Abstract
Several endoplasmic reticulum proteins, including tapasin, play an important role in MHC class I assembly. In this study, we assessed the influence of the tapasin cytoplasmic tail on three mouse MHC class I allotypes (H2-Kb, -Kd, and -Ld), and demonstrated that the expression of truncated mouse tapasin in mouse cells resulted in very low Kb, Kd, and Ld surface expression. The surface expression of Kd also could not be rescued by human soluble tapasin, suggesting the surface expression phenotype of the mouse MHC class I molecules in the presence of soluble tapasin was not due to mouse/human differences in tapasin. Notably, soluble mouse tapasin was able to partially rescue HLA-B8 surface expression on human 721.220 cells. Thus, the cytoplasmic tail of tapasin (either mouse or human) has a stronger impact on the surface expression of murine MHC class I molecules on mouse cells than on the expression of HLA-B8 on human cells. A K408W mutation in the mouse tapasin transmembrane/cytoplasmic domain disrupted Kd folding and release from tapasin, but not interaction with TAP, indicating that the mechanism whereby the tapasin transmembrane/cytoplasmic domain facilitates MHC class I assembly is not limited to TAP stabilization. Our findings indicate that the C-terminus of mouse tapasin plays a vital role in enabling murine MHC class I molecules to be expressed at the surface of mouse cells.
Keywords: antigen presentation, antigen processing, major histocompatibility complex, mouse, tapasin
Introduction
MHC class I molecules assemble with peptide in the endoplasmic reticulum by the assistance of a multi-component protein complex (Ortmann et al. 1997; Pamer and Cresswell 1998; Dick et al. 2002; Paquet et al. 2004; Park et al. 2006). Within this complex, tapasin binds directly to the MHC class I heavy chain (Farmery et al. 2000; Rizvi and Raghavan 2006). Experiments with an insect cell model have shown that tapasin stabilizes the peptide-binding site of the transporter associated with antigen processing (TAP) and increases the thermostability of both of the TAP subunits (Raghuraman et al. 2002). Cells from mice lacking tapasin express fewer cell surface MHC class I molecules, and the mice have weaker T cell-mediated immunity than wild type mice (Grandea et al. 2000; Garbi et al. 2000). Tapasin deficiency in mouse cells reduces TAP expression by about 300 fold (Garbi et al. 2003).
Consistent with these findings in mouse cells, in the human cell line 721.220 (a tapasin-deficient lymphoblastoid cell line), the amount of TAP is decreased and TAP/MHC class I association is abrogated (Grandea et al. 1995; Sadasivan et al. 1996; Solheim et al. 1997; Lehner et al. 1998; Copeman et al. 1998; Bangia et al. 1999; Li et al. 2000). Comparison of tapasin transfectants of 721.220 and mouse tapasin-knockout cells suggests that the impact of tapasin absence on TAP expression is less pronounced in human cells than in mouse cells (Lehner et al. 1998; Garbi et al. 2003). The surface expression of HLA-B*4402 or -B8 on 721.220 transfectants is reduced, as compared to B*4402 or B8 expression on 721.220 transfectants that also express tapasin (Peh et al. 1998; Zarling et al. 2003). The surface level reduction for B*4402 or B8 contrasts with virtually no reduction for Kb or B27 and a lesser reduction for A2 when these molecules are expressed on 721.220, as compared to on 721.220+tapasin (Peh et al. 1998; Barnden et al. 2000; Zarling et al. 2003).
Tapasin is a type I transmembrane protein (Li et al. 1997; Ortmann et al. 1997; Grandea et al. 1998; Li et al. 1999). Truncation of human tapasin after position 393 (thereby omitting the transmembrane and cytoplasmic sequences) prevented tapasin bridging of an HLA class I heavy chain to TAP, but did not completely abrogate HLA class I surface expression (Lehner et al. 1998; Bangia et al. 1999; Everett and Edidin 2007). A later investigation of truncated forms of human tapasin and a tapasin point mutant further established the C-terminus of tapasin as a TAP interaction site, and also showed that tapasin truncation destabilized the assembled MHC class I molecules (Tan et al. 2002). Substitution of a conserved lysine at position 408 in the human tapasin transmembrane/cytoplasmic (TM/CYT) region has been shown to affect the interaction of human tapasin with TAP (Petersen et al. 2005). A recent study by Papadopoulos and Momburg (2007) further defined amino acids in the mouse tapasin connecting peptide and transmembrane region that contribute to TAP stabilization.
The TM/CYT region of murine tapasin varies substantially from that of human tapasin in length and sequence (Ortmann et al. 1997; Li et al. 1997; Li et al. 1999). These differences, as well as species-specific differences in the degree of TAP stabilization by tapasin (Lehner et al. 1998; Garbi et al. 2003), led us to speculate that the TM/CYT region in mouse tapasin might have a different impact on function than the corresponding region of human tapasin. To determine the role of the TM/CYT tail in the function of mouse tapasin in mouse cells, we investigated the ability of soluble murine tapasin to associate with TAP and the murine MHC class I heavy chain (Ld, Kd, or Kb) and to support MHC class I cell surface expression on mouse cells. To distinguish between the influence of soluble tapasin versus the influence of the MHC heavy chain on the soluble tapasin/MHC interaction, we also examined the interaction of human soluble tapasin with Kd, and the interaction of mouse soluble tapasin with HLA-B8. Our data showed that the murine soluble tapasin could not facilitate the surface expression of Ld, Kd, or Kb, even though it could partially restore B8 surface expression and folding. Human soluble tapasin proved to be unable to facilitate Kd expression at the plasma membrane of mouse cells. Overall, these studies suggest that mouse MHC class I molecules are very dependent on the influence of the TM/CYT domain for their assembly and expression on mouse cells.
Materials and methods
Cell lines
The mouse fibroblast (MF) cell line was derived from tapasin-/- animals generated by Drs. A. Grandea and L. Van Kaer and colleagues (Vanderbilt University, Nashville, TN) (Grandea et al. 2000). Dr. T. Hansen (Washington University, St. Louis, MO) provided the MF cell line transduced with soluble mouse tapasin in the pMIN retroviral vector (Lybarger et al. 2001). To create a control cell line, a mouse wild type tapasin cDNA (Li et al. 1999), a kind gift from Dr. P. Wang (Barts and London School of Medicine), was cloned into the pMIN vector. The construct was packaged using 293E cells, and transduced into the MF cell line. MF cells expressing no mouse tapasin, wild type mouse tapasin, or soluble mouse tapasin were also transduced with Kd, Kb, or Ld cDNAs in the Clontech pLXSH retroviral vector (Miller et al. 1993), and the resulting cell lines were screened by Western blotting to identify clones expressing similar levels of wild type or mutant tapasin and of Kd, Kb, or Ld. MF transductants were also generated that expressed the Kd cDNA and either wild type human tapasin or soluble human tapasin (all in the pMIN vector). The soluble human tapasin was made by the polymerase chain reaction, using as a template a human tapasin cDNA (Li et al. 1997) that was generously provided by Dr. P. Wang, and sequenced to ensure fidelity to the template. The Kd and Kb constructs were donated by Dr. T. Hansen and included an epitope tag for the 64-3-7 antibody, so that the open form of the molecules could be identified by 64-3-7 in immunoprecipitation and flow cytometry procedures and the molecules could be recognized on Western blots by 64-3-7. This epitope tag has been used previously, and shown not to disrupt the normal peptide binding and trafficking of MHC class I molecules (Yu et al. 1999; Myers et al. 2000; Harris et al. 2001a; Lybarger et al. 2001). The 721.220 cell line expresses a mutant form of tapasin that has a truncated signal sequence and is lacking 49 amino acids after the signal sequence (Greenwood et al. 1994). The 721.220 cell lines, 721.220-B8, and 721.220-B8+soluble human tapasin cell lines were kindly donated by Drs. T. Spies (Fred Hutchinson Cancer Research Center, Seattle, WA) and P. Cresswell (Yale University, New Haven, CT). The 721.220-B8 cell line expressing soluble mouse tapasin was made by stably transfecting the soluble mouse tapasin cDNA in the pIRES vector (Clontech) into 721.220-B8 using the Nucleofector (Amaxa). An accompanying control cell line was made by stably transfecting wild type mouse tapasin cDNA in the pREP10 vector (Invitrogen) into 721.220-B8 using the Nucleofector. All cell lines were cultured at 37°C in 5% CO2 in RPMI or DMEM containing 10% or 15% FBS, 4 mM HEPES, 2 mM L-glutamine, 1X sodium pyruvate, 1X non-essential amino acids, penicillin (100 U/ml), and streptomycin (100 μg/ml), and for some cell lines, hygromycin B or G418. All media reagents were purchased from Invitrogen except the FBS, which was purchased from Atlanta Biologicals.
