in CSF predicted future cognitive impairment [4]. Since the advent of cART, these relationships may be less robust [5, 6], perhaps because many patients who receive cART achieve undetectable CSF HIV RNA concentrations or because CSF virus may be suppressed even in patients who are failing a cART regimen [7].
An ongoing question of particular interest is whether cART regimens that include agents that penetrate the central nervous system (CNS) in therapeutic concentrations reduce CSF virus and improve neuropsychological test performance. These questions have been addressed in several cross sectional and prospective studies (reviewed in [8]), but limited research has been conducted in subjects who begin or change treatment [9, 10]. We report the results of ACTG 736, a multi-site longitudinal natural history study whose primary goals were to examine changes in CSF and plasma HIV RNA and in neuropsychological function in HIV-infected individuals who begin or change a cART regimen.
Methods
Eligibility
Subjects were required to have either peripheral blood CD4+ T cells < 200/uL with plasma HIV RNA > 2000 copies/mL, or plasma HIV RNA > 50,000 copies/mL, regardless of CD4 count. All subjects were either initiating a new cART regimen or changing an existing regimen because of virologic failure. A cART regimen was defined as containing ≥ 3 antiretroviral agents (ARVs). The drug regimen was chosen by the subject's primary provider, or, if the subject was enrolling into a treatment trial, by the randomization arm. The study protocol was reviewed and approved by the Institutional Review Board at each participating site. Human experimentation guidelines of each site were followed in the conduct of this research.
Procedures
Within 21 days before beginning or changing the cART regimen, subjects underwent a structured medical history and neurological examination, venipuncture and lumbar puncture, and neuropsychological tests. At all sites, subjects underwent a brief neuropsychological test battery that included timed gait, grooved pegboard with the dominant hand, digit symbol, and finger tapping with the nondominant hand. At self-identified sites with additional expertise, subjects underwent a more extensive neuropsychological test battery that included the above tests and Rey auditory verbal learning test-trials I-VII, grooved pegboard with the non-dominant hand, Trail making parts A and B, finger tapping with the dominant hand, Rey auditory verbal learning test-trial VIII 30 minute delay, basic choice reaction time (CalCAP), and sequential reaction time (CalCAP). The short and the long batteries have been used routinely in studies of HIV-associated cognitive impairment [11, 12].
The same procedures were repeated 24 and 52 weeks after beginning therapy. If subjects discontinued their ARV therapy or withdrew from the study for any reason more than 4 weeks after a previous evaluation, they repeated the evaluation. Subjects who changed their regimen because of side effects continued in the study.
Laboratory Methods
HIV RNA in centrifuged CSF and plasma was measured by the Amplicor HIV-1 Monitor test with Ultrasensitive Specimen Preparation (Roche Molecular Systems, Pleasanton, CA). Samples with < 50 copies/mL were considered to be undetectable. Suppression of HIV RNA was defined as decline from detectable to undetectable. HIV RNA copies/mL were expressed as log10.
Statistical Methods
To estimate CNS penetration of a drug regimen, we used the CNS Penetration Effectiveness (CPE) rank, which assigns each ARV agent a value of 0 (low penetration), 0.5 (intermediate penetration) or 1.0 (good penetration) [8]. These ranks are summed to determine the CPE rank of a regimen. This method categorizes CNS drug penetration based on virologic and pharmacologic data and has been recently validated [8]. We constructed receiver operator characteristic curves to determine that the optimal cut-off point for CPE rank was ≥ 2. Ritonavir was not considered in the calculation of the CPE rank if it was used in low dose to increase drug levels of concomitantly administered protease inhibitors.
Z scores were calculated for each neuropsychological test using age-adjusted norms, and a composite Z score for the short battery (NPZ4) and the longer battery (NPZ8) was calculated at each visit, with lower scores reflecting poorer performance and higher scores reflecting better performance. CD4 estimates were based on a 50-cell increase.
Generalized estimating equations (GEE) with an autoregressive correlation structure were used to examine associations between variables. The initial multivariate models included CPE, controlling for covariates with p<0.15 in the univariate models. A backward covariate selection strategy was applied until p<0.15 for all covariates except CPE in the models. Results are expressed with 95% confidence intervals (CI = [LCL - UCL], where LCL is the lower confidence limit and UCL is the upper confidence limit). P-values ≤0.05 were considered to be significant.
