Abstract
Therapy with bronchodilators forms the pharmacologic foundation of the treatment of patients with COPD. Bronchodilators can significantly lessen dyspnea, increase airflow, improve quality of life, and enhance exercise performance. While bronchodilators decrease airway resistance and lessen dynamic hyperinflation in patients with COPD, they have not been shown to alter the rate of decline in FEV1 over time, or improve patient survival. Fairly recently, a long-acting, once-daily anticholinergic medication, tiotropium bromide, has been developed which may improve symptom management in COPD patients. This paper reviews anticholinergic pharmacologic therapy for patients with COPD focusing on tiotropium bromide, and discusses treatment strategies based on disease stage. It is important to recognize that while bronchodilators improve symptoms, a multimodality treatment approach including respiratory and rehabilitative therapy, nutrition services, psychosocial counseling, and surgical care, is often necessary for the best possible care of patients with COPD.
Keywords: COPD, tiotropium, anticholinergic, emphysema, pharmacology
Introduction
Chronic obstructive pulmonary disease (COPD) is an enormous health threat that currently ranks as the fourth leading cause of morbidity and mortality in the US (National Heart, Lung, and Blood Institute 1998), and is the only major disease with increasing mortality (Benson and Marano 1998; Centers for Disease Control and Prevention 1999). In the year 2000, COPD accounted for over 1.5 million visits to emergency departments in the US (Mannino et al. 2002). The prevalence of COPD is also exploding internationally. By the year 2020, the World Bank and the World Health Organization project that COPD will rank fifth as a cause of worldwide disability (Murray and Lopez 1997a, 1997b, 1997c). Given the increasing patient population, there has been a great push to develop medications that will appreciably affect the care of patients with this devastating and costly disease (Croxton et al 2003).
Anticholinergic medications are a class of drugs that have long been used to improve symptomatology of patients with COPD (Gross 1988; Dollery 1991; Bauer 1992). A new inhaled anticholinergic medication, tiotropium bromide (Boehringer Ingelheim, Ingelheim, Germany), is now being used in this patient population. This medication appears to be more effective in treating patients with COPD compared with older anticholinergics. This review will discuss the pharmacology of anticholinergic bronchodilators focusing on tiotropium bromide, and discuss treatment strategies based on disease stage.
Methods
A Cochrane Database and MEDLINE search from 1966 to October 2005, and a search of the PubMed database using the terms COPD, emphysema, tiotropium, anticholinergic, and autonomic nervous system was performed. Bibliographies of identified articles were then reviewed for further references. Non-English, and non-human studies were excluded. Bibliographies of review articles were examined and original research was then systematically evaluated.
Pharmacology of the autonomic nervous system
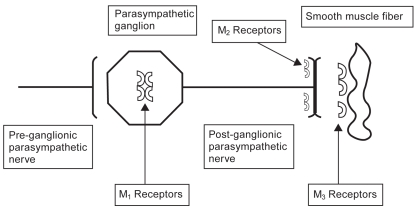
The autonomic nervous system through cholinergic nerve fibers is responsible for mediating mucus secretion and bronchial smooth muscle tone in the lung. Branches of the vagus nerve innervate muscarinic cholinergic (M) receptors in parasympathetic ganglia in the lung using acetylcholine as the primary neurotransmitter. No less than 5 muscarinic cholinergic receptor subtypes (M1–M5) have been identified in the lung. M1 and M3 mediate bronchoconstriction and mucus production. M2 is a presynaptic postganglionic autoreceptor in small airways that inhibits the M1 and M3 receptors via negative feedback mechanisms (Figure 1). Stimulation of the M2 receptor inhibits cholinergic action in the lung, so agonism of this receptor leads to inhibition of bronchoconstriction. At this time, the M4 and M5 receptors have poorly understood function in the lung (Mak et al 1993; Barnes 1993a, 1993b; Barnes et al. 1997; ZuWallack 2004).
Figure 1.
Pulmonary muscarinic cholinergic receptors. M1 and M3 receptors mediate bronchoconstriction and mucus production in the lung. M2 receptors inhibit M1 and M3 receptors via negative feedback. Ipratropium inhibits all three muscarinic receptors. Tiotropium quickly dissociates from the M2 receptor but continues to antagonize the M1 and M3 receptor. Thus, tiotropium blocks bronchoconstriction and allows inhibition of bronchoconstriction to continue. The slow dissociation of tiotropium from the M1 and M3 receptors accounts for its long half-life.
