Abstract
Theoretical considerations, and findings from computational modeling, comparative neuroanatomy and developmental neuroscience, motivate the hypothesis that a deviant brain growth trajectory will lead to deviant patterns of change in cortico-cortical connectivity. Differences in brain size during development will alter the relative cost and effectiveness of short- and long-distance connections, and should thus impact the growth and retention of connections. Reduced brain size should favor long-distance connectivity; brain overgrowth should favor short-distance connectivity; and inconsistent deviations from the normal growth trajectory – as occurs in autism – should result in potentially disruptive changes to established patterns of functional and physical connectivity during development. To explore this hypothesis, neural networks which modeled inter-hemispheric interaction were grown at the rate of either typically developing children or children with autism. The influence of the length of the inter-hemispheric connections was analyzed at multiple developmental time-points. The networks that modeled autistic growth were less affected by removal of the inter-hemispheric connections than those that modeled normal growth – indicating a reduced reliance on long-distance connections – for short response times, and this difference increased substantially at approximately 24 simulated months of age. The performance of the networks showed a corresponding decline during development. And direct analysis of the connection weights showed a parallel reduction in connectivity. These modeling results support the hypothesis that the deviant growth trajectory in autism spectrum disorders may lead to a disruption of established patterns of functional connectivity during development, with potentially negative behavioral consequences, and a subsequent reduction in physical connectivity. The results are discussed in relation to the growing body of evidence of reduced functional and structural connectivity in autism, and in relation to the behavioral phenotype, particularly the developmental aspects.
Introduction
Brain size has been found to be strongly correlated with relative long-distance cortico-cortical connectivity across species (Rilling & Insel, 1999; Zhang & Sejnowski, 2000), and also, though less strongly, within species (Jancke, Staiger, Schlaug, Huang & Steinmetz, 1997). Evidence that such a relationship also holds developmentally (Jancke, Preis & Steinmetz, 1999; Lewis & Courchesne, 2004; Lewis, Courchesne & Elman, 2003, 2004) suggests a link between findings of deviant brain growth trajectories in developmental disorders (Bailey, Luthbert, Dean, Harding, Janota, Montgomery, Rutter & Lantos, 1998; Bauman & Kemper, 1985; Courchesne, Carper & Akshoomoff, 2003; Courchesne, Karns, Davis, Ziccardi, Carper, Tigue, Chisum, Moses, Pierce, Lord, Lincoln, Pizzo, Schreibman, Haas, Akshoomoff & Courchesne, 2001; Hagberg, Stenbom & Engerström, 2001; Hazlett, Poe, Gerig, Smith, Provenzale, Ross, Gilmore & Piven, 2005) and findings of abnormalities in cortico-cortical connectivity in these disorders (Barnea-Goraly, Kwon, Menon, Eliez, Lotspeich & Reiss, 2004; Baron-Cohen, Knickmeyer & Belmonte, 2005; Egaas, Courchesne & Saitoh, 1995; Lewis & Courchesne, 2004; Lewis, Courchesne & Elman, 2003, 2004; Piven, Bailey, Ranson & Arndt, 1997; Vidal, Nicolson, DeVito, Hayashi, Geaga, Drost, Williamson, Rajakumer, Sul, Dutton, Toga & Thompson, 2006; Waiter, Williams, Murray, Gilchrist, Perrett & Whiten, 2005) – and possibly between these findings and behavior (Belmonte, Allen, Beckel-Mitchener, Boulanger, Carper & Webb, 2004; Castelli, Frith, Happe & Frith, 2002; Deutsch & Joseph, 2003; Just, Cherkassky, Keller & Minshew, 2004; Pelphrey, Morris & McCarthy, 2005; Tager-Flusberg & Joseph, 2003). Brain size is inversely correlated with the ratio of inter-hemispheric white-matter to gray-matter (Jancke et al., 1999; Jancke et al., 1997; Rilling & Insel, 1999), and presumably with the ratio of long-distance cortico-cortical connections, in general, to gray-matter (Zhang & Sejnowski, 2000). Thus developmental disorders in which brain size is abnormally small throughout development should show increased long-distance connectivity; and those in which brain size is abnormally large should show decreased long-distance connectivity. In cases in which brain size abnormalities are not consistent throughout development, more complex and possibly more detrimental effects on connectivity might be expected.
The relation between brain size and connectivity has been hypothesized to stem from the relations between brain size and conduction delay and between brain size and the cellular costs associated with long-distance connections (Lewis & Courchesne, 2004; Lewis et al., 2003, 2004; Ringo, Doty, Demeter & Simard, 1994). The conduction delay associated with either a myelinated or an unmyelinated axon is primarily a function of its diameter and length (Waxman, 1977); and differences in brain size necessarily correlate with differences in the lengths of long-distance connections, but appear to correlate only weakly with differences in axon diameters (Olivares, Montiel & Aboitiz, 2001). Thus, differences in brain size should alter the relative usefulness of short- and long-distance connections for tasks that require rapid responses. The cell maintenance costs associated with long-distance connections should also correlate with brain size, and so differences in brain size should also alter the relative costs of short- and long-distance connections.
Both intra- and inter-hemispheric connectivity appear to be arrived at via prenatal exuberance followed by postnatal pruning (LaMantia & Rakic, 1990; Rakic, Bourgeois, Eckenhoff, Zecevic & Goldman-Rakic, 1986), the latter of which is thought to be moderated by an activity dependent competition for neurotrophins (Barres & Raff, 1993; Callaway, Soha & Van Essen, 1987; Van Ooyen & Willshaw, 1999). Brain size differences during development should impact this competition, and so the growth and retention of connections (Lewis & Courchesne, 2004; Lewis et al., 2003, 2004). In abnormal development, in periods of time in which brain size is abnormally small, long-distance connections should be favored, and during periods of time in which brain size is abnormally large, short-distance connections should be favored. Brain development is not spatio-temporally homogeneous – evidence supports heterochronous development in typically developing children (Giedd, Blumenthal, Jeffries, Castellanos, Liu, Zijdenbos, Paus, Evans & Rapoport, 1999; Thompson, Giedd, Woods, MacDonald, Evans & Toga, 2000) – and so the effects of brain size abnormalities on connectivity should be expected to be temporally and spatially linked. A growth trajectory in which there is a developmentally inconsistent brain-size abnormality should thus show a corresponding fluctuation in the pattern of connectivity in spatial accord with the temporal aspects of the brain size abnormalities. And a very rapid change in the growth trajectory might be expected to alter established patterns of connectivity within a given brain area – abandoning connections in which early learning has been represented, potentially giving rise to abnormalities in the behaviors that relied on those connections.
There is now consistent evidence from post mortem studies (Bailey et al., 1998; Bauman & Kemper, 1985; Kemper & Bauman, 1998), head circumference measures (Aylward, Minshew, Field, Sparks & Singh, 2002; Bailey, Luthert, Bolton, LeCouteur, Rutter & Harding, 1993; Courchesne et al., 2003; Davidovitch, Patterson & Gartside, 1996; Dementieva, Vance, Donnelly, Elston, Wolpert, Ravan, DeLong, Abramson, Wright & Cuccaro, 2005; Fombonne, Roge, Claverie, County & Fremolle, 1999; Hazlett et al., 2005; Lainhart, Piven, Wzorek, Landa, Santangelo, Coon & Folstein, 1997; Miles, Hadden, Takahashi & Hillman, 2000; Woodhouse, Bailey, Rutter, Bolton, Baird & Couteur, 1996) and MRI volumetric analyses (Aylward et al., 2002; Courchesne et al., 2001; Piven, Arndt, Bailey, Havercamp, Andreason & Palmer, 1995; Sparks, Friedman, Shaw, Aylward, Echelard, Artru, Maravilla, Giedd, Munson, Dawson & Dager, 2002) that individuals with autism spectrum disorders have abnormally large brains after the second or third year of life, and that, early in development, this size difference can be multiple standard deviations above the norm (Courchesne et al., 2003; Courchesne et al., 2001; Dementieva et al., 2005; Hazlett et al., 2005; Sparks et al., 2002). Children later diagnosed with autism spectrum disorders, however, have head circumference measures at birth that are normal (Hazlett et al., 2005; Hultman, Sparen & Cnattingius, 2002), or even slightly smaller than normal (Courchesne et al., 2003; Redcay & Courchesne, 2005). That these results stem from accelerated growth over the first years of life is supported by longitudinal data (Courchesne et al., 2003; Dementieva et al., 2005; Hazlett et al., 2005). Thus, autism appears to be an example of a developmental disorder showing an abrupt increase in brain size after a period of normal or slightly reduced growth.
This pattern of growth is predicted to show disruptions in the initially established patterns of functional connectivity, abnormalities in the behaviors associated with these disruptions, and a subsequent reduction in physical connectivity.
There is a growing body of evidence of reduced large-scale functional and structural connectivity in adults with autism spectrum disorders (Castelli et al., 2002; Egaas et al., 1995; Just et al., 2004; Kana, Keller, Cherkassky, Minshew & Just, 2006; Koshino, Carpenter, Minshew, Cherkassky, Keller & Just, 2005; Manes, Piven, Vrancic, Nanclares, Plebst & Starkstein, 1999; Piven et al., 1997; Ring, Baron-Cohen, Wheelwright, Williams, Brammer, Andrew & Bullmore, 1999; Vidal et al., 2006). And it has been proposed that the deficits in autism are a result of reduced integration of information due to this underconnectivity (Herbert, 2005; Just et al., 2004). But there are no studies of the developmental changes in functional connectivity; and the relations between the trajectory of brain growth and the behavioral phenotype, and physical connectivity, are almost unknown. The growth of inter-hemispheric connectivity in children with autism spectrum disorders appears to be inversely related to brain size between 4 and 10 years of age (Lewis & Courchesne, 2004; Lewis et al., 2004), and by late childhood or adolescence, individuals with autism spectrum disorders show abnormal increases in gray-matter (Waiter, Williams, Murray, Gilchrist, Perrett & Whiten, 2004), decreases in inter-hemispheric white-matter (Lewis & Courchesne, 2004; Lewis et al., 2004; Waiter et al., 2005), and increased short-distance connectivity (Herbert, Ziegler, Makris, Filipek, Kemper, Normandin, Sanders, Kennedy & Caviness, 2004). And there is also some indication that the degree of abnormality in the rate of brain growth is related to the severity of the outcome (Akshoomoff, Lord, Lincoln, Courchesne, Carper, Townsend & Courchesne, 2004; Courchesne et al., 2003; Deutsch & Joseph, 2003; cf. Sparks et al., 2002; Tager-Flusberg & Joseph, 2003). But there is essentially no research that relates the shape of the brain growth trajectory in the first years of life to developmental changes in functional connectivity, structural connectivity, or to the particular aspects of the behavioral phenotype that might be vulnerable to reorganization.
This paper reports on a computational study of the possibility of such effects of brain growth on developmental patterns of connectivity and performance. Neural networks ‘grown’ in accord with either the brain-growth patterns of typically developing children or children with autism were used to explore this possibility.
Methods
Architecture and training
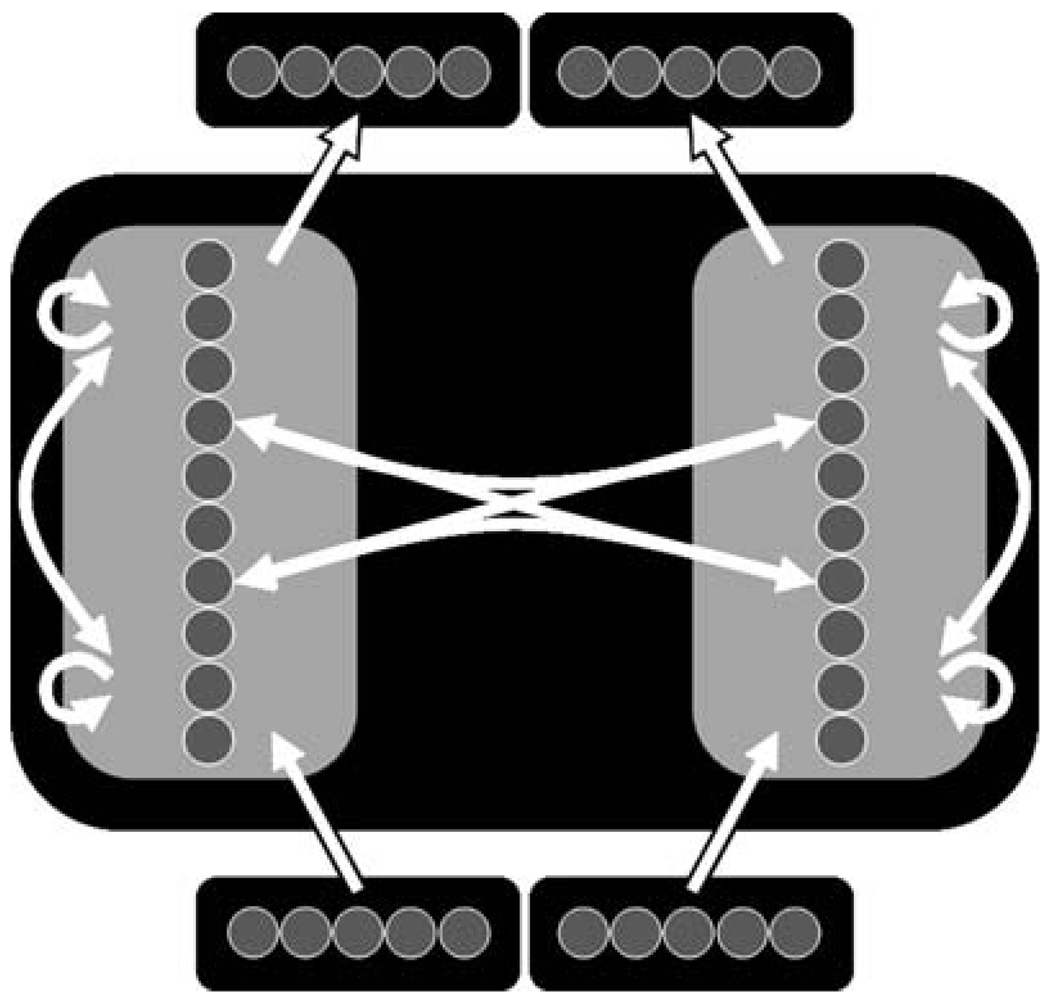
The simple two-hemisphere model shown in Figure 1 was used to explore this hypothesis. This architecture was based on that used by Ringo et al. (1994). The network comprised two ‘hemispheres’ of 10 units each, five input units and five output units for each hemisphere, and a number of units used to implement conduction delay in inter- and intra-hemispheric connections. Each unit in both hemispheres was fully connected with the nine other units in that hemisphere, and all of these units were recurrent. Two units in each hemisphere also had afferent and efferent connections with two units in the other hemisphere. The five input units for a hemisphere were fully connected with that hemisphere; and each hemisphere was fully connected with five output units.
Figure 1.
The network architecture. Five input units are fully connected to either ‘hemisphere’, and either hemisphere is fully connected to five output units. A hemisphere comprises 10 units and is fully recurrent. Two units from each hemisphere are used for inter-hemispheric connections; each of the two units in one hemisphere is connected to both units in the other hemisphere.