Results
Subject Characteristics
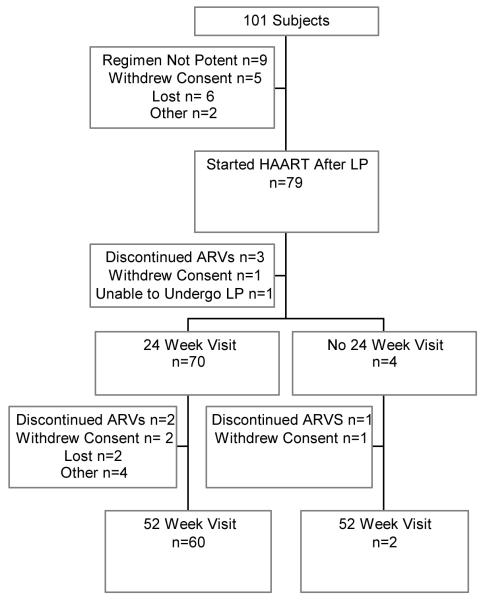
101 subjects were enrolled in the study. Twenty-two subjects were not included in the analysis (Figure 1). Sixty subjects were seen all three study visits (Figure 1). Subjects who prematurely ended study participation before the 24 or 52 week visits did not differ significantly from those who completed the study with regard to demographic and laboratory features.
Figure 1. Flow chart of number of subjects seen at each visit.
Twenty-two subjects did not start a cART regimen and thus did not contribute data. “Other” includes 1 subject who was too ill to participate, and 1 who died.
Five subjects did not return for the week 24 visit, and the reasons are shown in the Figure.
Twelve subjects did not return for the week 52 visit. “Other” includes 2 subjects who were not able to go to the study clinic, 1 who was too ill to participate and 1 who failed their appointments.
Three patients underwent study visits at 14, 44 and 48 weeks and then discontinued participation. For simplicity, these three visits are not included in the Figure.
The characteristics of the 79 study subjects included in the analyses are shown in Table 1. Eight subjects were receiving ARVs at study entry, before beginning their new regimens. In 7 subjects, this regimen was potent and in 1 it was not. Thirty-five subjects had taken ARVs in the past (“experienced”) and 44 had never taken ARVs (“naïve”). Subjects who were ARV naïve had significantly higher peripheral blood CD4+ T cell counts/uL (median 127 vs. 106, p=0.04) compared to ARV experienced subjects, but did not differ with respect to entry plasma or CSF HIV RNA concentration or neuropsychological function. In subjects with detectable CSF HIV RNA at baseline, median (interquartile range [IQR]) baseline plasma HIV RNA was 4.88 (4.66-5.34) copies/mL.
Table 1.
Baseline demographic and laboratory characteristics (n=79)
| Characteristic | Value |
|---|---|
| Female (number, percent) | 13 (16.5%) |
| Non-white (number, percent) | 40 (50.6%) |
| Age (median years, IQR) | 39 (33 to 45) |
| Years of education (median years, IQR) | 13 (12 to 15) |
| Peripheral blood CD4+ T cells/uL (median, IQR), n=77 | 111 (49 to 224) |
| Log CSF HIV RNA copies/mL (median IQR), n=74 | 3.33 (2.50 to 3.85) |
| CSF WBC/uL (median, IQR), n=70 | 3 (0 to 7) |
| Log plasma HIV RNA copies/mL (median, IQR), n=78 | 4.86 (4.55 to 5.30) |
| NPZ4 (median, IQR), n=75 | -0.29 (-0.96 to 0.14) |
| NPZ8 (median, IQR), n=52 | -0.24 (-0.70 to 0.15) |
Antiretroviral Regimens
Overall, subjects took 48 different drug regimens after study entry. Regimens with a CPE < 2 were prescribed at 57 (42%) of 135 visits. The median number of agents in a regimen was 3.0 (IQR 3.0-4.0), and the median CPE was 2.0 (IQR 1.5-2.5). Nonnucleoside reverse transcriptase inhibitor (NNRTI) based regimens, defined as an NNRTI plus at least two nucleoside reverse transcriptase inhibitors and no protease inhibitors were taken at 53 (39%) visits. The median CPE rank in the NNRTI-based regimens and in the remaining regimens was 2.0. Based on all visits, and adjusted for repeated measures, the number of ARVs agents in a regimen was greater when the CPE rank was higher (r=0.47, p<0.001) [13].