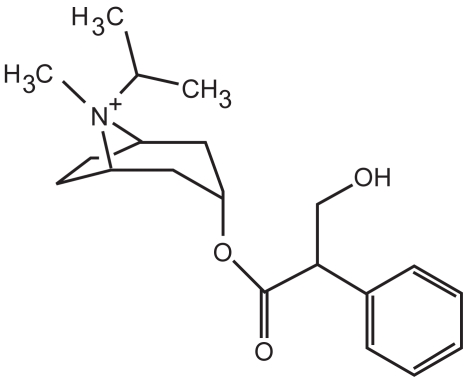
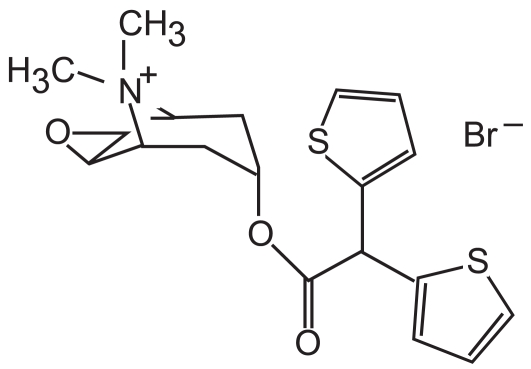
Older anticholinergic medications, such as ipratropium (Figure 2), block the M1, M2, and M3 receptors. Specifically, these medications decrease the bronchoconstrictive action of the M1 and M3 receptors, but also block the inhibitory action of the M2 receptor. Ipratropium then dissociates fairly quickly from these receptors. Thus, ipratropium is a weak bronchodilator because of its relatively short half-life compared with newer anticholinergics, and because of its inhibitory action on the M2 receptor. A new anticholinergic called tiotropium bromide (Figure 3) has been developed which also antagonizes M1, M2, and M3 receptors. However, tiotropium quickly dissociates from the M2 receptor, and thus its net effect is that of a selective M1 and M3 antagonist. Tiotropium, therefore, blocks the bronchoconstrictive action of the M1 and M3 muscarinic receptors while allowing M2 inhibition of bronchoconstriction to continue. Slow dissociation of tiotropium from the M1 and M3 receptors accounts for its long half-life.
Figure 2.
Chemical structure of ipratropium.
Figure 3.
Tiotropium bromide. Note the similarity to ipratropium (Figure 2) and the presence of the quaternary nitrogen group, which is responsible for its efficacy.
Anticholinergic medications
Ipratropium
Anticholinergic medications (Table 1) are some of the most common medications used in the treatment of COPD and have been used safely for decades (Anthonisen et al 1994). They are available for delivery by both metered dose inhaler and nebulizer. Their duration of action varies between 4 and 36 hours. Most anticholinergics, such as ipratropium, are quaternary ammonium derivatives that have difficulty crossing the blood–brain barrier and are poorly absorbed in the body (Ferguson 2000). The quaternary ammonium structure likely enhances their efficacy and minimizes the side-effects that beleaguer β-agonists. These medications have been shown to diminish the sense of dyspnea, and mucus hypersecretion via their inhibition of the cholinergic system (Ghafouri et al 1984). Anticholinergics have also been shown to improve oxyhemoglobin saturation with sleep (Martin et al 1999; McNicholas et al 2004), and provide similar, or greater, bronchodilation compared with β2-agonists (Karpel 1991; COMBIVENT Inhalation Aerosol Study Group 1994; Rennard et al 1996). Data suggest that in some patients who have COPD, ipratropium may be effective when β2-agonists are not effective (Braun and Levy 1991). Anticholinergics have a reasonable safety profile, and are not prone to receptor downregulation or tachyphylaxis (Rennard et al 1996). However, these medications may be associated with an increased number of adverse cardiovascular events in patients with COPD (Anthonisen et al 2002).
Table 1.
Anticholinergics used in the management of COPD
| Drug | Metered dose inhaler (μg) | Nebulizer (mg) | Time to peak effect (hr) | Duration (hr) |
|---|---|---|---|---|
| ipratropium | 40–80 | 0.25–0.5 | 1–2 | 4–6 |
| oxitropium | 200 | – | 1–2 | 6–7 |
| albuterol–ipratropiuma | 90/18 | – | 1–2 | 6–8 |
| tiotropium | 18 | – | 2–3 | 24–36 |
Adapted from Dollery (1991), Bauer (1992), Gross (1988), Lipson (2004).
combined β2-agonist and anticholinergic.
Combining ipratropium with long-acting β2-agonists may also have additive benefits for patients. Van Noord et al (2000) studied salmeterol compared with salmeterol plus ipratropium. The study found that both salmeterol alone or in combination with ipratropium improved bronchodilation compared with placebo, but the combination of the medications appeared to elicit a greater bronchodilator response and improvement in FEV1 than salmeterol alone. Dorinsky et al (1999) found that the combination of ipratropium and albuterol was superior to either medication alone in identifying patients with reversibility on spirometric testing of pulmonary function.
