Abstract
COPD represents one of the leading causes of mortality in the general population. This study aimed at evaluating the relationship between airway hyperresponsiveness (AHR) and COPD and its relevance for clinical practice. We performed a MEDLINE search that yielded a total of 1919 articles. Eligible studies were defined as articles that addressed specific aspects of AHR in COPD, such as prevalence, pathogenesis, or prognosis. AHR appears to be present in at least one out of two individuals with COPD. The occurrence of AHR in COPD is influenced by multiple mechanisms, among which impairment of factors that oppose airway narrowing plays an important role. The main determinants of AHR are reduction in lung function and smoking status. We envision a dual role of AHR: in suspected COPD, specific determinants of AHR, such as reactivity and the plateau response, may help the physician to discriminate COPD from asthma; in definite COPD, AHR may be relevant for the prognosis. Indeed, AHR is an independent predictor of mortality in COPD patients. Smoking cessation has been shown to reduce AHR. Further studies are needed to elucidate whether this functional change is associated with improvement in lung function and respiratory symptoms.
Keywords: bronchial hyperreactivity, airway hyperresponsiveness, bronchial provocation tests, COPD
Introduction
The hyperresponsive state of the airways, defined as a condition in which the airways narrow too easily or too much in response to a provoking stimulus, has been historically associated with the asthmatic phenotype, and a body of evidence has enabled definition of airway hyperresponsiveness (AHR) as one of the cardinal features of asthma. However, other inflammatory respiratory diseases, such as cystic fibrosis and COPD, may show an exaggerated airway response to spasmogens. The significance of AHR in diseases other than asthma is yet to be elucidated and deserves more attention. The so-called “Dutch hypothesis” postulates that asthma and COPD are two different aspects of the same disease, and that AHR predisposes to the development of both clinical conditions (Orie et al 1961). In this scenario, the increased airway responsiveness could be envisaged as a contributing factor to the development of COPD, rather than the consequence of this disease (Rijcken et al 1995), as proposed by the international guidelines for COPD (NHLBI/WHO 2001; Celli and MacNee 2004). However, the mechanisms underlying the relationship between AHR and COPD may not be the same as those between AHR and asthma.
COPD is characterized by progressive decline in lung function and impaired quality of life. It represents one of the leading causes of mortality in the general population, and its prevalence has increased dramatically in recent decades (Lopez and Murray 1998). Despite the impact of COPD in the healthcare system, the pathophysiological components of the disease and the multiple clinical manifestations are not fully understood. In this respect, some issues need to be addressed: first, the contribution of the presence (and the degree of severity) of AHR to the pathogenesis of COPD is not clear; second, the contribution of AHR to the accelerated decline in lung function and/or the increased mortality in COPD patients is still a matter of debate. Finally, if most of the clinical features and physiologic abnormalities of COPD are found to be linked to the hyperresponsive state, the potential of AHR as a target for COPD therapy emerges.
This article explores the relationship between AHR and COPD and its relevance for clinical practice with regard to the diagnosis, prognosis, and management of the chronic obstructive disease.
Methods
We performed a MEDLINE search, using the PubMed interface,1 to select articles that focus on AHR in patients with COPD. The search strategy initially included the Medical Subject Heading (MeSH) terms “bronchial hyperreactivity”, “respiratory hypersensitivity”, “bronchial provocation tests”, and “pulmonary disease, chronic obstructive”. Interestingly, “airway hyperresponsiveness” is not a MeSH term and is not recognized as an entry term for the MeSH “bronchial hyperreactivity”, which is the MeSH closest to the definition of AHR given above. To perform a more useful search, “free research” (ie, not restricted to the MeSH) was also undertaken using the following keywords: bronchial hyperreactivity, bronchial provocation tests, methacholine, histamine, adenosine, airway responsiveness, airway hyper-responsiveness, airway hyperresponsiveness, and COPD. The results were restricted to articles written in English and studies performed in humans. The combination of the PubMed searches provided a total of 1919 articles (updated May 2005), which were subsequently checked for inclusion in the present review. In addition, the reference lists from relevant eligible studies were hand-searched. All articles that were identified were examined for eligibility by three investigators independently (N Scichilone, S Battaglia, and A La Sala); any disagreement was discussed in a consensus form that included a senior investigator (V Bellia). Eligible studies were defined as the articles whose main objective was the evaluation of a specific aspect of AHR (prevalence, pathogenesis, prognosis) in individuals with COPD. Inclusion was also restricted to studies that used methacholine, histamine, or adenosine as bronchoconstrictor agents in the bronchoprovocation tests.
Prevalence of AHR in COPD
We have previously investigated the prevalence of AHR in the general population, focusing on the more advanced ages (Scichilone, Messina, et al 2005). However, information regarding the prevalence of AHR in COPD populations is scarce because of the paucity of studies that specifically address this aspect. AHR has been measured in case-series studies of smokers with various degrees of airway obstruction; heightened airway responses to methacholine or histamine have been documented in the majority of patients (Klein and Salvaggio 1966; Benson 1978; Ramsdell et al 1982; Bahous et al 1984; Ramsdale et al 1984). From these studies it can be inferred that AHR in COPD patients is more closely dependent on pre-challenge lung function and appears to be, on average, less severe than that recorded in subjects with asthma. To specifically establish the prevalence of AHR in individuals with COPD, studies with large numbers of subjects need to be performed. Tashkin and colleagues (1992) analyzed the prevalence of AHR in the context of the Lung Health Study. As part of this multicenter study, methacholine bronchoprovocation was performed in 5877 current cigarette smokers with borderline to moderate airflow limitation. The results demonstrated that about two-thirds of the population had AHR, expressed as PC20 (provocative concentration causing 20% decrease in FEV1), which was significantly more common in women than in men (85% vs 59%) and strongly related to the baseline degree of airflow obstruction.
In the context of a longitudinal study of COPD conducted in a small town in Northern Italy, methacholine bronchoprovocation was performed in 654 subjects, 50 of whom showed symptoms consistent with chronic bronchitis (Cerveri et al 1988). By using a cut-off value of 850 mg of methacholine and a PD15 (provocative dose to produce 15% decrease in FEV1), only nine subjects (23%) showed AHR. This increased to 41% when the cut-off dose was set at 1900 mg of methacholine. Yan and colleagues (1985) studied 922 adults living in Busselton, Western Australia. The results indicate that approximately half of the 59 subjects with COPD had AHR when challenged with histamine (PD20). When these results were compared with the prevalence of AHR detected in asthmatics in the same population, the geometric mean for PD20 was higher in COPD, supporting the concept of less severe AHR in this condition. Interestingly, there was a positive correlation between FEV1/FVC and PD20 values in subjects with COPD but not in those with asthma, thus confirming that in COPD AHR is dependent on the initial airway caliber. In this regard, the geometric factor (pre-challenge lung function) plays a controversial role in determining the degree of AHR (McParland et al 2003). As discussed below, the structural changes that occur in the airway walls could have beneficial effects on airway reactivity. In the study by Yan and co-workers (1985), the lack of a significant correlation in the asthmatic population could be simply attributed to a statistical weakness, or alternatively it could unveil the “protective” role of fixed bronchial obstruction.
The results on AHR prevalence in COPD from these studies are not superimposable. It seems conceivable that the discrepancy in terms of prevalence depends on the differences between studies. First, and probably most important, the different size of the sample populations limits comparison of the outcomes. Also, the different spasmogen used and the higher proportions of current smokers and women in the Lung Health Study may have contributed to the different outcomes. Smoking status strongly affects the occurrence of AHR. It is, therefore, not surprising that the results of the Lung Health Study show a higher prevalence of AHR. Similarly, the higher proportion of females could account for the discrepancy in AHR prevalence, in that women have smaller lung volumes and consequently smaller airway caliber, which in turn increases AHR, as demonstrated by Bakke and co-workers (1991).