Both inter- and intra-hemispheric connections were associated with conduction delays, implemented by constructing these connections as chains of copy units. The conduction delay was the number of links in an inter- or intra-hemispheric chain. The delay associated with an inter-hemispheric connection was a function of age, based on the head circumference growth trajectories for either typically developing children or children with autism. The delay function was:
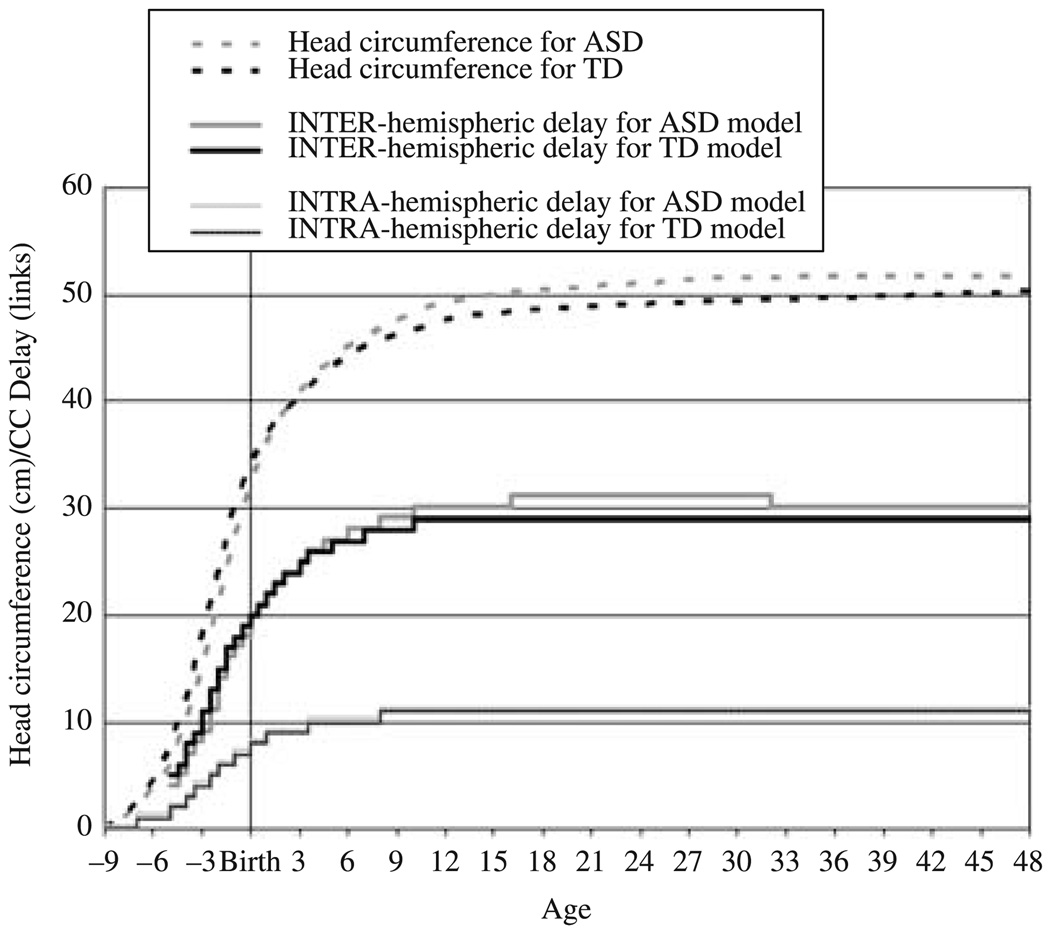
where HC(age, diagnosis) is the head circumference at the given age for the given diagnosis, and ConductionVelocity(age) is an increasing linear function of age. The inter-hemispheric delay was thus ⅔ of the diameter of a spherical head corresponding to HC(age, diagnosis) – a crude approximation of the length of the inter-hemispheric connections taking scalp, skull, cortical thickness, and cortical folding into account – divided by ConductionVelocity(age) – a crude approximation of the increases in axon diameter and myelination that occur over development. The delay associated with an intra-hemispheric connection was identical for both the networks modeling typical brain growth and the networks modeling autistic brain growth; it was ⅜ of the inter-hemispheric delay associated with the typical brain growth pattern. These functions are plotted in Figure 2. The developmental changes in the conduction delays associated with inter- and intra-hemispheric connections are, of course, a far more complex combination of non-linear functions which differ between regions; the connections may follow complex paths that are substantially greater than ⅔ of the diameter of the head; and heads are not spherical. But these functions are plausibly an index of the actual average conduction delays associated with the two types of connections.
Figure 2.
The growth trajectory for networks modeling typical development was based on head circumference measures from standardized growth charts (Kuczmarski, Ogden, Guo et al., 2002; Nellhaus, 1968) and on prenatal measures taken by ultrasound (Kurmanavicius, Wright, Royston, Wisser, Huch, Huch & Zimmermann, 1999; Schwärzler, Bland, Holden, Campbell & Ville, 2004). The growth trajectory for the networks modeling development in autism was derived from a meta-analysis of brain growth in children with autism (Redcay & Courchesne, 2005). Gompertz curves were fitted to these data to estimate the prenatal trajectories (Luecke, Wosilait & Young, 1995; Winsor, 1932; Wosilait, Luecke & Young, 1992).
As shown in Figure 2, network growth was discretized – i.e. the delay functions were approximated with step functions. Copy units were added to or subtracted from each of the inter- and intra-hemispheric connections in accord with the delay functions at the beginning of each simulated half-month. The network architecture was then held static for the duration of that simulated half-month. Training for 500 epochs constituted a simulated half-month. As shown in Figure 2, as well, the inter-hemispheric connections were connected at 5 months before birth; this is approximately the timing of the establishment of inter-hemispheric connections in prenatal development (Rakic & Yakovlev, 1968). Network training begins 5 months before birth as well.
The networks were trained to enumerate a set of input strings. The networks were provided with 32 input–output patterns, and were trained to associate each input pattern with its corresponding output pattern. The input patterns were randomly generated binary strings. The output patterns were the base 2 encodings of unique numbers assigned to the input patterns, duplicated for the output units of either hemisphere, e.g. the output for pattern number 1 was 00001 00001. The input patterns for both hemispheres together always corresponded to a unique output for all 32 input patterns. But, in order to explore the potential impact of the different growth trajectories on aspects of task performance that rely on inter-hemispheric communication and aspects that do not, half of the input–output pairs were unique within either hemisphere, and half of the input patterns for either hemisphere alone each corresponded to four different outputs. Thus, 16 of the input patterns could be associated with their outputs without inter-hemispheric communication; and the other 16 were four-way ambiguous within either hemisphere, and so to generate the correct output, the network had to make use of the inter-hemispheric connections. Each of these 32 inputs was randomly assigned a number between 0 and 31, and paired with the binary string comprising the five digit base 2 encoding of that number concatenated with itself. Table 1 and Table 2 show the two sorts of input–output patterns. Examples were drawn randomly from such training sets.
Table 1.
Sixteen example input-target patterns which are four-way ambiguous within each hemisphere. One half of one of the training sets comprises these examples. The four examples which are bolded are identical with respect to the inputs to the left hemisphere. The corresponding inputs to the right hemisphere are, however, unique. The left and right inputs together thus uniquely specify the outputs for both hemispheres. Note that this same within-hemisphere ambiguity exists for each of these 16 examples
| Left hemisphere |
Right hemisphere |
||||
|---|---|---|---|---|---|
| Input | Target | Input | Target | ||
| 1 0 0 0 1 | → | 0 0 0 0 1 | 0 1 0 0 0 | → | 0 0 0 0 1 |
| 0 1 0 0 0 | → | 0 1 1 1 0 | 0 0 1 0 0 | → | 0 1 1 1 0 |
| 1 1 1 1 0 | → | 1 0 0 0 1 | 1 0 1 0 1 | → | 1 0 0 0 1 |
| 1 0 0 0 1 | → | 0 1 0 1 1 | 1 0 1 0 1 | → | 0 1 0 1 1 |
| 1 1 0 1 1 | → | 1 0 0 1 1 | 0 0 1 0 0 | → | 1 0 0 1 1 |
| 1 1 1 1 0 | → | 1 0 1 1 0 | 0 1 0 0 0 | → | 1 0 1 1 0 |
| 0 1 0 0 0 | → | 1 0 1 1 1 | 1 0 1 0 1 | → | 1 0 1 1 1 |
| 1 1 1 1 0 | → | 1 1 0 0 1 | 0 0 0 0 0 | → | 1 1 0 0 1 |
| 1 1 0 1 1 | → | 1 1 0 1 0 | 0 1 0 0 0 | → | 1 1 0 1 0 |
| 1 0 0 0 1 | → | 0 1 0 0 0 | 0 0 1 0 0 | → | 0 1 0 0 0 |
| 1 1 0 1 1 | → | 1 1 1 0 0 | 1 0 1 0 1 | → | 1 1 1 0 0 |
| 0 1 0 0 0 | → | 1 1 1 0 1 | 0 1 0 0 0 | → | 1 1 1 0 1 |
| 1 0 0 0 1 | → | 0 1 0 0 1 | 0 0 0 0 0 | → | 0 1 0 0 1 |
| 0 1 0 0 0 | → | 1 1 1 1 1 | 0 0 0 0 0 | → | 1 1 1 1 1 |
| 1 1 1 1 0 | → | 0 0 1 0 0 | 0 0 1 0 0 | → | 0 0 1 0 0 |
| 1 1 0 1 1 | → | 0 0 1 1 0 | 0 0 0 0 0 | → | 0 0 1 1 0 |
Table 2.
The other half of the training set shown in Table 1. In these examples, the inputs to each hemisphere uniquely specify the outputs
| Left hemisphere |
Right hemisphere |
||||
|---|---|---|---|---|---|
| Input | Target | Input | Target | ||
| 0 0 0 1 0 | → | 0 0 0 0 0 | 0 0 0 1 0 | → | 0 0 0 0 0 |
| 0 1 0 1 0 | → | 0 0 0 1 0 | 1 0 1 1 0 | → | 0 0 0 1 0 |
| 0 0 0 0 0 | → | 0 0 0 1 1 | 1 0 0 0 1 | → | 0 0 0 1 1 |
| 1 0 0 1 1 | → | 0 0 1 0 1 | 0 0 0 1 1 | → | 0 0 1 0 1 |
| 0 1 1 0 1 | → | 0 0 1 1 1 | 1 1 0 1 0 | → | 0 0 1 1 1 |
| 1 1 0 1 0 | → | 0 1 0 1 0 | 0 0 1 1 1 | → | 0 1 0 1 0 |
| 0 0 0 0 1 | → | 0 1 1 0 0 | 0 1 0 1 0 | → | 0 1 1 0 0 |
| 1 1 1 0 1 | → | 0 1 1 0 1 | 1 1 1 0 1 | → | 0 1 1 0 1 |
| 0 1 1 0 0 | → | 0 1 1 1 1 | 1 0 0 1 1 | → | 0 1 1 1 1 |
| 1 1 0 0 0 | → | 1 0 0 0 0 | 0 1 0 1 1 | → | 1 0 0 0 0 |
| 1 0 1 1 1 | → | 1 0 0 1 0 | 1 0 0 0 0 | → | 1 0 0 1 0 |
| 1 0 0 0 0 | → | 1 0 1 0 0 | 1 1 1 0 0 | → | 1 0 1 0 0 |
| 0 0 1 0 0 | → | 1 0 1 0 1 | 0 1 1 0 1 | → | 1 0 1 0 1 |
| 0 0 1 1 0 | → | 1 1 0 0 0 | 0 0 1 0 1 | → | 1 1 0 0 0 |
| 0 1 0 0 1 | → | 1 1 0 1 1 | 0 1 0 0 1 | → | 1 1 0 1 1 |
| 1 0 1 0 1 | → | 1 1 1 1 0 | 1 1 0 0 1 | → | 1 1 1 1 0 |
The networks were constructed and trained on LENS (Rhode, 1999) using the backpropagation of error algorithm (Rumelhart, Hinton & Williams, 1986) and gradient descent with a learning rate of 0.01 and a momentum value of 0.9. A cross-entropy error function was used so that the activation levels of the output units could be interpreted as probabilities as well as confidence estimates.
To ensure the generality of the results, networks were trained on 10 different training sets, and for each training set were trained 20 times with different initial random connection weights. Additionally, the impact of the time between presentation of the input and evaluation of the response – i.e. the settling time – was assessed for each training set and each weight initialization. Settling time and conduction delay, together, determine how quickly, and for how long, the inter-hemispheric connections can influence the dynamics of the network, and so settling time was expected to have a strong influence on the development of functional and physical connectivity, and on performance. Each network was trained once with a short settling time, and once with a long settling time. The short settling time was 10 sweeps greater than the time required for activation to spread from one hemisphere to the other in the networks following the brain growth trajectory of typically developing children. The long settling time was 20 sweeps greater than the short settling time.
Measures of connectivity and performance
The impact of the difference in the two growth trajectories was analyzed for each network at multiple points during training: at the simulated equivalent of birth, 12, 24, 36, and 48 months of age. The impact on interhemispheric functional connectivity was measured. The impact on performance was measured. And the impact on inter-hemispheric physical connectivity was measured.
The functional analysis was done by removing all of the inter-hemispheric connections and measuring the impact of these lesions on the network’s performance on the training set. The measure of performance was the cross-entropy error. The difference between the pre- and post-lesion cross-entropy error – i.e. the increase in error caused by the lesion – was calculated. This will be referred to as the lesion induced error. This measure was taken as an indication of the functional role of the inter-hemispheric connections. A greater increase in the lesion induced error was assumed to indicate a greater reliance on inter-hemispheric connections. This was assessed separately for the two sorts of input patterns – the 16 binary strings that were unique within either hemisphere, and the 16 that were four-way ambiguous within either hemisphere.
The analysis of performance was done by measuring the cross-entropy error for the intact network on the training set, and on a version of the training set in which all targets were inverted. The network’s performance was taken to be the percent correct, calculated as the error produced on the inverted version of the training set as a percentage of the total possible error. As with the functional measure, this was assessed separately for the two sorts of input patterns – the 16 binary strings that were unique within either hemisphere, and the 16 that were four-way ambiguous within either hemisphere.
The analysis of the impact of the difference in the growth trajectories on inter-hemispheric physical connectivity was done by measuring connection weights. The analysis used the mean of the absolute value of the inter-hemispheric connection weights – i.e. (∑i,j|wij|)/N, where wij is the weight associated with the link between the ith and jth units, and the ith and jth units are in different hemispheres, and N is the number of such links – relative to the mean of the absolute value of the intra-hemispheric connection weights. This gave a measure of the physical connectivity across hemispheres relative to the physical connectivity within hemispheres.
To evaluate the effect of the growth trajectory on these measures, four sorts of analyses were conducted: (i) the estimated marginal means were computed for both growth conditions at each measured time-point; (ii) the estimated marginal means were compared between the two groups at each time-point; (iii) interactions between group and age were computed for successive pairs of time-points; and (iv) where the estimated marginal means decreased across time within a group, they were compared to assess the reduction. To compare the estimated marginal means, for each time-point and settling time, repeated measures ANOVAs were done with group and training set as within-subject factors – the different random initializations of a network being taken as different subjects. To evaluate interactions between the type of growth trajectory and age, for successive pairs of these time-points, repeated measures ANOVAs were done with group, training set, and age as within-subject factors. And to assess the possibility of a reduction in functional or physical connectivity, or a decline in performance, repeated measures ANOVAs were done for the group in question, for successive pairs of time-points, with training set, and age as within-subject factors.
Results
The results of the analysis of the impact of removing the inter-hemispheric connections show an overall reduction in functional connectivity at approximately ‘24 months’ in the networks that modeled autistic growth with short settling times, with considerable individual variation, and variation across training sets. The results of the analysis of performance show a corresponding decline in performance, with similar variability across individuals and training sets. And the results of the analysis of relative inter-hemispheric weight show a parallel reduction in physical connectivity. The results of the functional analyses are presented in Figure 3 and Figure 4, and in Table 3 and Table 4; the results of the analysis of performance during learning are presented in Figure 5 and Figure 6, and in Table 5 and Table 6; and the results of the analyses of the relative inter-hemispheric connection weight are presented in Figure 7 and Table 7.