Tiotropium
Tiotropium, with its extremely long duration of action, avoids one of the main limitations of first-generation anticholinergic medications – the necessity of frequent dosing. Casaburi et al (2002) showed that once-daily tiotropium, compared with placebo, improved bronchodilation, dyspnea scores, health status scores, and decreased COPD exacerbations and hospitalizations. The primary adverse side-effect observed in the study was dry mouth. In a fairly short 12-week study in patients with COPD, tiotropium was shown to provide a higher post-dose FEV1, and higher peak and FVC, compared with salmeterol (Briggs et al 2005). In a 6-month study of tiotropium compared with salmeterol, tiotropium produced a greater degree of bronchodilation, reduction in dyspnea scores, and improvement in health-related quality of life (Donohue 2002). In a longer 1-year study, Vincken et al (2002) demonstrated diminished salbutamol use and improvement in peak expiratory flow rates. Compared with ipratropium, tiotropium appeared to reduce the number of exacerbations, increase the time to a first exacerbation, and increase the time to first hospitalization in patients with COPD. Quality of life scores were also improved. More recently, a randomized, placebo-controlled study of tiotropium in mostly male veterans with COPD showed a decrease in exacerbation rate compared with placebo. While the effect was small, it was statistically significant (Niewoehner et al 2005). At this time, it is not known whether this effect would also be seen in women.
Anticholinergics and exercise tolerance
Numerous studies have demonstrated improvements in exercise tolerance in patients with COPD with the use of anticholinergic medications (Spence et al 1993). Hay et al (1992) studied the use of oxitropium in patients with COPD. The study showed significant improvements in breathlessness and walking distance, and an increased FEV1 with its use. Improvements in walking distances and symptoms were unrelated to changes in either FEV1 or FVC, which may suggest that routine reversibility testing is not a good predictor of symptomatic benefit in patients with COPD.
Tsukino et al (1998) investigated the combined effect of theophylline with ipratropium. The study found that both ipratropium and theophylline improved exercise tolerance but combination therapy with the two medications produced greater improvements in pulmonary function and exercise capacity than either drug alone. In this study the average serum theophylline levels were around 18.3 μg/mL, which increases the risk of adverse side-effects.
Casaburi et al (2005) showed that the combination of tiotropium in combination with pulmonary rehabilitation improved treadmill endurance and reduced dyspnea scores compared with rehabilitation alone. These effects seemed to be sustained for at least 3 months after the rehabilitation course was completed. Maltais et al (2005) demonstrated that tiotropium improved symptom-limited exercise tolerance compared with placebo in COPD patients up to 8 hours following dosing.
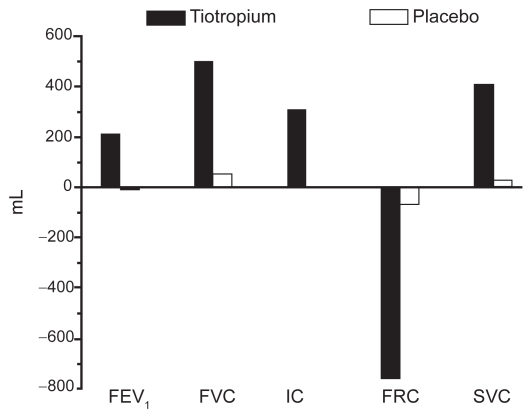
Tiotropium also appears to reduce lung hyperinflation at rest and during exertion (Celli et al 2003; Maltais et al 2005). This may be an important effect as dynamic hyperinflation likely contributes to the sense of dyspnea that often limits exercise in COPD patients (O’Donnell et al 1998; Taube et al 2000). Following 4 weeks of therapy, tiotropium clearly improves lung volumes compared with placebo (Figure 4) (Celli et al 2003).
Figure 4.
Tiotropium improves lung volumes compared with placebo. Reproduced from Celli B, ZuWallack R, Wang S, et al. 2003. Improvement in resting inspiratory capacity and hyperinflation with tiotropium in COPD patients with increased static lung volumes. Chest, 124:1743–8. Copyright © 2003, with permission from CHEST.
Abbreviations: FRC, functional residual capacity; IC, inspiratory capacity; SVC, slow vital capacity.
Recently, combination therapies using tiotropium have been shown to be beneficial in the treatment of patients with COPD. van Noord et al (2005) have demonstrated that tiotropium produces a greater improvement in daytime FEV1 than formoterol. Interestingly, night-time change in FEV1 was not different between the two medications. However, combination therapy with the two medications (with daily dosing) improved FEV1 to the greatest extent. While combination therapy appears to be a trend in caring for patients with more advanced disease, it is important to recognize that individual and combination pharmacologic medications are expensive, add significantly to the cost of healthcare, and have not been found to alter the overall decline in lung function seen in patients with COPD. Additional studies will be necessary to determine the best combination(s) of bronchodilators in the treatment of patients with COPD.