In conclusion, from the few available studies we can assume that at least one out of two COPD patients has some degree of AHR. In this context, major questions are raised and pose a challenge for future research: why do only a proportion of individuals with COPD show AHR, and what prevents AHR from occurring in the non-hyperresponsive patients with COPD?
Clinical relevance of AHR in COPD
In the diagnosis of asthma, the importance of AHR is well recognized, especially in cases in which baseline lung function is within the normal range. In addition, AHR can be important in determining the optimal inhaled steroid dosage, because therapy based only on symptoms and spirometry may be inadequate (Sont et al 1999). Furthermore, in asthma the degree of AHR may add useful information for the prognosis, in that the exacerbation rate appears to be highest in patients with severe hyperresponsiveness (Sont et al 1999). In contrast, the clinical relevance of AHR in COPD is less well defined, mainly because the bronchoprovocation test is often not ordered or not performed in individuals with COPD or in those with low lung function. Theoretically, the presence of AHR, or its degree of severity, can be important for diagnosis, prognosis, and/or therapy decisions. In our opinion, the evaluation of AHR could be indicated in two clinical settings, the first being represented by conditions in which the diagnosis of COPD and asthma may be confused. This is particularly true for mild forms of COPD, in which lung function is only mildly impaired and respiratory symptoms may not be fully descriptive of COPD; in this case, the diagnosis of COPD may be challenging. On the other hand, some asthmatic patients are characterized by airway obstruction that is not reversible and may pose diagnostic issues. In this regard, Fabbri and colleagues (2003), by investigating the differences in patients with fixed airflow limitation due to asthma or COPD, concluded that AHR (PD20 methacholine) does not distinguish between the two clinical entities. The task is, therefore, to detect specific characteristics of AHR in COPD that could enable the physician to solve the diagnostic dilemma. The second scenario includes individuals with a definite diagnosis of COPD, in whom the bronchoprovocation challenge could provide potential advantages in terms of treatment and prognosis.
AHR in suspected COPD
It is worth noting that the presentation of COPD can be heterogeneous and it can often overlap that of asthma. The heterogeneity of the clinical presentation implies that patients with COPD may show some characteristics that are more frequently observed in asthmatics. There are three possible reasons for this: real non-classic presentation of COPD, simultaneous presence of the two diseases, or erroneous diagnosis. In clinical settings, cases of asthma can be misdiagnosed as COPD, especially in the elderly and in individuals with disability (Bellia et al 2003). In clinical practice, the two conditions can coexist and this presentation is referred to as “asthmatic bronchitis” or “wheezy bronchitis”. Unfortunately, little is known about this condition, mainly because most studies exclude these patients to avoid confounding factors. Data from two epidemiological surveys in rural and urban areas of Italy demonstrated that up to 30% of patients with COPD had asthma, and up to 14% of patients with asthma had COPD (Viegi et al 2004). Further studies are needed to establish the role of AHR in the prognosis and management of asthmatic bronchitis and to evaluate the therapeutic response in this condition.
In the case of suspected COPD, a positive broncho- provocation challenge does not help to differentiate COPD from asthma, since, as discussed above, a degree of AHR is present in a large proportion of patients with COPD. However, given the high sensitivity and negative predictive value of the methacholine bronchoprovocation in asthma (ATS 2000), a negative test would rather favor the diagnosis of COPD. Studies in which agents other than methacholine have been used may help to distinguish COPD from asthma (Postma and Kerstjens 1998). For instance, despite showing a similar degree of bronchoconstriction to methacholine, smokers with chronic bronchitis and COPD generally do not show response to acetaldehyde (Sanchez-Toril et al 2000). Therefore, this test has been proposed as more specific than methacholine (95% vs 24%). In addition, COPD smokers show greater response to adenosine 5′-monophosphate (AMP) than ex-smokers (Oosterhoff et al 1993), and AHR to AMP is associated with a higher number of sputum eosinophils (Rutgers et al 2000). Therefore, the role of inflammation in the pathogenesis of AHR in COPD may be further explored by using this test. Perhaps AMP may better differentiate COPD from asthma and can contribute to selecting subgroups of COPD subjects who will benefit from antiinflammatory therapy.
Since the bronchoprovocation test cannot accurately distinguish between asthma and COPD, inclusion of the test in the diagnostic approach should not be routinely recommended. In this context, the advantage of assessing AHR in suspected cases of COPD could be provided by more specific aspects of AHR, such as exaggerated airway narrowing. Many investigators have clearly demonstrated that the outcome of the bronchoprovocation test depends on the pre-challenge lung function (Ryan et al 1982; Ramsdale et al 1984; Verma et al 1988; Sterk and Bel 1989). In other words, the lower the baseline FEV1, the higher the magnitude of AHR. This is true both in asthma and in COPD (Brand et al 1991) and can be purely attributed to geometric mechanisms. Using a mathematical model, Moreno and colleagues (1986) demonstrated that, for a given degree of airway smooth muscle (ASM) shortening, increased thickness of airway wall amplifies airway narrowing. Thickening of the airway wall can also uncouple the airways from the surrounding parenchyma, thus reducing the tethering forces that oppose airway narrowing. As a consequence, the assessment of AHR in individuals with low FEV1 (<70% of predicted value) has apparently no usefulness. However, structural changes of the asthmatic airway walls may have a protective effect against narrowing, in that the elastic load provided by collagen deposition and the folding of the ASM as it contracts may limit further shortening (Meiss 1999; McParland et al 2003). Therefore, the causes of reduction in airway caliber and the manifestations of AHR may differ between the two clinical conditions, as discussed below.
Airway narrowing in response to spasmogens is the result of opposing forces, ie, those related to ASM shortening and the distending forces applied to the outer airway wall surface by the alveolar septa. In the context of destroyed lung parenchyma, the load that counteracts the shortening of ASM is depressed and this favors maximal airway narrowing. In this respect, one should focus on the reactivity (the slope of the dose-response curve), which in COPD has been demonstrated to depend on the reduction of FEV1 (Verhoeven et al 2000) and on airway wall thickness (Corsico et al 2003). The maximal response on the dose-response curve describes a condition of increased risk of severe bronchoconstriction. Koh and colleagues (2002) showed that the elevated maximal response to methacholine (ie, the lack of plateau), but not the increased sensitivity to the stimulus (ie, the threshold dose), predicts the risk of developing asthma in individuals with allergic rhinitis. The plateau value, which is a measure of excessive maximal narrowing, is mainly determined by the lung elastic forces that oppose ASM contraction. A plateau response is usually not attained in asthma, especially in more severe cases (Woolcock et al 1984) because of inflammatory changes that uncouple the parenchyma and the airways. In COPD, the lack of a plateau is attributed to the structural changes in the lung that cause a reduction in the elastic recoil, thus impairing the tethering forces applied to the outer airway wall (Colebatch et al 1973). The study performed by Cheung et al (1997) provides a unique model of the relationship between the characteristics of AHR and the loss of elastic recoil. In this study, the authors recruited subjects with α1-antitrypsin deficiency and no current history of smoking, and found a close relationship between the degree of parenchymal destruction and the maximal airway narrowing. On the other hand, plateaus in the dose-response curve have been documented in some individuals with COPD (Verhoeven et al 2000). It can, however, be theorized that, given the structural alterations of the lung parenchyma, higher reactivity is likely to occur in COPD subjects. From the above observations, it can be argued that various components of airway responsiveness need to be combined in order to distinguish asthma from COPD. This would also shed light on the pathogenesis of AHR in chronic respiratory disorders, in that sensitivity is considered to be affected by pre-junctional mechanisms, such as inflammation, whereas the plateau value is mainly influenced by post-junctional factors, such as lung elastic recoil.