Antibodies
The 64-3-7 and 30-5-7 antibodies recognize the α1 domain of open, peptide-free and the α2 domain of folded, peptide-occupied Ld, respectively (Solheim et al. 1993; Smith et al. 1993). Both antibodies were used in this study for flow cytometry analysis and immunoprecipitation. The 64-3-7 antibody was also used in Western blot analysis to detect SDS-denatured total Ld (independent of the original Ld conformation prior to electrophoresis on SDS-PAGE). The 64-3-7 antibody can also detect open or denatured forms of other MHC class I molecules to which the 64-3-7 epitope has been added (Yu et al. 1999; Myers et al. 2000; Harris et al. 2001a; Lybarger et al. 2001). In this study, the 64-3-7 monoclonal antibody was used to detect open and denatured forms of epitope-tagged Kd and Kb, as well as Ld. The 34-1-2 monoclonal antibody binds to the α1 domain of folded Kd (Ozato et al. 1983). The Y3 and B8-24-3 monoclonal antibodies recognize folded Kb molecules (Jones et al. 1981; Allen et al. 1984). All of the anti-MHC class I antibodies used in this study were produced from hybridomas that were donated by Dr. T. Hansen. Rabbit antiserum specific for mouse TAP1 (Carreno et al. 1995) and hamster anti-mouse tapasin monoclonal antibody (Harris et al. 2001b), both gifts from Dr. T. Hansen, were used for Western blotting detection of mouse TAP and tapasin, respectively. Human HLA class I molecules were detected with HC10 (which recognizes unfolded human HLA class I molecules) or W6/32 (which recognizes folded, β2m-bound HLA class I) (Stam et al. 1986; Sernee et al. 1998; Carreno et al. 1994; Parham et al. 1979; Ladasky et al. 1999). Monoclonal antibody 148.3 (Meyer et al. 1994), used to immunoprecipitate human TAP1, was donated by Dr. R. Tampé (Goethe-University, Frankfurt/Main, Germany). Human tapasin was detected on Western blots with a monoclonal antibody made against a peptide corresponding to the N-terminal sequence of human tapasin (BD Transduction Laboratories).
Immunoprecipitations and Western blots
Immunoprecipitations and Western blotting were done similarly to a published protocol (Turnquist and Solheim, 2001). Prior to immunoprecipitations, cells were washed in PBS with 20 mM iodoacetamide (Sigma-Aldrich) three times and lysed in 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) lysis buffer. The CHAPS buffer consisted of 1% CHAPS (Sigma) in Tris-buffered saline (pH 7.4), to which PMSF (to 0.2 mM) and iodoacetamide (to 20 mM) and excess antibody were added immediately before it was used to lyse the cells. Following 1 h on ice, lysates were centrifuged to remove cell nuclei and the supernatants were incubated with Protein A-Sepharose beads (Amersham Biosciences) for 45 min on ice. (For the immunoprecipitations with the anti-human TAP1 antibody 148.3, Protein G-Sepharose (GE Healthcare) was used instead of Protein A-Sepharose.) The beads were then washed in 0.1% CHAPS/20 mM iodoacetamide in TBS (pH 7.4) four times and boiled for 5 min in 0.125 M Tris (pH 6.8)/2% SDS/12% glycerol/0.02% bromphenol blue to elute proteins. For cross-linking experiments, immunoprecipitations were performed as described above, except that cells were solubilized in Tris-buffered saline (pH 7.4) with freshly added 1% digitonin (Wako), 200 μM DSP (Tocis Bioscience), 0.2 mM PMSF, 20 mM iodoacetamide, and excess antibody, and washes were done in 0.1% digitonin/20 mM iodoacetamide/20 μM DSP in TBS (pH 7.4) rather than in a CHAPS-buffered solution.
Eluted immunoprecipitates were electrophoresed on SDS-PAGE gels (Invitrogen) and the proteins were transferred to Immobilon-P membranes (Millipore) for Western blotting. After blocking overnight in reconstituted dry milk, the membranes were incubated in dilutions of antibody for 2 h, washed three times with 0.05% Tween 20/PBS, and incubated for 1 h in diluted biotin-conjugated goat anti-mouse or anti-rabbit IgG (Invitrogen). Following three washes in 0.05% Tween 20/PBS, the membranes were incubated with streptavidin-conjugated horseradish peroxidase (Invitrogen) for 1 h, washed three times with 0.3% Tween 20/PBS, and incubated with enhanced chemiluminescence Western blot developing reagents (Pierce Chemical Co.). To visualize the bands, the membranes were exposed to Kodak BioMax film (Eastman Kodak).
In the endoglycosidase (Endo) H assay, immunoprecipitations were done as described above, except proteins were eluted from Protein A-Sepharose by boiling in 25 mM Tris (pH 8.3)/0.2 M glycine/0.1% SDS) for 5 min. After boiling, the samples were centrifuged and supernatants were transferred to new tubes. A 10X glycoprotein denaturing buffer (New England Biolabs) was added to 9 μl of supernatant to a final concentration of 1X, and the sample was boiled for 10 min. After boiling, the reaction volume of each sample was raised by addition of 2 μl of 10X G5 reaction buffer (New England Biolabs) and either 2 μl of Endo H (New England Biolabs) or 2 μl of water (for the mock digestion), plus a volume of water sufficient to produce a final volume of 20 μl. The tubes were incubated at 37°C for 1 h, and 5 μl of 0.5 M Tris (pH 6.8)/8% SDS/48% glycerol/0.08% bromophenol blue/8% β-mercaptoethanol were then added. The samples were electrophoresed and Western blots were performed as described above.
For Western blots without a preceding immunoprecipitation step, samples were processed in the following manner. Cells were washed with 20 mM iodoacetamide (Sigma-Aldrich) in PBS three times and lysed in buffer consisting of 0.125M Tris (pH 6.8), 2% (w/v) SDS, 12% (v/v) glycerol, 0.02% (w/v) bromophenol blue and fresh 0.2 mM PMSF for experiments that included soluble tapasin, and 150 mM NaCl, 20 mM Tris (pH 7.5), 5 mM EDTA, and 0.5% Triton X-100 for the other Western blotting experiments. The lysates were incubated for 1 hour on ice and centrifuged to remove nuclear material. Samples of the supernatants were boiled for 5 min before loading onto SDS-PAGE gels. Transfer of the proteins onto blotting membranes and the processing of the membranes were done as described above.
Flow cytometry
For flow cytometry analysis, cells were suspended at 5 × 106/ml in PBS with 0.5% BSA and 2 mM EDTA, and 0.1 ml aliquots were placed into a 96-well plate. After centrifugation and removal of the supernatant, excess antibody or BSA/EDTA/PBS alone (as a control) was added and the cells were incubated at 4°C for 30 min. The cells were then washed twice with BSA/EDTA/PBS, and incubated at 4°C for 30 min with PE-conjugated, Fc-specific F(ab′)2 goat anti-mouse IgG (Jackson ImmunoResearch). After the cells were washed 3 times in BSA/EDTA/PBS, they were resuspended in BSA/EDTA/PBS and assayed on a FACSCalibur flow cytometer (BD Biosciences). The Cell Quest software (BD Biosciences) was used for statistical analysis. For brefeldin A (Bfa) assays, Bfa (Sigma-Aldrich) was added at 2 μg/ml to the cell culture medium for varied amounts of time prior to cell harvest.
Results
Effect of mouse tapasin truncation on the surface expression of mouse MHC class I molecules
To determine the importance of the murine tapasin TM/CYT region to mouse MHC class I molecule assembly, we used a mouse tapasin mutant cDNA truncated after position 384. This mutant cDNA (and wild type tapasin, as a control) were expressed in mouse tapasin knockout cells (MF, MF-Ld, MF-Kd, and MF-Kb). The murine Ld, Kd, and Kb class I heavy chains that were transfected into the MF cells included a 64-3-7 epitope, which allows immunoprecipitation of the open, peptide-free heavy chain form. This epitope was either present naturally (in the case of Ld) or was added by site-directed mutagenesis (in the case of the Kd and Kb). The MF cells are H2b, and so also express some endogenous Kb.