Suppression of CSF HIV RNA
Cerebrospinal fluid HIV RNA was never detectable when plasma HIV RNA was undetectable. This relationship held in experienced and naïve subjects (data not shown). CSF HIV RNA was rarely detectable when plasma HIV RNA was < 1000 copies/mL (data not shown).
In univariate analysis, the odds of suppression of CSF HIV RNA were high in subjects who were ARV naïve compared to those who were ARV experienced (Odds Ratio [OR] 6.06, 95% CI 1.54-23.77) (Table 2). Compared to those prescribed a regimen with a CPE rank <2, there was a trend toward greater odds of suppression of CSF virus in subjects who were prescribed a regimen with a CPE rank ≥ 2 (OR 3.22, 95% CI 0.95-10.87). There was no significant relationship between suppression of CSF HIV RNA and use of an NNRTI-containing regimen.
Table 2.
Univariate Odds of Suppression of CSF HIV RNA by Characteristics at Study Entry
| Characteristic | OR | LCL | UCL | P Value |
|---|---|---|---|---|
| ARV Naive at Entry | 6.06 | 1.54 | 23.77 | 0.01 |
| Entry Log10 Plasma HIV RNA copies/mL | 0.83 | 0.26 | 2.66 | 0.75 |
| Entry Log10 CSF HIV RNA copies/mL | 0.86 | 0.25 | 2.99 | 0.81 |
| Entry CD4+ T cells per 50 cells/uL | 1.20 | 0.96 | 1.49 | 0.10 |
| NNRTI Regimen | 1.71 | 0.51 | 5.73 | 0.39 |
| Number of ARV Agents | 0.70 | 0.44 | 1.12 | 0.14 |
| CPE rank ≥ 2 | 3.22 | 0.95 | 10.87 | 0.06 |
In multivariate models, the odds of suppression of CSF virus were greater in subjects who were ARV naïve at study entry compared to those who were ARV experienced (OR 4.86, 95% CI 1.11-21.27, p=0.04). Taking into account being naïve and total number of ARV agents, subjects who were prescribed a regimen with CPE rank ≥ 2 had significantly greater odds of suppression of CSF virus (OR 4.10, 95% CI 1.06-15.91, p=0.04). There was a trend toward lower odds of suppression of CSF virus in subjects who were prescribed more ARV agents (OR 0.64, 95% CI 0.40-1.01, p=0.06).
Suppression of Plasma HIV RNA
In univariate analysis, the odds of suppression of plasma HIV RNA were greater in subjects who were ARV naïve (3.87, 95% CI 1.63-9.20, p=0.002) and in those who were prescribed an NNRTI-based regimen (OR 2.71, 95% CI 1.13-6.47, p=0.03). For every 50-cell increase in entry CD4, the odds of suppression of plasma HIV RNA were 1.24 fold (95% CI 1.03-1.49, p=0.02) higher. There were no significant associations between suppression of plasma HIV RNA and CPE rank ≥ 2 or number of agents in a regimen.
Neuropsychological Tests
Seventy-five subjects underwent the 4-test battery and 52 subjects underwent the 8-test battery (Table 1). Based on clinical convention and prior experience, a Z score ≤ -0.5 was chosen as the definition of cognitive impairment [14]. Twenty-six subjects were impaired based on their entry NPZ4 score, and 17 subjects were impaired based on their entry NPZ8 score. We restricted analyses of the effect of entry characteristics on neurocognitive performance over the course of the study to those subjects who were cognitively impaired at study entry. Data from 39 follow-up visits were available for analysis of NPZ4, and data from 26 follow-up visits were available for analysis of NPZ8. Subjects who were included in these analyses differed from those who were not included. As expected, compared to subjects not included in the NPZ4 analysis, subjects included in the NPZ4 analysis had lower entry peripheral blood CD4+ T cells/uL (median 94 vs. 132, p=0.05). They also had fewer years of education (median 12 yrs vs. 14 years, p=0.01), and were less likely to be white (23% vs. 62%, p=0.002). Compared to subjects not included in the NPZ8 analysis, subjects included in the NPZ8 analysis had lower median peripheral blood CD4+ T cells (54 cells/uL vs. 126 cell/uL, p=0.002) but did not differ in years of education or ethnicity.