How can this information be translated into clinical practice? The level of expertise required to measure the above-described variables precludes extensive applicability of the bronchoprovocation test. To overcome this limitation, assessment of the fall in FVC at the level of PC20 could provide an indirect measure of excessive airway narrowing and should be encouraged among physicians who are responsible for the management of COPD. Since elastic recoil is considered a limiting factor for the maximum decrease in airway caliber during bronchoconstriction (Ding et al 1987), structural alterations in lung elasticity may result in enhanced bronchoconstriction. In this context, the senile lung is characterized by structural and functional alterations (Verbeken et al 1992a, 1992b) that could favor airway narrowing and increased airway responsiveness. The results from an analysis conducted on studies specifically addressing the relationship between age and AHR are in favor of a positive correlation between these two factors (Scichilone, Messina, et al 2005). This is also suggested by the observation that, despite comparable pulmonary function and PC20 FEV1, elderly patients with asthma show higher AHR than young asthmatics, when expressed by metha-choline-induced changes in FVC (Cuttitta et al 2001). This evidence implies that age-associated structural alterations in elderly asthmatic patients contribute to determining a higher risk of early airway closure. As suggested by Macklem (1989), this phenomenon is amplified in COPD patients, in whom parenchymal destruction occurs.
AHR in definite COPD
In cases in which the diagnosis of COPD is already established, or in individuals at risk of developing more severe COPD (smokers and stage 0 in the GOLD classification) (NHLBI/WHO 2001), the presence of AHR may be important for the prognosis. The most important clinical question is whether AHR can be an independent predictor of mortality. To our knowledge, only one study has addressed the association between AHR and COPD mortality on a longitudinal basis (Hospers et al 2000). The authors presented findings from the Dutch epidemiological surveys of Vlagtwedde, Vlardingen, and Meppel and analyzed 526 deaths. After 24-year follow-up, they identified 60 patients who died with COPD as the primary or secondary cause of death. The study showed that the severity of AHR was associated with higher risk of mortality. These results corroborate the role of AHR as a predictor of mortality, although these observations were, to some extent, criticized (Vestbo and Hansen 2001). Indeed, it has been hypothesized that the “AHR factor” could be reduced to an insignificant level by already established predictors, such as lung function (Vestbo and Hansen 2001). In the Hospers et al study (2000), there was a correction for different subsets of baseline FEV1 (>100%, 80–100%, and <80% of predicted values). However, the category of <80% of predicted seems too wide and could be insufficient to control for this factor. Moreover, the contribution of smoking status and the presence of asthma should probably have been evaluated in more detail.
Is the assessment of AHR in patients with COPD important for the management of the disease? It is known that asthmatic patients show improvement in AHR after inhaled steroid therapy as assessed by either direct (Overbeek et al 1996) or indirect (van den Berge et al 2001) stimuli. Conversely, conflicting evidence has been reported in COPD. One large longitudinal randomized, placebo-controlled clinical trial on COPD (559 cases vs 557 controls) has demonstrated that inhaled corticosteroids improved AHR after long-term treatment (Lung Health Study Research Group 2000). The results showed that, at 9 and 33 months, the triamcinolone group had less reactivity in response to methacholine than the placebo group. Despite this improvement, pulmonary function decline was not influenced. In contrast, other authors reported that inhaled corticosteroid treatment had no effect on AHR. A randomized, placebo-controlled, double-blind trial in 59 nonasthmatic COPD patients who completed a 2-year follow-up demonstrated that inhaled beclomethasone did not change AHR to histamine (Weir et al 1999). Verhoeven et al (2002) demonstrated that 6 months of inhaled corticosteroids (fluticasone) did not modify PC20 or other indices of AHR, such as the slope of the dose-response curve, the plateau value (maximal bronchoconstriction), or the concentration of the stimulus causing 50% of maximal bronchoconstriction. These data confirm previous results showing that, in smokers with chronic bronchitis or COPD, treatment with inhaled corticosteroids (budesonide) for 6–12 weeks did not improve AHR (PC20 or PD20 histamine) to any clinically significant extent (Engel et al 1989; Auffarth et al 1991; Watson et al 1992; Weir and Burge 1993). Several factors may be advocated to explain these differences. The Lung Health Study (Lung Health Study Research Group 2000) accounts for a total of 1116 patients and has more statistical power in detecting small differences than the other trials, which consisted of groups of 14 to 105 subjects (Engel et al 1989; Auffarth et al 1991; Watson et al 1992; Weir and Burge 1993; Weir et al 1999; Verhoeven et al 2002). Six months (Verhoeven et al 2002) and 2 years (Weir et al 1999) of treatment should be a suitable length of time to evaluate modification in the histamine challenge test. It is well known that in asthma a period of 4 weeks is needed to detect any beneficial effect of corticosteroids on AHR assessed by methacholine (Prosperini et al 2002), whereas only 72 hours is required to improve histamine responsiveness (Sovijarvi et al 2003).
Finally, the difference in bronchoconstrictor agents used seems of minor importance, because both methacholine and histamine act as direct stimuli. It is interesting to note that corticosteroids do not reduce AHR, even when indirect stimuli are used. It has been demonstrated that AHR to AMP in smoker and non-smoker COPD subjects is significantly lower than in smoker healthy controls (Oosterhoff et al 1993). Treatment with inhaled corticosteroids did not significantly change PC20 AMP (Rutgers et al 1998). In the active treatment group of 22 non-atopic current smokers with COPD, who were equally reactive to AMP and methacholine under baseline conditions, 6-week therapy with inhaled budesonide did not improve AHR to AMP, and the sensitivity to the indirect stimulus produced by AMP was not greater than that of methacholine (Rutgers et al 1998). This may be explained by various hypotheses: (1) the reduction in mast cell numbers in the airway wall mucosa observed after treatment with inhaled corticosteroids (Hattotuwa et al 2002) is too small to result in changes in AHR to AMP in patients with COPD; (2) mast cells are not greatly activated and/or involved in the inflammatory process of COPD.
Mechanisms of AHR in COPD
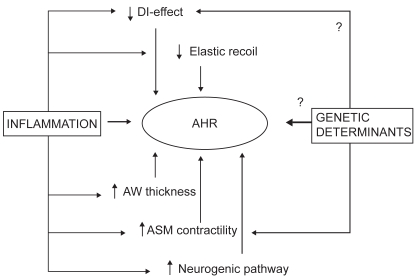
AHR in COPD may occur through multiple mechanisms, which are summarized in Figure 1. Although the contribution of each factor to the development of increased airway response to stimuli has been a subject of extensive investigation, the interaction between them remains largely disputed. In particular, recent evidence from in vivo and mathematical modeling revives the theory of cigarette smoking as the promoting factor of the cascade of events which, ultimately, induce AHR. On the other hand, evidence from transgenic technology and genetic screening provides support for genetic determinants of AHR, although, to our knowledge, candidate genes in COPD have not been replicated or performed in sufficiently powered populations.
Figure 1.
Schematic of the potential mechanisms underlying the relationship between AHR and COPD. Abbreviations: AHR, airway hyperresponsiveness; ASM, airway smooth muscle; AW, airway wall; DI, deep inspiration.