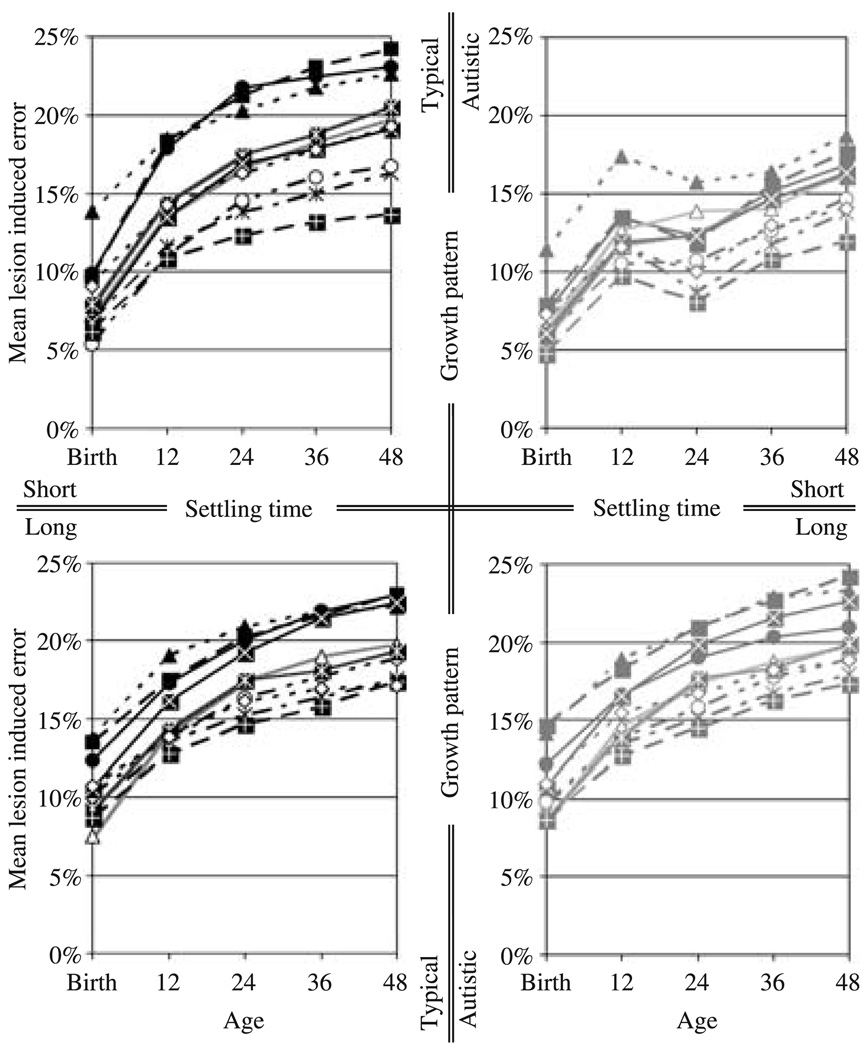
Figure 3.
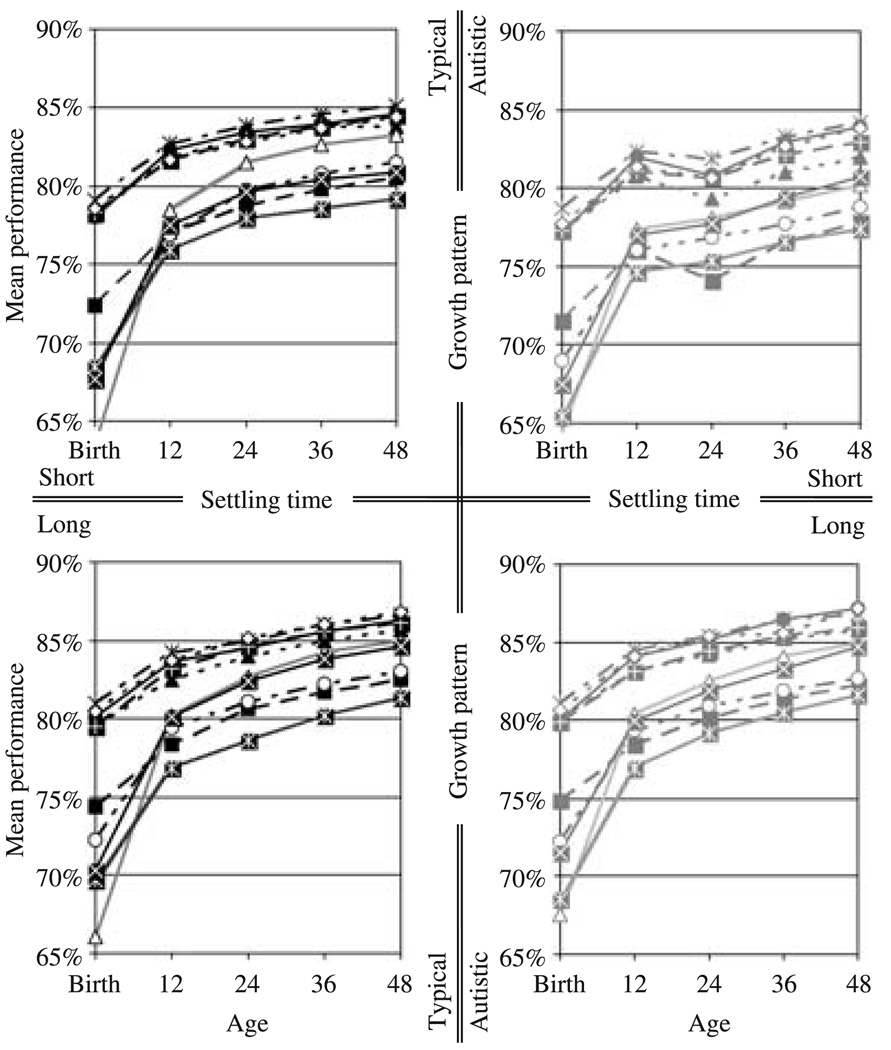
The impact of disconnecting the inter-hemispheric connections on the portion of the task which required inter-hemispheric communication. The left and right columns show the impact on the networks following the growth pattern in typical development and in autism, respectively. The top and bottom rows show the outcome for short and long settling times, respectively. Each curve represents the mean lesion induced error for a single training set.
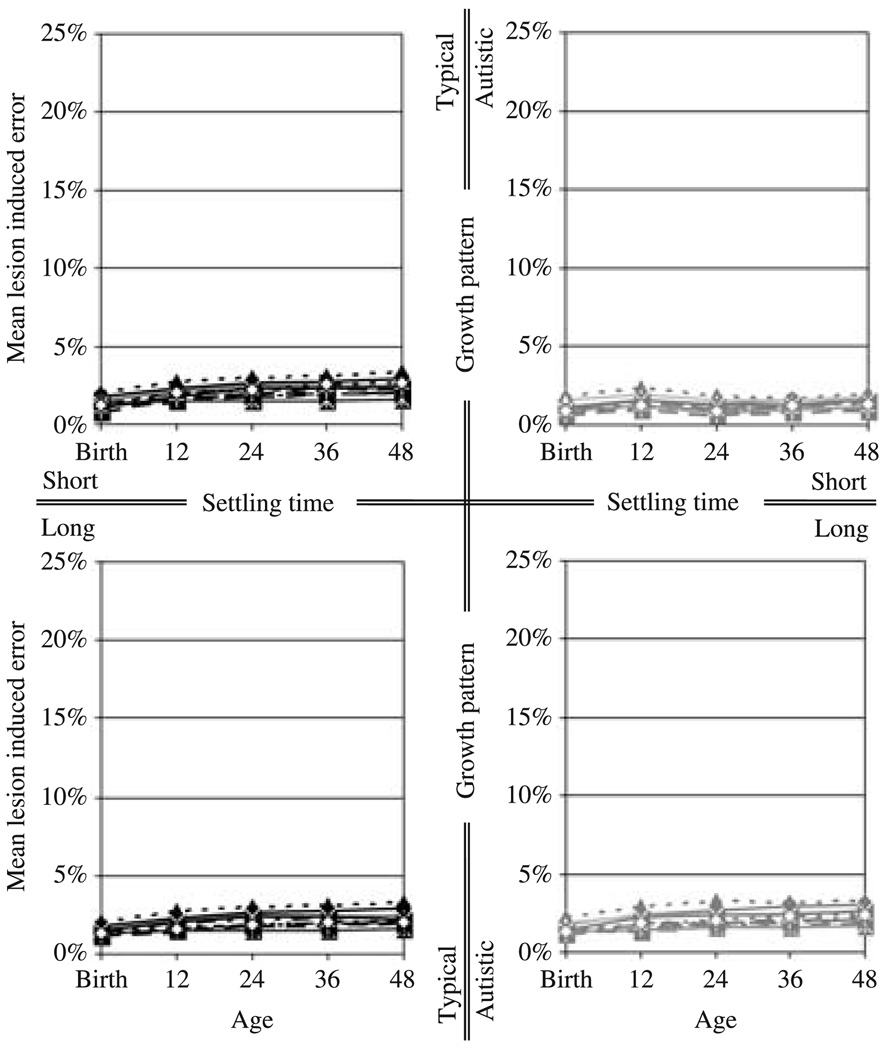
Figure 4.
The impact of disconnecting the inter-hemispheric connections on the portion of the training set for which the inputs for either hemisphere uniquely specify the outputs for that hemisphere. The left and right columns show the impact on the networks following the growth pattern in typical development and in autism, respectively. The top and bottom rows show the outcome for short and long settling times, respectively. Each curve represents the mean lesion induced error for a single training set.
Table 3.
The impact of removing the inter-hemispheric connections on the portion of the task which required inter-hemispheric communication. Three statistics are reported for both short and long settling times: (i) the estimated marginal means at each of the time-points; (ii) the main effect of group at each time-point; (iii) the interactions of group and age between successive pairs of time-points. These statistics complement the results graphed in Figure 3
| Birth | 12 | 24 | 36 | 48 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Short settling time | ||||||||||
| i | µASD | 6.9 | 12.4 | 11.7 | 13.8 | 15.6 | ||||
| µTD | 8.1 | 14.1 | 16.8 | 18.1 | 19.3 | |||||
| ii | F(1, 19) | 74.235 | 59.767 | 109.932 | 49.361 | 31.996 | ||||
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| partial-η2 | 0.796 | 0.759 | 0.853 | 0.722 | 0.627 | |||||
| iii | F(1, 19) | 4.913 | 68.224 | 4.102 | 4.224 | |||||
| p | 0.039 | <0.001 | 0.057 | 0.054 | ||||||
| partial-η2 | 0.205 | 0.782 | 0.178 | 0.182 | ||||||
| Long settling time | ||||||||||
| i | µASD | 10.6 | 15.3 | 17.7 | 19.3 | 20.3 | ||||
| µTD | 10.7 | 15.1 | 17.5 | 17.9 | 19.1 | |||||
| ii | F(1, 19) | 0.175 | 0.178 | 0.138 | 15.778 | 11.190 | ||||
| p | 0.681 | 0.678 | 0.714 | 0.001 | 0.003 | |||||
| partial-η2 | 0.009 | 0.009 | 0.007 | 0.454 | 0.371 | |||||
| iii | F(1, 19) | 1.283 | 0.012 | 22.790 | 1.434 | |||||
| p | 0.271 | 0.913 | <0.001 | 0.246 | ||||||
| partial-η2 | 0.063 | 0.001 | 0.545 | 0.070 | ||||||
Table 4.
The impact of removing the inter-hemispheric connections on the portion of the training sets comprising examples that do not require inter-hemispheric communication. Three statistics are reported for both short and long settling times: (i) the estimated marginal means at each of the time-points; (ii) the main effect of group at each time-point; (iii) the interactions of group and age between successive pairs of time-points. These statistics complement the results graphed in Figure 4
| Birth | 12 | 24 | 36 | 48 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Short settling time | ||||||||||
| i | µASD | 1.0 | 1.5 | 1.1 | 1.2 | 1.4 | ||||
| µTD | 1.3 | 2.1 | 2.6 | 2.8 | 3.0 | |||||
| ii | F(1, 19) | 23.613 | 50.316 | 103.864 | 106.377 | 98.270 | ||||
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| partial-η2 | 0.554 | 0.726 | 0.845 | 0.848 | 0.838 | |||||
| iii | F(1, 19) | 20.858 | 89.721 | 2.333 | 0.203 | |||||
| p | <0.001 | <0.001 | 0.143 | 0.658 | ||||||
| partial-η2 | 0.523 | 0.825 | 0.109 | 0.011 | ||||||
| Long settling time | ||||||||||
| i | µASD | 1.5 | 2.0 | 2.1 | 2.2 | 2.3 | ||||
| µTD | 1.4 | 1.9 | 2.1 | 2.2 | 2.3 | |||||
| ii | F(1, 19) | 3.851 | 1.560 | 0.033 | <0.001 | 0.049 | ||||
| p | 0.065 | 0.227 | 0.858 | 0.989 | 0.827 | |||||
| partial-η2 | 0.169 | 0.076 | 0.002 | <0.001 | 0.003 | |||||
| iii | F(1, 19) | 0.027 | 3.269 | 3.269 | 0.407 | |||||
| p | 0.872 | 0.087 | 0.536 | 0.532 | ||||||
| partial-η2 | 0.001 | 0.154 | 0.022 | 0.022 | ||||||
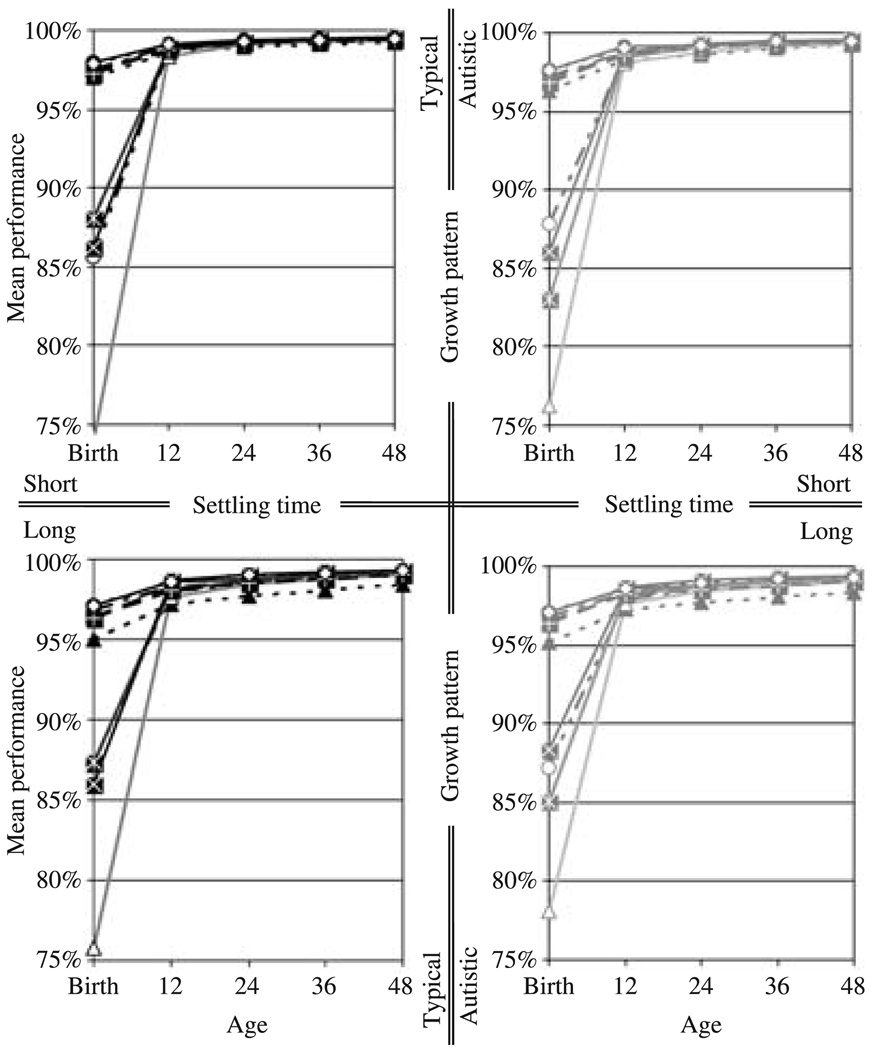
Figure 5.
