Abstract
COPD is associated with an increased load on the diaphragm. Since chronic muscle loading results in changes in antioxidant capacity and formation of reactive oxygen and reactive nitrogen species, we hypothesized that COPD has a similar effect on the diaphragm, which is related to the severity of COPD. Catalase activity was determined spectrophotometrically. Levels of 4-hydroxy-2-nonenal (HNE)-protein adducts and 3-nitrotyrosine (NT) formation were measured using western blotting. Levels of malondialdehyde (MDA) were assessed by high-performance liquid chromatography. We found that catalase activity was ~89% higher in the diaphragm of severe COPD patients (FEV1 37 ± 5% predicted) compared with non-COPD patients. MDA levels, a marker for lipid peroxidation, were significantly lower in the diaphragm of COPD patients compared with non-COPD patients, whereas the level of HNE-protein adducts was equal in both groups. NT formation was not different between groups. However, increasing hyperinflation and NT formation were inversely correlated. These results indicate that in COPD the diaphragm adapts to a higher work load by increasing catalase activity, resulting in a reduction in oxidative damage to lipids and tyrosine nitration of proteins.
Keywords: COPD, respiratory muscles, oxidants, NO, antioxidants
Introduction
In patients with COPD, the diaphragm is exposed to a chronically increased work load, due to several factors including airflow obstruction and geometrical changes of the thorax derived from pulmonary hyperinflation. As a result, several adaptations occur in diaphragm muscle, eg, a shift towards type I fibers, an increase in oxidative enzyme activity (Levine et al 2002), loss of fiber myosin heavy chain content, and reduced in vitro force generation (Ottenheijm et al 2005).
In rat diaphragm, in vitro studies have shown that the production of reactive oxygen species (ROS) is enhanced with increased contractile activity (Reid, Haack, et al 1992; Reid, Shoji, et al 1992; Kolbeck et al 1997). ROS are required for optimal contractile function (Reid, Haack, et al 1992), but overproduction of ROS may result in a disturbance between the pro-oxidant and antioxidant balance in favor of the former. The resulting so-called oxidative stress is associated with impaired force generation of skeletal and respiratory muscle in animal models (Kolbeck et al 1997; Reid, Shoji, et al 1992). In line with these observations, in vitro force generation of the emphysematous hamster diaphragm is inversely correlated with glutathione oxidation in the diaphragm (Heunks et al 2000).
Oxidative stress has been implicated in the involvement in muscle dysfunction of the quadriceps of COPD patients (Barreiro et al 2003). When a muscle is chronically exposed to oxidants, which can be hypothesized to occur in the diaphragm of patients with COPD, it may be expected that this chronic exposure provokes an increase in antioxidant defense. Whether this occurs in the diaphragm of patients with COPD is unknown. However, exercise training in healthy subjects, a condition known to enhance skeletal muscle ROS generation, has been shown to upregulate skeletal muscle antioxidant enzymes including superoxide dismutase (Zidoni and Kremer 1974).
In addition to ROS, nitric oxide (NO) has been shown to modulate muscle performance (for a review, see Reid 2001). NO is produced in skeletal muscle fibers by NO synthases (NOS). The main source of NO synthesis is the neuronal (nNOS) isoform, which is localized in close proximity to the sarcolemma (Kobzik et al 1994). There is increasing evidence that NO plays a role in regulating muscle glucose uptake (Roy et al 1998), sarcoplasmic Ca2+ release (Suko et al 1999), and blood flow (Hickner et al 1997). Excessive NO production inside skeletal muscle fibers, however, exerts deleterious effects on sarcolemmal integrity and contractile function (Stamler and Meissner 2001). NO can react with the superoxide anion (·O2−) to form the highly reactive oxidant peroxynitrite (·ONOO) (for a review see Beckman and Koppenol 1996). Peroxynitrite modifies protein tyrosine residues to create 3-nitrotyrosine (NT).