Airway smooth muscle
Since the primary mechanism of airway obstruction is contraction of ASM, the behavior of this tissue should primarily be assessed. Abnormal ASM contraction can be attributed to increased velocity of shortening, or simply to the increased amount of muscle, although increased force generation as assessed in vitro does not necessarily translate into in vivo ASM shortening. The possibility should also be taken into account that ASM has a “synthetic” function, producing and/or releasing factors that promote inflammation and increase contractility (Howarth et al 2004). The evaluation of ASM contractility from COPD subjects has yielded conflicting results (Taylor et al 1985; De Jongste et al 1987; Black 1991). De Jongste and colleagues (1987) showed that isolated bronchioles from COPD subjects exhibited greater maximal isometric forces than that recorded from subjects without COPD. These observations have been recently confirmed by Opazo-Saez and co-workers (2000), suggesting that, together with loss of elastic recoil and remodeling of small airways (see below), altered smooth muscle function plays a role in the development of AHR. Alternatively, smooth muscle may contract normally, but the increase in its mass, per se or by increasing the thickening of the airway wall, leads to excessive airway narrowing. ASM content increases significantly in the small airways in COPD (Jeffery 2001) and contributes to the overall thickness of the airway wall. Computational modeling of the bronchial tree has revealed that increased thickness of the airways augments reactivity, rather than sensitivity. By dissecting the different components of the airways, Lambert and colleagues (1993) and Tiddens and colleagues (1999) demonstrated that the ASM content is the most important factor responsible for the bronchoconstrictive response to stimuli in COPD. Against the airway thickening hypothesis is the evidence that thicker airways may have a protective role against broncho- constrictors (Milanese et al 2001; Niimi et al 2003). Niimi and colleagues (2003) showed that in asthmatics, thickened airway walls, as assessed by high-resolution computed tomography (HRCT) scans, inversely correlated with airway reactivity. The thickening of airway smooth muscle is also determined by accumulation of matrix which opposes airway contraction. As Pare (2003) has recently pointed out, an increased amount of muscle may not mean greater contractile capacity. The possibility should also be considered that, in COPD, ASM reaches a “frozen” state (Fredberg 2000), in which actin and myosin filaments are tightly bound. According to this theory (Fredberg et al 1997; Fredberg 2000), the frozen state is attained when the smooth muscle is not subjected to periodic stretches, such as in the condition of reduced excursions that are observed in the airways in COPD. This phenomenon would lead to increased responsiveness, as inferred by a pivotal study by Skloot and colleagues (1995), who demonstrated that prohibiting deep inspiratory maneuvers in healthy individuals produced a reaction to methacholine of the same magnitude as that in asthmatics.
Deep inspiration
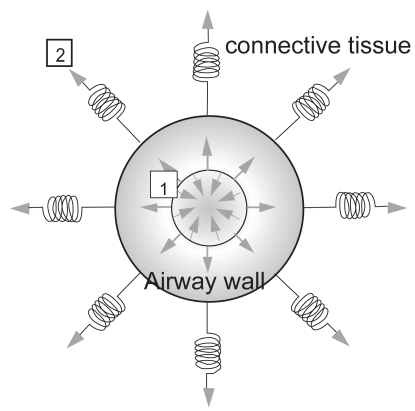
Deep inspiratory maneuvers have been demonstrated to exert beneficial effects on the airways of healthy individuals (Pellegrino et al 1996, 1998; King et al 1999; Kapsali et al 2000; Scichilone et al 2000, 2004a; Brown et al 2001; Scichilone, Pyrgos, et al 2001; Sundblad and Larsson 2002; Jackson et al 2004; Salerno et al 2005). It has been widely demonstrated that deep inspirations (DI) are able to revert bronchial obstruction that has been experimentally provoked (Pellegrino et al 1996, 1998; King et al 1999; Scichilone et al 2000, 2004a; Brown et al 2001; Scichilone, Pyrgos, et al 2001; Sundblad and Larsson 2002; Jackson et al 2004). For this reason, a DI is considered among the most potent bronchodilators. Using single-dose methacholine bronchoprovocation, we have established the methodology to measure the bronchodilatory effect of DI (Scichilone et al 2000; Scichilone, Permutt, et al 2001), which allows us to explore the phenomenon of airway distensibility and its impairment; indeed, we have demonstrated that the bronchodilatory effect decreases with increasing severity of asthma (Scichilone et al 2002) and is abolished in subjects with COPD (Scichilone et al 2004b). The mechanisms through which a deep inspiratory maneuver acts are mediated by the tethering forces that are applied to the airway walls, and any factor affecting the interdependence between airways and the surrounding parenchyma can blunt the ability of DI to further distend airways (Figure 2). At least two conditions could result in the loss of lung inflation-induced bronchodilation. On one hand, increased thickness of the airway wall, such as that observed in the more severe stages of asthma, could induce greater stiffness of airways, which in turn would oppose airway distension (Figure 2[1]). On the other hand, reduction of elastic recoil could diminish the radial traction of the airway walls, allowing for loss of supporting tissue for peripheral airways with ease of airway closure (Figure 2[2]) (Cheung et al 1997). This would explain, for example, why bronchodilation by DI appears to be essentially absent in individuals with structural changes in lung parenchyma, such as emphysema (Scichilone et al 2004b). Recent observations from our group (Scichilone, Bruno, et al 2005) indicate that the attenuation of the bronchodilatory effect of DI in COPD is proportional to the parenchymal damage, as assessed by the content of destroyed alveolar septa. We propose that the different magnitudes of DI-induced bronchodilation could, to some extent, account for the variance in the degree of AHR. In other words, whereas in healthy individuals the bronchodilatory effect of DI is present and manages to adequately counteract airway tone so that bronchoconstriction does not take place, in patients with COPD, the bronchodilatory effect of DI is blunted and may not be sufficient to balance the additional airway tone induced by an extrinsic stimulus, thus affecting the magnitude of induced bronchoconstriction.
Figure 2.
Schematic of the interdependence between the airway and the surrounding parenchyma, showing the opposing forces, ie, those distending the airway and those causing narrowing. The former are sustained by increased lung volumes and mediated by the connective tissue; the latter are induced by airway smooth muscle contraction. The bronchodilatory effect of deep inspiration, which is mediated by the tethering forces that are applied to the airway walls, can be affected by two mechanisms: thickness of the airway wall due to remodeling changes (1), or structural damage to the parenchymal tissue (2).
In addition to the bronchodilatory effect, lung inflation exerts a bronchoprotective role in healthy individuals; ie, a series of DI taken before inhalation of the spasmogen is able to prevent airways from narrowing. We have demonstrated that asthmatic and rhinitic subjects with AHR show impairment in bronchoprotection induced by DI (Scichilone, Permutt, et al 2001), irrespective of respiratory symptoms. Therefore, reduced bronchoprotection by DI could result from very early inflammation-induced functional or structural alterations in the airways. Whether this applies to COPD is yet to be proven. To date, the effects of DI on AHR in COPD patients have not been specifically addressed. We suggest that lung inflation is responsible for maintenance of airway patency in healthy humans, and that the loss of this function in COPD contributes to the occurrence of AHR. We recently showed that DI-induced bronchoprotection can be restored by inhaled glucocorticosteroids only in asthmatics with mild hyperresponsiveness (Scichilone, Permutt, et al 2005). To date, we do not know whether the lack of DI-induced bronchoprotection is predictive of the response to pharmacological and nonpharmacological therapy in COPD.
Inflammation
In asthma, AHR has been associated with airway inflammation (NHLBI/WHO 2001). The same paradigm could be applied in COPD, although the nature of inflammation differs. Postma et al (1988) demonstrated, in COPD subjects, a close association between AHR to histamine and superoxide anion production from peripheral leukocytes. In assessing the inflammatory aspect of the relationship between AHR and COPD, the inflammation-induced abnormalities that occur in the parenchymal compartment must be discussed. In COPD, the elastolytic destruction of the parenchyma and the consequent loss of elastic recoil decrease the support to the smaller airways, thus increasing airway collapsibility and early closure. This can be enhanced by the structural changes that occur in the small airways (Jeffery 2001). On the other hand, the asthmatic inflammation is confined to the airways, with little or no effect on the surrounding parenchyma; therefore, the increase in airway resistance is predominantly attributed to remodeling changes of the airway walls. The differences in small airway pathology between asthma and COPD may support this hypothesis. It has been demonstrated that the small airways from asthmatic subjects contain a significantly greater density of CD45+ lymphocytes in the outer adventitial region as opposed to the inner submucosal region (Haley et al 1998). In COPD subjects, the opposite phenomenon occurs, in that the density of CD45+ lymphocytes is higher in the inner than in the outer wall region (Saetta et al 2000). These observations are consistent with mathematical models examining the role of submucosal thickening, adventitial thickening, and increase in smooth muscle mass as contributors to the AHR of asthma and COPD (Lambert et al 1993). Using a mathematical model, Lambert and colleagues (1993) postulated that the increase in muscle mass appears to be the most important feature accounting for AHR. However, the increases in the adventitial and the submucosal areas also play an important role (Lambert et al 1993). Although this computer-generated model needs to be confirmed, it is conceivable that in asthma the increased cellular density in the adventitia would promote airway constriction by reducing the effectiveness of airway to parenchyma interdependence, whereas the increased cellular density observed in the submucosa of individuals with COPD would promote airway constriction by amplifying the effect of ASM shortening (Lambert et al 1993; Saetta et al 2000).