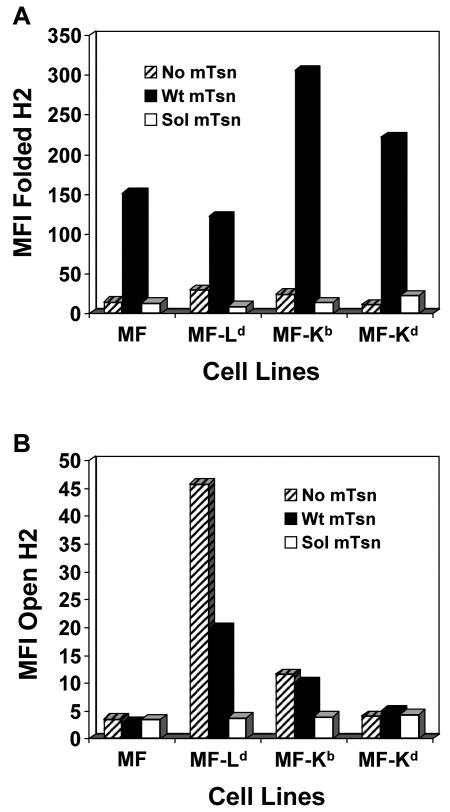
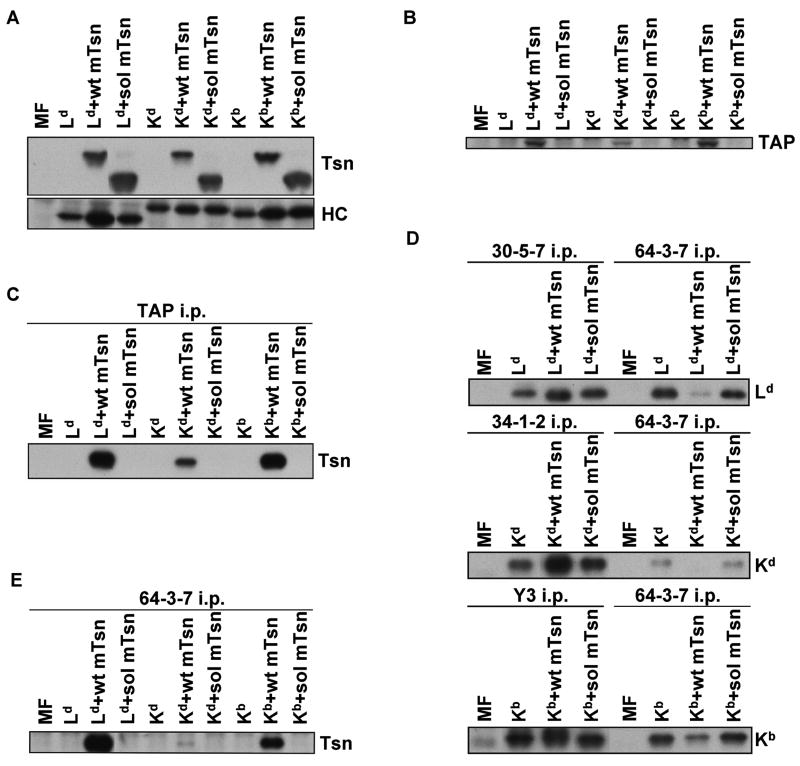
The levels of cell surface Ld, Kd, and Kb molecules on the MF transfectants expressing no tapasin, wild type tapasin, or soluble tapasin were analyzed by flow cytometry. Folded Ld, Kd, and Kb molecules were readily detected on cells expressing wild type tapasin (Fig. 1A). The proportion of open forms (detected by 64-3-7) at the surface of the wild type tapasin-transfected MFs was low for Kb and even lower for Kd, relative to Ld (Fig. 1B), as previously shown for 64-3-7-epitope-tagged Kb and Kd expressed in 721.221 (Myers et al. 2000). As expected, the quantity of each of these mouse MHC allotypes (Ld, Kd, and Kb) on cells lacking any tapasin was very low (Fig. 1), consistent with previous findings by others of weak Kb expression on non-activated tapasin knockout mouse cells (Grandea et al. 2000; Garbi et al. 2000; Garbi et al. 2003). Notably, the expression of Ld, Kb, and Kd was also found to be very low on cells that expressed truncated murine tapasin, similar to the level of these molecules on cells that expressed no tapasin at all (Fig. 1). As shown in Figure 2A, mutant tapasin was expressed in the transfectants in quantity similar to wild type tapasin, and the amount of total MHC class I heavy chain was reasonably matched among the transfectants (with slightly stronger heavy chain expression noted in the Ld+wt mTsn cell line). Thus, these experiments demonstrated that soluble mouse tapasin does not facilitate cell surface expression of Ld, Kd, or Kb on mouse cells, and the flow cytometry results were not merely due to low levels of soluble tapasin or MHC class I heavy chain expression in the transfected cells.
Figure 1.
The murine soluble tapasin mutant was not able to facilitate normal H2-Ld, -Kb, and -Kd surface expression. MF, MF+Ld, MF+Kb, or MF+Kd cells transfected with no tapasin, wild type mouse tapasin (Wt mTsn) or soluble mouse tapasin (Sol mTsn) were incubated with secondary antibody only, or with an antibody against the folded form of the transfected mouse MHC class I molecule (30-5-7 for Ld, Y3 for Kb, and 34-1-2 for Kd) or with 64-3-7 against the open form of each epitope-tagged mouse MHC class I molecule. Results obtained with the antibody against the folded form are shown in (A), and results obtained with 64-3-7 are shown in (B). Results obtained with secondary antibody only were all less than 5.0. Values on the y axes are relative mean fluorescence intensity (MFI) units. Very similar results were obtained with independent transfectant clones, with alternative antibodies (B8-24-3 for folded Kb), and in separate flow cytometry experiments.
Figure 2.
(A) Western blotting for mouse tapasin showed that soluble tapasin was present at a level similar to (or slightly exceeding) wild type mouse tapasin in transfected MF cells, and Western blotting for the transfected mouse MHC class I heavy chain demonstrated that Ld, Kd, or Kb heavy chains were expressed at a roughly similar level in the MF cells. After electrophoresis of samples of cell lysates on a 10% acrylamide Tris-glycine gel for tapasin or on a 4→20% acrylamide Tris-glycine gel for MHC class I heavy chain, the proteins were blotted and probed with Ab specific for mouse tapasin (Tsn) or with 64-3-7 (for HC). The Ld heavy chain naturally has the 64-3-7 epitope, and the epitope was introduced into the Kd and Kb heavy chains by site-directed mutagenesis. (B) Murine soluble tapasin did not stabilize mouse TAP. Lysate samples were electrophoresed on a 10% acrylamide Tris-glycine gel before blotting and probing with antibody specific for mouse TAP. (C) Soluble mouse tapasin did not associate with TAP. Immunoprecipitates of TAP molecules were electrophoresed on a 10% acrylamide Tris-glycine gel. The proteins were then transferred to a blotting membrane and probed with antiserum specific for mouse tapasin (Tsn). (D) The folding of Ld, Kd, and Kb heavy chains was improved by the intracellular presence of wild type tapasin but not soluble tapasin. Immunoprecipitations were performed with mAb 30-5-7, 34-1-2, and Y3 (for folded Ld, Kd, and Kb, respectively) on lysates of MF cells that expressed each of the aforementioned MHC class I heavy chains along with no tapasin, wild type tapasin, or soluble tapasin. Immunoprecipitations with the 64-3-7 mAb were also done on corresponding cell lysates. The immunoprecipitates were electrophoresed on 4→20% acrylamide Tris-glycine gels, transferred to membranes, and probed with 64-3-7. (E) Tapasin association with the mouse MHC class I heavy chain was abrogated by the deletion of the tapasin TM/CYT region. Open MHC class I heavy chains were immunoprecipitated from a lysate of each of the indicated cell types with the 64-3-7 mAb. The immunoprecipitates were electrophoresed on a 10% acrylamide Tris-glycine gel, transferred to a membrane, and probed with an anti-tapasin antibody (Tsn).
Murine soluble tapasin did not stabilize TAP or aid in Ld, Kd, or Kb intracellular assembly
A known function of wild type tapasin is to stabilize TAP (Lehner et al. 1998; Garbi et al. 2003). To test the ability of soluble murine tapasin to stabilize TAP, we probed lysates of tapasin-deficient MF cells transfected with Ld, Kd, or Kb along with no tapasin, wild type mouse tapasin or soluble mouse tapasin on a Western blot, using an antiserum against the mouse TAP1 subunit. The truncated murine tapasin molecule was unable to stabilize TAP (Fig. 2B). We also immunoprecipitated TAP from lysates of the transfected MF cells and probed with antibody to detect co-immunoprecipitated tapasin. Soluble mouse tapasin was unable to bind to TAP (Fig. 2C). Weaker bands were noted for TAP (Fig. 2B) and co-immunoprecipitated tapasin (Fig. 2C) for Kd+wt mTsn because this cell line expressed slightly less tapasin than Ld+wtTsn or Kb+wtTsn (Fig. 2A). Thus, loss of the mouse tapasin TM/CYT region prevented tapasin stabilization of TAP and interaction with TAP.