There was no significant relationship between impaired NPZ4 at study entry and the CPE rank of the initial ARV regimen. Specifically, 14 (54%) of 26 subjects with impaired NPZ4 at entry were prescribed an initial ARV regimen with CPE rank ≥ 2, and 12 (46%) were prescribed a regimen with CPE rank < 2. Similarly, 25 (56%) of 45 subjects with unimpaired NPZ4 at entry were prescribed an initial ARV regimen with CPE rank ≥ 2, and 20 (44%) were prescribed a regimen with CPE rank < 2.
Among the 26 patients with impaired NPZ4 at study entry, there was no significant difference in the baseline NPZ4 score in subjects who were ARV-experienced vs. ARV-naïve (median [IQR] -1.10 [-1.19 to -0.67] Z vs. -1.09 [-1.89 to -0.84] Z). Over the course of the study, there was no significant difference in the number of ARVs prescribed in the 10 experienced vs. 16 naïve subjects (data not shown).
Table 3 shows the results of univariate analyses of NPZ4 for subjects with entry NPZ4 ≤ -0.5. For every 1 Z score higher entry NPZ4, NPZ4 was 0.86 Z score higher over the course of the study. Compared to subjects who were prescribed an ARV regimen with a CPE rank < 2, subjects prescribed a regimen with a CPE rank ≥ 2 had 1.08 Z score lower NPZ4 over the course of the study. For every one agent added to a regimen, NPZ4 was subsequently 0.38 Z lower. There was no significant relationship between undetectable CSF HIV RNA at entry or use of efavirenz and NPZ4 score. In multivariate models, the association with CPE ≥ 2 remained significant after controlling for entry NPZ4. Compared to subjects who were prescribed an ARV regimen with a CPE rank < 2, subjects prescribed a regimen with a CPE rank ≥ 2 had 0.66 Z (0.14-1.19, p=0.01) score lower NPZ4 over the course of the study.
Table 3.
Univariate Estimates of Entry Characteristics on NPZ4 Over the Course of the Study in Subjects with Entry NPZ4 ≤ -0.5 Z
| Characteristic | Estimate | LCL | UCL | P Value |
|---|---|---|---|---|
| Entry NPZ4 | 0.86 | 0.61 | 1.12 | <0.001 |
| Entry Log10 Plasma HIV RNA Copies/mL | -0.11 | -0.54 | 0.33 | 0.63 |
| Undetectable CSF HIV RNA at Entry | 0.27 | -0.25 | 0.79 | 0.31 |
| Entry CD4+ T cells per 50 Cells/uL | 0.07 | -0.04 | 0.17 | 0.21 |
| NNRTI Regimen | -0.30 | -1.10 | 0.50 | 0.46 |
| Number of ARV Agents | -0.38 | -0.59 | -0.17 | 0.001 |
| CPE rank ≥ 2 | -1.08 | -1.66 | -0.50 | <0.001 |
| ARV regimen includes efavirenz | -0.29 | -1.09 | 0.51 | 0.48 |
| ARV Naive | -0.59 | -1.24 | -0.06 | 0.08 |
Among those subjects with entry NPZ4 ≤ -0.5, we examined change in NPZ4 over the course of the study in those who were prescribed 4 agents vs. 3, and in those who were prescribed regimens with CPE ≥ 2 compared to < 2. There was a trend toward improvement in NPZ4 in subjects who were prescribed 3 ARVs (median [IQR] 0.36 [0.06-0.79] Z, p=0.07), but NPZ4 did not change significantly in those prescribed 4 agents (median [IQR] -0.58 [-0.82-0.59] Z, p=0.47). NPZ4 improved significantly in subjects who were prescribed a regimen with CPE rank < 2 (median [IQR] 0.28 [0.19-0.87] Z, p=0.02), but not in those prescribed regimens with CPE rank ≥ 2 (median [IQR] 0.01 [-0.64-0.59] Z, p=0.86).