Allergy
It has been postulated that allergic or immune factors play a role in modulating AHR in COPD. In non-allergic (negative skin tests) patients with COPD, higher serum total IgE levels were independently associated with a lower PC20 histamine at baseline (Renkema et al 1998). This observation has been confirmed by Mitsunobu and colleagues (2001), who demonstrated that AHR to methacholine is enhanced by the presence of specific IgE antibodies against inhalant allergens in patients with COPD. Data from a 2-year follow-up study showed that higher initial serum total IgE levels were associated with a slower annual decline of PC20, regardless of treatment and pre-challenge FEV1 (Renkema et al 1998). The observation that the initial serum total IgE level predicts a more favorable course with regard to annual decline of PC20histamine could be in line with the assumption that COPD patients with features of asthma have a better prognosis than those with “pure emphysema” conditions. Thus, one would have expected that increased total IgE would indicate the presence of a “responsive group” to inhaled corticosteroids. However, regardless of total IgE levels, corticosteroid therapy did not influence changes in PC20 over time (Dow 1998; Renkema et al 1998). Perhaps one explanation is that elevated IgE levels do not always imply the presence of atopic asthma; for instance, they could be related to tobacco smoking (Dow 1998).
Smoking status
Since cigarette smoking is the greatest independent risk factor for the development of COPD, studies on the role of AHR in COPD have concentrated on the effect of smoke. If a relationship does exist, it can be expected that smoking cessation strategies would improve AHR in COPD. Airway response to cigarette smoking shows a dose-dependent relationship with the consumption of cigarettes (Jensen et al 1998). The effect of smoking on AHR is mediated by inflammatory or neurogenic mechanisms, which are intertwined with the effect on lung geometry. Smoking stimulates nerve endings in the airway walls, with consequent edema, mucus hypersecretion, and recruitment of inflammatory cells. Willemse et al (2004) recently demonstrated that 1-year smoking cessation led to improvement in AHR in individuals with COPD. This may result from reduced stimulation of the irritant receptors, reduction in mucus secretion, or changes in the inflammatory cells. The results from the Lung Health Study also showed an increase in AHR over a 5-year period in COPD subjects who continued to smoke, but not in those who quit (Wise et al 2003). Since ex-smokers also improved, or stabilized, their lung function, it cannot be excluded that the benefit of smoking cessation was merely due to geometric changes of the airways.
Genetic determinants
It is generally assumed that an interaction between genetic determinants and environmental factors initiates the development of COPD. Several candidate genes have been identified as related to COPD, but some of them have not been confirmed or replicated in different populations. Given the role of AHR in the development and the course of COPD, the genetic predisposition to AHR in these individuals should also be assessed. Genetic susceptibility to AHR has been largely analyzed in asthma, and it has been demonstrated to be coinherited with genetic markers on chromosome 5q31–q33 (Postma et al 1995). Coding genes for interleukin (IL)-4 and IL-13, which are implicated in the development of AHR, are also located in chromosome 5q31. Hegab and colleagues (2004) recently investigated polymorphisms within these genes in two different populations consisting of Japanese (88 COPD and 61 healthy controls) and Egyptian (106 COPD and 72 healthy controls) subjects, and they were able to demonstrate significant group-specific differences between COPD individuals and healthy controls. In conclusion, the few studies performed do support a genetic basis for the development of AHR in COPD, but the precise role of genetic determinants in this condition is still uncertain.
Conclusions
AHR may be envisaged as an important component of COPD. In the clinical setting, the importance of assessing AHR in COPD individuals is still underestimated, mainly because physicians tend to avoid bronchoprovocation challenges in individuals with COPD or with low lung function. In cases of suspected COPD, specific determinants of the dose-response curve might provide additional information to the diagnostic approach. In the context of COPD, AHR has been demonstrated to be a predictor of mortality; thus, reduction in the severity of AHR by smoking cessation strategies could potentially influence the natural history of COPD. Understanding of the pathophysiology of AHR in COPD may contribute to better characterization of patients who might benefit from specific treatments.
Footnotes
References
- [ATS] American Thoracic Society. Guidelines for methacholine and exercise challenge testing-1999. Am J Respir Crit Care Med. 2000;161:309–29. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- Auffarth B, Postma DS, de Monchy JG, et al. Effects of inhaled budesonide on spirometric values, reversibility, airway responsiveness, and cough threshold in smokers with chronic obstructive lung disease. Thorax. 1991;46:372–7. doi: 10.1136/thx.46.5.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahous J, Cartier A, Pineau L, et al. Pulmonary function tests and airway responsiveness to methacholine in chronic bronchiectasis of the adult. Bull Eur Physiopathol Respir. 1984;20:375–80. [PubMed] [Google Scholar]
- Bakke PS, Baste V, Gulsvik A. Bronchial responsiveness in a Norwegian community. Am Rev Respir Dis. 1991;143:317–22. doi: 10.1164/ajrccm/143.2.317. [DOI] [PubMed] [Google Scholar]
- Bellia V, Battaglia S, Catalano F, et al. Aging and disability affect misdiagnosis of COPD in elderly asthmatics: The SARA study. Chest. 2003;123:1066–72. doi: 10.1378/chest.123.4.1066. [DOI] [PubMed] [Google Scholar]
- Benson MK. Bronchial responsiveness to inhaled histamine and iso-prenaline in patients with airway obstruction. Thorax. 1978;33:211–13. doi: 10.1136/thx.33.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JL. Pharmacology of airway smooth muscle in chronic obstructive pulmonary disease and in asthma. Am Rev Respir Dis. 1991;143:1177–81. doi: 10.1164/ajrccm/143.5_Pt_1.1177. [DOI] [PubMed] [Google Scholar]
- Brand PL, Postma DS, Kerstjens HA, et al. Relationship of airway hyperresponsiveness to respiratory symptoms and diurnal peak flow variation in patients with obstructive lung disease. The Dutch CNSLD Study Group. Am Rev Respir Dis. 1991;143:916–21. doi: 10.1164/ajrccm/143.5_Pt_1.916. [DOI] [PubMed] [Google Scholar]
- Brown RH, Scichilone N, Mudge B, et al. High-resolution computed tomographic evaluation of airway distensibility and the effects of lung inflation on airway caliber in healthy subjects and individuals with asthma. Am J Respir Crit Care Med. 2001;163:994–1001. doi: 10.1164/ajrccm.163.4.2007119. [DOI] [PubMed] [Google Scholar]
- Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- Cerveri I, Bruschi C, Zoia MC, et al. Distribution of bronchial non-specific reactivity in the general population. Chest. 1988;93:26–30. doi: 10.1378/chest.93.1.26. [DOI] [PubMed] [Google Scholar]
- Cheung D, Schot R, Zwinderman A, et al. Relationship between loss in parenchymal elastic recoil pressure and maximal airway narrowing in subjects with αl-antitrypsin deficiency. Am J Respir Crit Care Med. 1997;155:135–40. doi: 10.1164/ajrccm.155.1.9001302. [DOI] [PubMed] [Google Scholar]
- Colebatch HJ, Finucane KE, Smith MM. Pulmonary conductance and elastic recoil relationships in asthma and emphysema. J Appl Physiol. 1973;34:143–53. doi: 10.1152/jappl.1973.34.2.143. [DOI] [PubMed] [Google Scholar]
- Corsico A, Milanese M, Baraldo S, et al. Small airway morphology and lung function in the transition from normality to chronic airway obstruction. J Appl Physiol. 2003;95:441–7. doi: 10.1152/japplphysiol.01018.2002. discussion 435. [DOI] [PubMed] [Google Scholar]
- Cuttitta G, Cibella F, Bellia V, et al. Changes in FVC during methacoline-induced bronchoconstriction in elderly patients with asthma. Chest. 2001;119:1685–90. doi: 10.1378/chest.119.6.1685. [DOI] [PubMed] [Google Scholar]
- De Jongste JC, Mons H, Block R, et al. Increased in vitro histamine responses in human small airways smooth muscle from patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1987;135:549–53. doi: 10.1164/arrd.1987.135.3.549. [DOI] [PubMed] [Google Scholar]
- Ding D, Martin J, Macklem P. Effects of lung volume on maximal methacholine-induced bronchoconstriction in normal humans. J Appl Physiol. 1987;62:1324–30. doi: 10.1152/jappl.1987.62.3.1324. [DOI] [PubMed] [Google Scholar]
- Dow L. Predicting the impairment and accelerated decline of lung function – the role of immunoglobulin E. Clin Exp Allergy. 1998;28:1174–7. doi: 10.1046/j.1365-2222.1998.00389.x. [DOI] [PubMed] [Google Scholar]
- Engel T, Heinig JH, Madsen O, et al. A trial of inhaled budesonide on airway responsiveness in smokers with chronic bronchitis. Eur Respir J. 1989;2:935–9. [PubMed] [Google Scholar]
- Fabbri LM, Romagnoli M, Corbetta L, et al. Differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:418–24. doi: 10.1164/rccm.200203-183OC. [DOI] [PubMed] [Google Scholar]
- Fredberg J. Frozen objects: small airways, big breaths, and asthma. J Allergy Clin Immunol. 2000;106:615–24. doi: 10.1067/mai.2000.109429. [DOI] [PubMed] [Google Scholar]
- Fredberg J, Inouye D, Miller B, et al. Airway smooth muscle, tidal stretches, and dynamically determined contractile states. Am J Respir Crit Care Med. 1997;156:1752–9. doi: 10.1164/ajrccm.156.6.9611016. [DOI] [PubMed] [Google Scholar]
- Haley KJ, Sunday ME, Wiggs BR, et al. Inflammatory cell distribution within and along asthmatic airways. Am J Respir Crit Care Med. 1998;158:565–72. doi: 10.1164/ajrccm.158.2.9705036. [DOI] [PubMed] [Google Scholar]
- Hattotuwa KL, Gizycki MJ, Ansari TW, et al. The effects of inhaled fluticasone on airway inflammation in chronic obstructive pulmonary disease: a double-blind, placebo-controlled biopsy study. Am J Respir Crit Care Med. 2002;165:1592–6. doi: 10.1164/rccm.2105025. [DOI] [PubMed] [Google Scholar]
- Hegab AE, Sakamoto T, Saitoh W, et al. Polymorphisms of IL4, IL13, and ADRB2 genes in COPD. Chest. 2004;126:1832–9. doi: 10.1378/chest.126.6.1832. [DOI] [PubMed] [Google Scholar]
- Hospers JJ, Postma DS, Rijcken B, et al. Histamine airway hyper-responsiveness and mortality from chronic obstructive pulmonary disease: a cohort study. Lancet. 2000;356:1313–17. doi: 10.1016/S0140-6736(00)02815-4. [DOI] [PubMed] [Google Scholar]
- Howarth PH, Knox AJ, Amrani Y, et al. Synthetic responses in airway smooth muscle. J Allergy Clin Immunol. 2004;114:S32–50. doi: 10.1016/j.jaci.2004.04.041. [DOI] [PubMed] [Google Scholar]
- Jackson AC, Murphy MM, Rassulo J, et al. Deep breath reversal and exponential return of methacholine-induced obstruction in asthmatic and nonasthmatic subjects. J Appl Physiol. 2004;96:137–42. doi: 10.1152/japplphysiol.00504.2003. [DOI] [PubMed] [Google Scholar]
- Jeffery P. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164:S28–38. doi: 10.1164/ajrccm.164.supplement_2.2106061. [DOI] [PubMed] [Google Scholar]
- Jensen EJ, Dahl R, Steffensen F. Bronchial reactivity to cigarette smoke in smokers: repeatability, relationship to methacholine reactivity, smoking and atopy. Eur Respir J. 1998;11:670–6. [PubMed] [Google Scholar]
- Kapsali T, Permutt S, Laube B, et al. Potent bronchoprotective effect of deep inspiration and its absence in asthma. J Appl Physiol. 2000;89:711–20. doi: 10.1152/jappl.2000.89.2.711. [DOI] [PubMed] [Google Scholar]
- King GG, Moore BJ, Seow CY, et al. Time course of increased airway narrowing caused by inhibition of deep inspiration during methacholine challenge. Am J Respir Crit Care Med. 1999;160:454–7. doi: 10.1164/ajrccm.160.2.9804012. [DOI] [PubMed] [Google Scholar]
- Klein R, Salvaggio J. Nonspecificity of the bronchoconstricting effect of histamine and acety-beta-methylcholine in patients with obstructive airway disease. J Allergy. 1966;37:158–68. doi: 10.1016/0021-8707(66)90090-6. [DOI] [PubMed] [Google Scholar]
- Koh YY, Kang EK, Min YG, et al. The importance of maximal airway response to methacholine in the prediction of asthma development in patients with allergic rhinitis. Clin Exp Allergy. 2002;32:921–7. doi: 10.1046/j.1365-2222.2002.01399.x. [DOI] [PubMed] [Google Scholar]
- Lambert RK, Wiggs BR, Kuwano K, et al. Functional significance of increased airway smooth muscle in asthma and COPD. J Appl Physiol. 1993;74:2771–81. doi: 10.1152/jappl.1993.74.6.2771. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Murray CC. The global burden of disease, 1990–2020. Nat Med. 1998;4:1241–3. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343:1902–9. doi: 10.1056/NEJM200012283432601. [DOI] [PubMed] [Google Scholar]
- Macklem P. Mechanical factors determining maximum bronchoconstriction. Eur Respir J. 1989;2:516S–519S. [PubMed] [Google Scholar]
- McParland BE, Macklem PT, Pare PD. Airway wall remodeling: friend or foe? J Appl Physiol. 2003;95:426–34. doi: 10.1152/japplphysiol.00159.2003. [DOI] [PubMed] [Google Scholar]
- Meiss RA. Influence of intercellular tissue connections on airway muscle mechanics. J Appl Physiol. 1999;86:5–15. doi: 10.1152/jappl.1999.86.1.5. [DOI] [PubMed] [Google Scholar]
- Milanese M, Crimi E, Scordamaglia A, et al. On the functional consequences of bronchial basement membrane thickening. J Appl Physiol. 2001;91:1035–40. doi: 10.1152/jappl.2001.91.3.1035. [DOI] [PubMed] [Google Scholar]
- Mitsunobu F, Mifune T, Hosaki Y, et al. Enhanced production of leukotrienes by peripheral leukocytes and specific IgE antibodies in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2001;107:492–8. doi: 10.1067/mai.2001.112694. [DOI] [PubMed] [Google Scholar]