Immunoprecipitations of the open forms of each of these mouse MHC class I heavy chains were done with the 64-3-7 monoclonal antibody, and immunoprecipitations of the folded forms were performed with the monoclonal antibody 30-5-7, 34-1-2, or Y3 (for folded Ld, Kd, or Kb, respectively) from lysates of mouse MF cells that expressed no tapasin, wild type tapasin, or soluble murine tapasin. The samples were electrophoresed, transferred to a membrane, and probed with 64-3-7 (which can recognize all of these heavy chains after SDS-PAGE and membrane transfer, whether originally open or folded). The amounts of open heavy chains, and the relative levels of open and folded heavy chains in the cell lines were compared (Fig. 2D). Levels of Ld precipitable by 30-5-7, of Kd precipitable by 34-1-2, and of Kb precipitable by Y3 were relatively high from lysates of cells expressing wild type tapasin, but as low from cells with soluble mouse tapasin as from tapasin-negative cells. Thus, these immunoprecipitation results revealed that soluble mouse tapasin did not facilitate folding of Ld, Kd, and Kb. Figure 2D also shows that Kd assembly was highly efficient in the presence of wild type tapasin, such that the amount of open (64-3-7+) form relative to folded (34-1-2+) form was quite small.
Murine soluble tapasin did not detectably associate with the Ld, Kd, or Kb allotypes
We next sought to ascertain whether soluble murine tapasin interacted with mouse MHC class I heavy chains. Open forms of Ld, Kd, and Kb were immunoprecipitated from lysates of MF cells expressing each murine MHC class I heavy chain along with no tapasin, wild type murine tapasin, or soluble murine tapasin. The immunoprecipitates were probed on a Western blot with antibody against tapasin, and no association was detected between truncated murine tapasin and Ld, Kd, or Kb (Fig. 2E). The same result was obtained when a cross-linking agent (DSP) was added to the immunoprecipitation mixture and digitonin rather than CHAPS was used as the detergent (data not shown). These findings suggest that in the absence of the mouse tapasin TM/CYT domain there is no detectable association of mouse tapasin with mouse MHC class I heavy chains in mouse cell lysates.
Human soluble tapasin associated very weakly with Kd, and mouse soluble tapasin did not detectably interact with HLA-B8
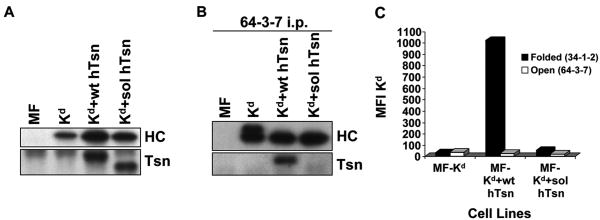
To determine whether the inability of soluble mouse tapasin to associate with the mouse MHC class I heavy chain was attributable to the nature of the soluble mouse tapasin or to the nature of the mouse MHC class I molecule, we generated transfectants with mixed combinations of mouse and human molecules. MF-Kd+wt hTsn and MF-Kd+sol hTsn transfectants were made that expressed similar levels of tapasin (Fig. 3A). Figure 3B shows data derived with these transfectants that demonstrates wild type human tapasin co-immunoprecipitated much more strongly with the Kd heavy chain than did soluble human tapasin. (These experiments were done using the DSP cross-linker so that any weak interactions would be revealed.) Therefore, the weak/absent interaction of soluble tapasin with Kd in the mouse cells was generalizable from mouse to human soluble tapasin. Flow cytometric analysis also showed that soluble human tapasin was unable to support Kd cell surface expression on MF cells (Fig. 3C).
Figure 3.
(A) Tapasin and Kd were expressed at similar levels in Kd+wt hTsn relative to Kd+sol hTsn. Samples of lysates from MF, MF+Kd, MF+Kd+wt hTsn, and MF+Kd+sol hTsn were electrophoresed on a 10% acrylamide Tris-glycine gel for tapasin (Tsn) or on a 4→20% acrylamide Tris-glycine gel for Kd, the proteins were transferred to a blotting membrane and probed with Ab specific for human tapasin (Tsn) or with 64-3-7 to identify the total epitope-tagged Kd heavy chain (HC). (B) Soluble human tapasin binds very weakly to Kd. Open Kd heavy chains were immunoprecipitated from a digitonin lysate of each of the indicated cell types with the 64-3-7 mAb in the presence of 200 μM DSP. The immunoprecipitates were electrophoresed on acrylamide Tris-glycine gels, transferred to membranes, and probed with 64-3-7 to identify the immunoprecipitated open Kd heavy chain (HC) or with anti-tapasin mAb (Tsn) to identify the co-immunoprecipitated tapasin. (C) Soluble human tapasin did not enable Kd to be expressed at the surface of mouse cells. Cells were incubated with secondary antibody only, or an antibody against the folded form of Kd (34-1-2), or an antibody against the open form of Kd (64-3-7). Results obtained with the secondary antibody only were all less than 5.0. Values on the y axis are relative mean fluorescence intensity (MFI) units. Wt hTsn = wild type human tapasin; Sol hTsn = soluble human tapasin. Very similar results were obtained in a separate flow cytometry experiment.
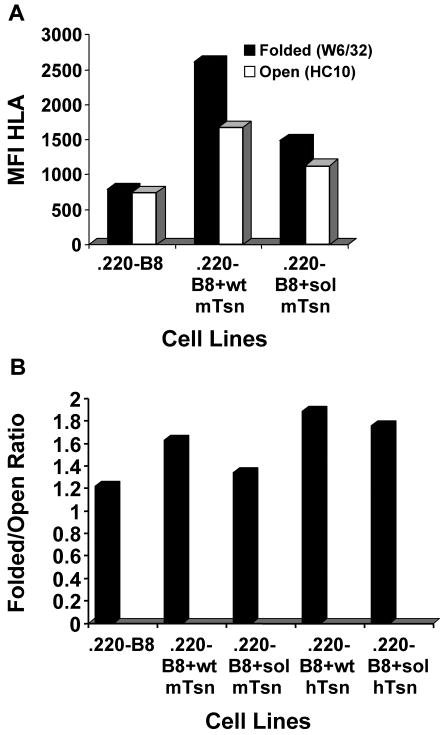
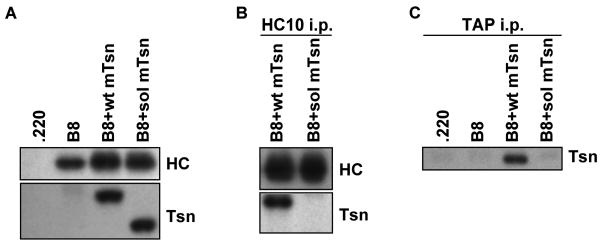
Transfected 721.220 cell lines were made to assess the effect of mouse soluble tapasin on the human HLA class I molecule B8. With these cell lines, we found that soluble mouse tapasin could not facilitate the surface expression of folded B8 as well as wild type mouse tapasin (Fig. 4A). The level of surface B8 (either open or folded) was lower for B8 expressed in the presence of no tapasin versus soluble tapasin or wild type tapasin (Fig. 4A), perhaps due in part to a slightly lower level of B8 expressed in .220-B8. The ratio of folded to open HLA class I molecules on .220-B8+sol mTsn was consistently somewhat higher than the ratio of folded to open HLA on .220-B8 (Fig. 4B). In this analysis, we also included .220-B8+wt hTsn and .220-B8+sol hTsn for comparison, and found that the ratio of folded to open HLA class I molecules was higher for both of these cell lines than for .220-B8+wt mTsn or .220-B8+sol mTsn (Fig. 4B). Furthermore, the HLA class I folded/open ratio was decreased 18% by expression of soluble mouse tapasin compared to wild type mouse tapasin, but only by 7% by expression of soluble human tapasin compared to wild type human tapasin (Fig. 4B).
Figure 4.
Soluble mouse tapasin partially restored B8 expression at the surface of 721.220 cells. (A) Cells (.220-B8, .220-B8+wt mTsn, and .220-B8+sol mTsn) were incubated with secondary antibody only (data not shown), or with an antibody against the folded form of the HLA class I molecule (W6/32), or with an antibody against the open form of the HLA class I molecule (HC10). Results obtained with the secondary antibody only were all less than 11.0. Values on the y axis are relative mean fluorescence intensity (MFI) units. Wt mTsn = wild type mouse tapasin; Sol mTsn = soluble mouse tapasin. Very similar results were obtained in a separate flow cytometry experiment. (B) Cells (.220-B8, .220-B8+wt mTsn, .220-B8+sol mTsn, .220-B8+wt hTsn, .220-B8+sol hTsn) were incubated with secondary antibody only, W6/32, or HC10. Results obtained with the secondary antibody only were all less than 7.0. The ratio of the mean fluorescence intensity for W6/32 versus HC10 for each cell type is displayed.
