Abstract
In this review we consider the therapeutic potential of targeting Akt for the treatment of COPD. Akt is a serine/threonine protein kinase that functions as a signaling intermediate linked to multiple signaling programs involved in survival, inflammation, and growth. Akt is closely associated with key membrane-bound receptors and represents a convergent integration point for multiple stimuli implicated in COPD pathogenesis. Persistent activation of Akt secondary to somatic mutations in regulatory oncogenes, such as PTEN, may explain why inflammation in COPD does not resolve when smoking is ceased. Akt is also implicated in the systemic manifestations of COPD such as skeletal muscle wasting and metabolic disturbances. Furthermore, targeting Akt may provide a useful means of limiting the severity and duration of disease exacerbations in COPD. As such, Akt represents a particularly attractive therapeutic target for the treatment of COPD. Interestingly, current knowledge suggests that both inhibitors and activators of Akt may be useful for treating different clinical subpopulations of COPD patients.
Keywords: Akt, COPD, inflammation, apoptosis
Chronic obstructive pulmonary disease will be the third most common cause of death worldwide by 2020 (Murray and Lopez 1996), and costs the global healthcare system tens of billions of dollars annually. For reasons that are largely unknown, COPD is only marginally responsive to all contemporary drugs, even powerful antiinflammatory glucocorticosteroids (Keatings et al 1997; Barnes 2000). COPD is diverse and encompasses emphysema, the proteolytic destruction of alveolar units; bronchitis, associated with massive goblet cell and mucous gland proliferation; and bronchiolitis, an inflammatory condition of small airways associated with fibroblast proliferation and fibrosis. The cause of most COPD is cigarette smoking, but the molecular pathogenesis of COPD is obscure. Inhaled smoke or irritants are thought to trigger alveolar macrophages and the epithelium to secrete tumor necrosis factor-alpha (TNF-α), interleukin 8 (IL-8), and chemokines such as macrophage inflammatory proteins (MIPs). These factors are chemotactic and activating factors for neutrophils, macrophages, and other inflammatory cells. Over time the lung also accumulates increased numbers of CD8+ lymphocytes, which are capable of triggering macrophage-dependent lung proteolysis. Emphysema results from destruction of alveolar units by proteases such as neutrophil elastase (NE; also a potent goblet cell secretagogue), macrophage metalloelastases like MMP-12 (Finkelstein et al 1995; Hautamaki et al 1997), and possibly also by apoptosis of alveolar wall cells.
In the small airways, fibroblast proliferation and collagen deposition cause fixed airway obstruction (Hogg et al 2004). The resulting airflow limitation is compounded in many patients by mucus hypersecretion and inflammation. Lung destruction in COPD is well correlated with the intensity of inflammation and once inflammation is established in COPD, removing the provocative stimulus through smoking cessation does not resolve disease (Turato et al 1995). Furthermore, it is unknown why COPD is associated with a very high prevalence of both viral and bacterial exacerbations (known triggers of the innate immune system, specifically macrophages and natural killer cells), prompting further damage to the lungs. It is believed that much of the deterioration that accompanies exacerbations is due to flaring of inflammation. This interpretation is supported by spikes in inflammatory markers during exacerbations measured in sputum and in breath condensates.
Although there remains much to be understood, our current understanding of molecular pathways in COPD pathogenesis implicates Akt as a central regulator. Akt, (also previously referred to as protein kinase B [PKB]), is an intracellular serine/threonine protein kinase that is activated by a broad range of cytokines (eg, TNFα) (Murao et al 2000), growth factors (eg, PDGF, GM-CSF, CTGF) (Klein et al 2000; Rauch et al 2000; Crean et al 2002), and cigarette smoke components, including nicotine (Nakayama et al 2002; West et al 2003). In particular, Akt is a major target of PI3-kinase (PI3K) dependent signaling pathways (Figure 1). On activation, Akt is recruited to membrane associated signaling complexes and activated by phosphorylation. In addition to Akt, PI3K activates multiple signaling kinases (PKC, MAPK, Btk, ILK) involved in key processes. Hence, targeting PI3K directly may be detrimental due to its pleiotropic activities.
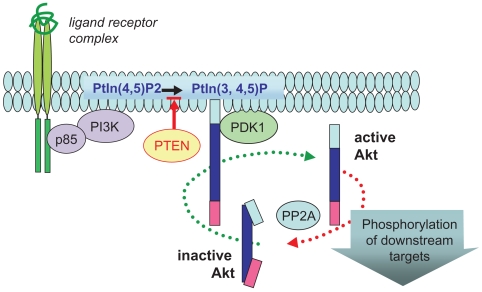
Figure 1.