- Moreno RH, Hogg JC, Pare PD. Mechanics of airway narrowing. Am Rev Respir Dis. 1986;133:1171–80. doi: 10.1164/arrd.1986.133.6.1171. [DOI] [PubMed] [Google Scholar]
- [NHLBI/WHO] National Institutes of Health; National Heart, Lung and Blood Institute/World Health Organization. Update of the management sections. National Institutes of Health; National Heart, Lung and Blood Institute; 2001. Workshop report: Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for diagnosis, management, and prevention of chronic obstructive pulmonary disease. updated 1 Jul 2003. URL: http://www.goldcopd.com. [PubMed] [Google Scholar]
- Niimi A, Matsumoto H, Takemura M, et al. Relationship of airway wall thickness to airway sensitivity and airway reactivity in asthma. Am J Respir Crit Care Med. 2003;168:983–8. doi: 10.1164/rccm.200211-1268OC. [DOI] [PubMed] [Google Scholar]
- Oosterhoff Y, de Jong JW, Jansen MA, et al. Airway responsiveness to adenosine 5′-monophosphate in chronic obstructive pulmonary disease is determined by smoking. Am Rev Respir Dis. 1993;147:553–8. doi: 10.1164/ajrccm/147.3.553. [DOI] [PubMed] [Google Scholar]
- Opazo-Saez AM, Seow CY, Pare PD. Peripheral airway smooth muscle mechanics in obstructive airways disease. Am J Respir Crit Care Med. 2000;161:910–17. doi: 10.1164/ajrccm.161.3.9903138. [DOI] [PubMed] [Google Scholar]
- Orie NG, Slutter HJ, de Vries, et al. Chronic nonspecific respiratory diseases [in Dutch] Ned Tijdschr Geneeskd. 1961;105:2136–9. [PubMed] [Google Scholar]
- Overbeek SE, Rijnbeek PR, Vons C, et al. Effects of fluticasone propionate on methacholine dose-response curves in nonsmoking atopic asthmatics. Eur Respir J. 1996;9:2256–62. doi: 10.1183/09031936.96.09112256. [DOI] [PubMed] [Google Scholar]
- Pare PD. Airway hyperresponsiveness in asthma: geometry is not everything! Am J Respir Crit Care Med. 2003;168:913–14. doi: 10.1164/rccm.2307005. [DOI] [PubMed] [Google Scholar]
- Pellegrino R, Sterk P, Sont J, et al. Assessing the effect of deep inhalation on airway calibre: a novel approach to lung function in bronchial asthma and COPD. Eur Respir J. 1998;12:1219–27. doi: 10.1183/0903.1936.98.12051219. [DOI] [PubMed] [Google Scholar]
- Pellegrino R, Violante B, Brusasco V. Maximal bronchoconstriction in humans – relationship to deep inhalation and airway sensitivity. Am J Respir Crit Care Med. 1996;153:115–21. doi: 10.1164/ajrccm.153.1.8542103. [DOI] [PubMed] [Google Scholar]
- Postma D, Bleecker E, Amelung P, et al. Genetic susceptibility to asthma-bronchial hyperresponsiveness coinherited with a major gene for atopy. N Engl J Med. 1995;333:894–900. doi: 10.1056/NEJM199510053331402. [DOI] [PubMed] [Google Scholar]
- Postma D, Renkema T, Noordhoek J, et al. Association between nonspecific bronchial hyperreactivity and superoxide anion production by polymorphonuclear leukocytes in chronic air-flow obstruction. Am Rev Respir Dis. 1988;137:57–61. doi: 10.1164/ajrccm/137.1.57. [DOI] [PubMed] [Google Scholar]
- Postma DS, Kerstjens HA. Characteristics of airway hyperresponsiveness in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:S187–92. doi: 10.1164/ajrccm.158.supplement_2.13tac170. [DOI] [PubMed] [Google Scholar]
- Prosperini G, Rajakulasingam K, Cacciola RR, et al. Changes in sputum counts and airway hyperresponsiveness after budesonide: monitoring anti-inflammatory response on the basis of surrogate markers of airway inflammation. J Allergy Clin Immunol. 2002;110:855–61. doi: 10.1067/mai.2002.130050. [DOI] [PubMed] [Google Scholar]
- Ramsdale EH, Morris MM, Roberts RS, et al. Bronchial responsiveness to methacholine in chronic bronchitis: relationship to airflow obstruction and cold air responsiveness. Thorax. 1984;39:912–18. doi: 10.1136/thx.39.12.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsdell JW, Nachtwey FJ, Moser KM. Bronchial hyperreactivity in chronic obstructive bronchitis. Am Rev Respir Dis. 1982;126:829–32. doi: 10.1164/arrd.1982.126.5.829. [DOI] [PubMed] [Google Scholar]
- Renkema TE, Kerstjens HA, Schouten JP, et al. The importance of serum IgE for level and longitudinal change in airways hyperresponsiveness in COPD. Clin Exp Allergy. 1998;28:1210–18. doi: 10.1046/j.1365-2222.1998.00382.x. [DOI] [PubMed] [Google Scholar]
- Rijcken B, Schouten JP, Xu X, et al. Airway hyperresponsiveness to histamine associated with accelerated decline in FEV1. Am J Respir Crit Care Med. 1995;151:1377–82. doi: 10.1164/ajrccm.151.5.7735588. [DOI] [PubMed] [Google Scholar]
- Rutgers SR, Koeter GH, van der Mark TW, et al. Short-term treatment with budesonide does not improve hyperresponsiveness to adenosine 5′-monophosphate in COPD. Am J Respir Crit Care Med. 1998;157:880–6. doi: 10.1164/ajrccm.157.3.9709100. [DOI] [PubMed] [Google Scholar]
- Rutgers SR, Timens W, Tzanakis N, et al. Airway inflammation and hyperresponsiveness to adenosine 5′-monophosphate in chronic obstructive pulmonary disease. Clin Exp Allergy. 2000;30:657–62. doi: 10.1046/j.1365-2222.2000.00819.x. [DOI] [PubMed] [Google Scholar]
- Ryan G, Latimer KM, Dolovich J, et al. Bronchial responsiveness to histamine: relationship to diurnal variation of peak flow rate, improvement after bronchodilator, and airway calibre. Thorax. 1982;37:423–9. doi: 10.1136/thx.37.6.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saetta M, Turato G, Baraldo S, et al. Goblet cell hyperplasia and epithelial inflammation in peripheral airways of smokers with both symptoms of chronic bronchitis and chronic airflow limitation. Am J Respir Crit Care Med. 2000;161:1016–21. doi: 10.1164/ajrccm.161.3.9907080. [DOI] [PubMed] [Google Scholar]
- Salerno FG, Pellegrino R, Trocchio G, et al. Attenuation of induced bronchoconstriction in healthy subjects: effects of breathing depth. J Appl Physiol. 2005;98:817–21. doi: 10.1152/japplphysiol.00763.2004. [DOI] [PubMed] [Google Scholar]
- Sanchez-Toril F, Prieto L, Peris R, et al. Differences in airway responsiveness to acetaldehyde and methacholine in asthma and chronic bronchitis. Eur Respir J. 2000;15:260–5. doi: 10.1034/j.1399-3003.2000.15b07.x. [DOI] [PubMed] [Google Scholar]
- Scichilone N, Bruno A, Marchese R, et al. Association between reduced bronchodilatory effect of deep inspiration and loss of alveolar attachments. Respir Res. 2005;6:55. doi: 10.1186/1465-9921-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scichilone N, Kapsali T, Permutt S, et al. Deep inspiration-induced bronchoprotection is stronger than bronchodilation. Am J Respir Crit Care Med. 2000;162:910–16. doi: 10.1164/ajrccm.162.3.9907048. [DOI] [PubMed] [Google Scholar]
- Scichilone N, Marchese R, Catalano F, et al. The bronchodilatory effect of deep inspiration diminishes with aging. Respir Med. 2004a;98:838–43. doi: 10.1016/j.rmed.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Scichilone N, Marchese R, Catalano F, et al. Bronchodilatory effect of deep inspiration is absent in subjects with mild COPD. Chest. 2004b;125:2029–35. doi: 10.1378/chest.125.6.2029. [DOI] [PubMed] [Google Scholar]