The staging of COPD
Various respiratory societies, including the American Thoracic Society (ATS) and the European Respiratory Society (ERS), have developed staging systems to help identify patients at risk for COPD and to aid in standardization of therapies and clinical trials. The ATS guidelines for staging COPD are shown in Table 2 (ATS 1995). “Stage 0” disease describes a smoker with normal spirometry who has symptoms of chronic mucus production or cough. “Stage I” or “Mild COPD” describes the patient with objective spirometric evidence of airflow obstruction. Their FEV1/FVC ratio is < 70%, and their post-bronchodilator FEV1 is ≥ 80% of predicted. They may or may not have symptoms of airflow obstruction, chronic bronchitis, or dyspnea. “Stage II” or “Moderate COPD” is characterized by typical symptoms, airflow obstruction with the FEV1/FVC ratio < 70%, and the FEV1 between 30% and 80% of predicted. It is considered “IIA” disease if the FEV1 is ≥ 50% of predicted, and “IIB” disease if the FEV1 falls between 30% and 50% of predicted. “Stage III” or “Severe COPD” is characterized by appropriate symptomatology, a FEV1/FVC ratio < 70%, and a FEV1 < 30% of predicted. Evidence of respiratory failure or cor pulmonale, with a FEV1 < 50% of predicted, is also consistent with “Severe” disease.
Table 2.
Stages of COPD
| Stage | Findings |
|---|---|
| 0: At Risk | Normal spirometry |
| Chronic cough, sputum production, dyspnea | |
| I: Mild COPD | FEV1/FVC < 70% |
| FEV1a ≥ 80% predicted | |
| May have chronic cough, sputum production, dyspnea | |
| IIA: Moderate COPD | FEV1/FVC < 70% |
| 50% ≤ FEV1< 80% predicted | |
| May have chronic cough, sputum production, dyspnea | |
| IIB: Moderate COPD | FEV1/FVC < 70% |
| 30% ≤ FEV1 < 50% predicted | |
| May have chronic cough, sputum production, dyspnea | |
| III: Severe COPD | FEV1/FVC < 70% |
| FEV1 < 30% predicted or FEV1 < 50% predicted and either respiratory failureb or cor pulmonale |
Adapted from ATS (1995).
all FEV1 values refer to post bronchodilator measurements.
PaO2 < 60 mmHg.
Treatment according to disease stage
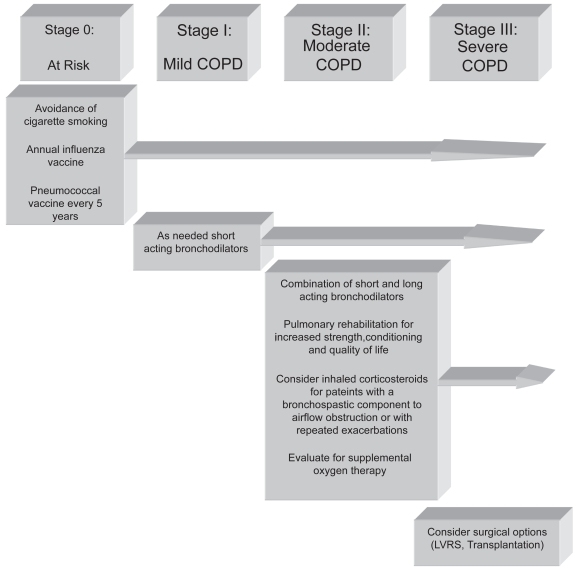
Spirometry should be used to help identify and stage patients with COPD as their treatment is often predicated upon disease stage (Figure 5) (Lipson 2004). However, following stable patients with multiple repeat spirometric tests may not be useful (Wilt et al 2005). “At risk” patients must refrain from cigarette smoking, and should obtain annual influenza vaccination and should be vaccinated with the pneumococcal vaccine every 5 years. Bronchodilator therapy on an “as needed” basis with short-acting bronchodilators may be used for managing mild COPD patients (Vathenen et al 1988).
Figure 5.
Treatment of COPD by stage. Reproduced from Lipson DA. 2004. Redefining treatment in COPD: new directions in bronchodilator therapy. Treat Respir Med, 3:89–95. Erratum in Treat Respir Med, 3:181. Copyright © 2004, with permission from Adis International Ltd.
Abbreviations: LVRS, lung volume reduction surgery.