Recently, Barreiro et al (2005) observed increased oxidative stress as measured by 4-hydroxy-2-nonenal (HNE) levels and protein carbonyl groups, but unchanged 3-NT levels in the diaphragm of moderate and severe COPD patients. In contrast, in a pilot report (Wijnhoven et al 2004) we showed that NT levels were higher in the diaphragm of COPD patients, whereas the level of HNE was not changed. The present study extended the data of this pilot study. Specifically, we hypothesized that in the diaphragm of COPD patients: (1) The activity of antioxidant enzymes is increased; (2) Indirect markers for ROS production and reactive nitrogen species (RNS) production are elevated; and (3) These changes are related to the severity of hyperinflation. To test these hypotheses, we determined catalase activity as a marker for antioxidative capacity, levels of lipid peroxidation as a marker for oxidative stress, and NT formation as a marker for RNS production.
Methods
Subjects
Diaphragm muscle biopsies were obtained from subjects undergoing thoracotomy for lung cancer or lung volume reduction surgery. These patients were divided into 3 groups: non-COPD (n = 15; mean FEV1 = 110 ± 3 % predicted), mild-to-moderate COPD (n = 17; mean FEV1 = 67 ± 3% predicted). and severe COPD (n = 7; mean FEV1 = 37 ± 5% predicted). General characteristics and pulmonary function data are shown in Table 1. Informed consent was obtained from each of the subjects, and the study was approved by the ethical committee of our hospital.
Table 1.
Patient characteristics
| Mild-to-moderate | |||
|---|---|---|---|
| Non-COPD (n = 15) | COPD (n = 17) | Severe COPD (n = 7) | |
| Age (years) | 58 ± 3 | 63 ±V2 | 59 ± 4 |
| BMI (kg/m2) | 25 ± 1 | 26 ± 1 | 23 ± 1 |
| FEV1 | 3.30 ± 0.13 | 1.91 ± 0.09 aa | 1.12 ± 0.19 aabb |
| FEV1 % predicted | 110 ± 3 | 67 ± 3 aa | 37 ± 5 aabb |
| FEV1/VC | 78 ± 1 | 53 ± 2 aa | 33 ± 5 aabb |
| TLC % predicted | 103 ± 3 | 90 ± 5 | 114 ± 8 b |
| FRC % predicted | 98 ± 19 | 93 ± 6 | 130 ± 15 b |
| KCO % predicted | 95 ± 6 | 76 ± 4 a | 48 ± 8 aab |
Values are means ± SEM.
p < 0.05 and
p < 0.001 vs non-COPD patients.
p < 0.05 and
p < 0.001 between mild-to-moderate COPD and severe COPD patients.
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in 1 second; VC, vital capacity; TLC, total lung capacity; KCO, diffusion coefficient for carbon monoxide; FRC, functional residual capacity.
Biopsy handling
Biopsies were immediately cut into 2 pieces. One part was directly frozen in liquid nitrogen for biochemical determinations. The remaining part was directly frozen in isopentane precooled by liquid nitrogen for immunohistochemistry. Both samples were stored at −80°C until further analysis. Due to limitation of the biopsy size, all the biochemical determinations could not be performed in each patient.
Catalase activity determination
Fresh frozen biopsies were thawed in ice-cooled buffer containing 250 mM sucrose, 2 mM EDTA, and 10 mM Tris-HCl (pH 7.4). In this buffer muscle homogenates (5% wt/vol) were prepared using a Potter-Elvehjem glass-teflon homogenizer. The catalase activity was measured using the method of Aebi (1984). Briefly, the above-mentioned homogenates were used, and H2O2 decomposition was measured spectrophotometrically. The difference in absorbance (ΔA240) per minute was a measure of the catalase activity. The assay was performed in duplicate.