In univariate analysis of NPZ8 over the course of the study in subjects with entry NPZ8 ≤ -0.5, the negative relationship between more ARV agents in a regimen and neurocognitive performance remained highly significant, but the magnitude of the effect was small (Table 4). For every one agent added to a regimen, NPZ8 was 0.11 Z lower. CPE rank was not significantly related to NPZ8 over the course of the study. The small number of observations in this analysis precluded construction of multivariate models.
Table 4.
Univariate Estimates of Entry Characteristics on NPZ8 Over the Course of the Study in Subjects with Entry NPZ8 ≤ -0.5
| Parameter | Estimate | LCL | UCL | P Value |
|---|---|---|---|---|
| Entry NPZ8 | 0.53 | -0.11 | 1.18 | 0.11 |
| Entry Log10 Plasma HIV RNA Copies/mL | -0.38 | -0.67 | -0.08 | 0.01 |
| Baseline Suppression of CSF RNA HIV RNA | -0.15 | -0.74 | 0.45 | 0.63 |
| Entry CD4+ T cells per 50 Cells/uL | -0.01 | -0.31 | 0.28 | 0.94 |
| Patient Was on NNRTI Regimen | -0.10 | -0.97 | 0.78 | 0.83 |
| Number of ARV Agents | -0.11 | -0.11 | -0.11 | <0.001 |
| CPE rank ≥ 2 | -0.29 | -1.00 | 0.41 | 0.42 |
| ARV Naive | -0.32 | -1.02 | 0.38 | 0.37 |
Discussion
The goal of this study was to determine if patients who begin or change to an ARV regimen with “good” CNS penetration, defined as a CPE rank ≥ 2, have better CSF virologic and neurocognitive outcomes than individuals who are not prescribed such regimens. Our study population was chosen to have advanced disease to increase the number of subjects with cognitive impairment, and, as expected, we found that subjects with neurocognitive impairment had lower median peripheral blood CD4+ T cells.
We found that regimens with CPE rank ≥ 2 conveyed greater odds of suppression of CSF HIV RNA. Use of an NNRTI-based regimen was not significantly related to the odds of suppression of CSF HIV RNA. No significant relationship between CPE rank and suppression of plasma HIV RNA was seen. This finding suggests that the benefit of CNS penetrating agents on suppression of CSF viral replication may be independent of the overall efficacy of a regimen. The findings in our longitudinal study are in agreement with those of a cross-sectional study [8].
We also found that subjects who were ARV naïve had significantly greater odds of suppression of CSF virus compared to experienced subjects, even taking into account CPE rank or number of ARVs in a regimen. A longitudinal study of 29 individuals who started or changed cART also found that being ARV naïve predicted greater decline in CSF HIV RNA [10].
In contrast to our hypothesis that subjects who were prescribed ARV regimens with good CNS penetration would have better neurocognitive performance, we found the opposite. In univariate analysis of NPZ4, compared to use of a regimen with CPE rank < 2, use of a regimen with CPE rank ≥ 2 was significantly associated with poorer neurocognitive performance in subjects who were cognitively impaired at study entry. The magnitude of the effect was substantial. In the same analysis, use of ARV regimens that contained more drugs was also significantly associated with poorer neurocognitive performance, although the magnitude of the effect was smaller. In multivariate analyses, CPE rank ≥ 2 remained significantly associated with poorer NPZ4 scores over the course of the study.
Previous studies have shown that neuropsychological test performance in HIV-infected individuals improves with repeat testing, consistent with a practice effect [9]. Among subjects who were neuropsychologically impaired at study entry, we saw significant improvement in NPZ4 in subjects who were prescribed regimens with CPE rank < 2 and a trend toward improvement in those prescribed 3 agents (instead of 4). In contrast, we saw no significant change in performance in the subjects prescribed a regimen with CPE rank ≤ 2 or who were prescribed 4 drugs. Thus we cannot say that the latter group simply “improved less” than the former group.