A combination of short- and long-acting bronchodilators is often used to treat moderate emphysema. Inhaled anticholinergic medications and combinations of short- and long-acting β2-agonists are standard treatments. Inhaled corticosteroids may be useful for patients with more severe disease, or those patients with a partially reversible, bronchospastic component to airflow obstruction. These medications may also be useful in patients who have repeated exacerbations (Burge et al 2000; The Lung Health Study Research Group 2000; Sin and Tu 2001; Hattotuwa et al 2002). Medications combining an inhaled steroid and a long-acting β-agonist, such as fluticasone and salmeterol, may also be useful in this patient population (Mahler et al 2002).
Pulmonary rehabilitation is an important addition to pharmacologic therapy in patients with COPD because severe dyspnea leads to a sedentary lifestyle, subsequent deconditioning, and muscle weakness (ACCP/AACVPR Pulmonary Rehabilitation Guidelines Panel 1997). Pulmonary rehabilitation increases strength, quality of life, sense of well-being, and exercise tolerance. It is also useful in breaking the vicious cycle of progressive debilitation in patients with advanced lung disease (Fishman 1994; Celli 1997; ATS 1999).
All patients with moderate COPD should be evaluated for the need for supplemental oxygen. Patients who exhibit oxyhemoglobin desaturation at rest, or with exertion, should be prescribed supplemental oxygen to maintain oxyhemoglobin saturations greater than 90%. Long-term oxygen therapy, in COPD patients who require it, has been shown to improve survival, exercise tolerance, and quality of life (Nocturnal Oxygen Therapy Trial Group 1980; Medical Research Council Working Party 1981; Tarp and Celli 1995). A patient requires supplemental oxygen if they demonstrate an oxyhemoglobin saturation ≤ 88% or a PaO2 ≤ 55 mmHg. Additionally, an oxyhemoglobin saturation < 89% or a PaO2 < 60 mmHg also qualifies a patient for supplemental oxygen if there is evidence of cor pulmonale, a hematocrit > 56%, dependent edema, or other signs suggestive of heart failure.
The treatment of severe COPD is equivalent to that of moderate disease, except patients in this stage should also be evaluated for potential surgical treatments such as lung volume reduction surgery or lung transplantation (Cooper et al 1996; Sciurba et al 1996; Arcasoy and Kotloff 1999; Criner et al 1999; Geddes et al 2000; Flaherty et al 2001; Kotloff et al 2001; National Emphysema Treatment Trial Research Group 2001, 2003).
Conclusions
Anticholinergic medications are safe and useful adjuncts in the care of patients with COPD. While they have not been shown to alter the rate of decline in the FEV1, or alter survival, they improve exercise tolerance, dynamic hyperinflation, and breathlessness. These medications improve quality of life measures and reduce the risk of exacerbation (Barr et al 2005). Newer anticholinergics, such as tiotropium, have the added advantage of once-daily dosing and more specific cholinergic receptor targets.
Clearly, the best treatment of COPD remains smoking cessation and abstinence from smoking. Evaluation and care of the patient with COPD must include respiratory and rehabilitative therapy, nutrition services, psychosocial counseling, and evaluation for the need for long-term oxygen therapy. Physicians, and persons charged with caring for patients with COPD, await the development of novel therapies that will further improve survival, and the quality of the lives of patients with lung disease.
Footnotes
Disclosures
Grant Support: NIH K23 HL04486. The author has no conflict of interest to declare.