Soluble and wild type tapasin were expressed at equivalent levels in .220-B8+wt mTsn and .220-B8+sol mTsn transfectants, and equal amounts of open HLA class I heavy chains were immunoprecipitated from .220-B8+wt mTsn and .220-B8+sol mTsn cell lysates (Fig. 5A and B). Untransfected .220 cells express some endogenous HLA class I, but our analyses and the findings of others (Purcell et al. 2001; Momburg and Tan 2002) have indicated that the level is low relative to the level of HLA class I expressed in HLA-transfected .220 cells. Therefore, the majority of immunoprecipitated HLA class I heavy chain in this case is B8. Wild type mouse tapasin was co-immunoprecipitated with B8, but soluble mouse tapasin did not co-immunoprecipitate with B8 (Fig. 5B). Co-immunoprecipitation of soluble mouse tapasin with B8 was also not observed when DSP cross-linking was used (data not shown). These data indicate that the strength of interaction with B8 may be even weaker with mouse soluble tapasin than with human soluble tapasin, since we could not detect soluble mouse tapasin binding to B8 even after cross-linking. (Note that the flow cytometry data mentioned above indicated that soluble mouse tapasin was nevertheless still able to assist B8 folding and surface expression.) In addition, we demonstrated that soluble mouse tapasin did not co-immunoprecipitate with human TAP1 (Fig. 5C).
Figure 5.
(A) Tapasin and B8 were expressed at similar levels in B8+wt mTsn relative to B8+sol mTsn. Samples of lysates from .220, .220-B8, .220-B8+wt mTsn, and .220-B8+sol mTsn were electrophoresed on a 10% acrylamide Tris-glycine gel for tapasin (Tsn) or on a 4→20% acrylamide Tris-glycine gel for B8, the proteins were transferred to a blotting membrane and probed with Ab specific for mouse tapasin (Tsn) or with HC10 to identify the open B8 heavy chain (HC). (B) Soluble mouse tapasin does not associate with B8. Open B8 heavy chains were immunoprecipitated from a lysate of each of the indicated cell types with the HC10 mAb. The immunoprecipitates were electrophoresed on acrylamide Tris-glycine gels, transferred to membranes, and probed with HC10 to identify the immunoprecipitated open B8 heavy chain (HC) or with anti-mouse tapasin antibody (Tsn) to identify the co-immunoprecipitated tapasin. (C) Soluble mouse tapasin does not associate with human TAP. TAP1 was immunoprecipitated from cell lysates with antibody 148.3. After electrophoresis of the immunoprecipitated proteins on a 10% acrylamide Tris-glycine gel, the proteins were blotted and probed with anti-mouse tapasin antibody (Tsn).
Mouse tapasin K408W increases the proportion of open, immature Kd molecules
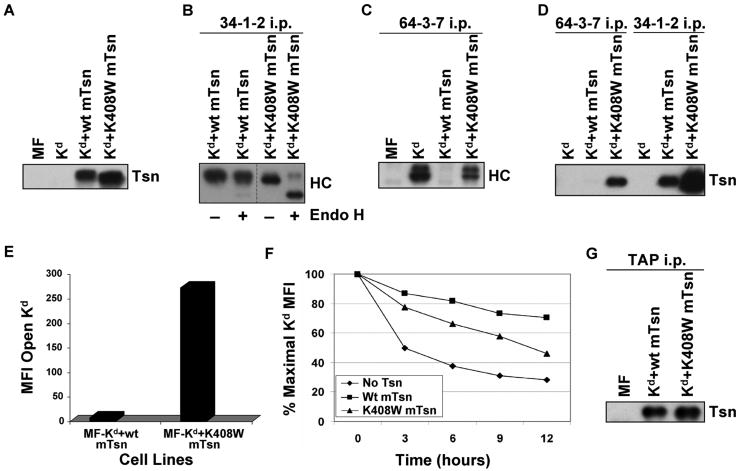
In a previous study from our laboratory, we identified a lysine in the TM/CYT region of human tapasin as influential in HLA-B8 assembly by mutating the lysine to either an alanine or a tryptophan (Petersen et al. 2005). To assess whether this lysine in mouse tapasin has similar effects on a mouse MHC class I molecule, we mutated this position in mouse tapasin to a tryptophan and transfected the K408W mutant into MF cells expressing Kd (Fig. 6A). Folded forms of Kd were immunoprecipitated by 34-1-2 from lysates of MF-Kd+wt mTsn and MF-Kd+K408W mTsn, either with or without Endo H treatment. In both cell types, there were folded Kd molecules, but a majority of Kd molecules folded in the presence of mouse tapasin K408W were Endo H sensitive (Fig. 6B), and therefore had not passed through the Golgi (Krangel et al. 1979; Owen et al. 1980). Open forms of Kd were immunoprecipitated by 64-3-7 and the immunoprecipitates were electrophoresed and probed on a Western blot with 64-3-7 to reveal the amount of open Kd. As shown in Figure 6C, in the presence of wild type tapasin, Kd assembly was highly efficient and the amount of intracellular 64-3-7+ forms was very low. In contrast, there was a considerable quantity of 64-3-7+ Kd detectable in the cell line expressing tapasin K408W, indicating that the folding of Kd in the presence of tapasin K408W was much less efficient than in the presence of wild type tapasin. Thus, the intracellular folding of Kd was affected by this mouse tapasin mutation.
Figure 6.
(A) Tapasin K408W was detected in similar or slightly greater amount than wild type tapasin in the transfected cells. Samples of cell lysates were electrophoresed on a 10% acrylamide Tris-glycine gel, and the proteins were transferred to a blotting membrane and probed with an antibody for mouse tapasin (Tsn). (B) A majority of the Kd molecules folded in the presence of mouse tapasin K408W were Endo H sensitive. Folded Kd molecules were immunoprecipitated with 34-1-2, electrophoresed on 4→20% acrylamide Tris-glycine gel, blotted, and probed with 64-3-7 (HC). Samples were treated (+) or untreated (-) with Endo H as indicated. (C) Mouse tapasin K408W was found to increase the amount of Kd in the open conformation. Immunoprecipitations were performed with antibody 64-3-7 on lysates of the indicated cell types. The immunoprecipitated proteins were electrophoresed on a 4→20% acrylamide Tris-glycine gel, transferred, and probed on a blot with 64-3-7 (HC). (D) Kd was more strongly associated with tapasin K408W than with wild type tapasin, and both wild type tapasin and tapasin K408W were predominantly associated with the folded, and not the open, form of Kd. Immunoprecipitations were performed with mAb 64-3-7 and 34-1-2 on lysates of the indicated cell types. The immunoprecipitated proteins were electrophoresed on a 10% acrylamide Tris-glycine gel, transferred to a membrane, and probed with anti-tapasin antibody (Tsn). (E) The amount of open Kd at the cell surface was increased by expression of tapasin K408W compared to wild type tapasin. Cells were incubated with secondary antibody only, or with an antibody against the open form of the Kd molecule (64-3-7). Background staining with PE-conjugated secondary antibody only was <5.0 for both MF-Kd+wt mTsn and MF-Kd+K408W mTsn (not shown). Values on the y axis are relative mean fluorescence intensity (MFI) units. Similar results were obtained in a separate flow cytometry experiment. (F) Kd molecules assembled in cells expressing mouse tapasin K408W were less stable than those assembled in the presence of wild type mouse tapasin. Cells were treated with 2 μg/ml Bfa in complete medium for 0, 3, 6, 9, or 12 hours, then stained with 34-1-2 and analyzed by flow cytometry. (G) The K408W mutation in mouse tapasin does not abrogate the association of tapasin with TAP. Immunoprecipitations were performed with an anti-TAP antiserum on lysates of the cell lines indicated. The immunoprecipitated proteins were electrophoresed on a 10% acrylamide Tris-glycine gel, and probed on a Western blot with an anti-mouse tapasin antibody.
The association of the open (64-3-7+) form of Kd with wild type mouse tapasin and with tapasin K408W was also assessed, and a greater quantity of tapasin K408W than wild type tapasin co-immunoprecipitated with open Kd (Fig. 6D). Surprisingly, wild type tapasin was demonstrated to be strongly associated with folded (34-1-2+) forms of Kd, and even more folded Kd molecules were bound to tapasin K408W than to wild type tapasin (Fig. 6D). Thus, there was interaction between folded Kd and wild type tapasin that was increased further by the tapasin K408W mutation.