The impact of the different patterns of growth on performance on the portion of the task which required inter-hemispheric communication. The left and right columns show the values for the networks following the growth pattern in typical development and in autism, respectively. The top and bottom rows show the outcome for short and long settling times, respectively. Each curve represents the mean performance for a single training set.
Figure 6.
The impact of the different patterns of growth on performance on the portion of the training set comprising examples for which the inputs for either hemisphere uniquely specify the outputs for that hemisphere. The left and right columns show the values for the networks following the growth pattern in typical development and in autism, respectively. The top and bottom rows show the outcome for short and long settling times, respectively. Each curve represents the mean performance for a single training set.
Table 5.
Performance on the portion of the task which required inter-hemispheric communication. Three statistics are reported for both short and long settling times: (i) the estimated marginal means of the performance error at each of the time-points; (ii) the main effect of group at each time-point; (iii) the interactions of group and age between successive pairs of time-points. These statistics complement the results graphed in Figure 5
| Birth | 12 | 24 | 36 | 48 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Short settling time | ||||||||||
| i | µASD | 72.6 | 78.8 | 78.5 | 80.1 | 81.2 | ||||
| µTD | 73.2 | 79.6 | 81.4 | 82.2 | 82.8 | |||||
| ii | F(1, 19) | 9.060 | 21.364 | 193.165 | 101.767 | 67.116 | ||||
| p | 0.007 | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| partial-η2 | 0.323 | 0.529 | 0.910 | 0.843 | 0.779 | |||||
| iii | F(1, 19) | 0.556 | 181.463 | 18.721 | 35.910 | |||||
| p | 0.465 | <0.001 | <0.001 | <0.001 | ||||||
| partial-η2 | 0.028 | 0.905 | 0.496 | 0.654 | ||||||
| Long settling time | ||||||||||
| i | µASD | 75.6 | 81.4 | 82.9 | 84.1 | 84.9 | ||||
| µTD | 75.3 | 81.2 | 82.9 | 84.0 | 84.8 | |||||
| ii | F(1, 19) | 3.361 | 3.823 | 0.112 | 0.382 | 0.969 | ||||
| p | 0.082 | 0.065 | 0.741 | 0.544 | 0.337 | |||||
| partial-η2 | 0.150 | 0.168 | 0.006 | 0.020 | 0.049 | |||||
| iii | F(1, 19) | 0.373 | 7.277 | 0.286 | 0.881 | |||||
| p | 0.548 | 0.014 | 0.599 | 0.360 | ||||||
| partial-η2 | 0.019 | 0.277 | 0.015 | 0.044 | ||||||
Table 6.
Performance on the portion of the training sets comprising examples that do not require inter-hemispheric communication. Three statistics are reported for both short and long settling times: (i) the estimated marginal means of the performance error at each of the time-points; (ii) the main effect of group at each time-point; (iii) the interactions of group and age between successive pairs of time-points. These statistics complement the results graphed in Figure 6
| Birth | 12 | 24 | 36 | 48 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Short settling time | ||||||||||
| i | µASD | 91.5 | 98.6 | 99.0 | 99.3 | 99.4 | ||||
| µTD | 91.9 | 98.8 | 99.2 | 99.4 | 99.4 | |||||
| ii | F(1, 19) | 0.884 | 36.465 | 54.152 | 3.145 | 0.144 | ||||
| p | 0.359 | <0.001 | <0.001 | 0.092 | 0.709 | |||||
| partial-η2 | 0.044 | 0.657 | 0.740 | 0.142 | 0.007 | |||||
| iii | F(1, 19) | 0.221 | 0.876 | 98.760 | 24.273 | |||||
| p | 0.644 | 0.361 | <0.001 | <0.001 | ||||||
| partial-η2 | 0.011 | 0.044 | 0.839 | 0.561 | ||||||
| Long settling time | ||||||||||
| i | µASD | 91.8 | 98.2 | 98.6 | 98.9 | 99.1 | ||||
| µTD | 91.5 | 98.1 | 98.6 | 98.9 | 99.0 | |||||
| ii | F(1, 19) | 1.208 | 2.477 | 0.121 | 0.963 | 0.289 | ||||
| p | 0.285 | 0.132 | 0.732 | 0.339 | 0.597 | |||||
| partial-η2 | 0.060 | 0.115 | 0.006 | 0.048 | 0.015 | |||||
| iii | F(1, 19) | 1.027 | 8.124 | 5.59 | 2.519 | |||||
| p | 0.324 | 0.010 | 0.029 | 0.129 | ||||||
| partial-η2 | 0.051 | 0.300 | 0.227 | 0.117 | ||||||
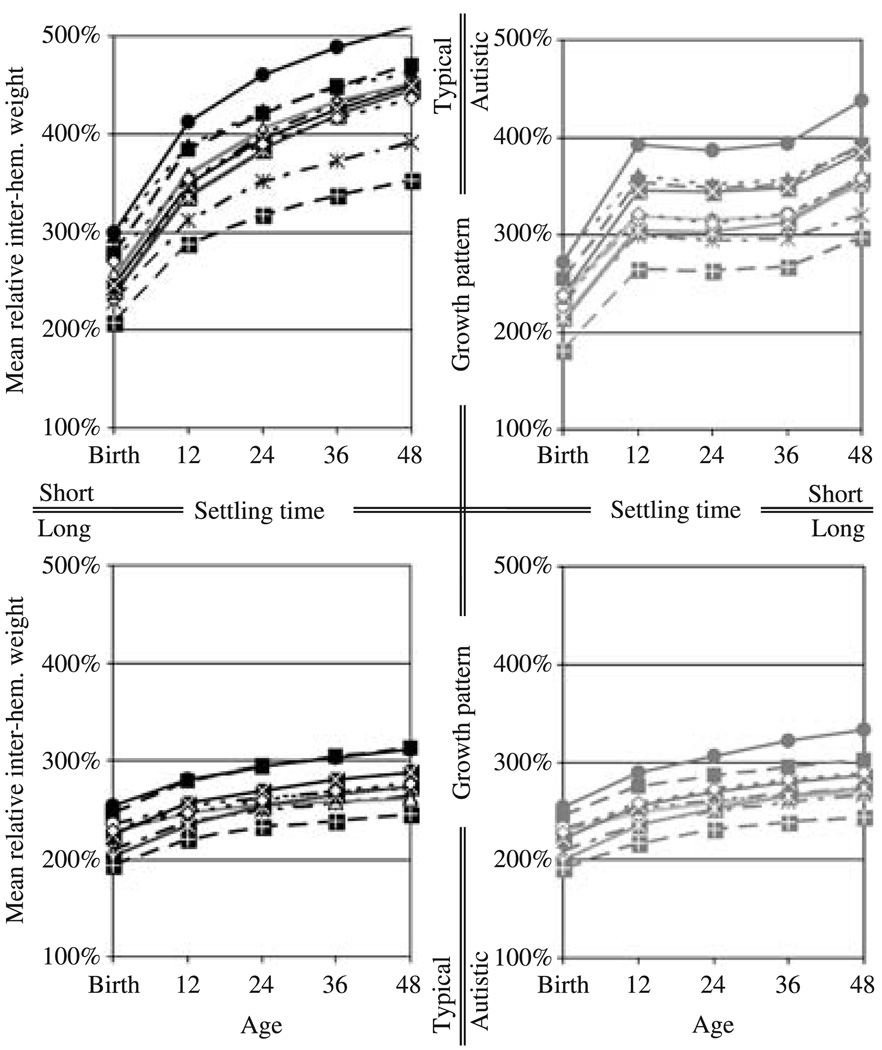
Figure 7.
The mean inter-hemispheric connection weight as a percentage of the mean intra-hemispheric connection weight. The left and right columns show the values for the networks following the growth pattern in typical development and in autism, respectively. The top and bottom rows show the outcome for short and long settling times, respectively. Each curve represents the mean relative inter-hemispheric weight for a single training set.
Table 7.
Relative inter-hemispheric connection weight. Three statistics are reported for both short and long settling times: (i) the estimated marginal means at each of the time-points; (ii) the main effect of group at each time-point; (iii) the interactions of group and age between successive pairs of time-points. These statistics complement the results graphed in Figure 7
| Birth | 12 | 24 | 36 | 48 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Short settling time | ||||||||||
| i | µASD | 235.2 | 327.8 | 323.2 | 328.5 | 364.2 | ||||
| µTD | 255.5 | 353.3 | 394.9 | 422.1 | 442.2 | |||||
| ii | F(1, 19) | 91.287 | 40.725 | 203.527 | 272.155 | 182.560 | ||||
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| partial-η2 | 0.828 | 0.682 | 0.915 | 0.935 | 0.906 | |||||
| iii | F(1, 19) | 2.758 | 690.058 | 302.471 | 132.939 | |||||
| p | 0.133 | <0.001 | <0.001 | <0.001 | ||||||
| partial-η2 | 0.127 | 0.973 | 0.941 | 0.875 | ||||||
| Long settling time | ||||||||||
| i | µASD | 224.8 | 252.1 | 265.0 | 274.2 | 281.2 | ||||
| µTD | 225.7 | 251.2 | 262.9 | 270.9 | 277.3 | |||||
| ii | F(1, 19) | 0.624 | 0.635 | 2.118 | 3.748 | 4.388 | ||||
| p | 0.439 | 0.435 | 0.162 | 0.068 | 0.050 | |||||
| partial-η2 | 0.032 | 0.032 | 0.100 | 0.165 | 0.188 | |||||
| iii | F(1, 19) | 3.539 | 2.653 | 4.447 | 1.544 | |||||
| p | 0.075 | 0.120 | 0.048 | 0.229 | ||||||
| partial-η2 | 0.157 | 0.123 | 0.190 | 0.075 | ||||||
Averaging over individual variation, and different outcomes for different training sets, the networks with short settling times grown at the rate of children with autism showed a reduction in functional connectivity – in terms of the impact of removing the inter-hemispheric connections – at approximately the simulated equivalent of 24 months of age. These networks showed less impact of the lesions than their typically developing instantiations at all measured time-points, but the difference was smaller at ‘birth’ than at ‘12 months’, and much smaller at ‘12 months’ than thereafter. This is reflected in the differences in the estimated marginal means (Table 3 and Table 4), and in the fact that there were significant interactions between group and age for these time-points (Table 3 and Table 4). This reduction occurred both for the portion of the task which required inter-hemispheric communication, and for the portion that did not – the latter presumably due to reorganization caused by the former. And, as is evident from the estimated marginal means and the graphs of the mean lesion induced error for each training set (Figure 3 and Figure 4), the interaction between group and age between ‘12 months’ and ‘24 months’ indicates not just a decrease relative to the networks following the growth pattern in typical development, but an actual decrease in functional connectivity. A repeated measures ANOVA on the lesion impact measures from the networks grown at the rate of children with autism yielded a marginal main effect of age (F(1, 19) = 3.264, p = .087, partial η2 = 0.147) for the portion of the task that requires inter-hemispheric communication. But there was considerable variation in the timing of the reduction in functional connectivity. Adjusting for the latency of this reduction by taking, for each network, either the period from ‘12 months’ to ‘24 months’, or ‘24 months’ to ‘36 months’, whichever showed the greater decrease in functional connectivity, yielded a significant main effect of age (F(1, 19) = 29.016, p < .001, partial η2 = 0.604). For the portion of the task that does not require inter-hemispheric communication, a repeated measures ANOVA on the lesion impact measures from the networks grown at the rate of children with autism yielded a significant main effect of age for this time period (F(1, 19) = 30.331, p < .001, partial η2 = 0.615). In both cases, however, there was substantial individual variation, with some networks showing a continual increase in functional connectivity, some showing a period of little change, and others showing substantial reductions. And, as is evident from the estimated marginal means for individual training sets (Figure 3 and Figure 4), some training sets also resulted in continual increases in functional connectivity overall, some in a period of little change, and others in reductions.
The networks with long settling times grown at the rate of children with autism showed no such reduction in functional connectivity. Rather, these networks showed greater functional connectivity at the simulated equivalent of 36 and 48 months of age for the portion of the task that required inter-hemispheric communication (Table 3), though the effect was small. It is unclear why this occurred, but perhaps it indicates that the longer settling times allowed the networks to compensate for the increased conduction delay in the networks following the growth trajectory in autism – a difference that is maximal between 16 and 32 simulated months of age, and is thus diminished at 36 and 48 simulated months of age.
The performance of the networks grown at the rate of children with autism shows a similar pattern of development. On the portion of the task which required inter-hemispheric communication, averaging over individual variation and different outcomes for different training sets, the networks with short settling times grown at the rate of children with autism showed a fall-off in performance – i.e. an increase in cross-entropy error – at approximately the simulated equivalent of 24 months of age (Figure 5 and Table 5). These networks, overall, performed worse on that portion of the task than their typically developing counterparts at all measured time-points, but the difference was smaller at ‘birth’ than at ‘12 months’, and much smaller at ‘12 months’ than thereafter. This is reflected in the differences in the estimated marginal means (Table 5), and in the fact that there was a significant interaction between group and age between ‘12 months’ and ‘24 months’ (F(1, 19) = 181.463, p < .001, partial η2 = 0.905). As indicated by the greater estimated marginal mean at ‘12 months’ than at ‘24 months’, this is a decline in performance paralleling the reduction in functional connectivity. Due to variation in the timing of the decline in performance, a main effect of age was not significant (F(1, 19) = 2.073, p < .166, partial η2 = 0.098). But adjusting for the latency of the decline by taking, for each network, either the period from ‘12 months’ to ‘24 months’, or ‘24 months’ to ‘36 months’, whichever showed the greater increase in performance error, yielded a significant main effect of age (F(1, 19) = 43.515, p < .001, partial η2 = 0.696).
As with functional connectivity, however, there was substantial individual variation, with some networks showing a continual increase in performance, some showing a period of little change, and others showing substantial declines in performance. And, as is evident from the estimated marginal means for individual training sets (Figure 5), some training sets also resulted in continual improvement in performance, overall, some in a period of little change, and others in overall declines in performance.
On the portion of the task which does not require inter-hemispheric communication (Figure 6 and Table 6), the networks with short settling times grown at the rate of children with autism showed significantly inferior performance at ‘12 months’ (F(1, 19) = 36.465, p < .001, partial η2 = 0.657) and at ‘24 months’ (F(1, 19) = 54.152, p < .001, partial η2 = 0.740) in comparison to the networks grown at the rate of typically developing children. But there was no actual decline in performance over time. Rather, following this initial inferior performance, the performance of the networks grown at the rate of children with autism improved rapidly, and by ’48 months’, was comparable to the performance of the networks grown at the rate of typically developing children.
The networks with long settling times grown at the rate of children with autism showed no such fall-off in performance, neither for the portion of the task that required inter-hemispheric interaction (Figure 5 and Table 5), nor for the portion of the task that did not (Figure 6 and Table 6).
This pattern of development of functional connectivity, and of performance, is directly reflected in the patterns of development of physical connectivity as measured by relative inter-hemispheric connection weight (Figure 7 and Table 7). Averaging over individual variation, and different outcomes for different training sets, the networks with short settling times grown at the rate of children with autism showed a reduction in physical connectivity at approximately the simulated equivalent of 24 months of age. These networks, overall, had reduced relative inter-hemispheric connection weights at all measured time-points, but the difference increases substantially at ‘12 months’. This is reflected in the differences in the estimated marginal means (Table 7), and in the fact that there was a significant interaction between group and age between ‘12 months’ and ‘24 months’ (Table 7). And, as with the pattern of development of functional connectivity, and of performance, the interaction between group and age between ‘12 months’ and ‘24 months’ indicates not just a decrease relative to the networks following the growth pattern in typical development, but an actual reduction in physical connectivity. A repeated measures ANOVA on the relative inter-hemispheric weight measures from the networks trained with short settling times and grown at the rate of children with autism yielded a significant main effect of age for this time period (F(1, 19) = 68.470, p < .001, partial η2 = 0.783).