Ligand-targeted activation of Akt. Ligand-mediated activation of a broad range of receptors promotes recruitment of PI3K (p85 and p110 complex) to the plasma membrane, where this lipid kinase catalyzes the production of phosphatidylinositol-3,4,5-phosphate (PtIn3,4,5)-P. PTEN (lipid phosphatase) limits this reaction by reverting PtIns(3,4,5)-P to PtIns(3,4)-P. This phospholipid acts as a docking molecule for both Akt and its activator PDK-1, which activates Akt by direct phosphorylation of the critical T(activation)-loop residue (Thr-308). Once active, Akt is released from the membrane to target multiple cellular substrates and is subsequently inactivated by protein phosphatase2A (PP2A) dephosphorylation.
There are three known homologs of Akt that display a high level of homology at the amino acid level (Table 1). The most characterized isoform, Akt1, is expressed in various tissues including lung, and targets diverse substrates involved in critical cellular events such as cell survival, proliferation, and transcription.
Table 1.
Mammalian Akt homologs
| Isoform | Homology to Akt1 | Distribution |
|---|---|---|
| Akt1 | 100% | Ubiquitous |
| Akt 2 | 90% | Prominent in heart, liver, muscle, kidney, and adipose tissue |
| Akt 3 | 83% | Prominent in brain, kidney, lung, testis |
Hence, the multifunctional activities of Akt have the potential to coordinate cellular mechanisms that are strongly implicated in the destructive pathogenesis of COPD (Figure 2). As Akt is a serine/threonine kinase it is amenable to selective pharmacological inhibition (Glazer 1998).
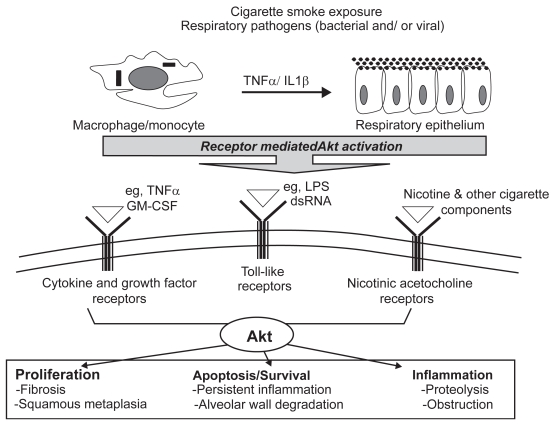
Figure 2.
Akt is a molecular mediator of cellular processes central to COPD pathogenesis. Cell surface receptors on alveolar macrophages and the bronchial epithelium recognize and respond to cigarette smoke components and respiratory pathogens. These unique signaling modules converge onto and activate multiple pathways including Akt. Once active, Akt contributes to key cellular processes including proliferation, apoptosis, and inflammation. Therefore, dysregulated activation of Akt in a chronically inflamed environment has the potential to disrupt homeostasis, leading to an altered pathology responsible for dramatic and progressive decline in lung function.
Rationale for inhibiting Akt activity in COPD
Role in cell survival
Apoptosis governs the persistence and severity of inflammation. Akt promotes inflammatory cell survival in vivo. More specifically, oxidative stress and/or GM-CSF- mediated activation of Akt enhances cell survival of macrophages, neutrophils, and T lymphocytes (Jones et al 2000; Klein et al 2000; Wang et al 2000). Akt inhibits apoptosis by four main mechanisms: (1) phosphorylation and inactivation of BAD (Datta et al 1997, 1999), which normally binds to and inactivates antiapoptotic Bcl2 and BclXL, a critical step in the activation of the caspase protease pathway. However, since Akt displays pro-survival properties in tissues that do not express detectable levels of BAD, alternative mechanisms must exist. Akt also promotes survival by: (2) phosphorylation of lytic pro-caspase-9 (Cardone et al 1998). This event blocks the activity of pro-caspase-9 by altering the conformation of its substrate-binding pocket, thereby inhibiting apoptosis. Apoptosis can also be induced by expression of key genes involved in cell death. For example, withdrawal of survival factors promotes transcription of the Fas ligand gene involved in positively regulating the caspase protease pathway. Akt controls their expression by: (3) maintaining Forkhead family of transcriptional factors (AFX) in a transcriptionally silent form (Biggs et al 1999; Brunet et al 1999). In the absence of Akt signaling, AFX members are directed to the nucleus where they bind to insulin-responsive sequence (Irs) found in a number of promoters responsible for controlling apoptosis gene expression. When Akt activity is maintained for prolonged periods, Forkhead members are sequestered away from the nucleus by Akt-mediated phosphorylation. Phosphorylated AFX members are kept transcriptionally silent due to a physical interaction with the structural cytoplasmic protein, 14-3-3. (4) More recently, Akt has been shown to promote activation of the transcription factor CREB, which enhances transcription of cell survival genes such as the Bcl-2 family member, Mcl-1 (Wang et al 1999). Therefore, in a chronically inflamed COPD lung that is constantly exposed to cigarette smoke and airborne pathogens, Akt activity will be maintained for prolonged periods. The persistence of Akt-mediated survival of inflammatory cells may prove to be an integral process responsible for the accumulation of macrophages, neutrophils, and T lymphocytes in the airways, parenchyma, and pulmonary vasculature. A paradox that cannot be explained with current knowledge is that the epithelium and T cells from COPD patients have a somewhat increased rate of apoptosis (Hodge et al 2005). Majo et al (2001) have reported enhanced parenchymal apoptosis in COPD in association with lymphocytic infiltration. The increased rate of apoptosis needs to be balanced against evidence for enhanced proliferation (Yokohori et al 2004). Tomita and colleagues (2002) found that alveolar macrophage apoptosis was decreased in macrophages from smokers in association with increased p21(Cip/Waf) BCLxL expression. Neutrophil apoptosis is markedly reduced during COPD exacerbations (Pletz et al 2004). It has also been suspected for some time that vascular endothelial cell death may be a pathogenic contributor to the development of emphysema in COPD (Kasahara et al 2000).