References
- ACCP/AACVPR Pulmonary Rehabilitation Guidelines Panel. Pulmonary rehabilitation: Joint ACCP/AACVPR evidence-based guidelines. Chest. 1997;112:1363–96. [PubMed] [Google Scholar]
- [ATS] American Thoracic Society. Statement: Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Care Med. 1995;152(Suppl):77–121. [PubMed] [Google Scholar]
- [ATS] American Thoracic Society. Official Statement: Pulmonary rehabilitation-1999. Am J Respir Crit Care Med. 1999;159:1666–82. doi: 10.1164/ajrccm.159.5.ats2-99. [DOI] [PubMed] [Google Scholar]
- Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272:1497–505. [PubMed] [Google Scholar]
- Anthonisen NR, Connett JE, Enright PL, et al. Lung Health Study Research Group. Hospitalizations and mortality in the Lung Health Study. Am J Respir Crit Care Med. 2002;166:333–9. doi: 10.1164/rccm.2110093. [DOI] [PubMed] [Google Scholar]
- Arcasoy SM, Kotloff RM. Medical progress: lung transplantation. N Engl J Med. 1999;340:1081–91. doi: 10.1056/NEJM199904083401406. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Muscarinic receptor subtypes in airways. Eur Resp J. 1993a;6:328–31. [PubMed] [Google Scholar]
- Barnes PJ. Muscarinic receptor subtypes in airways. Life Sci. 1993b;52:521–7. doi: 10.1016/0024-3205(93)90310-y. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Haddad EB, Rousell J. Regulation of muscarinic M2 receptors. Life Sci. 1997;60:1015–21. doi: 10.1016/s0024-3205(97)00042-8. [DOI] [PubMed] [Google Scholar]
- Barr RG, Bourbeau J, Camargo CA, et al. Tiotropium for stable chronic obstructive pulmonary disease. The Cochrane Database of Systematic Reviews. 2005;2 doi: 10.1002/14651858.CD002876.pub2.. Art. No. CD002876.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R. The pharmacology of oxitropium bromide. Rev Contemp Pharmacother. 1992;3:197–203. [Google Scholar]
- Benson V, Marano MA. Current estimates from the National Health Interview Survey, 1995. Vital Health Stat 10. 1998;199:1–428. [PubMed] [Google Scholar]
- Braun SR, Levy SF. Comparison of ipratropium bromide and albuterol in chronic obstructive pulmonary disease: a three-center study. Am J Med. 1991;91:S28–32. doi: 10.1016/0002-9343(91)90259-z. [DOI] [PubMed] [Google Scholar]
- Briggs DD, Jr, Covelli H, Lapidus R, et al. Improved daytime spirometric efficacy of tiotropium compared with salmeterol in patients with COPD. Pulm Pharmacol Ther. 2005;18:397–404. doi: 10.1016/j.pupt.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Burge PS, Calverley PM, Jones PW, et al. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320:1297–303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217–24. doi: 10.1183/09031936.02.00269802. [DOI] [PubMed] [Google Scholar]
- Casaburi R, Kukafka D, Cooper CB, et al. Improvement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPD. Chest. 2005;127:809–17. doi: 10.1378/chest.127.3.809. [DOI] [PubMed] [Google Scholar]
- Celli B. Is pulmonary rehabilitation an effective treatment for chronic obstructive pulmonary disease? Yes. Am J Respir Crit Care Med. 1997;155:781–3. doi: 10.1164/ajrccm.155.3.9117007. [DOI] [PubMed] [Google Scholar]
- Celli B, ZuWallack R, Wang S, et al. Improvement in resting inspiratory capacity and hyperinflation with tiotropium in COPD patients with increased static lung volumes. Chest. 2003;124:1743–8. doi: 10.1378/chest.124.5.1743. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Achievements in Public Health, 1900–1999: decline in deaths from heart disease and stroke – United States, 1900–1999. MMWR CDC Surveill Summ. 1999;48:649–56. [PubMed] [Google Scholar]
- COMBIVENT Inhalation Aerosol Study Group. In chronic obstructive pulmonary disease, a combination of ipratropium and albuterol is more effective than either agent alone. An 85-day multicenter trial. Chest. 1994;105:1411–19. doi: 10.1378/chest.105.5.1411. [DOI] [PubMed] [Google Scholar]
- Cooper JD, Patterson GA, Sanderson RS, et al. Results of 150 consecutive bilateral lung volume reduction procedures in patients with severe emphysema. J Thorac Cardiovasc Surg. 1996;112:1319–30. doi: 10.1016/S0022-5223(96)70147-2. [DOI] [PubMed] [Google Scholar]
- Criner GJ, Cordova FC, Furukawa S, et al. Prospective randomized trial comparing bilateral lung volume reduction surgery to pulmonary rehabilitation in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:2018–27. doi: 10.1164/ajrccm.160.6.9902117. [DOI] [PubMed] [Google Scholar]
- Croxton TL, Weinmann GG, Senior RM, et al. Clinical research in chronic obstructive pulmonary disease. needs and opportunities. Am J Respir Crit Care Med. 2003;167:1142–9. doi: 10.1164/rccm.200207-756WS. [DOI] [PubMed] [Google Scholar]