As with functional connectivity and performance, however, there was substantial individual variation, with some networks showing a continual increase in physical connectivity, some showing a period of little change, and others showing reductions. As is evident from the estimated marginal means for individual training sets (Figure 7), however, the input did not have the same sort of influence on physical connectivity as it did on performance and functional connectivity. This may indicate that there was reorganization in all cases, but that some training sets posed greater problems for this process than others, and so the physical changes resulted in better functional recovery in some cases than in others.
The networks with long settling times grown at the rate of children with autism showed no such reduction in physical connectivity. Rather, similar to the pattern of development in functional connectivity, these networks showed a small increase in physical connectivity at 48 simulated months of age (Table 7). This increase in relative inter-hemispheric weight reflects only an increase in the inter-hemispheric weights; the mean intra-hemispheric weights are virtually identical to the networks following the typical growth trajectory. Again, it is unclear why this occurred, but perhaps the longer settling times allowed the networks to compensate for the increased conduction delay in the networks following the growth trajectory in autism.
Discussion
Autism is a developmental disorder defined by impairments in reciprocal social interactions, impairments in verbal and nonverbal communication, and a restricted repertoire of activities and interests (American Psychiatric Association, 1994). In addition to these core symptoms, however, there are usually deficits in multiple other areas, e.g. imitation (Aldridge, Stone, Sweeney & Bower, 2000; Charman, Swettenham, Baron-Cohen, Cox, Baird & Drew, 1997; DeMyer, Alpern, Barton, DeMeyer, Churchill, Hingtgen, Bryson, Pontius & Kimberlin, 1972; Hobson & Lee, 1999; V. Jones & Prior, 1985; Rogers, Bennetto, McEvoy & Pennington, 1996; Royeurs, Van Oost & Bothuyne, 1998; Sigman & Ungerer, 1984; Smith & Bryson, 1998; Stone, Lemanek, Fishel, Fernandez & Altemeier, 1990; Stone, Ousley & Littleford, 1997), motor coordination (Dawson, Osterling, Meltzoff & Kuhl, 2000; Kanner, 1943; Teitelbaum, Teitelbaum, Nye, Fryman & Maurer, 1998), perception of rapid auditory transitions (Oram Cardy, Flagg, Roberts, Brian & Roberts, 2005), and of visual motion (Bertone, Mottron, Jelenic & Faubert, 2003; Gepner & Mestre, 2002; Gepner, Mestre, Masson & de Schonen, 1995; Milne, Swettenham, Hansen, Campbell, Jeffries & Plaisted, 2002; Spencer, O’Brien, Riggs, Braddick, Atkinson & Wattam-Bell, 2000). There are also areas of strength, e.g. discrimination and detection of simple perceptual patterns, and analysis of visuospatial details (Bonnel, Mottron, Peretz, Trudel, Gallun & Bonnel, 2003; Happé, 1999; Jolliffe & Baron-Cohen, 1997; O’Riordan, Plaisted, Driver & Baron-Cohen, 2001; Plaisted, O’Riordan & Baron-Cohen, 1998; Shah & Frith, 1983). But autism is heterogeneous in its presentation – six of the 12 aspects of the three core symptoms suffice for a diagnosis of autism, so individuals present with different sets of behaviors. There is also substantial variation in terms of the subsets of non-core deficits that are present and strengths that are present. And individuals show differing degrees of superiority in their strengths, and differing degrees of severity in both core and non-core deficits. Additionally, the onset of the disorder varies: some children exhibit a failure to progress appropriately and show gradual development of any aberrant behaviors; others appear to develop normally for one or two years and then show sudden losses in acquired behaviors and the appearance of aberrant behaviors (Bailey, Phillips & Rutter, 1996; Filipek, Accardo, Baranek, Cook, Dawson, Gordon, Gravel, Johnson, Kallen, Levy, Minshew, Prizant, Rapin, Rogers, Stone, Teplin, Tuchman & Volkmar, 1999; Kolvin, 1971; Lainhart, Ozonoff, Coon, Krasny, Dinh, Nice & McMahon, 2002; Luyster, Richler, Risi, Hsu, Dawson, Bernier, Dunn, Hepburn, Hyman, McMahon, Nice-Goudie, Minshew, Rogers, Sigman, Spence, Goldberg, Tager-Flusberg, Volkmar & Lord, 2005; Short & Schopler, 1988; Siperstein & Volkmar, 2004).
Associated with this behavioral profile are findings of increased head and brain size (Aylward et al., 2002; Bailey et al., 1993; Bailey et al., 1998; Bauman & Kemper, 1985; Courchesne et al., 2003; Courchesne et al., 2001; Davidovitch et al., 1996; Dementieva et al., 2005; Fombonne et al., 1999; Hazlett et al., 2005; Hultman et al., 2002; Kemper & Bauman, 1998; Lainhart et al., 1997; Miles et al., 2000; Piven et al., 1995; Redcay & Courchesne, 2005; Sparks et al., 2002; Waiter et al., 2004; Woodhouse et al., 1996), and reduced large-scale functional connectivity (Castelli et al., 2002; Just et al., 2004; Kana et al., 2006; Koshino et al., 2005; Ring et al., 1999) and structural connectivity (Chung, Dalton, Alexander & Davidson, 2004; Egaas et al., 1995; Lewis & Courchesne, 2004; Lewis et al., 2003, 2004; Manes et al., 1999; Piven et al., 1997; Vidal et al., 2006; Waiter et al., 2005).
The modeling results presented here suggest that the abnormal brain growth trajectory in autism, via the influence of conduction delay, may provide an explanation for the findings of reduced large-scale functional and structural connectivity, and perhaps even for several aspects of the behavioral phenotype.
The decline in inter-hemispheric functional connectivity seen in the models with short settling times grown at the rate of children with autism provides almost directly for the findings of reduced large-scale functional connectivity in autism (Castelli et al., 2002; Just et al., 2004; Kana et al., 2006; Koshino et al., 2005; Ring et al., 1999). These reductions have been found in language tasks (Just et al., 2004; Kana et al., 2006), visual motion processing tasks (Castelli et al., 2002), and tasks involving working memory (Koshino et al., 2005). Language requires very rapid integration of prosodic and syntactic information in the acoustic signal, and possibly also the dynamic visual information in the face and in gesture. Working memory appears to rely on verbal rehearsal (Baddeley & Hitch, 1974). And motion processing utilizes delay circuits, which are inherently dependent on temporal precision. These temporal processing demands for language and motion processing tasks make it reasonable to speak of such networks as having short settling times. There are, however, no longitudinal data on functional connectivity in children with autism, or even cross-sectional data.
The decline in inter-hemispheric physical connectivity seen in the models with short settling times grown at the rate of children with autism, similarly, provides almost directly for the findings of reduced large-scale structural connectivity in autism (Chung et al., 2004; Egaas et al., 1995; Lewis & Courchesne, 2004; Lewis et al., 2003, 2004; Manes et al., 1999; Piven et al., 1997; Vidal et al., 2006; Waiter et al., 2005). Cross-sectional and longitudinal data indicate that this is a reduction that occurs developmentally (Lewis & Courchesne, 2004; Lewis et al., 2004), and is related to brain-size (Lewis & Courchesne, 2004; Lewis et al., 2004). But the functionality of the networks involved, and their temporal demands, must be inferred from the correspondence with the findings of functional reductions.
The fall-off of functional and physical connectivity seen in some of the models with short settling times grown at the rate of children with autism indicates that the brain growth trajectory in autism may result in an initial near-normal commitment to representations involving long-distance connections, followed by substantial reorganization that abandons these large-scale networks in favor of functionally localized representations. This process of reorganization is plausibly extremely functionally disruptive, and as suggested by the increase in performance error seen in these models, might be expected to have negative behavioral consequences. The modeling results indicate that, via such a process of reorganization, the brain overgrowth in autism may explain a number of aspects of the phenotype: the timing of the onset of the behavioral symptoms, the possibility of regression, the sorts of behaviors that are affected, and the heterogeneity.
Autism is generally reported to have a gradual onset with subtle signs presenting over the first year, and more obvious behavioral symptoms usually appearing late in the second year, or early in the third (Lord, Rutter, Goode, Heemsbergen, Jordan, Mawhood & Schopler, 1989; Volkmar, Stier & Cohen, 1985). But children later diagnosed with autism may, until around 18 to 30 months, develop normally or with subtle abnormalities, and then simply fail to progress, or may even regress – i.e. lose skills that they had already acquired (Davidovitch, Glick, Holtzman, Tirosh & Safir, 2000; Fombonne & Chakrabarti, 2001; Goldberg, Osann, Filipek, Laulhere, Jarvis, Modahl, Flodman & Spence, 2003; Kurita, 1985; Lainhart et al., 2002; Lord, Shulman & DiLavore, 2004; Luyster et al., 2005; Rapin & Katzman, 1998; Rogers, 2004; Rutter & Lord, 1987; Shinnar, Rapin, Arnold, Tuchman, Shulman, Ballaban-Gil, Maw, Deuel & Volkmar, 2001; Simons & Oishi, 1987; Wilson, Djukic, Shinnar, Dharmani & Rapin, 2003). Both patterns of development thus show a decline in performance during the latter part of the second year, or early in the third. At all simulated ages, the networks with short settling times grown at the rate of children with autism showed a reduction in performance in comparison to their typically developing counterparts. This difference, however, became much greater between 12 and 24 simulated months of age, or in some cases, between 24 and 36 simulated months of age. The correspondence, in terms of the age at which performance is most affected, between the modeling results and the patterns of development in autism suggests that the brain overgrowth in autism may force reorganization, and thereby bring about the appearance of the behavioral symptoms. In the models following the growth trajectory in autism, inter-hemispheric conduction delay is maximal between 16 and 32 months.
Regression occurs in 20% to 50% of children with autism, and is characterized by a significant loss of language and nonverbal communication skills (Davidovitch et al., 2000; Goldberg et al., 2003; Kurita, 1985; Rapin & Katzman, 1998; Rutter & Lord, 1987; Tuchman, Rapin & Shinnar, 1991). Many of these children show significant cognitive impairments, and many become nonverbal (Volkmar & Cohen, 1989; Wilson et al., 2003). There is generally gradual abatement of behavioral abnormalities in autism (DeMyer, Barton, DeMeyer, Norton, Allen & Steele, 1973; Gillberg, 1991; Kanner, 1943; Kobayashi, Murata & Yoshinaga, 1992; Lotter, 1978; Wolf & Goldberg, 1986), but the prognosis seems to be at least partially determined by the severity of the early symptoms (Coplan & Jawad, 2005). Children with late onset autism with regression generally show limited recovery (Volkmar & Cohen, 1989; Wilson et al., 2003). The modeling results suggest that cases of autism with regression may have the same etiology as cases of autism in which there is a failure to progress, or in which the onset is more gradual – i.e. the reorganization is driven by brain overgrowth. The reduction in performance at around 24 simulated months of age for the networks grown at the rate of children with autism was, in some cases, just a reduction relative to the typically developing networks; on average, there was a slight decline in performance – perhaps akin to a failure to progress in autism; and in some cases, this decline was substantial. Some of the networks showed a fall-off in performance of almost 10% – or, in terms of performance above chance, a decline of almost 25%. And this decline in performance would most likely be substantially larger if the networks followed the more extreme growth trajectory of some children with autism; and the impact on performance would most likely be larger still if the training sets increased in complexity over time – more in keeping with, for example, the problem of language acquisition. With a more extreme growth trajectory and more complex input, the number of networks showing such declines would also probably increase. A lesser degree of recovery would probably be seen as well, though realistic recovery results would probably, at a minimum, require the models to become less plastic over time, and to include pruning; the models used here have the same capacity to learn at all points in development, and permit weights to be reduced to zero, and then become non-zero again.
The characteristic loss of language and communication skills in cases of autism with regression may be in part due to the complexity of the input for those particular tasks, but the modeling results suggest that the rapid temporal demands of tasks such as these, and their reliance on inter-hemispheric connectivity, will make them particularly likely to be affected. As suggested by the far smaller impact on functional and physical connectivity, and on performance, for the models with long settling times, or for the portion of the task for which inter-hemispheric communication was not necessary, the consequences of the growth trajectory depend on the nature of the task. Networks should be expected to be negatively affected to the extent that those networks must rapidly integrate their inputs, to the extent that those networks comprise connections with substantially different conduction delays, and to the extent that the rapidity of the brain growth outpaces the ability of the system to compensate for the changes. The failure of such networks may underlie many of the behavioral deficits in autism, either directly or indirectly – indirectly in that behaviors that rely developmentally on the functioning of affected circuits cannot be expected to develop normally. Some of the core behavioral symptoms of autism, as well as many of the other associated abnormalities, may simply reflect network disruptions caused by unequal changes in conduction delay as a result of rapid changes in brain size.
Language abilities, for example, rely on the rapid integration of syntactic and prosodic information in the acoustic signal, as well as the information available in facial dynamics and gesture. These networks span the brain. And the long-distance interactions may be particularly important in language acquisition. Language acquisition appears to make substantially greater use of long-distance connections than does the adult system. Language acquisition, for instance, appears to rely on prosodic cues to a much greater extent than does adult language processing (Gerken, 1996; Hirsh-Pasek, Kemler Nelson, Jusczyk, Cassidy, Druss & Kennedy, 1987), and so on the interactions between the hemispheres. Studies of language processing show more bi-lateral activation in infants than in adults (Dehaene-Lambertz, 2000; Dehaene-Lambertz & Dehaene, 1994; Dehaene-Lambertz, Dehaene & Hertz-Pannier, 2002; Sachs & Gaillard, 2003). Moreover, disruptions in the networks responsible for processing any of the linguistic, and supra-linguistic, input – e.g. the networks responsible for low-level auditory or visual processing, for integration of low-level information, or for processing at more abstract levels – may contribute to a language deficit.
The networks that underlie auditory speech perception extend from the cochlea to the brain stem to anterior and posterior regions of the temporal cortex (Belin, Zatorre, LaFaille, Ahad & Pike, 2000; Binder, Frost, Hammeke, Bellgowan, Springer, Kaufman & Possing, 2000; Wise, Scott, Blank, Mummery, Murphy & Warburton, 2001), prefrontal areas (Fiez, Tallal, Raichle, Miezin, Katz & Petersen, 1995; Schubotz & von Cramon, 2001), the cerebellum (Hazeltine, Grafton & Ivry, 1997; Ivry & Keele, 1989), and the basal ganglia (Meck & Benson, 2002; Rammsayer & Classen, 1997). The rapid temporal processing demands of speech (Benasich, Thomas, Choudhury & Leppänen, 2002) place high demands on such a widely distributed system. Thus auditory speech perception should be expected to be impacted by the early brain overgrowth in autism. Results from EEG studies of auditory brain stem responses to acoustic stimuli indicate that there are increased conduction times from the cochlear nerve to the contralateral lateral lemniscus and inferior colliculus in individuals with autism (Maziade, Merette, Cayer, Roy, Szatmari, Cote & Thivierge, 2000; Rosenhall, Nordin, Brantberg & Gillberg, 2003; Wong & Wong, 1991); and delayed cortical responses to sinusoidal tones have been found in MEG studies (Gage, Siegel & Roberts, 2003). The question of whether or not these abnormalities translate into deficits in acoustic speech perception in autism is largely unaddressed, but deficits in rapid temporal processing have been reported (Oram Cardy et al., 2005), and tone and phoneme changes have been found to evoke a delayed cortical response compared to normal controls (Kasai, Hashimoto, Kawakubo, Yumoto, Kamio, Itoh, Koshida, Iwanami, Nakagome, Fukuda, Yamasue, Yamada, Abe, Aoki & Kato, 2005), with a significant positive relation between this latency delay and symptom severity. The ability to process brief acoustic transitions is critical for speech perception, and such impairments in this ability during development are likely to interfere with language acquisition. And deficits elsewhere may also interfere.