At first inspection, our findings seem to be inconsistent with previous studies. For example, the incidence of HIV-associated dementia has significantly decreased in the era of cART [15, 16]. Although few studies have examined change in neuropsychological function after starting or changing ARV therapy, cognitive improvement has been documented, even when the regimen would not be considered “potent” [9, 17, 18].
Nonetheless, the observation that neurocognitive impairment may be associated with specific components of a cART regimen is not without precedent. For example, a longitudinal study showed that patients whose ARV regimens contained ritonavir with another protease inhibitor or a regimen that contained ≥ 3 CNS penetrant drugs had poorer motor performance than patients who did not receive the protease inhibitor combination or who were prescribed regimens with < 3 penetrant drugs [19]. The authors suggested that complex drug interactions could contribute to CNS toxicity. A proton magnetic resonance spectroscopy (MRS) study showed that subjects who took didanosine or stavudine had decreased frontal white matter N-acetyl aspartate concentrations compared to HIV-uninfected controls [20]. This difference was not seen in patients who took zidovudine and lamivudine compared to controls. The authors speculated that the negative effect could be mediated by mitochondrial toxicity of didanosine and stavudine, a known effect of these drugs in the peripheral nervous system.
Several explanations could be advanced to explain our findings regarding the relationship between neurocognitive performance, number of ARV agents in a regimen, and measures of CNS drug penetration. First, the findings could be spurious. We limited our analysis of neurocognitive performance to subjects with abnormal performance at study entry to eliminate a “ceiling effect.” As a consequence, small numbers limited our analyses.
Poorer adherence to treatment, and hence untreated or poorly treated HIV, which we know is associated with poorer cognitive function, could also be raised as a possible explanation for why subjects who were prescribed CNS penetrant agents or a higher number of agents had poorer neurocognitive performance. We did not specifically address adherence, but plasma HIV RNA is a good surrogate for adherence. As in the group as a whole, in the subgroup of subjects included in the neurocognitive analyses, we did not see a relationship between number of ARV agents in a regimen or CPE rank and suppression of plasma HIV RNA (data not shown). These findings suggest that suboptimal adherence to treatment does not explain poorer cognitive performance. Similarly, the lack of association between number of drugs in a regimen and suppression of plasma HIV RNA argues against the possibility that subjects who took more ARVs did so because they had less well controlled HIV.
Subjects who took regimens that contained more drugs may have had more drug-related side effects, which could have impacted their cognitive performance. This possibility is particularly relevant to use of efavirenz, which has known cognitive adverse effects. However, we found no significant relationship between use of efavirenz and neuropsychological performance over the course of the study.
Our study has limitations. We assume that, because it takes into account virologic and pharmacologic data, the CPE score reflects CNS drug penetration rather than simply CSF drug penetration. We were unable to directly test this hypothesis, as we did not have access to pre- or postmortem brain tissue. We were not able to include data from 22 of the 101 enrolled subjects. However, our study population is, to our knowledge, the largest of any longitudinal investigation of subjects beginning or changing ARV therapy. Antiretroviral regimens were not randomly allocated, and were chosen by the treating provider or by the co-enrolling clinical trial. It is thus possible that subjects who were enrolled from clinical care and who were more cognitively impaired may have been preferentially prescribed a more penetrant regimen. Our finding that there was no significant relationship between cognitive impairment at baseline and the CPE rank of the initially prescribed regimen does not support this hypothesis. Another possibility is that highly experienced subjects may have been more likely to be cognitively impaired and to have received more ARV agents. However, we found that among those subjects with baseline NPZ4 ≤ -0.5, there was no significant difference in baseline NPZ4 score in experienced compared to naïve subjects. Similarly, there was no significant difference in the number of ARV agents prescribed experienced compared to naïve subjects over the course of the study. Nonetheless, confounding could have occurred based on subject characteristics that were not measured in the study.
Finally, it is possible that agents with good CNS penetration are neurotoxic in the population of subjects with advanced HIV. A mechanism for neurotoxicity is a matter of speculation. Patients with advanced disease might be more susceptible to drug-related neurotoxicity because of disruption of the blood-brain-barrier or because of decreased cognitive reserve due to chronic brain HIV infection or comorbidities such as age or vascular disease.