Role in proliferation
Akt may promote in situ macrophage and fibroblast proliferation. Macrophage proliferation in COPD has been recognized for several decades (Bitterman et al 1984) notwithstanding recent evidence suggesting increased apoptotic rates. Most in vitro evidence suggests that smoke directly suppresses fibroblast proliferation in COPD but the pattern of growth factor expression in thickened small airways suggests the opposite occurs in vivo. The in vivo response of parenchymal fibroblasts is less certain. Macrophage and fibroblast proliferation in COPD worsens inflammation and promotes airway fibrosis, which leads to fixed obstruction in small airways. Inhaled smoke and pathogen products released during exacerbations cause lung tissue damage, which triggers tissue repair mechanisms releasing multiple growth factors including PDGF, TGFβ, IGF1, EGF, CTGF, and bFGF, all of which activate Akt.
Recently, Akt has been implicated in the regulation of cellular proliferation and cell cycle control, a function more usually associated with MAP kinases. Akt inactivates the cell cycle regulator GSK3 by phosphorylation (Shaw et al 1997), promoting cell cycle entry by targeting D-type cyclins for proteolysis (Diehl et al 1998). Hence, mitogen-induced activation of cyclin D ultimately leads to the G1→S transition of the cell cycle. Furthermore, by phosphorylating and inactivating AFX transcription factor, Akt also blocks expression of the cell cycle inhibitor p27kip1, whose expression is AFX dependent (Medema et al 2000). This prevents p27kip1 from binding to the cyclinD/CDK2 complex and derepressing cell cycle arrest.
Role in inflammation
Akt is directly activated by gram-negative bacterial cell wall lipopolysaccharide (LPS) (Bozinovski et al 2002, 2004) and other innate immunity triggers implicated in COPD exacerbations (Salh et al 1998). In addition, Akt is activated by oxidative stress, in part via the EGF receptor (EGFR) (Wang et al 2000), both of which are strongly implicated in COPD (MacNee 2000). Akt selectively triggers NFκB p65/RelA heterodimer activation to coordinate monocyte and neutrophil recruitment in response to smoke and infection. The transduction pathways and inflammatory responses activated by cigarette smoke and infectious pathogen products such as LPS trigger innate immunity-mediated exacerbations in a similar manner, as inferred by coordinated expression of chemoattractant factors following activation of NFκB. This is not just because LPS is a biologically active component of cigarette smoke occurring in concentrations known to cause lung disease (Hasday et al 1999). It is also because cigarette smoke consists of multiple components capable of stimulating signal transduction pathways that are similar to those triggered by receptors of the innate immune system. Bacterial endotoxins stimulate lung epithelial cells to release neutrophil and monocyte chemotactic peptides (Koyama et al 1999, 2000) by coordinated activation of Toll-like receptors (TLRs) that respond to LPS (gram-negative cell wall component; TLR4) and lipoteichoic acid (gram-positive cell wall component; TLR2) (Brightbill et al 1999; Takeuchi et al 1999; Underhill et al 1999). Consistent with LPS, cigarette smoke extract also promotes release of chemotactic factors (Masubuchi et al 1998). Binding sites for NFκB are present in the promoter region of many inflammatory mediators including GM-CSF (Thomas et al 1997), MCP-1 (Takaya et al 2000), and IL-8 (Ozes et al 1999); and NFκB cooperates as part of a higher order complex to promote transcription. NFκB is normally sequestered in the cytoplasm through an association with inhibitor of NFκB (IκB). When cells are exposed to activation signals such as LPS, oxidative stresses, and TNFα, IκB is phosphorylated and targeted for proteolytic destruction. This process is promoted by Akt, which triggers IκB kinase alpha (IKKa) to phosphorylate IκB (Ozes et al 1999). Akt can also enhance the DNA binding activity of NFκB by targeting the transactivation domain of the p65 subunit for phosphorylation (Sizemore et al 1999; Madrid et al 2000).