We also evaluated whether the tapasin K408W mutation resulted in greater numbers of open Kd molecules at the cell surface. As shown in Figure 6E, flow cytometric analysis demonstrated that the amount of open Kd at the plasma membrane was increased by expression of tapasin K408W compared to wild type tapasin. Consistent with the increase observed in open Kd at the cell surface, the drop in folded Kd molecules at the cell surface following Bfa treatment of cells expressing tapasin K408W was faster compared to wild type tapasin (Fig. 6F). The effect on the level of open MHC class I at the cell surface was much stronger in this mouse model than we had observed with the equivalent human tapasin mutant in human cells, which caused no increase in open forms or instability at the cell surface (Petersen et al. 2005). These findings with mouse tapasin K408W suggested that expression of this mutant might cause deficiencies in peptide binding or supply. To test whether the mouse tapasin K408W mutation affected tapasin/TAP interaction, we immunoprecipitated TAP from lysates of MF-Kd+wt mTsn or MF-Kd+K408W mTsn, and probed the immunoprecipitates for tapasin. We found tapasin/TAP interaction to be similar in both cell types (Fig. 6G), which was surprising because the K408W mutation in human tapasin weakens tapasin/TAP association in human cells (Petersen et al. 2005). Hence, the phenotype of the mouse tapasin K408W mutant in mouse cells does not result from abrogation of TAP interaction.
Discussion
In this study, we found that soluble murine tapasin transfected into mouse tapasin knockout fibroblasts had no association with TAP and no detectable association with mouse MHC class I heavy chains (Ld, Kd, or Kb). Furthermore, in contrast to previous findings by others using B8 and soluble human tapasin in human cells (Lehner et al. 1998), we found that mouse cells with soluble murine tapasin expressed virtually no mouse MHC class I molecules at the plasma membrane. Thus, we found that the TM/CYT domain of murine tapasin has a significant impact on the ability of the tapasin protein to support expression of mouse MHC class I molecules on mouse cells.
Notably, a previous study from our laboratory on a tapasin mutant unable to bind to the Ld heavy chain (tapasin Δ334-342) demonstrated that, although the mutant allowed Ld to be expressed at the cell surface, the ratio of Ld folded (peptide-occupied) forms to open (peptide-free) forms was decreased by the mutant relative to wild type tapasin (Turnquist et al. 2004). Because the tapasin Δ334-342 mutant is able to bind to TAP and stabilize TAP (Turnquist et al. 2001), this rise in the number of open forms argues that tapasin binding to the heavy chain, apart from its binding to TAP, is likely also a factor in tapasin's normal ability to assist in mouse MHC class I assembly. We found that, like Ld, Kd is not bound by tapasin Δ334-342, and on MF cells that expressed tapasin Δ334-342 along with soluble tapasin, Kd surface expression was not increased at all, relative to Kd on cells transfected with soluble tapasin (data not shown). Furthermore, the tapasin K408W mutation affected Kd folding, but not TAP association. These findings further support the importance of tapasin interaction with the heavy chain, rather than TAP, as contributing to tapasin's effect on MHC class I assembly.
However, the ability of mouse soluble tapasin to facilitate B8 surface expression in part (Fig 4) suggests that the MHC class I assembly activity of mouse soluble tapasin in human cells goes beyond interacting with the MHC class I heavy chain or TAP, since soluble mouse tapasin expressed in 721.220 can neither bind B8 nor TAP (Figs. 5B and C). Consistent with our findings, the expression of B*4402 has been noted to be greatly increased at the surface of 721.220 cells by the transfection of soluble mouse tapasin (Peh et al. 2000), and expression of soluble human tapasin facilitated the maturation of B*4405 molecules and B8 in 721.220 cells, though not as well as wild type tapasin (Zarling et al. 2003; Purcell et al. 2004). Everett and Edidin (2007) did not observe a difference in cell surface HLA class I stability on 721.220 transfected with soluble human tapasin compared to wild type when they used a directly conjugated primary antibody for flow cytometry analysis, but they did observe a difference in some assays when they used W6/32 and a secondary antibody, as we have. The binding site on tapasin for another protein with which it interacts in the peptide-loading complex, ERp57, is at position 95 (Dick et al. 2002), which is distant from the TM/CYT domain. Therefore, the binding of tapasin to ERp57 should not be disrupted by tapasin truncation, and soluble tapasin's ability to at least partially restore normal MHC class I assembly and surface expression on human cells may be related to interactions with ERp57.
An interesting contrast to the ability of soluble mouse tapasin to partially assist B8 surface expression can be found by close examination of the data in Figure 1. In Figure 1, comparison of MHC class I expression in the presence of no tapasin versus soluble tapasin shows that the expression of soluble tapasin reduced the cell surface expression of Ld and Kb below the level seen for cells with no tapasin. The effect is most pronounced in the reduction of the amount of Ld at the cell surface. In Figure 1A, the level of folded (30-5-7+) Ld is 30.0 mean fluorescence intensity units on MF-Ld, and only 9.6 on MF-Ld+sol mTsn. In Figure 1B, the level of open (64-3-7+) Ld drops from 45.8 on MF-Ld to only 3.8 on MF-Ld+sol mTsn. This observation was made in other separate experiments for Ld and Kb, and was seen in some experiments with Kd, suggesting that the mouse soluble tapasin interfered with surface expression of the mouse MHC class I molecules.
We found that wild type tapasin was easily detectable associated with folded Kd, and that the interaction of either open or folded Kd with tapasin was increased dramatically by the K408W mutation (Fig. 6D). In our studies, folded forms of Kd were identified with the 34-1-2 antibody. This antibody binds to the Kd α1 domain (Ozato et al. 1983) and cross-reacts weakly with Ld (Nieto et al. 1989). It is known that the reactivity of 34-1-2 with Ld is increased strongly if the Ld groove is occupied by certain synthetic peptide ligands, or if Ld is mutated at certain positions within the groove floor in a position to contact bound peptide (Solheim et al. 1995), which indicates that this antibody is conformationally sensitive and specific for folded forms of the MHC class I molecules that it recognizes. With W6/32+ (folded, β2m-associated) forms of HLA-B8, there is also some association with wild type human tapasin and even stronger association with human tapasin K408A (Petersen et al. 2005). Thus, our results with 34-1-2 and Kd are reminiscent of our previous findings with W6/32 and HLA-B8. However, the amount of folded Kd associated with wild type tapasin is surprising compared to previous findings with Ld, because in the case of Ld there is very little association with tapasin or other members of the peptide-loading complex after folding with peptide (Carreno et al. 1995; Harris et al. 1998; data not shown).
Our studies have examined how mouse tapasin's TM/CYT domain affects mouse MHC class I assembly, and our results suggest that the mouse tapasin TM/CYT domain is crucial to mouse tapasin's interactions involving the MHC class I heavy chain as well as TAP, and that it is required for the mouse MHC class I molecules Ld, Kd, and Kb to be expressed on the plasma membrane of mouse cells. In contrast, although the human tapasin TM/CYT region has been shown to be necessary for TAP association, previous studies have not indicated that it is necessary for HLA class I surface expression. We discovered that Kd was affected by a K408W mutation in the tapasin TM/CYT region; this substitution resulted in a large increase in open forms of Kd within the cell and at the cell surface. The increase in open forms for Kd in the presence of this mutant was much greater than that found for HLA-B8 (Petersen et al. 2005). The Kd molecule was unable to be expressed at the surface of mouse cells in the presence of soluble human tapasin, but mouse soluble tapasin could partially support B8 surface expression on human cells. Our results, together with the results of other tapasin studies, suggest a model in which the mouse TM/CYT domain normally provides interactions with the MHC class I molecule and TAP, and of these two interactions the one with the MHC class I heavy chain may be the more crucial. In total, our analyses have increased our comprehension of the diversity of tapasin function in cells from different species.
Acknowledgments
This work was supported by NIH Grant GM57428 (to J.C.S.), by an NIH/NCI Training Grant T32 CA009476 Fellowship (to L.S.), and by UNMC Graduate Studies Fellowships (to L.S. and A.T.). Core facilities at the University of Nebraska Medical Center receive support from the NIH/NCI Cancer Center Support Grant P30CA036727 (to the Eppley Cancer Center) and the Nebraska Research Initiative. The UNMC DNA Sequencing Core Facility receives partial support from the NIH/NCRR INBRE Program P20 RR016469 Grant. We thank Dr. Ping Wang for the gift of the tapasin cDNAs, Drs. T. Hansen, A. Grandea, L. Van Kaer, T. Spies, and P. Cresswell for gifts of cell lines, and Dr. T. Hansen for gifts of antibodies. We also gratefully acknowledge the assistance of Briana Ormsbee and the personnel at the University of Nebraska Medical Center Cell Analysis Facility, Monoclonal Antibody Facility, and DNA Sequencing Core Facility.