On a practical basis, our data support the contention that ARV regimens with estimated good CNS penetration are more effective than regimens with poorer CNS penetration in controlling CSF (and presumably CNS) viral replication, regardless of the number of agents in a regimen and whether the regimen is NNRTI-based or not. However, we are not able to say that better CNS penetration translates into better cognition. In fact, our data suggest that the opposite may be true. Our results should be interpreted in light of the limitations of the study. A larger, controlled trial that addresses the impact of CNS penetrant ARV regimens on cognition is required.
Acknowledgements
Award Number AI38858 and U01AI068636 from the National Institute of Allergy and Infectious Diseases supported this research.
Participating personnel, sites and grant support: Mary Gould, RN, BA and Teresa Spitz, RN, CCRC, Washington University in St. Louis, Grant # AI69495 and NS32228
Margot Perrin, RN and N. Jeanne Conley, RN, University of Washington, Seattle, Grant #AI 69434
Joan Dragavon, University of Washington Center for AIDS Research Grant #AI-27757, and AIDS Clinical Trials Group Virology Support Laboratory Grant # AI-38858
Margia Vasquez, RN and Maura Laverty RN, New York University/NYC HHC at Bellevue, Grant #AI -69532 and M01RR00096
Paula Potter, RN and Dee Dee Pacheco, University of California, San Diego, Grant # AI69432
Diane Gochnour, RN, The Ohio State University, Grant # AI69474
Patricia Walton RN BSN, Case Western Reserve University, Grant #AI69501
Colin Hall, MD, Cheryl Marcus, Wendy Robertson, University of North Carolina, Grant # AI50410, AI69423, RR00046
Anne Weisbeck, RN, University of Rochester, Grant # AI69511 and Grant #5-MO1 RR00044
Kathryn Carter, PA-C, Johns Hopkins University, Grant # AI-69465 and RR-00052
Karen T Tashima, MD and Helen B Sousa, The Miriam Hospital, Grant # AI69472
Bruce Cohen, MD and Linda Reisberg, RN, Northwestern ACTU, Grant #AI69471
Nancy Hanks, RN, University of Hawai'i at Mano'a and Cecilia M. Shikuma, MD, University of Hawai'i at Mano'a- University of Hawaii, Grant # AI34853
Dennis Kolson MD, PhD and Keith Mickelberg, RN, University of Pennsylvania, Grant # AI69467 and CFAR Grant #5-P30-AI-045008-07
Jorge L. Santana, MD and Olga Mendez, MD, University of Puerto Rico, Grant #AI 69415
Sharon Shriver, AACTG Operations Office, Rockville, MD
Linda Millar, Frontier Sciences and Research Technology Research Foundation, Amherst, NY
References
- 1.Brew BJ, Pemberton L, Cunningham P, Law MG. Levels of human immunodeficiency virus type 1 RNA in cerebrospinal fluid correlate with AIDS dementia stage. J Infect Dis. 1997;175:963–966. doi: 10.1086/514001. [DOI] [PubMed] [Google Scholar]
- 2.McArthur JC, McClernon DR, Cronin MF, et al. Relationship between human immunodeficiency virus-associated dementia and viral load in cerebrospinal fluid and brain. Ann Neurol. 1997;42:689–698. doi: 10.1002/ana.410420504. [DOI] [PubMed] [Google Scholar]
- 3.Ellis RJ, Hsia K, Spector SA, et al. Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. HIV Neurobehavioral Research Center Group. Ann Neurol. 1997;42:679–688. doi: 10.1002/ana.410420503. [DOI] [PubMed] [Google Scholar]
- 4.Ellis RJ, Moore DJ, Childers ME, et al. Progression to neuropsychological impairment in human immunodeficiency virus infection predicted by elevated cerebrospinal fluid levels of human immunodeficiency virus RNA. Arch Neurol. 2002;59:923–928. doi: 10.1001/archneur.59.6.923. [DOI] [PubMed] [Google Scholar]
- 5.McArthur JC, McDermott MP, McClernon D, et al. Attenuated central nervous system infection in advanced HIV/AIDS with combination antiretroviral therapy. Arch Neurol. 2004;61:1687–1696. doi: 10.1001/archneur.61.11.1687. [DOI] [PubMed] [Google Scholar]