Akt, lung cancer, and acquired somatic mutations of the lung epithelium
Lung cancer is the most frequent cause of tumor-associated deaths in the US and is strongly associated with chronic smoking. Lung cancer arises from a series of pathological changes linked to both gross genetic alterations and quite discrete acquired gene changes known as somatic mutations. Airflow obstruction is commonly present in patients with bronchial carcinoma (Congleton and Muers 1995), and the presence of moderate to severe airflow obstruction is a significant predictor of incident lung cancer (Mannino et al 2003). Therefore, a logical and widely accepted corollary is that chronic inflammation predisposes to the development of cancer (Marx 2004). Since the acquisition of somatic mutations predisposes to lung cancer, we have previously proposed that non-inherited genetic abnormalities also contribute to the pathogenesis of COPD (Anderson and Bozinovski 2003). As only 15% of smokers develop COPD, much recent research has focused on identifying inherited genetic risk factors such as α1-antitrypsin deficiency associated with early onset COPD. However, less then 1% of COPD patients display α1-antitrypsin deficiencies, and searches for other heritable mutations including TNFα, IL-10, MMPs, and IL-8 have yielded limited success. We have proposed the novel hypothesis that a fundamental determinant of COPD is the acquisition of mutations (caused by mutagens in cigarette smoke) in regulatory oncogenes that control Akt activation status in the epithelium. This hypothesis is supported by cytogenetic studies on the molecular pathogenesis of lung cancer that have unequivocally demonstrated very frequent loss of heterozygosity (LOH) somatic mutations in preneoplastic/dysplastic epithelium of smokers (Wistuba et al 1997; 2002).
Indeed it is now apparent that the epithelium can become a mosaic of epithelial lineages, each carrying one or more somatic mutations. Although histological abnormalities in the epithelium associated with smoking can resolve after cessation, these somatic mutations persist as much as inflammation persists in the mucosa of ex-smokers (Wistuba et al 1997). Multiple gene mutations appear necessary to develop lung cancer, but single gene loci changes may be sufficient to trigger COPD. Of the known oncogenic mutations in the epithelium, three of the most frequent directly promote Akt activation. These are: (1) oncogenic mutations of Ras (p21); a GTP bound form of Ras interacts with PI3K, that in turn activates Akt. Ras mutations occur in approximately 30% of all human malignancies with a >30% mutation frequency in the bronchial epithelium of human lung cancer patients (Scott et al 1997). Ras-transformed adherent epithelial cells display elevated levels of Akt activity, and inhibition of this activity blocks the transforming potential of Ras by inducing cell death (Khwaja et al 1997). (2) The lipid phosphatase tumor suppressor PTEN degrades phospholipid products generated by PI3K, thereby negatively regulating the activity of Akt. PTEN is mutated in a large fraction of all human cancers, with a mutation frequency approaching that of p53. More recently, PTEN mutations have been found in 6/15 (40%) of small cell lung carcinomas (SCLC) (Kohno et al 1998). Loss of PTEN activity attributed to homozygous deletions of the PTEN gene correlate with elevated levels of Akt activity, and reconstitution of PTEN restores normal Akt regulation (Stambolic et al 1998). (3) A constitutively active form of PI3K has been reported to exist in a small-scale screen of SCLC cell lines. Of the five cell lines tested, all exhibited high basal constitutive PI3K activity, which resulted in elevated basal Akt activity (Moore et al 1998).
Preneoplastic changes also occur more commonly in smokers and may manifest as a phenotypic abnormality in the bronchial epithelium known as squamous metaplasia. Here, ciliated epithelial cells that normally trap and remove airborne particulates and mucus are replaced by flat, non-ciliated cells. The consequences of this altered phenotype are believed to include airway obstruction mediated by mucus accumulation. In addition, impaired pathogen clearance has been implicated in lower airway bacterial colonization and is prominent in advanced COPD. The mechanism that drives this altered phenotype is obscure and thought to be associated with an ongoing damage/repair cycle triggered by chronic smoking; however, Akt may prove to be an important molecular regulator of this process. Conceptually, sustained activation of Akt in the bronchial epithelium as a consequence of exposure to cigarette smoke favors an ongoing proliferative process that prevents differentiation into normal epithelial cells. Consistent with this hypothesis, nicotine has been shown to modulate the phenotype of normal airway epithelial cells via an Akt dependant mechanism (Nakayama et al 2002; West et al 2003).