Footnotes
The original publication is available at www.springerlink.com.
References
- Allen H, Wraith D, Pala P, Askonas B, Flavell RA. Domain interactions of H-2 class I antigens alter cytotoxic T-cell recognition sites. Nature. 1984;309:279–281. doi: 10.1038/309279a0. [DOI] [PubMed] [Google Scholar]
- Bangia N, Lehner PJ, Hughes EA, Surman M, Cresswell P. The N-terminal region of tapasin is required to stabilize the MHC class I loading complex. Eur J Immunol. 1999;29:1858–1870. doi: 10.1002/(SICI)1521-4141(199906)29:06<1858::AID-IMMU1858>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Barnden MJ, Purcell AW, Gorman JJ, McCluskey J. Tapasin-mediated retention and optimization of peptide ligands during the assembly of class I molecules. J Immunol. 2000;165:322–330. doi: 10.4049/jimmunol.165.1.322. [DOI] [PubMed] [Google Scholar]
- Carreno BM, Hansen TH. Exogenous peptide ligand influences the expression and half-life of free HLA class I heavy chains ubiquitously detected at the cell surface. Eur J Immunol. 1994;24:1285–1292. doi: 10.1002/eji.1830240607. [DOI] [PubMed] [Google Scholar]
- Carreno B, Solheim JC, Harris M, Stroynowski I, Connolly JM, Hansen TH. TAP associates with a unique class I conformation, whereas calnexin associates with multiple class I forms in mouse and man. J Immunol. 1995;155:4726–4733. [PubMed] [Google Scholar]
- Copeman J, Bangia N, Cross JC, Cresswell P. Elucidation of the genetic basis of the antigen presentation defects in the mutant cell line .220 reveals polymorphism and alternative splicing of the tapasin gene. Eur J Immunol. 1998;28:3788–3791. doi: 10.1002/(SICI)1521-4141(199811)28:11<3783::AID-IMMU3783>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Dick TP, Bangia N, Peaper DR, Cresswell P. Disulfide bond isomerization and the assembly of MHC class I-peptide complexes. Immunity. 2002;16:87–98. doi: 10.1016/s1074-7613(02)00263-7. [DOI] [PubMed] [Google Scholar]
- Everett MW, Edidin M. Tapasin increases efficiency of MHC I assembly in the endoplasmic reticulum but does not affect MHC I stability at the cell surface. J Immunol. 2007;179:7646–7652. doi: 10.4049/jimmunol.179.11.7646. [DOI] [PubMed] [Google Scholar]
- Farmery MR, Allen S, Allen AJ, Bulleid NJ. The role of ERp57 in disulfide bond formation during the assembly of major histocompatibility complex class I in a synchronized semipermeabilized cell translation system. J Biol Chem. 2000;275:14933–14938. doi: 10.1074/jbc.275.20.14933. [DOI] [PubMed] [Google Scholar]
- Garbi N, Tan P, Diehl AD, Chambers BJ, Ljunggren HG, Momburg F, Hämmerling GJ. Impaired immune responses and altered peptide repertoire in tapasin-deficient mice. Nature Immunol. 2000;3:234–238. doi: 10.1038/79775. [DOI] [PubMed] [Google Scholar]
- Garbi N, Tiwari N, Momburg F, Hammerling GJ. A major role for tapasin as a stabilizer of the TAP peptide transporter and consequences for MHC class I expression. Eur J Immunol. 2003;33:264–273. doi: 10.1002/immu.200390029. [DOI] [PubMed] [Google Scholar]
- Grandea AG, III, Androlewicz MJ, Athwal RS, Geraghty DE, Spies T. Dependence of peptide binding by MHC class I molecules on their interaction with TAP. Science. 1995;270:105–108. doi: 10.1126/science.270.5233.105. [DOI] [PubMed] [Google Scholar]
- Grandea AG, III, Comber PG, Wenderfer SE, Schoenhals G, Fruh K, Monaco JJ, Spies T. Sequence, linkage to H2-K, and function of mouse tapasin in MHC class I assembly. Immunogenetics. 1998;48:260–265. doi: 10.1007/s002510050430. [DOI] [PubMed] [Google Scholar]
- Grandea AG, III, Golovina TN, Hamilton SE, Sriram V, Spies T, Brutkiewicz RR, Harty JT, Eisenlohr LC, Van Kaer L. Impaired assembly yet normal trafficking of MHC class I molecules in tapasin mutant mice. Immunity. 2000;13:213–222. doi: 10.1016/s1074-7613(00)00021-2. [DOI] [PubMed] [Google Scholar]
- Greenwood R, Shimizu Y, Sehon GS, DeMars R. Novel, allele-specific post-translational reduction in HLA class I surface expression in a mutant human B cell line. J Immunol. 1994;153:5525–5536. [PubMed] [Google Scholar]
- Harris MR, Yu YYL, Kindle CS, Hansen TH, Solheim JC. Calreticulin and calnexin interact with different protein and glycan determinants during the assembly of MHC class I. J Immunol. 1998;160:5404–5409. [PubMed] [Google Scholar]
- Harris MR, Lybarger L, Myers NB, Hilbert C, Solheim JC, Hansen TH, Yu YY. Interactions of HLA-B27 with the peptide loading complex as revealed by heavy chain mutations. Int Immunol. 2001a;13:1275–1282. doi: 10.1093/intimm/13.10.1275. [DOI] [PubMed] [Google Scholar]
- Harris MR, Lybarger L, Yu YYL, Myers NB, Hansen TH. Association of ERp57 with mouse MHC class I molecules is tapasin dependent and mimics that of calreticulin and not calnexin. J Immunol. 2001b;166:6686–6692. doi: 10.4049/jimmunol.166.11.6686. [DOI] [PubMed] [Google Scholar]
- Jones B, Janeway CA., Jr Cooperative interaction of B lymphocytes with antigen-specific helper T lymphocytes is MHC restricted. Nature. 1981;292:547–549. doi: 10.1038/292547a0. [DOI] [PubMed] [Google Scholar]
- Krangel MS, Orr HT, Strominger JL. Assembly and maturation of HLA-A and HLA-B antigens. Cell. 1979;18:979–991. doi: 10.1016/0092-8674(79)90210-1. [DOI] [PubMed] [Google Scholar]
- Ladasky JJ, Shum BP, Canavez F, Seuanez HN, Parham P. Residue 3 of β2-microglobulin affects binding of class I MHC molecules by the W6/32 antibody. Immunogenetics. 1999;49:312–320. doi: 10.1007/s002510050498. [DOI] [PubMed] [Google Scholar]
- Lehner PJ, Surman MJ, Cresswell P. Soluble tapasin restores MHC class I expression and function in the tapasin-negative cell line .220. Immunity. 1998;8:221–231. doi: 10.1016/s1074-7613(00)80474-4. [DOI] [PubMed] [Google Scholar]
- Li S, Sjögren HO, Hellman U, Pettersson RF, Wang P. Cloning and functional characterization of a subunit of the transporter associated with antigen processing. Proc Natl Acad Sci USA. 1997;94:8708–8713. doi: 10.1073/pnas.94.16.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Paulsson KM, Sjogren HO, Wang P. Peptide-bound major histocompatibility complex class I molecules associate with tapasin before dissociation from transporter associated with antigen processing. J Biol Chem. 1999;274:8649–8654. doi: 10.1074/jbc.274.13.8649. [DOI] [PubMed] [Google Scholar]
- Li S, Paulsson KM, Chen S, Sjögren HO, Wang P. Tapasin is required for efficient peptide binding to transporter associated with antigen processing. J Biol Chem. 2000;275:1581–1586. doi: 10.1074/jbc.275.3.1581. [DOI] [PubMed] [Google Scholar]
- Lybarger L, Yu YYL, Chun T, Wang CR, Grandea AG, III, Van Kaer L, Hansen TH. Tapasin enhances peptide-induced expression of H2-M3 molecules, but is not required for the retention of open conformers. J Immunol. 2001;167:2097–2105. doi: 10.4049/jimmunol.167.4.2097. [DOI] [PubMed] [Google Scholar]
- Meyer TH, van Endert PM, Uebel S, Ehring B, Tampé R. Functional expression and purification of the ABC transporter complex associated with antigen processing (TAP) in insect cells. FEBS Lett. 1994;351:443–447. doi: 10.1016/0014-5793(94)00908-2. [DOI] [PubMed] [Google Scholar]
- Miller AD, Miller DG, Garcia JV, Lynch CM. Use of retroviral vectors for gene transfer and expression. Meth Enzymol. 1993;217:581–599. doi: 10.1016/0076-6879(93)17090-r. [DOI] [PubMed] [Google Scholar]