Immunoblotting
NT formation was detected using western immunoblotting with a monoclonal anti-NT antibody (clone 1A6; Upstate Biotechnology Inc, Lake Placid, NY, USA), as published previously (Zhu et al 2005). Briefly, crude muscle homogenate proteins (10 μg) were separated by electrophoresis (100 V for 1 hour) on 10% sodium dodecylsulfate polyacrylamide gels. Proteins were transferred electrophoretically (100 V, 300 mA for 1.5 hours) to nitrocellulose membranes. The nitrocellulose membranes were subsequently incubated with primary monoclonal antibodies against NT. After a washing step, the nitrocellulose membranes were incubated with a secondary antibody (polyclonal anti-mouse IgG horseradish peroxidase conjugated; Pierce, Rockford, IL, USA). After a second washing step, protein bands were visualized using an enhanced chemiluminescence detection kit (Amersham Biosciences Europe GmbH, The Netherlands). The blots were scanned with an imaging densitometer and optical densities (OD) of positive NT protein bands were quantified with GeneTools software (Syngene, UK). Total NT OD was calculated for each sample by adding OD of individual positive protein bands.
HNE was detected with the same method as NT, with a few alterations. The primary antibody was a polyclonal rabbit antibody against 4-hydroxy-2-nonenal (Calbiochem, San Diego, CA, USA). The secondary antibody was a polyclonal anti-rabbit IgG antibody (polyclonal anti-rabbit IgG horseradish peroxidase conjugated; Pierce, Rockford, IL, USA).
Immunohistochemistry
A polyclonal rabbit antibody against 4-hydroxy-2-nonenal used (Calbiochem, San Diego, CA, USA). NT was detected with a polyclonal rabbit anti-NT antibody (Upstate Biotechnology Inc., Lake Placid, NY, USA). Tissue sections (5 μm) were incubated with the antibodies in 0.5% (w/v) BSA in PBS with 0.05% Tween-20 (BSA/PBST) for 60 minutes. Bound antibodies were detected using Alexa-labelled (488) anti-rabbit antibodies. Finally, tissue sections were fixed in methanol, air-dried, and embedded in mowiol (10% [w/v] in 0.1 M Tris-HCl, pH 8.5, 25% [v/v] glycerol), and 2.5% [w/v] NaN3). As a control, primary antibodies were omitted.
Malondialdehyde determination
Malondialdehyde (MDA) levels were assessed by high-performance liquid chromatography, as previously reported (Wong et al 1987; Zhu et al 2005). Muscle samples (40 mg) were homogenized and centrifuged, and the supernatants were collected and stored on ice. Samples were hydrolyzed by boiling in diluted phosphoric acid. MDA, one of the low-molecular-weight end products formed via the decomposition of lipid-peroxidation products, was reacted with thiobarbituric acid (TBA) to form MDA-TBA adducts (Janero 1990). The adduct was eluted from the column with methanol-phosphate buffer and quantified spectrophotometrically at 532 nm.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM) when appropriate. Data were analyzed with SPSS for Windows®, version 12.0.1. A oneway ANOVA test was used to compare results between groups. To establish the relationship between the oxidative stress parameters and lung function, Pearson correlation was determined. Significance was set at the 0.05 level.
Results
Patient characteristics
Patient characteristics are shown in Table 1. The patients with COPD did not differ significantly from the non-COPD patients with respect to age and body mass index (BMI).
Catalase activity
Diaphragm muscle catalase activity was significantly higher in severe COPD patients (n = 3) compared with both non-COPD patients (n = 5) and with patients with mild-to-moderate COPD (n = 5) (Figure 1).
Figure 1.
Catalase activity (mean ± SEM) in diaphragms of non-COPD patients and both mild-to-moderate and severe COPD patients. Catalase activity was significantly elevated in the diaphragm of severe COPD patients.
ap < 0.05 vs non-COPD patients; bp < 0.05 vs mild-to-moderate COPD patients.