Visual and auditory integration is also likely to be important in early language acquisition. In typically developing children, and in adults, pairing visually presented speech – i.e. an image sequence of a face that is mouthing words – with noisy auditory stimuli results in improved perceptual accuracy in comparison to performance on either modality alone (Calvert, Brammer & Iversen, 1998; Massaro, 1998; Summerfield & McGrath, 1984). And visually presented speech has been found to activate both visual cortex and auditory cortex (Calvert, Bullmore, Brammer, Campbell, Williams, McGuire, Woodruff, Iversen & David, 1997; Calvert & Campbell, 2003; Campbell, MacSweeney, Surguladze, Calvert, McGuire, Suckling, Brammer & David, 2001; MacSweeney, Amaro, Calvert, Campbell, David, McGuire, Williams, Woll & Brammer, 2000; Pekkola, Ojanen, Autti, Jääskeläinen, Möttönen, Tarkiainen & Sams, 2005; Santi, Servos, Vatikiotis-Bateson, Kuratate & Munhall, 2003). But this integration relies on long-distance connections, and must occur rapidly, and so is likely to be impacted by the brain overgrowth in autism. Children with autism show the expected effect: they show a much smaller influence of visually presented speech on their performance in identifying noisy auditory stimuli (Massaro, 1987; Massaro & Bosseler, 2003). And visual speech processing may be particularly important for language acquisition in the context of impairments in speech-related auditory processing abilities.
The integration of visual and auditory information, moreover, involves the amygdala (E.G. Jones & Powell, 1970; Turner, Mishhkin & Knapp, 1980; Webster, Ungerleider & Bachevalier, 1991), which has been reported as a possible neural basis of the affect deficits in autism (Aylward, Minshew, Goldstein, Honeycutt, Augustine, Yates, Barta & Pearlson, 1999; Baron-Cohen, Ring, Bullmore, Wheelwright, Ashwin & Williams, 2000; Bauman & Kemper, 1985; Schumann, Hamstra, Goodlin-Jones, Lotspeich, Kwon, Buonocore, Lammers, Reiss & Amaral, 2004).
The processing of visually presented speech also relies on visual motion processing, which should also be expected to be negatively impacted by the early brain overgrowth in autism. The visual system extends from the retina to the lateral geniculate nucleus to striate cortex, and then to higher-level processing areas in occipital, parietal, and temporal cortex, and to frontal cortex and numerous subcortical areas (Van Essen & Gallant, 1994). Delay circuits are at the core of visual motion processing networks, and at multiple levels of processing the precisely timed activation of pools of neurons is critical. These delay circuits calculate the difference in the timing of the activation of the receptive fields corresponding to two different spatial locations on the retina. Uneven alterations to the conduction delays of the axons that comprise such delay circuits will alter the motion that is detected and the delay with which the detector fires. So the pools of neurons that detect any given motion will be negatively affected in two ways. And the neurons that integrate the outputs of these delay circuits will be similarly vulnerable. The early brain overgrowth in autism should thus be expected to impact visual motion perception, and the effect should be greater for faster motion and complex patterns. Children with autism are reported to be less sensitive to visual full-field radiating flow fields than typically developing children (Gepner et al., 1995), and the deficit is reported to increase with speed (Gepner & Mestre, 2002). And children with autism are reported to have significantly higher motion coherence thresholds with global motion stimuli (Spencer et al., 2000), random dot kinematograms (Milne et al., 2002) and texture-defined motion stimuli (Bertone et al., 2003) than typically developing children, but not for luminance-defined motion stimuli (Bertone et al., 2003).
Visual motion processing is, moreover, required to process social cues such as dynamic emotional expressions, eye gaze shifts, and gesture. Children with autism are reported to perform significantly worse than typically developing children on tasks involving the processing of facial dynamics, such as emotional expressions and movements of the lips and eyes (Gepner, Deruelle & Grynfeltt, 2001). And this performance difference is reported to be substantially reduced when the stimuli are displayed more slowly (Gepner et al., 2001).
Deficits in motion processing should also be expected to impact an individual’s ability to learn about, and respond to, the dynamic properties of the environment. Motor skills show the expected effect: in the first description of autism, Kanner (1943) described children with autism as ‘clumsy in gait and gross motor performances’; and subsequent research has established that motor deficits are, perhaps reliably, present in children with autism (Dawson et al., 2000; Teitelbaum et al., 1998).
And deficits in motion processing should also be expected to impair an individual’s ability to perceive the actions of others. Autistic children are reported to be impaired in the perception of biological motion presented as point-light animations (Blake, Turner, Smoski, Pozdol & Stone, 2003). This deficit might in turn be expected to lead to an impaired ability to learn motor behaviors which are modeled by others.
Additionally, the long-distance connections that link visual cortex with pre-motor cortex – the mirror neuron system (di Pellegrino, Fadiga, Fogassi, Gallese & Rizzolatti, 1992; Rizzolatti, Fadiga, Gallese & Fogassi, 1996) – should be negatively affected by the early brain overgrowth in autism. Neurons in premotor cortex that discharge when executing an action also discharge when observing that action (di Pellegrino et al., 1992; Rizzolatti et al., 1996; Rizzolatti, Fogassi & Gallese, 2001). The ability to carry out such internal simulation may play a critical role in the ability to understand other individuals’ movements, and the ability to understand social interactions (Rizzolatti & Craighero, 2004; Williams, Whiten & Singh, 2004). Thus imitation, and imitation learning, should be negatively affected in autism. Individuals with autism spectrum disorders have been reported to lack a mu rhythm response when observing another performing an action (Oberman, Hubbard, McCleery, Altschuler, Ramachandran & Pineda, 2005) – carrying the implication of a dysfunctional mirror neuron system. Imitation ability in autism is well researched, and support for a deficit in autism is unequivocal. Toddlers with autism have been found to have a deficit in meaningful and non-meaningful action imitation compared to typically developing toddlers (Charman et al., 1997; Stone et al., 1997). Young children with autism show clear deficits on body and motor-object imitation tasks (DeMyer et al., 1972), on simple imitation tasks from the Motor Imitation Scale (Sigman & Ungerer, 1984), on motor imitation tasks (V. Jones & Prior, 1985), on imitative ‘pretend’ actions and body movements (Stone et al., 1990), and on gestural (Aldridge et al., 2000; Royeurs et al., 1998) and procedural imitation (Royeurs et al., 1998). And adolescents continue to show deficits in symbolic and non-symbolic imitation (Rogers et al., 1996; Smith & Bryson, 1998), pantomime tasks (Rogers et al., 1996), and the imitation of the style in which an action is performed (Hobson & Lee, 1999).
Disruptions to networks such as these, which operate at a relatively low level, may also interfere with higher-level processing. And as suggested by the modeling results, the reorganization required for one task may have negative consequences for others. But this reorganization may also underlie some of the areas of strength seen in autism. Individuals with autism perform well on visual search tasks (O’Riordan et al., 2001; Plaisted et al., 1998), the block design and object assembly subtests of the Wechsler Intelligence Scale (Frith, 1989; Shah & Frith, 1983), the embedded figures task of the Wechsler intelligence test (Jolliffe & Baron-Cohen, 1997; Mitchell & Ropar, 2004; Shah & Frith, 1983), perceptual learning tasks (Plaisted et al., 1998), and global precedence tasks (Plaisted, Swettenham & Rees, 1999). Individuals with autism appear to employ a cognitive strategy for such tasks which relies more heavily on low-level processes implemented in relatively local circuits (Koshino et al., 2005; Ring et al., 1999). In the embedded figures task – a task requiring detection and discrimination of simple stimuli, and analysis and memory of perceptual detail – fMRI results show less extensive activation overall in individuals with autism compared to normal controls, no activation of prefrontal areas normally related to memory, and more activation in visual cortex (Ring et al., 1999). Visual cortex, in fact, appears to play a greater role than normal in satisfying the memory requirements of such tasks (Koshino et al., 2005). In an n-back task, individuals with autism were found to activate visual cortex to a greater extent than normal controls (Koshino et al., 2005). And measures of functional connectivity from fMRI indicate that this altered pattern of activation involves relatively smallscale networks (Castelli et al., 2002; Just et al., 2004; Koshino et al., 2005; Ring et al., 1999). For tasks in which high-level processing normally interferes with low-level processing – e.g. the global precedence task, the embedded figures task, and the block design task – the reduced influence of high-level processing on low-level processing should logically result in superior performance. And in the block design task, for instance, children with autism show superior performance only when the designs are presented whole, but not when the designs are presented as segmented pieces (Shah & Frith, 1993). The Gestalt design appears to have less impact on children with autism than on typically developing children. This has been taken as support for the theory of weak central coherence (Shah & Frith, 1993); the hypothesis presented here can be seen, for these cases, as providing a mechanism which explains why children with autism exhibit weak central coherence.
This mechanism, and the modeling results presented above, also provide an explanation for the heterogeneity in autism. The developmental performance measures for the networks grown at the rate of children with autism showed considerable between-subject variability in both the degree to which their performance was reduced relative to the networks grown at the rate of typically developing children, and the time at which the greatest reduction occurred. There was also considerable variability between training sets. The individual variation suggests that the precise impact of the brain growth trajectory on functional connectivity, behavior, and physical connectivity is likely to depend on potentially small differences in brain structure. The variation for different training sets indicates that this relationship is also likely to be modulated by differences in the environment. And these sources of variability can affect the networks subserving different functions relatively independently. Thus the same growth trajectory can impact different subsets of behaviors differentially in different individuals. Additionally, variability in brain size prenatally, and early in postnatal development, should lead to differences in the degree of commitment to widely distributed networks, and therefore in the amount of functional disruption that later rapid brain growth would produce. Variability in the amount of brain development that occurs before the period of accelerated brain growth should have a similar impact, and will also interact with developmental changes in neuroplasticity. And more rapid brain growth is more likely to be disruptive, as are differences in maximum brain size achieved.
Core aspects of the behavioral phenotype in autism spectrum disorders, as well as many of the ostensibly peripheral aspects, may thus be related to the effect that abnormalities in the growth trajectory have on conduction delay and, in turn, functional and physical connectivity and performance. The modeling results presented here take this beyond pure speculation; they provide an in-principle demonstration. The different outcomes for the two growth patterns reflect differences solely in inter-hemispheric conduction delay. But these models, and the input–output pairs they were trained on, are obviously very far from reality. The results are thus simply suggestive with respect to autism; and the above speculations are merely an initial exploration of the aspects of autism that might, via this mechanism, be related to the early brain overgrowth. But the relations between the trajectory of brain growth and functional connectivity, the behavioral phenotype, and physical connectivity, are almost unknown; and the empirical research that tests the hypothesis outlined here, for the most part, remains to be done.
Acknowledgement
This research was supported by grant NIH/NIMHR01-MH60517 to the second author.
References
- Akshoomoff N, Lord C, Lincoln AJ, Courchesne RYBA, Carper RA, Townsend J, Courchesne E. Outcome classification of preschool children with autism spectrum disorders using MRI brain measures. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(3):349–357. doi: 10.1097/00004583-200403000-00018. [DOI] [PubMed] [Google Scholar]
- Aldridge MA, Stone KR, Sweeney MH, Bower TGR. Preverbal children with autism understand the intentions of others. Developmental Science. 2000;3:294–301. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edn. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Aylward EH, Minshew NJ, Field K, Sparks BF, Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002;59:175–183. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Minshew NJ, Goldstein G, Honeycutt NA, Augustine AM, Yates KO, Barta PE, Pearlson GD. MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology. 1999;53:2145–2150. doi: 10.1212/wnl.53.9.2145. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch GJ. Working memory. In: Bower GA, editor. Recent advances in learning and motivation. Vol. 8. New York: Academic Press; 1974. pp. 47–90. [Google Scholar]
- Bailey A, Luthert P, Bolton P, LeCouteur A, Rutter M, Harding B. Autism and megalencephaly. Lancet. 1993;341:1225–1226. doi: 10.1016/0140-6736(93)91065-t. [DOI] [PubMed] [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P. A clinicopathological study of autism. Brain. 1998;121:889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Bailey A, Phillips W, Rutter M. Autism: towards an integration of clinical, genetic, neuro-psychological, and neurobiological perspectives. Journal of Child Psychology and Psychiatry. 1996;37(1):89–126. doi: 10.1111/j.1469-7610.1996.tb01381.x. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biological Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Knickmeyer RC, Belmonte MK. Sex differences in the brain: implications for explaining autism. Science. 2005;310:819–823. doi: 10.1126/science.1115455. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC. The amygdala theory of autism. Neuroscience and Biobehavioral Reviews. 2000;24:355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361:258–260. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- Bauman M, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35:866–874. doi: 10.1212/wnl.35.6.866. [DOI] [PubMed] [Google Scholar]
- Belin P, Zatorre RJ, LaFaille P, Ahad P, Pike B. Voice-selective areas in human auditory cortex. Nature. 2000;403:309–312. doi: 10.1038/35002078. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. The Journal of Neuroscience. 2004;24(42):9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benasich AA, Thomas JJ, Choudhury N, Leppänen PHT. The importance of rapid auditory processing abilities to early language development: evidence from converging methodologies. Developmental Psychobiology. 2002;40(3):278–292. doi: 10.1002/dev.10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertone A, Mottron L, Jelenic P, Faubert J. Motion perception in autism: a ‘complex’ issue. Journal of Cognitive Neuroscience. 2003;15(2):218–225. doi: 10.1162/089892903321208150. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Springer JA, Kaufman JN, Possing ET. Human temporal lobe activation by speech and nonspeech sounds. Cerebral Cortex. 2000;10:512–528. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Blake R, Turner LM, Smoski MJ, Pozdol SL, Stone WL. Visual recognition of biological motion is impaired in children with autism. Psychological Science. 2003;14(2):151–157. doi: 10.1111/1467-9280.01434. [DOI] [PubMed] [Google Scholar]