- Momburg F, Tan P. Tapasin – the keystone of the loading complex optimizing peptide binding by MHC class I molecules in the endoplasmic reticulum. Mol Immunol. 2002;39:217–233. doi: 10.1016/s0161-5890(02)00103-7. [DOI] [PubMed] [Google Scholar]
- Myers NB, Harris MR, Connolly JM, Lybarger L, Yu YY, Hansen TH. Kb, Kd, and Ld molecules share common tapasin dependencies as determined using a novel epitope tag. J Immunol. 2000;165:5656–5663. doi: 10.4049/jimmunol.165.10.5656. [DOI] [PubMed] [Google Scholar]
- Nieto MC, Song ES, McKinney D, McMillan M, Goodenow RS. The association of H-2Ld with human β-2 microglobulin induces localized conformational changes in the α-1 and -2 superdomain. Immunogenetics. 1989;30:361–369. doi: 10.1007/BF02425276. [DOI] [PubMed] [Google Scholar]
- Ortmann B, Copeman J, Lehner PJ, Sadasivan B, Herberg JA, Grandea AG, Riddell SR, Tampé R, Spies T, Trowsdale J, Cresswell P. A critical role for tapasin in the assembly and function of multimeric MHC class I-TAP complexes. Science. 1997;277:1306–1309. doi: 10.1126/science.277.5330.1306. [DOI] [PubMed] [Google Scholar]
- Ozato K, Evans GA, Shykind B, Margulies D, Seidman JG. Hybrid H-2 histocompatibility gene products assign domains recognized by alloreactive T cells. Proc Natl Acad Sci USA. 1983;80:2040–2043. doi: 10.1073/pnas.80.7.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen MJ, Kissonerghis AM, Lodish HF. Biosynthesis of HLA-A and HLA-B antigens in vivo. J Biol Chem. 1980;255:9678–9684. [PubMed] [Google Scholar]
- Pamer E, Cresswell P. Mechanisms of MHC class I-restricted antigen processing. Annu Rev Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- Papadopoulos M, Momburg F. Multiple residues in the transmembrane helix and connecting peptide of mouse tapasin stabilize the transporter associated with antigen processing TAP2 subunit. J Biol Chem. 2007;282:9401–9410. doi: 10.1074/jbc.M610429200. [DOI] [PubMed] [Google Scholar]
- Paquet ME, Cohen-Doyle M, Shore GC, Williams DB. Bap29/31 influences the intracellular traffic of MHC class I molecules. J Immunol. 2004;172:7548–7555. doi: 10.4049/jimmunol.172.12.7548. [DOI] [PubMed] [Google Scholar]
- Parham P, Barnstable CJ, Bodmer WF. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C antigens. J Immunol. 1979;123:342–349. [PubMed] [Google Scholar]
- Park B, Lee S, Kim E, Cho K, Riddell SR, Cho S, Ahn K. Redox regulation facilitates optimal peptide selection by MHC class I during antigen processing. Cell. 2006;127:369–382. doi: 10.1016/j.cell.2006.08.041. [DOI] [PubMed] [Google Scholar]
- Peh CA, Burrows SR, Barnden M, Khanna R, Cresswell P, Moss D, McCluskey J. HLA-B27 restricted antigen presentation in the absence of tapasin reveals polymorphism in mechanisms of HLA class I-peptide loading. Immunity. 1998;8:531–542. doi: 10.1016/s1074-7613(00)80558-0. [DOI] [PubMed] [Google Scholar]
- Petersen JL, Hickman-Miller HD, McIlhaney MM, Vargas SE, Purcell AW, Hildebrand WH, Solheim JC. A charged amino acid residue in the transmembrane/cytoplasmic region of tapasin influences MHC class I assembly and maturation. J Immunol. 2005;174:962–969. doi: 10.4049/jimmunol.174.2.962. [DOI] [PubMed] [Google Scholar]
- Purcell AW, Gorman JJ, Garcia-Peydro M, Paradela A, Burrows SR, Talbo GH, Laham N, Peh CA, Reynolds EC, Lopez de Castro JA, McCluskey J. Quantitative and qualitative influences of tapasin on the class I peptide repertoire. J Immunol. 2001;166:1016–1027. doi: 10.4049/jimmunol.166.2.1016. [DOI] [PubMed] [Google Scholar]
- Raghuraman G, Lapinski PE, Raghavan M. Tapasin interacts with the membrane-spanning domains of both TAP subunits and enhances the structural stability of TAP1 •TAP2 complexes. J Biol Chem. 2002;277:41786–41794. doi: 10.1074/jbc.M207128200. [DOI] [PubMed] [Google Scholar]
- Rizvi SM, Raghavan M. Direct peptide-regulatable interactions between MHC class I molecules and tapasin. Proc Natl Acad Sci USA. 2006;103:18220–18225. doi: 10.1073/pnas.0605131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadasivan B, Lehner PJ, Ortmann B, Spies T, Cresswell P. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity. 1996;5:103–114. doi: 10.1016/s1074-7613(00)80487-2. [DOI] [PubMed] [Google Scholar]
- Sernee MF, Ploegh HL, Schust DJ. Why certain antibodies cross-react with HLA-A and HLA-G: epitope mapping of two common MHC class I reagents. Mol Immunol. 1998;35:177–188. doi: 10.1016/s0161-5890(98)00026-1. [DOI] [PubMed] [Google Scholar]
- Smith JD, Myers NB, Gorka JJ, Hansen TH. Model for the in vivo assembly of nascent Ld class I molecules and for the expression of unfolded Ld molecules at the cell surface. J Exp Med. 1993;178:2035–2046. doi: 10.1084/jem.178.6.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solheim JC, Carreno BM, Smith JD, Gorka J, Myers NB, Wen Z, Martinko JM, Lee DR, Hansen TH. Binding of peptides lacking consensus anchor residue alters H-2Ld serologic recognition. J Immunol. 1993;151:5387–5397. [PubMed] [Google Scholar]
- Solheim JC, Carreno BM, Myers NB, Lee DR, Hansen TH. Peptide-induced rescue of serologic epitopes on class I MHC molecules. J Immunol. 1995;154:1188–1197. [PubMed] [Google Scholar]
- Solheim JC, Harris MR, Kindle CS, Hansen TH. Prominence of β2-microglobulin, class I heavy chain conformation, and tapasin in the interactions of class I heavy chain with calreticulin and the transporter associated with antigen processing. J Immunol. 1997;158:2236–2241. [PubMed] [Google Scholar]
- Stam N, Spit H, Ploegh H. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986;137:2299–2306. [PubMed] [Google Scholar]
- Tan P, Kropshofer H, Mandelboim O, Bulbuc N, Hämmerling GJ, Momburg F. Recruitment of MHC class I molecules by tapasin into the transporter associated with antigen processing-associated complex is essential for optimal peptide loading. J Immunol. 2002;168:1950–1960. doi: 10.4049/jimmunol.168.4.1950. [DOI] [PubMed] [Google Scholar]
- Turnquist HR, Solheim JC. Analysis of MHC class I interactions with endoplasmic reticulum proteins. Methods Mol Biol. 2001;156:165–173. doi: 10.1385/1-59259-062-4:165. [DOI] [PubMed] [Google Scholar]
- Turnquist HR, Vargas SE, Reber AJ, McIlhaney MM, Li S, Wang P, Sanderson SD, Gubler B, van Endert P, Solheim JC. A region of tapasin that affects Ld binding and assembly. J Immunol. 2001;157:4443–4449. doi: 10.4049/jimmunol.167.8.4443. [DOI] [PubMed] [Google Scholar]
- Turnquist HR, Petersen JL, Vargas SE, McIlhaney MM, Bedows E, Mayer WE, Grandea AG, III, Van Kaer L, Solheim JC. The Ig-like domain of tapasin influences intermolecular interactions. J Immunol. 2004;172:2976–2984. doi: 10.4049/jimmunol.172.5.2976. [DOI] [PubMed] [Google Scholar]
- Yu YYL, Myers NB, Hilbert CH, Harris MR, Balendiran GK, Hansen TH. Definition and transfer of a serological epitope specific for peptide-empty forms of MHC class I. Int Immunol. 1999;11:1897–1906. doi: 10.1093/intimm/11.12.1897. [DOI] [PubMed] [Google Scholar]
- Zarling AL, Luckey CJ, Marto JA, White FM, Brame CJ, Evans AM, Lehner PJ, Cresswell P, Shabanowitz J, Hunt DF, Engelhard VH. Tapasin is a facilitator, not an editor, of class I MHC peptide binding. J Immunol. 2003;171:5287–5295. doi: 10.4049/jimmunol.171.10.5287. [DOI] [PubMed] [Google Scholar]