Rationale for selective restoration of Akt activity in COPD
Akt and apoptosis-mediated emphysema
The long-standing paradigm for alveolar septal wall destruction in emphysema involves an imbalance between proteases and antiproteases that results in digestion of elastin and other structural proteins. However, alternate mechanisms may also exist as alveolar matrix destruction is not prevalent in cystic fibrosis, acute asthma, and other lung diseases associated with excessive inflammation and proteolysis. A recent model for the loss of lung tissue in emphysema implicates enhanced apoptosis as supported by animal models and a small number of human studies. Targeting disruption of vascular endothelial growth factor (VEGF) in mice results in increased alveolar and bronchial cell apoptosis, airspace enlargement, and persistent changes in lung elastic recoil (Tuder et al 2000, 2003; Tang et al 2004). Akt plays a critical role in VEGF-mediated cell survival (Gerber et al 1998) and, hence, would most likely prevent apoptosis in VEGF depleted lungs, although this hypothesis has yet to be tested. Even though these findings are quite striking in animal models, the role of VEGF in human COPD disease has not been clearly established. Despite there being no strong evidence implicating a defect in VEGF expression in emphysema, oxidative stress has been shown to impair VEGF survival signaling via inhibition of PI3K/Akt (el-Remessy et al 2005). More recently, marked alveolar apoptosis has been observed in end-stage emphysema patients (Yokohori et al 2004; Calabrese et al 2005). Selective restoration of Akt in VEGF dependent endothelial lung cells would therefore prevent accelerated alveolar apoptosis associated with cellular destruction in emphysema.
Akt and muscle wasting in COPD
COPD elicits a persistent local inflammatory process that has quite marked peripheral consequences such as the wasting syndrome seen in advanced COPD. In COPD, even clinically obese patients have loss of lean muscle mass identified on MRI imaging. Locally, cytokines such as IL-6, TNFα, and IL-1β are released in chronically inflamed COPD lungs, which can act on multiple organ systems, tissues, and cell types. Such inflammatory cytokines are known to impair activity of anabolic hormones such as insulin growth factor-1 (IGF-1) (Spate and Schulze 2004). Akt is activated by IGF-1, an important mediator of signaling programs in skeletal muscle. For instance, increasing Akt activation has the potential to induce hypertrophic pathways while repressing atrophic processes (Bodine et al 2001; Rommel et al 2001). Akt promotes skeletal muscle hypertrophy via a number of downstream signaling pathways; direct and indirect targets include the mammalian target of rapamycin (mTOR), p70 S6 kinase (p70S6K), PHAS-1 (4EBP-1), and glycogen synthase kinase 3β (Glass 2003). Reduced IGF-1 expression is observed in most wasting syndromes (Spate and Schulze 2004), which will impact on Akt activity in muscle tissue. In rodents, selective overexpression of Akt1 in skeletal muscle cells causes hypertrophy and protects against denervation-induced atrophy, while mice with genetically disrupted Akt1 display growth defects (Chen et al 2001). Thus, the IGF-1/Akt signaling pathway provides a potential therapeutic target for promoting muscle hypertrophy in chronic diseases where wasting manifests. However, since Akt is ubiquitously expressed and promotes cell survival, proliferation, and inflammation, selective targeting of Akt in affected tissue would be most efficacious.
Summary
COPD is a heterogenous disease that is pathologically diverse, complex in nature, and is associated with defects in cell growth, survival, and inflammation. Consequently, developing an effective therapeutic strategy is difficult as multiple cellular processes need to be targeted. Dysregulation of Akt activity will impact on all these essential cellular processes, therefore implicating Akt as an attractive therapeutic target. The strategy of modulating Akt activity will develop as our understanding of COPD pathogenesis progresses, since there is evidence for either inhibiting or restoring Akt activity. The significance of Akt will become more apparent as we evolve a better understanding of the cellular processes that drive the altered phenotype that leads to reduced lung function. The challenge that lies ahead is that of generating highly specific therapies that either block or restore Akt activity in discrete tissue compartments.