- Bonnel A, Mottron L, Peretz I, Trudel M, Gallun E, Bonnel A-M. Enhanced pitch sensitivity in individuals with autism: a signal detection analysis. Journal of Cognitive Neuroscience. 2003;15(2):226–235. doi: 10.1162/089892903321208169. [DOI] [PubMed] [Google Scholar]
- Callaway EM, Soha JM, Van Essen DC. Competition favouring inactive over active motor neurons during synapse elimination. Nature. 1987;382:422–426. doi: 10.1038/328422a0. [DOI] [PubMed] [Google Scholar]
- Calvert GA, Brammer MJ, Iversen SD. Crossmodal identification. Trends in Cognitive Sciences. 1998;2:247–253. doi: 10.1016/S1364-6613(98)01189-9. [DOI] [PubMed] [Google Scholar]
- Calvert GA, Bullmore ET, Brammer MJ, Campbell R, Williams SC, McGuire PK, Woodruff PW, Iversen SD, David AS. Activation of auditory cortex during silent lipreading. Science. 1997;276:593–596. doi: 10.1126/science.276.5312.593. [DOI] [PubMed] [Google Scholar]
- Calvert GA, Campbell R. Reading speech from still and moving faces: the neural substrates of visible speech. Journal of Cognitive Neuroscience. 2003;15:57–70. doi: 10.1162/089892903321107828. [DOI] [PubMed] [Google Scholar]
- Campbell R, MacSweeney M, Surguladze S, Calvert GA, McGuire P, Suckling J, Brammer MJ, David AS. Cortical substrates for the perception of face actions: an fMRI study of the specificity of activation for seen speech and for meaningless lower-face acts (gurning) Brain Research. Cognitive Brain Research. 2001;12:233–243. doi: 10.1016/s0926-6410(01)00054-4. [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Charman T, Swettenham J, Baron-Cohen S, Cox A, Baird G, Drew A. Infants with autism: an investigation of empathy, pretend play, joint attention, and imitation. Developmental Psychology. 1997;33:781–789. doi: 10.1037//0012-1649.33.5.781. [DOI] [PubMed] [Google Scholar]
- Chung MK, Dalton K, Alexander AL, Davidson RJ. Less white matter concentration in autism: 2D voxel-based morphometry. NeuroImage. 2004;23:242–251. doi: 10.1016/j.neuroimage.2004.04.037. [DOI] [PubMed] [Google Scholar]
- Coplan J, Jawad AF. Modeling clinical outcome of children with autistic spectrum disorders. Pediatrics. 2005;116:117–122. doi: 10.1542/peds.2004-1118. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. Journal of the American Medical Association. 2003;290(3):337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff NA, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Davidovitch M, Glick L, Holtzman G, Tirosh E, Safir MP. Developmental regression in autism: maternal perception. Journal of Autism and Developmental Disorders. 2000;30(2):113–119. doi: 10.1023/a:1005403421141. [DOI] [PubMed] [Google Scholar]
- Davidovitch M, Patterson B, Gartside P. Head circumference measurements in children with autism. Journal of Child Neurology. 1996;11:389–393. doi: 10.1177/088307389601100509. [DOI] [PubMed] [Google Scholar]
- Dawson G, Osterling J, Meltzoff AN, Kuhl P. Case study of the development of an infant with autism from birth to two years of age. Journal of Applied Developmental Psychology. 2000;21:299–313. doi: 10.1016/S0193-3973(99)00042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G. Cerebral specialization for speech and non-speech stimuli in infants. Journal of Cognitive Neuroscience. 2000;12(3):449–460. doi: 10.1162/089892900562264. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S. Speed and cerebral correlates of syllable discrimination in infants. Nature. 1994;370:292–295. doi: 10.1038/370292a0. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- Dementieva YA, Vance DD, Donnelly SL, Elston LA, Wolpert CM, Ravan SA, DeLong GR, Abramson RK, Wright HH, Cuccaro ML. Accelerated head growth in early development of individuals with autism. Pediatric Neurology. 2005;32(2):102–108. doi: 10.1016/j.pediatrneurol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- DeMyer MK, Alpern GD, Barton S, DeMyer WE, Churchill DW, Hingtgen JN, Bryson CQ, Pontius W, Kimberlin C. Imitation in autistic, early schizophrenic, and non-psychotic subnormal children. Journal of Autism and Childhood Schizophrenia. 1972;2(3):264–287. doi: 10.1007/BF01537618. [DOI] [PubMed] [Google Scholar]
- DeMyer MK, Barton S, DeMyer WE, Norton JA, Allen J, Steele R. Prognosis in autism: a follow-up study. Journal of Autism and Child Schizophrenia. 1973;3:199–246. doi: 10.1007/BF01538281. [DOI] [PubMed] [Google Scholar]
- Deutsch CK, Joseph RM. Brief report: cognitive correlates of enlarged head circumference in children with autism. Journal of Autism and Developmental Disorders. 2003;33(2):209–215. doi: 10.1023/a:1022903913547. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Experimental Brain Research. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Egaas B, Courchesne E, Saitoh O. Reduced size of corpus callosum in autism. Archives of Neurology. 1995;52:794–801. doi: 10.1001/archneur.1995.00540320070014. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Tallal P, Raichle ME, Miezin FM, Katz WF, Petersen SE. PET studies of auditory and phonological processing: effects of stimulus characteristics and task demands. Journal of Cognitive Neuroscience. 1995;7:357–375. doi: 10.1162/jocn.1995.7.3.357. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Accardo PJ, Baranek GT, Cook EH, Jr, Dawson G, Gordon B, Gravel JS, Johnson CP, Kallen RJ, Levy SE, Minshew NJ, Pizant BM, Rapin I, Rogers SJ, Stone WL, Teplin S, Tuchman RF, Volkmar FR. The screening and diagnosis of autistic spectrum disorders. Journal of Autism and Developmental Disorders. 1999;29(6):439–484. doi: 10.1023/a:1021943802493. [DOI] [PubMed] [Google Scholar]
- Fombonne E, Chakrabarti S. No evidence for a new variant of measles-mumps-rubella-induced autism. Pediatrics. 2001;108(4):58–64. doi: 10.1542/peds.108.4.e58. [DOI] [PubMed] [Google Scholar]
- Fombonne E, Roge B, Claverie J, County S, Fremolle J. Microcephaly and macrocephaly in autism. Journal of Autism and Developmental Disorders. 1999;29:113–119. doi: 10.1023/a:1023036509476. [DOI] [PubMed] [Google Scholar]
- Frith U. Autism: Explaining the enigma. Oxford: Blackwell; 1989. [Google Scholar]
- Gage NM, Siegel B, Roberts TPL. Cortical auditory system maturational abnormalities in children with autism disorder: an MEG investigation. Developmental Brain Research. 2003;144:201–209. doi: 10.1016/s0165-3806(03)00172-x. [DOI] [PubMed] [Google Scholar]
- Gepner B, Deruelle C, Grynfeltt S. Motion and emotion: a novel approach to the study of face processing by young autistic children. Journal of Autism and Developmental Disorders. 2001;31:37–45. doi: 10.1023/a:1005609629218. [DOI] [PubMed] [Google Scholar]
- Gepner B, Mestre D. Postural reactivity to fast visual motion differentiates autistic from children with Asperger syndrome. Journal of Autism and Developmental Disorders. 2002;32:231–238. doi: 10.1023/a:1015410015859. [DOI] [PubMed] [Google Scholar]
- Gepner B, Mestre D, Masson G, de Schonen S. Postural effects of motion vision in young autistic children. NeuroReport. 1995;6(8):1211–1214. doi: 10.1097/00001756-199505300-00034. [DOI] [PubMed] [Google Scholar]
- Gerken L. Prosody’s role in language acquisition and adult parsing. Journal of Psycholinguistic Research. 1996;25(2):345–356. doi: 10.1007/BF01708577. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gillberg C. Outcome in autism and autistic-like conditions. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30:375–382. doi: 10.1097/00004583-199105000-00004. [DOI] [PubMed] [Google Scholar]
- Goldberg WA, Osann K, Filipek PA, Laulhere T, Jarvis K, Modahl C, Flodman P, Spence MA. Language and other regression: assessment and timing. Journal of Autism and Developmental Disorders. 2003;33(6):607–616. doi: 10.1023/b:jadd.0000005998.47370.ef. [DOI] [PubMed] [Google Scholar]
- Hagberg G, Stenbom Y, Engerström IW. Head growth in Rett Syndrome. Brain and Development. 2001;23:S227–S229. doi: 10.1016/s0387-7604(01)00375-8. [DOI] [PubMed] [Google Scholar]
- Happé F. Autism: cognitive deficit or cognitive style? Trends in Cognitive Sciences. 1999;3(6):216–222. doi: 10.1016/s1364-6613(99)01318-2. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Grafton ST, Ivry R. Attention and stimulus characteristics determine the locus of motor-sequence encoding: a PET study. Brain. 1997;120:123–140. doi: 10.1093/brain/120.1.123. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J. Magnetic resonance imaging and head circumference study of brain aize in autism: birth through age 2 years. Archives of General Psychiatry. 2005;62(12):1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Herbert MR. Large brains in autism: the challenge of pervasive abnormality. The Neuroscientist. 2005;11(5):417–440. doi: 10.1177/0091270005278866. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, Sanders HA, Kennedy DN, Caviness VS. Localization of white matter volume increase in autism and developmental language disorder. Annals of Neurology. 2004;55(4):530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Hirsh-Pasek K, Kemler Nelson DG, Jusczyk PW, Cassidy KW, Druss B, Kennedy L. Clauses are perceptual units for young infants. Cognition. 1987;26(3):269–286. doi: 10.1016/s0010-0277(87)80002-1. [DOI] [PubMed] [Google Scholar]
- Hobson RP, Lee A. Imitation and identification in autism. Journal of Child Psychology and Psychiatry. 1999;40:649–659. [PubMed] [Google Scholar]
- Hultman CM, Sparen P, Cnattingius S. Perinatal risk factors for infantile autism. Epidemiology. 2002;13(4):417–423. doi: 10.1097/00001648-200207000-00009. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Keele SW. Timing functions of the cerebellum. Journal of Cognitive Neuroscience. 1989;1:136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- Jancke L, Preis S, Steinmetz H. The relation between forebrain volume and midsagittal size of the corpus callosum in children. NeuroReport. 1999;10:2981–2985. doi: 10.1097/00001756-199909290-00020. [DOI] [PubMed] [Google Scholar]
- Jancke L, Staiger JF, Schlaug G, Huang Y, Steinmetz H. The relationship between corpus callosum size and forebrain volume. Cerebral Cortex. 1997;7(1):48–56. doi: 10.1093/cercor/7.1.48. [DOI] [PubMed] [Google Scholar]
- Jolliffe T, Baron-Cohen S. Are people with autism and Asperger syndrome faster than normal on the Embedded Figures Test? Journal of Child Psychology and Psychiatry. 1997;38:527–534. doi: 10.1111/j.1469-7610.1997.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Jones EG, Powell TPS. An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain. 1970;83:793–820. doi: 10.1093/brain/93.4.793. [DOI] [PubMed] [Google Scholar]
- Jones V, Prior M. Motor imitation abilities and neurological signs in autistic children. Journal of Autism and Developmental Disorders. 1985;15(1):37–46. doi: 10.1007/BF01837897. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127(8):1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective content. Nervous Child. 1943;2:217–250. [Google Scholar]
- Kasai K, Hashimoto O, Kawakubo Y, Yumoto M, Kamio S, Itoh K, Koshida I, Iwanami A, Nakagome K, Fukuda M, Yamasue H, Yamada H, Abe O, Aoki S, Kato N. Delayed automatic detection of change in speech sounds in adults with autism: a magnetoencephalographic study. Clinical Neurophysiology. 2005;116(7):1655–1664. doi: 10.1016/j.clinph.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Kemper TL, Bauman M. Neuropathology of infantile autism. Journal of Neuropathology and Experimental Neurology. 1998;57:645–652. doi: 10.1097/00005072-199807000-00001. [DOI] [PubMed] [Google Scholar]
- Kobayashi R, Murata T, Yoshinaga K. A follow-up study of 201 children with autism in Kyushu and Yamaguchi areas, Japan. Journal of Autism and Developmental Disorders. 1992;22:395–411. doi: 10.1007/BF01048242. [DOI] [PubMed] [Google Scholar]
- Kolvin I. The phenomenology of childhood psychosis. British Journal of Psychiatry. 1971;118:385–395. doi: 10.1192/bjp.118.545.385. [DOI] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. NeuroImage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Statistics. 2002;11:1–190. [PubMed] [Google Scholar]
- Kurita H. Infantile autism with speech loss before the age of thirty months. Journal of the American Academy of Child Psychiatry. 1985;24:191–196. doi: 10.1016/s0002-7138(09)60447-7. [DOI] [PubMed] [Google Scholar]
- Kurmanavicius J, Wright EM, Royston P, Wisser J, Huch R, Huch A, Zimmermann R. Fetal ultrasound biometry: 1. Head reference values. British Journal of Obstetrics and Gynaecology. 1999;106(2):126–135. doi: 10.1111/j.1471-0528.1999.tb08212.x. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Ozonoff S, Coon H, Krasny L, Dinh E, Nice J, McMahon W. Autism, regression, and the broader autism phenotype. American Journal of Medical Genetics. 2002;113(3):231–237. doi: 10.1002/ajmg.10615. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Piven J, Wzorek M, Landa R, Santangelo SL, Coon H, Folstein S. Macrocephaly in children and adults with autism. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(2):282–290. doi: 10.1097/00004583-199702000-00019. [DOI] [PubMed] [Google Scholar]
- LaMantia AS, Rakic P. Axon overproduction and elimination in the corpus callosum of the developing rhesus monkey. Neuroscience. 1990;10(7):2156–2175. doi: 10.1523/JNEUROSCI.10-07-02156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Courchesne E. Pathological brain overgrowth leads to reduced interhemispheric connectivity. Paper presented at the the 4th International Meeting for Autism Research; Sacremento, CA. 2004. [Google Scholar]
- Lewis JD, Courchesne E, Elman JL. Rate of growth and hemispheric specialization; Paper presented at the the 23rd International Summer School of Brain Research; 2003. [Google Scholar]
- Lewis JD, Courchesne E, Elman JL. Growth trajectories and cortico-cortical connections; Paper presented at the the 37th Annual Gatlinburg Conference: On research and theory in intellectual and developmental disabilities; 2004. [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, Schopler E. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. Journal of Autism and Developmental Disorders. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- Lord C, Shulman C, DiLavore P. Regression and word loss in autistic spectrum disorders. Journal of Child Psychology and Psychiatry. 2004;45(5):936–955. doi: 10.1111/j.1469-7610.2004.t01-1-00287.x. [DOI] [PubMed] [Google Scholar]
- Lotter V. Follow-up studies. In: Rutter M, Schopler E, editors. Autism: A reappraisal of concepts and treatment. New York: Plenum; 1978. pp. 475–495. [Google Scholar]
- Luecke RH, Wosilait WD, Young JF. Mathematical representation of organ growth in the human embryo/fetus. International Journal of Bio-Medical Computing. 1995;39(3):337–347. doi: 10.1016/0020-7101(95)01115-u. [DOI] [PubMed] [Google Scholar]
- Luyster R, Richler J, Risi S, Hsu W-L, Dawson G, Bernier R, Dunn M, Hepburn S, Hyman SL, McMahon WM, Nice-Goudie J, Minshew N, Rogers S, Sigman M, Spence MA, Goldberg WA, Tager-Flusberg H, Volkmar FR, Lord C. Early regression in social communication in autism spectrum disorders: a CPEA study. Developmental Neuropsychology. 2005;27(3):311–336. doi: 10.1207/s15326942dn2703_2. [DOI] [PubMed] [Google Scholar]
- MacSweeney M, Amaro E, Calvert GA, Campbell R, David AS, McGuire P, Williams SC, Woll B, Brammer MJ. Silent speechreading in the absence of scanner noise: an event-related fMRI study. NeuroReport. 2000;11:1729–1733. doi: 10.1097/00001756-200006050-00026. [DOI] [PubMed] [Google Scholar]
- Manes F, Piven J, Vrancic D, Nanclares V, Plebst C, Starkstein SE. An MRI study of the corpus callosum and cerebellum in mentally retarded autistic individuals. Journal of Neuropsychiatry and Clinical Neurosciences. 1999;11:470–474. doi: 10.1176/jnp.11.4.470. [DOI] [PubMed] [Google Scholar]
- Massaro DW. Speech perception by ear and eye: A paradigm for psychological inquiry. Hillsdale, NJ: Erlbaum; 1987. [Google Scholar]