- Dollery C. Oxitropium bromide. In: Dollery C, editor. Therapeutic drugs . Edinburgh; Livingstone: 1991. pp. 168–70. [Google Scholar]
- Donohue JF, van Noord JA, Bateman ED, et al. A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest. 2002;122:47–55. doi: 10.1378/chest.122.1.47. [DOI] [PubMed] [Google Scholar]
- Dorinsky PM, Reisner C, Ferguson GT, et al. The combination of ipratropium and albuterol optimizes pulmonary function reversibility testing in patients with COPD. Chest. 1999;115:966–71. doi: 10.1378/chest.115.4.966. [DOI] [PubMed] [Google Scholar]
- Ferguson GT. Update on pharmacologic therapy for chronic obstructive pulmonary disease. Clin Chest Med. 2000;21:723–38. doi: 10.1016/s0272-5231(05)70180-5. [DOI] [PubMed] [Google Scholar]
- Fishman AP. Pulmonary rehabilitation research: NIH workshop summary. Am J Respir Crit Care Med. 1994;149:825–33. doi: 10.1164/ajrccm.149.3.8118655. [DOI] [PubMed] [Google Scholar]
- Flaherty KR, Cameroon EA, Curtis JL, et al. Short-term and long-term outcomes after bilateral lung volume reduction surgery: prediction by quantitative CT. Chest. 2001;119:1337–46. doi: 10.1378/chest.119.5.1337. [DOI] [PubMed] [Google Scholar]
- Geddes D, Davies M, Koyama H, et al. Effect of lung-volume-reduction surgery in patients with severe emphysema. N Engl J Med. 2000;343:239–45. doi: 10.1056/NEJM200007273430402. [DOI] [PubMed] [Google Scholar]
- Ghafouri MA, Patil KD, Kass I. Sputum changes associated with the use of ipratropium bromide. Chest. 1984;86:387–93. doi: 10.1378/chest.86.3.387. [DOI] [PubMed] [Google Scholar]
- Gross NJ. Ipratropium bromide. N Engl J Med. 1988;319:486–94. doi: 10.1056/NEJM198808253190806. [DOI] [PubMed] [Google Scholar]
- Hattotuwa KL, Gizycki MJ, Ansari TW, et al. The effects of inhaled fluticasone on airway inflammation in chronic obstructive pulmonary disease: a double-blind, placebo-controlled biopsy study. Am J Respir Crit Care Med. 2002;165:1592–6. doi: 10.1164/rccm.2105025. [DOI] [PubMed] [Google Scholar]
- Hay JG, Stone P, Carter J, et al. Bronchodilator reversibility, exercise performance and breathlessness in stable chronic obstructive pulmonary disease. Eur Respir J. 1992;5:659–64. [PubMed] [Google Scholar]
- Karpel JP. Bronchodilator responses to anticholinergic and beta-adrenergic agents in acute and stable COPD. Chest. 1991;99:871–6. doi: 10.1378/chest.99.4.871. [DOI] [PubMed] [Google Scholar]
- Kotloff RM, Hansen-Flaschen J, Lipson DA, et al. Apical perfusion fraction as a predictor of short term functional outcome following bilateral lung volume reduction surgery. Chest. 2001;120:1609–15. doi: 10.1378/chest.120.5.1609. [DOI] [PubMed] [Google Scholar]
- Lipson DA. Redefining treatment in COPD: new directions in bronchodilator therapy. Treat Respir Med. 2004;3:89–95. doi: 10.2165/00151829-200403020-00003. Erratum in Treat Respir Med, 3:181. [DOI] [PubMed] [Google Scholar]
- The Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343:1902–9. doi: 10.1056/NEJM200012283432601. [DOI] [PubMed] [Google Scholar]
- Mahler DA, Wire P, Horstman D, et al. Effectiveness of fluticasone propionate and salmeterol combination delivered via the diskus device in the treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:1084–91. doi: 10.1164/rccm.2112055. [DOI] [PubMed] [Google Scholar]
- Mak JC, Haddad EB, Buckley NJ, et al. Visualization of muscarinic M4 mRNA and M4 receptor subtype in rabbit lung. Life Sci. 1993;53:1501–8. doi: 10.1016/0024-3205(93)90624-c. [DOI] [PubMed] [Google Scholar]
- Maltais F, Hamilton A, Marciniuk D, et al. Improvements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPD. Chest. 2005;128:1168–78. doi: 10.1378/chest.128.3.1168. [DOI] [PubMed] [Google Scholar]
- Mannino DM, Homa DM, Akinbami LJ, et al. Chronic obstructive pulmonary disease surveillance – United States, 1971–2000. MMWR Surveill Summ. 2002;51:1–16. [PubMed] [Google Scholar]
- Martin RJ, Bartelson BL, Smith P, et al. Effect of ipratropium bromide treatment on oxygen saturation and sleep quality in COPD. Chest. 1999;115:1338–45. doi: 10.1378/chest.115.5.1338. [DOI] [PubMed] [Google Scholar]
- McNicholas WT, Calverley PMA, Lee A, et al. Long-acting inhaled anticholinergic therapy improves sleeping oxygen saturation in COPD. Eur Resp J. 2004;23:825–31. doi: 10.1183/09031936.04.00085804. [DOI] [PubMed] [Google Scholar]
