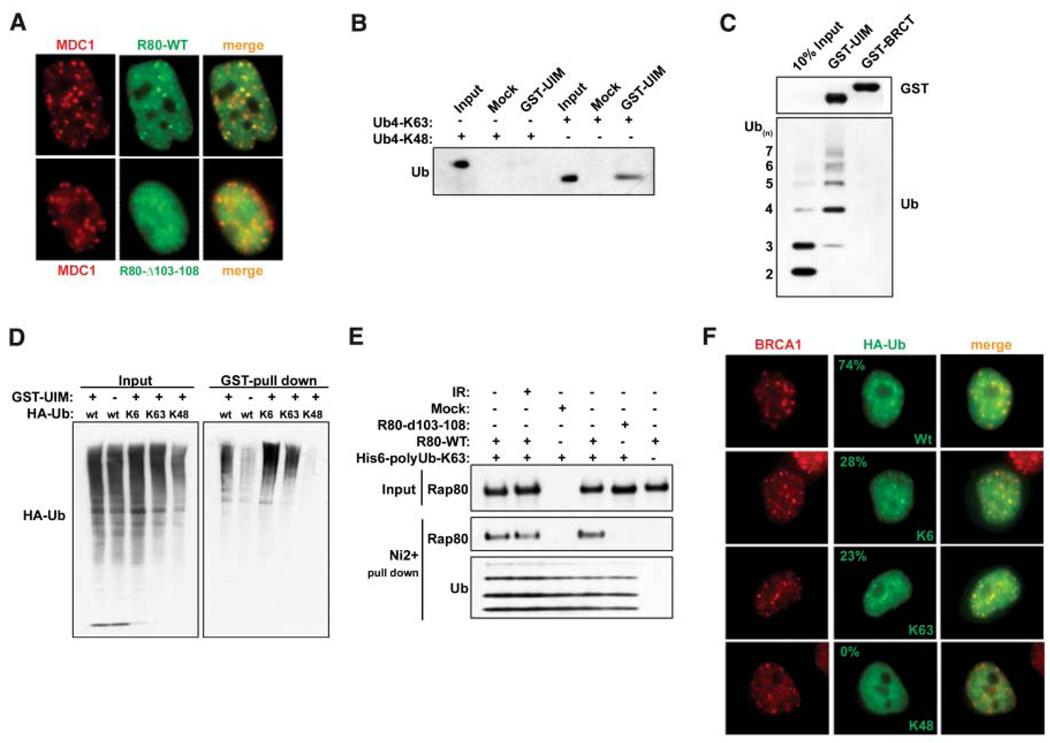
Fig. 2.
RAP80 forms IRIF by binding to non-K48–linked ubiquitin. (A) HeLa cells stably expressing FLAG-HA– tagged WT or Δ103–108 RAP80 were treated with 6 Gy IR, and IF was performed 1 hour later. (B) K63- or K48-linked tetraubiquitin was incubated with the RAP80 GST-UIM domain. GST precipitations were analyzed by IB. (C) A mixture of K63-linked ubiquitin polymers containing two to seven molecules of ubiquitin (Ub2–7) was incubated with RAP80 GST-UIM or GST-BRCT. IB was performed as indicated after purification on glutathione-conjugated sepharose beads. (D) 293T cells transfected with the indicated HA- tagged ubiquitin expression vectors were treated with 10 µM MG132 for 2 hours before lysis and then incubated with RAP80 GST-UIM protein. The bound ubiquitin species were analyzed by IB. (E) FLAG-HA–tagged WT or Δ103–108 RAP80 were purified before and after IR from stably expressing HeLa-S3 cells by FLAG IP followed by FLAG-peptide elution. These proteins were then incubated with His-tagged, K63-Ub2–7, followed by purification on Ni2+- agarose beads. Ubiquitin-associated proteins were detected by IB. (F) HeLa cells were transfected with the same ubiquitin-expression vectors as in (D) and analyzed by IF 1 hour after IR. The percentage of transfected cells that display colocalization of epitope-tagged ubiquitin and BRCA1 is indicated (N > 200).