- Massaro DW. Perceiving talking faces: From speech perception to a behavioral principle. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Massaro DW, Bosseler A. Perceiving speech by ear and eye: multimodal integration by children with autism. Journal of Developmental and Learning Disorders. 2003;7:111–146. [Google Scholar]
- Maziade M, Merette C, Cayer M, Roy M-A, Szatmari P, Cote R, Thivierge J. Prolongation of brain-stem auditory-evoked responses in autistic probands and their unaffected relatives. Archives of General Psychiatry. 2000;57:1077–1083. doi: 10.1001/archpsyc.57.11.1077. [DOI] [PubMed] [Google Scholar]
- Meck WH, Benson AM. Dissecting the brain’s internal clock: how frontal–striatal circuitry keeps time and shifts attention. Brain and Cognition. 2002;48(1):195–211. doi: 10.1006/brcg.2001.1313. [DOI] [PubMed] [Google Scholar]
- Miles JH, Hadden LL, Takahashi TN, Hillman RE. Head circumference is an independent clinical finding associated with autism. American Journal of Medical Genetics. 2000;95:339–350. [PubMed] [Google Scholar]
- Milne E, Swettenham J, Hansen P, Campbell R, Jeffries H, Plaisted K. High motion coherence thresholds in children with autism. Journal of Child Psychology and Psychiatry. 2002;43(2):255–263. doi: 10.1111/1469-7610.00018. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Ropar D. Visuo-spatial abilities in autism. Infant and Child Development. 2004;13:185–198. [Google Scholar]
- Nellhaus G. Head circumference from birth to eighteen years: practical composite international and interracial graphs. Pediatrics. 1968;41:106–114. [PubMed] [Google Scholar]
- Oberman LM, Hubbard EM, McCleery JP, Altschuler EL, Ramachandran VS, Pineda JA. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cognitive Brain Research. 2005;24:190–198. doi: 10.1016/j.cogbrainres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Olivares R, Montiel J, Aboitiz F. Species differences and similarities in the fine structure of the mammalian corpus callosum. Brain, Behavior and Evolution. 2001;57(2):98–105. doi: 10.1159/000047229. [DOI] [PubMed] [Google Scholar]
- Oram Cardy JE, Flagg EJ, Roberts W, Brian J, Roberts T. Magnetoencephalography identifies rapid temporal processing deficit in autism and language impairment. NeuroReport. 2005;16(4):329–332. doi: 10.1097/00001756-200503150-00005. [DOI] [PubMed] [Google Scholar]
- O’Riordan MA, Plaisted KC, Driver J, Baron-Cohen S. Superior visual search in autism. Journal of Experimental Psychology: Human Perception and Performance. 2001;27(3):719–730. doi: 10.1037//0096-1523.27.3.719. [DOI] [PubMed] [Google Scholar]
- Pekkola J, Ojanen V, Autti T, Jääskeläinen IP, Möttönen R, Tarkiainen A, Sams M. Primary auditory activation by visual speech: an fMRI study at 3 Tesla. NeuroReport. 2005;16(2):125–128. doi: 10.1097/00001756-200502080-00010. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128(5):1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Piven J, Arndt S, Bailey J, Havercamp S, Andreasen NC, Palmer P. An MRI study of brain size in autism. American Journal of Psychiatry. 1995;12:1145–1149. doi: 10.1176/ajp.152.8.1145. [DOI] [PubMed] [Google Scholar]
- Piven J, Bailey J, Ranson BJ, Arndt S. An MRI study of the corpus callosum in autism. American Journal of Psychiatry. 1997;154(8):1051–1056. doi: 10.1176/ajp.154.8.1051. [DOI] [PubMed] [Google Scholar]
- Plaisted K, O’Riordan M, Baron-Cohen S. Enhanced visual search for a conjunctive target in autism: a research note. The Journal of Child Psychology and Psychiatry and Allied Disciplines. 1998;39:777–783. [PubMed] [Google Scholar]
- Plaisted K, O’Riordan M, Baron-Cohen S. Enhanced discrimination of novel, highly similar stimuli by adults with autism during a perceptual learning task. Journal of Child Psychology and Psychiatry. 1998;39:765–775. [PubMed] [Google Scholar]
- Plaisted K, Swettenham J, Rees L. Children with autism show local precedence in a divided attention task and global precedence in a selective attention task. Journal of Child Psychology and Psychiatry. 1999;40:733–742. [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Rakic P, Yakovlev PI. Development of the corpus callosum and cavum septi in man. Journal of Comparative Neurology. 1968;132:45–72. doi: 10.1002/cne.901320103. [DOI] [PubMed] [Google Scholar]
- Rammsayer T, Classen W. Impaired temporal discrimination in Parkinson’s disease: temporal processing of brief durations as an indicator of degeneration of dopaminergic neurons in the basal ganglia. International Journal of Neuroscience. 1997;91(1–2):45–55. doi: 10.3109/00207459708986364. [DOI] [PubMed] [Google Scholar]
- Rapin I, Katzman R. Neurobiology of autism. Annals of Neurology. 1998;43:7–14. doi: 10.1002/ana.410430106. [DOI] [PubMed] [Google Scholar]
- Redcay E, Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biological Psychiatry. 2005;58(1):1–9. doi: 10.1016/j.biopsych.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Rhode D. LENS: The light, efficient network simulator (No. CMU-CS-99-164) Pittsburgh, PA: Department of Computer Science, Carnegie Mellon University; 1999. [Google Scholar]
- Rilling JK, Insel TR. Differential expansion of neural projection systems in primate brain evolution. NeuroReport. 1999;10(7):1453–1459. doi: 10.1097/00001756-199905140-00012. [DOI] [PubMed] [Google Scholar]
- Ring HA, Baron-Cohen S, Wheelwright S, Williams SCR, Brammer M, Andrew C, Bullmore ET. Cerebral correlates of preserved cognitive skills in autism. Brain. 1999;122:1305–1315. doi: 10.1093/brain/122.7.1305. [DOI] [PubMed] [Google Scholar]
- Ringo JL, Doty RW, Demeter S, Simard PY. Time is of the essence: a conjecture that hemispheric specialization arises from interhemispheric conduction delay. Cerebral Cortex. 1994;4(4):331–343. doi: 10.1093/cercor/4.4.331. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Cognitive Brain Research. 1996;3:131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying imitation and the understanding of action. Nature Reviews Neuroscience. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Rogers SJ. Developmental regression in autism spectrum disorders. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10(2):139–143. doi: 10.1002/mrdd.20027. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Bennetto L, McEvoy R, Pennington BF. Imitation and pantomime in high-functioning adolescents with autism spectrum disorders. Child Development. 1996;67:2060–2073. [PubMed] [Google Scholar]
- Rosenhall U, Nordin V, Brantberg K, Gillberg C. Autism and auditory brain stem responses. Ear and Hearing. 2003;24(3):206–214. doi: 10.1097/01.AUD.0000069326.11466.7E. [DOI] [PubMed] [Google Scholar]
- Royeurs H, Van Oost P, Bothuyne S. Immediate imitation and joint attention in young children with autism. Developmental Psychopathology. 1998;10:441–450. doi: 10.1017/s0954579498001680. [DOI] [PubMed] [Google Scholar]
- Rumelhart DE, Hinton GE, Williams RJ. Learning representations by back-propagating errors. Nature. 1986;323:533–536. [Google Scholar]
- Rutter M, Lord C. Language disorders associated with psychiatric disturbance. In: Rule W, Rutter M, editors. Language development and disorders. Philadelphia, PA: J.B. Lippincott; 1987. [Google Scholar]
- Sachs BC, Gaillard WD. Organization of language networks in children: functional magnetic resonance imaging studies. Current Neurology and Neuroscience Reports. 2003;3:157–162. doi: 10.1007/s11910-003-0068-z. [DOI] [PubMed] [Google Scholar]
- Santi A, Servos P, Vatikiotis-Bateson E, Kuratate T, Munhall K. Perceiving biological motion: dissociating visible speech from walking. Journal of Cognitive Neuroscience. 2003;15:800–809. doi: 10.1162/089892903322370726. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY. Interval and ordinal properties of sequences are associated with distinct premotor areas. Cerebral Cortex. 2001;11(3):210–222. doi: 10.1093/cercor/11.3.210. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, Lammers CR, Reiss AL, Amaral DG. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. Journal of Neuroscience. 2004;24(28):6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwärzler P, Bland JM, Holden D, Campbell S, Ville Y. Sex-specific antenatal reference growth charts for uncomplicated singleton pregnancies at 15–40 weeks of gestation. Ultrasound in Obstetrics and Gynecology. 2004;23(1):23–29. doi: 10.1002/uog.966. [DOI] [PubMed] [Google Scholar]
- Shah A, Frith U. An islet of ability in autistic children: a research note. Journal of Child Psychology and Psychiatry. 1983;24:613–620. doi: 10.1111/j.1469-7610.1983.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Shah A, Frith U. Why do autistic individuals show superior performance on the block design task? Journal of Child Psychology and Psychiatry. 1993;34(8):1351–1364. doi: 10.1111/j.1469-7610.1993.tb02095.x. [DOI] [PubMed] [Google Scholar]
- Shinnar S, Rapin I, Arnold S, Tuchman RF, Shulman L, Ballaban-Gil K, Maw M, Deuel RK, Volkmar FR. Language regression in childhood. Pediatric Neurology. 2001;24:185–191. doi: 10.1016/s0887-8994(00)00266-6. [DOI] [PubMed] [Google Scholar]
- Short AB, Schopler E. Factors relating to age of onset in autism. Journal of Autism and Developmental Disorders. 1988;18:207–216. doi: 10.1007/BF02211947. [DOI] [PubMed] [Google Scholar]
- Sigman M, Ungerer JA. Cognitive and language skills in autistic, mentally retarded and normal children. Developmental Psychology. 1984;20:293–302. [Google Scholar]
- Simons J, Oishi S. The hidden child. Rockville, MD: Woodbine House; 1987. [Google Scholar]
- Siperstein R, Volkmar F. Brief report: Parental reporting of regression in children with pervasive developmental disorders. Journal of Autism and Developmental Disorders. 2004;34(6):731–734. doi: 10.1007/s10803-004-5294-y. [DOI] [PubMed] [Google Scholar]
- Smith IM, Bryson SE. Gesture imitation in autism I: Nonsymbolic postures and sequences. Cognitive Neuropsychology. 1998;15:747–770. doi: 10.1080/026432998381087. [DOI] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, Dager SR. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Spencer J, O’Brien J, Riggs K, Braddick O, Atkinson J, Wattam-Bell J. Motion processing in autism: evidence for a dorsal stream deficiency. NeuroReport. 2000;11(12):2765–2767. doi: 10.1097/00001756-200008210-00031. [DOI] [PubMed] [Google Scholar]
- Stone WL, Lemanek KL, Fishel PT, Fernandez MC, Altemeier WA. Play and imitation skills in the diagnosis of autism in young children. Pediatrics. 1990;86:267–272. [PubMed] [Google Scholar]
- Stone WL, Ousley OY, Littleford CD. Motor imitation in young children with autism: what’s the object? Journal of Abnormalities in Child Psychology. 1997;25:475–485. doi: 10.1023/a:1022685731726. [DOI] [PubMed] [Google Scholar]
- Summerfield AQ, McGrath M. Detection and resolution of audio-visual incompatibility in the perception of vowels. Quarterly Journal of Experimental Psychology. 1984;36A:51–74. doi: 10.1080/14640748408401503. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, Joseph RM. Identifying neurocognitive phenotypes in autism. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences. 2003;358:303–314. doi: 10.1098/rstb.2002.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum P, Teitelbaum O, Nye J, Fryman J, Maurer RG. Movement analysis in infancy may be useful for early diagnosis of autism. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13982–13987. doi: 10.1073/pnas.95.23.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404:190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- Tuchman RF, Rapin I, Shinnar S. Autistic and dysphasic children. I: Clinical characteristics. Pediatrics. 1991;88:1211–1218. [PubMed] [Google Scholar]
- Turner BH, Mishhkin M, Knapp M. Organization of the amygdalopetal projections from modality-specific cortical association areas in the monkey. Journal of Comparative Neurology. 1980;191:515–543. doi: 10.1002/cne.901910402. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Gallant J. Neural mechanisms of form and motion processing in the primate visual system. Neuron. 1994;13:1–10. doi: 10.1016/0896-6273(94)90455-3. [DOI] [PubMed] [Google Scholar]
- Van Ooyen A, Willshaw DJ. Competition for neurotrophic factor in the development of nerve connections. Proceedings of the Royal Society of London, Series B: Biological Sciences. 1999;266:883–892. doi: 10.1098/rspb.1999.0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal CN, Nicolson R, DeVito TJ, Hayashi KM, Geaga JA, Drost DJ, Williamson PC, Rajakumar N, Sul Y, Dutton RA, Toga AW, Thompson PM. Mapping corpus callosum deficits in autism: an index of aberrant cortical connectivity. Biological Psychiatry. 2006;60(3):218–225. doi: 10.1016/j.biopsych.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Volkmar FR, Cohen DJ. Disintegrative disorder or ‘late onset’ autism. Journal of Child Psychology and Psychiatry. 1989;5:717–724. doi: 10.1111/j.1469-7610.1989.tb00784.x. [DOI] [PubMed] [Google Scholar]
- Volkmar FR, Stier D, Cohen D. Age of recognition of pervasive developmental disorder. American Journal of Psychiatry. 1985;142:1450–1452. doi: 10.1176/ajp.142.12.1450. [DOI] [PubMed] [Google Scholar]
- Waiter GD, Williams JHG, Murray AD, Gilchrist A, Perrett DI, Whiten A. A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. NeuroImage. 2004;22:619–625. doi: 10.1016/j.neuroimage.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Waiter GD, Williams JHG, Murray AD, Gilchrist A, Perrett DI, Whiten A. Structural white matter deficits in high-functioning individuals with autistic spectrum disorder: a voxel-based investigation. NeuroImage. 2005;24:455–461. doi: 10.1016/j.neuroimage.2004.08.049. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Conduction in myelinated, unmyelinated, and demyelinated fibers. Archives of Neurology. 1977;34:585–589. doi: 10.1001/archneur.1977.00500220019003. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Ungerleider LG, Bachevalier J. Connections of inferior temporal areas TE and TEO with medial temporal-lobe structures in infant and adult monkeys. Journal of Neuroscience. 1991;11:1095–1116. doi: 10.1523/JNEUROSCI.11-04-01095.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JHG, Whiten A, Singh T. A systematic review of action imitation in autistic spectrum disorder. Journal of Autism and Developmental Disorders. 2004;34(3):285–299. doi: 10.1023/b:jadd.0000029551.56735.3a. [DOI] [PubMed] [Google Scholar]
- Wilson S, Djukic A, Shinnar S, Dharmani C, Rapin I. Clinical characteristics of language regression in children. Developmental Medicine and Child Neurology. 2003;45:508–514. doi: 10.1017/s0012162203000951. [DOI] [PubMed] [Google Scholar]
- Winsor CP. The Gompertz curve as a growth curve. Proceedings of the National Academy of Sciences of the United States of America. 1932;18(1):1–8. doi: 10.1073/pnas.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RJ, Scott SK, Blank SC, Mummery CJ, Murphy K, Warburton EA. Separate neural subsystems within ‘Wernicke’s area’. Brain. 2001;124:83–95. doi: 10.1093/brain/124.1.83. [DOI] [PubMed] [Google Scholar]
- Wolf L, Goldberg B. Autistic children grow up: an eight to twenty-four year follow-up study. Canadian Journal of Psychiatry. 1986;31:550–556. doi: 10.1177/070674378603100613. [DOI] [PubMed] [Google Scholar]
- Wong V, Wong SN. Brainstem auditory evoked potential study in children with autistic disorder. Journal of Autism and Developmental Disorders. 1991;21:329–340. doi: 10.1007/BF02207329. [DOI] [PubMed] [Google Scholar]
- Woodhouse W, Bailey A, Rutter M, Bolton P, Baird G, Couteur AL. Head circumference in autism and other pervasive developmental disorders. Journal of Child Psychology and Psychiatry. 1996;37:665–671. doi: 10.1111/j.1469-7610.1996.tb01458.x. [DOI] [PubMed] [Google Scholar]
- Wosilait WD, Luecke RH, Young JF. A mathematical analysis of human embryonic and fetal growth data. Growth, Development, and Aging. 1992;56:249–257. [PubMed] [Google Scholar]
- Zhang K, Sejnowski TJ. A universal scaling law between gray matter and white matter of cerebral cortex. Proceedings of the National Academy of Sciences, USA. 2000;97(10):5621–5626. doi: 10.1073/pnas.090504197. [DOI] [PMC free article] [PubMed] [Google Scholar]