References
- Anderson GP, Bozinovski S. Acquired somatic mutations in the molecular pathogenesis of COPD. Trends Pharmacol Sci. 2003;24:71–6. doi: 10.1016/S0165-6147(02)00052-4. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–80. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- Biggs WH, Meisenhelder J, Hunter T, et al. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR 1. Proc Natl Acad Sci U S A. 1999;96:7421–6. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman PB, Saltzman LE, Adelberg S, et al. Alveolar macrophage replication. One mechanism for the expansion of the mononuclear phagocyte population in the chronically inflamed lung. J Clin Invest. 1984;74:460–9. doi: 10.1172/JCI111443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–19. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Bozinovski S, Jones J, Beavitt SJ, et al. Innate immune responses to LPS in mouse lung are suppressed and reversed by neutralization of GM-CSF via repression of TLR-4. Am J Physiol Lung Cell Mol Physiol. 2004;286:L877–85. doi: 10.1152/ajplung.00275.2003. [DOI] [PubMed] [Google Scholar]
- Bozinovski S, Jones JE, Vlahos R, et al. Granulocyte/macrophage-colony-stimulating factor (GM-CSF) regulates lung innate immunity to lipopolysaccharide through Akt/Erk activation of NFkappa B and AP-1 in vivo. J Biol Chem. 2002;277:42808–14. doi: 10.1074/jbc.M207840200. [DOI] [PubMed] [Google Scholar]
- Brightbill HD, Libraty DH, Krutzik SR, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–6. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Calabrese F, Giacometti C, Beghe B, et al. Marked alveolar apoptosis/proliferation imbalance in end-stage emphysema. Respir Res. 2005;6:14. doi: 10.1186/1465-9921-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–21. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Chen WS, Xu PZ, Gottlob K, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–8. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congleton J, Muers MF. The incidence of airflow obstruction in bronchial carcinoma, its relation to breathlessness, and response to bronchodilator therapy. Respir Med. 1995;89:291–6. doi: 10.1016/0954-6111(95)90090-x. [DOI] [PubMed] [Google Scholar]
- Crean JK, Finlay D, Murphy M, et al. The role of p42/44 MAPK and protein kinase B in connective tissue growth factor induced extracellular matrix protein production, cell migration, and actin cytoskeletal rearrangement in human mesangial cells. J Biol Chem. 2002;277:44187–94. doi: 10.1074/jbc.M203715200. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, et al. Glycogen synthase kinase-3 beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Remessy AB, Bartoli M, Platt DH, et al. Oxidative stress inactivates VEGF survival signaling in retinal endothelial cells via PI 3-kinase tyrosine nitration. J Cell Sci. 2005;118:243–52. doi: 10.1242/jcs.01612. [DOI] [PubMed] [Google Scholar]
- Finkelstein R, Fraser RS, Ghezzo H, et al. Alveolar inflammation and its relation to emphysema in smokers. Am J Respir Crit Care Med. 1995;152:1666–72. doi: 10.1164/ajrccm.152.5.7582312. [DOI] [PubMed] [Google Scholar]
- Gerber HP, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–43. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- Glass DJ. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol. 2003;5:87–90. doi: 10.1038/ncb0203-87. [DOI] [PubMed] [Google Scholar]
- Glazer RI. The protein kinase ABC’s of signal transduction as targets for drug development. Curr Pharm Des. 1998;4:277–90. [PubMed] [Google Scholar]
- Hasday JD, Bascom R, Costa JJ, et al. Bacterial endotoxin is an active component of cigarette smoke. Chest. 1999;115:829–35. doi: 10.1378/chest.115.3.829. [DOI] [PubMed] [Google Scholar]
- Hautamaki RD, Kobayashi DK, Senior RM, et al. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–4. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- Hodge S, Hodge G, Holmes M, et al. Increased airway epithelial and T-cell apoptosis in COPD remains despite smoking cessation. Eur Respir J. 2005;25:447–54. doi: 10.1183/09031936.05.00077604. [DOI] [PubMed] [Google Scholar]
- Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–53. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- Jones RG, Parsons M, Bonnard M, et al. Protein kinase B regulates T lymphocyte survival, nuclear factor kappaB activation, and Bcl-X(L) levels in vivo. J Exp Med. 2000;191:1721–34. doi: 10.1084/jem.191.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara Y, Tuder RM, Taraseviciene-Stewart L, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106:1311–19. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keatings VM, Jatakanon A, Worsdell YM, et al. Effects of inhaled and oral glucocorticoids on inflammatory indices in asthma and COPD. Am J Respir Crit Care Med. 1997;155:542–8. doi: 10.1164/ajrccm.155.2.9032192. [DOI] [PubMed] [Google Scholar]