- Scichilone N, Marchese R, Minà C, et al. Asthma severity is related to the bronchodilatory effect of deep inspiration (DI) Am J Respir Crit Care Med. 2002;167:A:181. [Google Scholar]
- Scichilone N, Messina M, Battaglia S, et al. Airway hyperresponsiveness in the elderly: prevalence and clinical implications. Eur Respir J. 2005;25:364–75. doi: 10.1183/09031936.05.00080204. [DOI] [PubMed] [Google Scholar]
- Scichilone N, Permutt S, Bellia V, et al. Inhaled corticosteroids and the beneficial effect of deep inspiration in asthma. Am J Respir Crit Care Med. 2005;172:693–9. doi: 10.1164/rccm.200407-955OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scichilone N, Permutt S, Togias A. The lack of the bronchoprotective and not the bronchodilatory ability of deep inspiration is associated with airway hyperresponsiveness. Am J Respir Crit Care Med. 2001;163:413–19. doi: 10.1164/ajrccm.163.2.2003119. [DOI] [PubMed] [Google Scholar]
- Scichilone N, Pyrgos G, Kapsali T, et al. Airways hyperresponsiveness and the effects of lung inflation. Int Arch Allergy Immunol. 2001;124:262–6. doi: 10.1159/000053728. [DOI] [PubMed] [Google Scholar]
- Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J Clin Invest. 1995;96:2393–403. doi: 10.1172/JCI118296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sont JK, Willems LN, Bel EH, et al. Clinical control and histopathologic outcome of asthma when using airway hyperresponsiveness as an additional guide to long-term treatment. The AMPUL Study Group. Am J Respir Crit Care Med. 1999;159:1043–51. doi: 10.1164/ajrccm.159.4.9806052. [DOI] [PubMed] [Google Scholar]
- Sovijarvi AR, Haahtela T, Ekroos HJ, et al. Sustained reduction in bronchial hyperresponsiveness with inhaled fluticasone propionate within three days in mild asthma: time course after onset and cessation of treatment. Thorax. 2003;58:500–4. doi: 10.1136/thorax.58.6.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterk PJ, Bel EH. Bronchial hyperresponsiveness: the need for a distinction between hypersensitivity and excessive airway narrowing. Eur Respir J. 1989;2:267–74. [PubMed] [Google Scholar]
- Sundblad BM, Larsson K. Effect of deep inhalations after a bronchial methacholine provocation in asthmatic and non-asthmatic subjects. Respir Med. 2002;96:477–81. doi: 10.1053/rmed.2002.1339. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Altose MD, Bleecker ER, et al. The lung health study: airway responsiveness to inhaled methacholine in smokers with mild to moderate airflow limitation. The Lung Health Study Research Group. Am Rev Respir Dis. 1992;145:301–10. doi: 10.1164/ajrccm/145.2_Pt_1.301. [DOI] [PubMed] [Google Scholar]
- Taylor SM, Pare PD, Armour CL, et al. Airway reactivity in chronic obstructive pulmonary disease. Failure of in vivo methacholine responsiveness to correlate with cholinergic, adrenergic, or nonadrenergic responses in vitro. Am Rev Respir Dis. 1985;132:30–5. doi: 10.1164/arrd.1985.132.1.30. [DOI] [PubMed] [Google Scholar]
- Tiddens HA, Hofhuis W, Bogaard JM, et al. Compliance, hysteresis, and collapsibility of human small airways. Am J Respir Crit Care Med. 1999;160:1110–18. doi: 10.1164/ajrccm.160.4.9709004. [DOI] [PubMed] [Google Scholar]
- van den Berge M, Kerstjens H, Meijer R, et al. Corticosteroid-induced improvement in the PC20 of adenosine monophosphate is more closely associated with reduction in airway inflammation than improvement in the PC20 of methacholine. Am J Resp Crit Care Med. 2001;164:1127–32. doi: 10.1164/ajrccm.164.7.2102135. [DOI] [PubMed] [Google Scholar]
- Verbeken E, Cauberghs M, Mertens I, et al. The senile lung: comparison with normal and emphysematous lungs. Functional aspects. Chest. 1992a;101:800–9. doi: 10.1378/chest.101.3.800. [DOI] [PubMed] [Google Scholar]
- Verbeken E, Cauberghs M, Mertens I, et al. The senile lung: comparison with normal and emphysematous lungs. Structural aspects. Chest. 1992b;101:793–9. doi: 10.1378/chest.101.3.793. [DOI] [PubMed] [Google Scholar]
- Verhoeven GT, Hegmans JP, Mulder PG, et al. Effects of fluticasone propionate in COPD patients with bronchial hyperresponsiveness. Thorax. 2002;57:694–700. doi: 10.1136/thorax.57.8.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven GT, Verbraak AF, Boerevan der Straat S, et al. Influence of lung parenchymal destruction on the different indexes of the methacholine dose-response curve in COPD patients. Chest. 2000;117:984–90. doi: 10.1378/chest.117.4.984. [DOI] [PubMed] [Google Scholar]
- Verma VK, Cockcroft DW, Dosman JA. Airway responsiveness to inhaled histamine in chronic obstructive airways disease. Chronic bronchitis vs emphysema. Chest. 1988;94:457–61. doi: 10.1378/chest.94.3.457. [DOI] [PubMed] [Google Scholar]
- Vestbo J, Hansen EF. Airway hyperresponsiveness and COPD mortality. Thorax. 2001;56(Suppl 2):ii11–14. [PMC free article] [PubMed] [Google Scholar]
- Viegi G, Matteelli G, Angino A, et al. The proportional Venn diagram of obstructive lung disease in the Italian general population. Chest. 2004;126:1093–101. doi: 10.1378/chest.126.4.1093. [DOI] [PubMed] [Google Scholar]
- Watson A, Lim TK, Joyce H, et al. Failure of inhaled corticosteroids to modify bronchoconstrictor or bronchodilator responsiveness in middle-aged smokers with mild airflow obstruction. Chest. 1992;101:350–5. doi: 10.1378/chest.101.2.350. [DOI] [PubMed] [Google Scholar]
- Weir DC, Bale GA, Bright P, et al. A double-blind placebo-controlled study of the effect of inhaled beclomethasone dipropionate for 2 years in patients with nonasthmatic chronic obstructive pulmonary disease. Clin Exp Allergy. 1999;29(Suppl 2):125–8. doi: 10.1046/j.1365-2222.1999.00021.x. [DOI] [PubMed] [Google Scholar]
- Weir DC, Burge PS. Effects of high dose inhaled beclomethasone dipropionate, 750 micrograms and 1500 micrograms twice daily, and 40 mg per day oral prednisolone on lung function, symptoms, and bronchial hyperresponsiveness in patients with non-asthmatic chronic airflow obstruction. Thorax. 1993;48:309–16. doi: 10.1136/thx.48.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemse BW, ten Hacken NH, Rutgers B, et al. Smoking cessation improves both direct and indirect airway hyperresponsiveness in COPD. Eur Respir J. 2004;24:391–6. doi: 10.1183/09031936.04.00117603. [DOI] [PubMed] [Google Scholar]
- Wise RA, Kanner RE, Lindgren P, et al. The effect of smoking intervention and an inhaled bronchodilator on airways reactivity in COPD: the Lung Health Study. Chest. 2003;124:449–58. doi: 10.1378/chest.124.2.449. [DOI] [PubMed] [Google Scholar]
- Woolcock A, Salome C, Yan K. The slope of the dose-response curve to histamine in asthmatic and normal subjects. Am Rev Respir Dis. 1984;130:71–5. doi: 10.1164/arrd.1984.130.1.71. [DOI] [PubMed] [Google Scholar]
- Yan K, Salome CM, Woolcock AJ. Prevalence and nature of bronchial hyperresponsiveness in subjects with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1985;132:25–9. doi: 10.1164/arrd.1985.132.1.25. [DOI] [PubMed] [Google Scholar]