- 6.Sevigny JJ, Albert SM, McDermott MP, et al. Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology. 2004;63:2084–2090. doi: 10.1212/01.wnl.0000145763.68284.15. [DOI] [PubMed] [Google Scholar]
- 7.Spudich S, Lollo N, Liegler T, Deeks SG, Price RW. Treatment benefit on cerebrospinal fluid HIV-1 levels in the setting of systemic virological suppression and failure. J Infect Dis. 2006;194:1686–1696. doi: 10.1086/508750. [DOI] [PubMed] [Google Scholar]
- 8.Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Letendre SL, McCutchan JA, Childers ME, et al. Enhancing antiretroviral therapy for human immunodeficiency virus cognitive disorders. Ann Neurol. 2004;56:416–423. doi: 10.1002/ana.20198. [DOI] [PubMed] [Google Scholar]
- 10.Antinori A, Giancola ML, Grisetti S, et al. Factors influencing virological response to antiretroviral drugs in cerebrospinal fluid of advanced HIV-1-infected patients. Aids. 2002;16:1867–1876. doi: 10.1097/00002030-200209270-00003. [DOI] [PubMed] [Google Scholar]
- 11.Schifitto G, Navia BA, Yiannoutsos CT, et al. Memantine and HIV-associated cognitive impairment: a neuropsychological and proton magnetic resonance spectroscopy study. Aids. 2007;21:1877–1886. doi: 10.1097/QAD.0b013e32813384e8. [DOI] [PubMed] [Google Scholar]
- 12.Schifitto G, Zhang J, Evans SR, et al. A multicenter trial of selegiline transdermal system for HIV-associated cognitive impairment. Neurology. 2007 doi: 10.1212/01.wnl.0000268487.78753.0f. [DOI] [PubMed] [Google Scholar]
- 13.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: Part 2--Correlation between subjects. Bmj. 1995;310:633. doi: 10.1136/bmj.310.6980.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collie A, Darby DG, Falleti MG, Silbert BS, Maruff P. Determining the extent of cognitive change after coronary surgery: a review of statistical procedures. Ann Thorac Surg. 2002;73:2005–2011. doi: 10.1016/s0003-4975(01)03375-6. [DOI] [PubMed] [Google Scholar]
- 15.d'Arminio Monforte A, Cinque P, Mocroft A, et al. Changing incidence of central nervous system diseases in the EuroSIDA cohort. Ann Neurol. 2004;55:320–328. doi: 10.1002/ana.10827. [DOI] [PubMed] [Google Scholar]
- 16.Dore GJ, Correll PK, Li Y, Kaldor JM, Cooper DA, Brew BJ. Changes to AIDS dementia complex in the era of highly active antiretroviral therapy [In Process Citation] Aids. 1999;13:1249–1253. doi: 10.1097/00002030-199907090-00015. MEDLINE record in process. [DOI] [PubMed] [Google Scholar]
- 17.Price RW, Yiannoutsos CT, Clifford DB, et al. Neurological outcomes in late HIV infection: adverse impact of neurological impairment on survival and protective effect of antiviral therapy. AIDS Clinical Trial Group and Neurological AIDS Research Consortium study team. Aids. 1999;13:1677–1685. doi: 10.1097/00002030-199909100-00011. [DOI] [PubMed] [Google Scholar]
- 18.Marra CM, Lockhart D, Zunt JR, Perrin M, Coombs RW, Collier AC. Changes in CSF and plasma HIV-1 RNA and cognition after starting potent antiretroviral therapy. Neurology. 2003;60:1388–1390. doi: 10.1212/01.wnl.0000058768.73358.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cysique LA, Maruff P, Brew BJ. Antiretroviral therapy in HIV infection: are neurologically active drugs important? Arch Neurol. 2004;61:1699–1704. doi: 10.1001/archneur.61.11.1699. [DOI] [PubMed] [Google Scholar]
- 20.Schweinsburg BC, Taylor MJ, Alhassoon OM, et al. Brain mitochondrial injury in human immunodeficiency virus-seropositive (HIV+) individuals taking nucleoside reverse transcriptase inhibitors. J Neurovirol. 2005;11:356–364. doi: 10.1080/13550280591002342. [DOI] [PubMed] [Google Scholar]