- Khwaja A, Rodriguez-Viciana P, Wennstrom S, et al. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. Embo J. 1997;16:2783–93. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JB, Rane MJ, Scherzer JA, et al. Granulocyte-macrophage colony-stimulating factor delays neutrophil constitutive apoptosis through phosphoinositide 3-kinase and extracellular signal-regulated kinase pathways. J Immunol. 2000;164:4286–91. doi: 10.4049/jimmunol.164.8.4286. [DOI] [PubMed] [Google Scholar]
- Kohno T, Takahashi M, Manda R, et al. Inactivation of the PTEN/MMAC1/TEP1 gene in human lung cancers. Genes Chromosomes Cancer. 1998;22:152–6. doi: 10.1002/(sici)1098-2264(199806)22:2<152::aid-gcc10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Koyama S, Sato E, Nomura H, et al. The potential of various lipopolysaccharides to release monocyte chemotactic activity from lung epithelial cells and fibroblasts. Eur Respir J, 1999;14:545–52. doi: 10.1034/j.1399-3003.1999.14c11.x. [DOI] [PubMed] [Google Scholar]
- Koyama S, Sato E, Nomura H, et al. The potential of various lipopolysaccharides to release IL-8 and G-CSF. Am J Physiol Lung Cell Mol Physiol. 2000;278:L658–66. doi: 10.1152/ajplung.2000.278.4.L658. [DOI] [PubMed] [Google Scholar]
- MacNee W. Oxidants/antioxidants and COPD. Chest. 2000;117:303S–17S. doi: 10.1378/chest.117.5_suppl_1.303s-a. [DOI] [PubMed] [Google Scholar]
- Madrid LV, Wang CY, Guttridge DC, et al. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappaB. Mol Cell Biol. 2000;20:1626–38. doi: 10.1128/mcb.20.5.1626-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majo J, Ghezzo H, Cosio MG. Lymphocyte population and apoptosis in the lungs of smokers and their relation to emphysema. Eur Respir J. 2001;17:946–53. doi: 10.1183/09031936.01.17509460. [DOI] [PubMed] [Google Scholar]
- Mannino DM, Aguayo SM, Petty TL, et al. Low lung function and incident lung cancer in the United States: data From the First National Health and Nutrition Examination Survey follow-up. Arch Intern Med. 2003;163:1475–80. doi: 10.1001/archinte.163.12.1475. [DOI] [PubMed] [Google Scholar]
- Marx J. Inflammation and cancer: the link grows stronger. Science. 2004;306:966–8. doi: 10.1126/science.306.5698.966. [DOI] [PubMed] [Google Scholar]
- Masubuchi T, Koyama S, Sato E, et al. Smoke extract stimulates lung epithelial cells to release neutrophil and monocyte chemotactic activity. Am J Pathol. 1998;153:1903–12. doi: 10.1016/S0002-9440(10)65704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema RH, Kops GJ, Bos JL, et al. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–7. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- Moore SM, Rintoul RC, Walker TR, et al. The presence of a constitutively active phosphoinositide 3-kinase in small cell lung cancer cells mediates anchorage-independent proliferation via a protein kinase B and p70s6k-dependent pathway. Cancer Res. 1998;58:5239–47. [PubMed] [Google Scholar]
- Murao K, Ohyama T, Imachi H, et al. TNF-alpha stimulation of MCP-1 expression is mediated by the Akt/PKB signal transduction pathway in vascular endothelial cells. Biochem Biophys Res Commun. 2000;276:791–6. doi: 10.1006/bbrc.2000.3497. [DOI] [PubMed] [Google Scholar]
- Murray CJL, Lopez AD, editors. A comprehensive assessment of mortality and disability from diseases, injuries and risk factors in 1990 and projected to 2020. Cambridge, Massachusetts: Harvard School of Public Health on behalf of the World Health Organization and the World Bank; 1996. The global burden of disease. GBD Series Volume I . [Google Scholar]
- Nakayama H, Numakawa T, Ikeuchi T, et al. Nicotine-induced phosphorylation of Akt through epidermal growth factor receptor and Src in PC12h cells. J Neurochem. 2002;83:1372–9. doi: 10.1046/j.1471-4159.2002.01248.x. [DOI] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, et al. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–5. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- Pletz MW, Ioanas M, de Roux A, et al. Reduced spontaneous apoptosis in peripheral blood neutrophils during exacerbation of COPD. Eur Respir J. 2004;23:532–7. doi: 10.1183/09031936.04.00089004. [DOI] [PubMed] [Google Scholar]
- Rauch BH, Weber A, Braun M, et al. PDGF-induced Akt phosphorylation does not activate NF-kappa B in human vascular smooth muscle cells and fibroblasts. FEBS Lett. 2000;481:3–7. doi: 10.1016/s0014-5793(00)01957-8. [DOI] [PubMed] [Google Scholar]