- Medical Research Council Working Party. Long-term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet. 1981;1:681–6. [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Regional patterns of disability-free life expectancy and disability-adjusted life expectancy: Global Burden of Disease Study. Lancet. 1997a;349:1347–52. doi: 10.1016/S0140-6736(96)07494-6. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997b;349:1436–42. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997c;349:1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- National Emphysema Treatment Trial Research Group. Patients at high risk of mortality from lung volume reduction surgery. N Engl J Med. 2001;345:1075–83. doi: 10.1056/NEJMoa11798. [DOI] [PubMed] [Google Scholar]
- National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume–reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–73. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute. Morbidity and mortality: Chartbook on cardiovascular, lung, and blood diseases. Bethesda, MD: US Department of Health and Human Services, Public Health Service, NIH; 1998. [Google Scholar]
- Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease. Ann Int Med. 1980;93:391–8. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- Niewoehner DE, Rice K, Cote C, et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator. A randomized trial. Ann Int Med. 2005;143:317–26. doi: 10.7326/0003-4819-143-5-200509060-00007. [DOI] [PubMed] [Google Scholar]
- O’Donnell DE, Lam M, Webb KA. Measurements of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:1557–65. doi: 10.1164/ajrccm.158.5.9804004. [DOI] [PubMed] [Google Scholar]
- Rennard SI, Serby CW, Ghafouri, et al. Extended therapy with ipratropium is associated with improved lung function in patients with COPD. A retrospective analysis of data from seven clinical trials. Chest. 1996;110:62–70. doi: 10.1378/chest.110.1.62. [DOI] [PubMed] [Google Scholar]
- Sciurba FC, Rogers RM, Keenan RJ, et al. Improvement in pulmonary function and elastic recoil after lung-reduction surgery for diffuse emphysema. N Engl J Med. 1996;334:1095–9. doi: 10.1056/NEJM199604253341704. [DOI] [PubMed] [Google Scholar]
- Sin DD, Tu JV. Inhaled corticosteroids and the risk of mortality and readmission in elderly patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:580–4. doi: 10.1164/ajrccm.164.4.2009033. [DOI] [PubMed] [Google Scholar]
- Spence DP, Hay JG, Carter J, et al. Oxygen desaturation and breathlessness during corridor walking in chronic obstructive pulmonary disease: effect of oxitropium bromide. Thorax. 1993;48:1145–50. doi: 10.1136/thx.48.11.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarp SP, Celli BR. Long-term oxygen therapy. N Engl J Med. 1995;333:710–14. doi: 10.1056/NEJM199509143331107. [DOI] [PubMed] [Google Scholar]
- Taube C, Lehnigk B, Paasch K, et al. Factor analysis of changes in dyspnea and lung function parameters after bronchodilation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:216–20. doi: 10.1164/ajrccm.162.1.9909054. [DOI] [PubMed] [Google Scholar]
- Tsukino M, Nishimura K, Ikeda A, et al. Effects of theophylline and ipratropium bromide on exercise performance in patients with stable chronic obstructive pulmonary disease. Thorax. 1998;53:269–73. doi: 10.1136/thx.53.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vathenen AS, Britton JR, Ebden P, et al. High-dose inhaled albuterol in severe chronic airflow limitation. Am Rev Respir Dis. 1988;138:850–5. doi: 10.1164/ajrccm/138.4.850. [DOI] [PubMed] [Google Scholar]
- van Noord JA, de Munck DR, Bantje TA, et al. Long-term treatment of chronic obstructive pulmonary disease with salmeterol and the additive effect of ipratropium. Eur Respir J. 2000;15:878–85. doi: 10.1034/j.1399-3003.2000.15e11.x. [DOI] [PubMed] [Google Scholar]
- van Noord JA, Aumann J-L, Janssens E, et al. Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPD. Eur Resp J. 2005;26:214–22. doi: 10.1183/09031936.05.00140404. [DOI] [PubMed] [Google Scholar]
- Vincken W, van Noord JA, Greefhorst AP, et al. Dutch/Belgian Tiotropium Study Group. Improved health outcomes in patients with COPD during 1 year’s treatment with tiotropium. Eur Respir J. 2002;19:209–16. doi: 10.1183/09031936.02.00238702. [DOI] [PubMed] [Google Scholar]
- Wilt TJ, Niewoehner D, Kim C, et al. Evidence Report/Technology Assessment, nr 121:1–7. Rockville, MD, USA: Agency for Healthcare Research and Quality; 2005. Use of spirometry for case finding, diagnosis, and management of chronic obstructive pulmonary disease (COPD) AHRQ Publication No 05-E017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZuWallack AR, ZuWallack RL. Tiotropium bromide, a new once-daily inhaled anticholinergic bronchodilator for chronic-obstructive pulmonary disease. Expert Opin Pharmacother. 2004;5:1827–35. doi: 10.1517/14656566.5.8.1827. [DOI] [PubMed] [Google Scholar]