- Rommel C, Bodine SC, Clarke BA, et al. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–13. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- Salh B, Wagey R, Marotta A, et al. Activation of phosphatidylinositol 3-kinase, protein kinase B, and p70 S6 kinases in lipopolysaccharide-stimulated Raw 264.7 cells: differential effects of rapamycin, Ly294002, and wortmannin on nitric oxide production. J Immunol. 1998;161:6947–54. [PubMed] [Google Scholar]
- Scott FM, Modali R, Lehman TA, et al. High frequency of K-ras codon 12 mutations in bronchoalveolar lavage fluid of patients at high risk for second primary lung cancer. Clin Cancer Res. 1997;3:479–82. [PubMed] [Google Scholar]
- Shaw M, Cohen P, Alessi DR. Further evidence that the inhibition of glycogen synthase kinase-3beta by IGF-1 is mediated by PDK1/PKB-induced phosphorylation of Ser-9 and not by dephosphorylation of Tyr-216. FEBS Lett. 1997;416:307–11. doi: 10.1016/s0014-5793(97)01235-0. [DOI] [PubMed] [Google Scholar]
- Sizemore N, Leung S, Stark GR. Activation of phosphatidylinositol 3-kinase in response to interleukin- 1 leads to phosphorylation and activation of the NF-kappaB p65/RelA subunit. Mol Cell Biol. 1999;19:4798–805. doi: 10.1128/mcb.19.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spate U, Schulze PC. Proinflammatory cytokines and skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7:265–9. doi: 10.1097/00075197-200405000-00005. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Suzuki A, de la Pompa JL, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- Takaya H, Andoh A, Shimada M, et al. The expression of chemokine genes correlates with nuclear factor-kappaB activation in human pancreatic cancer cell lines. Pancreas. 2000;21:32–40. doi: 10.1097/00006676-200007000-00049. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- Tang K, Rossiter HB, Wagner PD, et al. Lung-targeted VEGF inactivation leads to an emphysema phenotype in mice. J Appl Physiol. 2004;97:1559–66. doi: 10.1152/japplphysiol.00221.2004. discussion 1549. [DOI] [PubMed] [Google Scholar]
- Thomas RS, Tymms MJ, McKinlay LH, et al. ETS1, NFkappaB and AP1 synergistically transactivate the human GM-CSF promoter. Oncogene. 1997;14:2845–55. doi: 10.1038/sj.onc.1201125. [DOI] [PubMed] [Google Scholar]
- Tomita K, Caramori G, Lim S, et al. Increased p21(CIP1/WAF1) and B cell lymphoma leukemia-x(L) expression and reduced apoptosis in alveolar macrophages from smokers. Am J Respir Crit Care Med. 2002;166:724–31. doi: 10.1164/rccm.2104010. [DOI] [PubMed] [Google Scholar]
- Tuder RM, Kasahara Y, Voelkel NF. Inhibition of vascular endothelial growth factor receptors causes emphysema in rats. Chest. 2000;117:281S. doi: 10.1016/s0012-3692(15)51033-7. [DOI] [PubMed] [Google Scholar]
- Tuder RM, Zhen L, Cho CY, Taraseviciene-Stewart L, et al. Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am J Respir Cell Mol Biol. 2003;29:88–97. doi: 10.1165/rcmb.2002-0228OC. [DOI] [PubMed] [Google Scholar]
- Turato G, Di Stefano A, Maestrelli P, et al. Effect of smoking cessation on airway inflammation in chronic bronchitis. Am J Respir Crit Care Med. 1995;152:1262–7. doi: 10.1164/ajrccm.152.4.7551380. [DOI] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A, Smith KD, et al. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci U S A. 1999;96:14459–63. doi: 10.1073/pnas.96.25.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JM, Chao JR, Chen W, et al. The antiapoptotic gene mcl-1 is up-regulated by the phosphatidylinositol 3-kinase/Akt signaling pathway through a transcription factor complex containing CREB. Mol Cell Biol. 1999;19:6195–206. doi: 10.1128/mcb.19.9.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, McCullough KD, Franke TF, et al. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem. 2000;275:14624–31. doi: 10.1074/jbc.275.19.14624. [DOI] [PubMed] [Google Scholar]
- West KA, Brognard J, Clark AS, et al. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistuba II, Lam S, Behrens C, et al. Molecular damage in the bronchial epithelium of current and former smokers. J Natl Cancer Inst. 1997;89:1366–73. doi: 10.1093/jnci/89.18.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistuba II, Mao L, Gazdar AF. Smoking molecular damage in bronchial epithelium. Oncogene. 2002;21:7298–306. doi: 10.1038/sj.onc.1205806. [DOI] [PubMed] [Google Scholar]
- Yokohori N, Aoshiba K, Nagai A. Increased levels of cell death and proliferation in alveolar wall cells in patients with pulmonary emphysema. Chest. 2004;125:626–32. doi: 10.1378/chest.125.2.626. [DOI] [PubMed] [Google Scholar]