NT formation
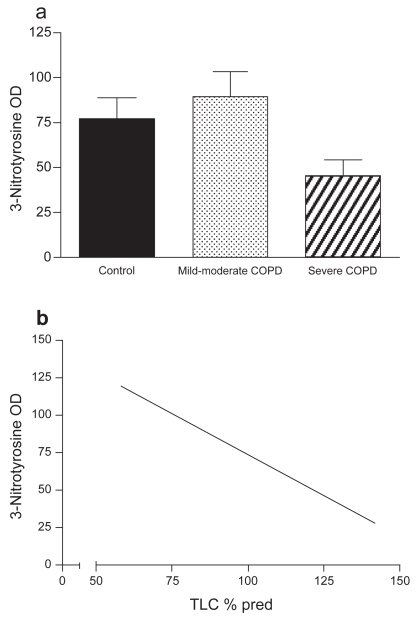
Diaphragm muscle NT level was assessed as a marker for peroxynitrite formation. As illustrated in Figure 2, no difference was found in muscle protein nitration between the groups (non-COPD: n = 7, mild-to-moderate COPD: n = 6, severe COPD: n = 3). Among all COPD patients, significant negative correlations were found between muscle protein nitration and total lung capacity (TLC) (r = −0.797, p = 0.018), TLC% predicted (r = −0.85, p = 0.007), and FRC% predicted (r = −0.85, p = 0.008). Immunofluorescence analysis showed positive staining for NT localized within the muscle fibers in both COPD patients and non-COPD patients, with some fibers staining more intensely than other fibers (Figure 3).
Figure 2.
(a) 3-Nitrotyrosine (NT) optical density (OD) (mean ± SEM) in diaphragms of non-COPD patients and both mild-to-moderate and severe COPD patients. NT levels were not different between the three groups.
(b) Relationship between total lung capacity (TLC) % predicted and NT OD in all COPD patients (r = −0.853, p = 0.007).
Figure 3.
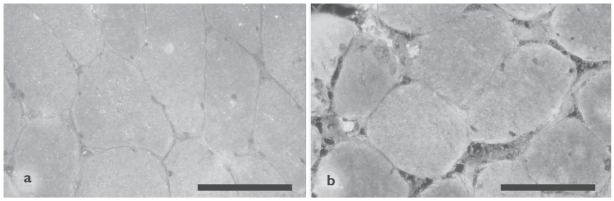
Immunofluorescence analysis of 3-nitrotyrosine localization in diaphragm muscle of 1 representative non-COPD patient (a) and 1 representative severe COPD patient (b). The staining is localized diffusely within the muscle fibers, with some fibers staining more intensely than other fibers. Scale bar = 100 μm.
Lipid peroxidation
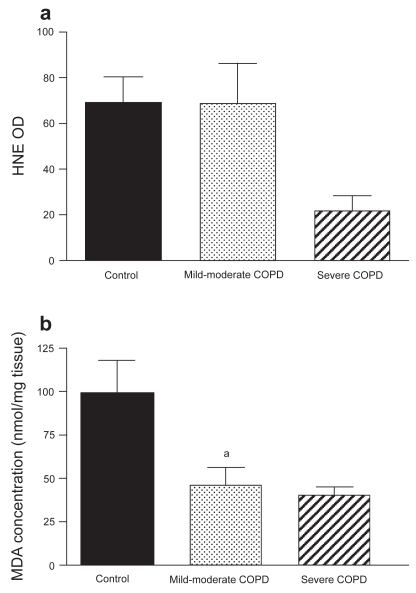
Figure 4 shows the effect of COPD on markers for lipid peroxidation in the diaphragm. HNE-protein adduct formation was not significantly different between severe COPD (n = 2), mild-to-moderate COPD (n = 6), and non-COPD patients (n = 7). No relationship was found between HNE-protein adducts and severity of hyperinflation in the COPD group. Immunofluorescence analysis showed positive staining for HNE-protein adducts localized within the muscle fibers in both COPD patients and non-COPD patients (Figure 5).
Figure 4.
(a) 4-Hydroxy-2-nonenal (HNE) optical density (OD) (mean ± SEM) in diaphragms of non-COPD patients and both mild-to-moderate and severe COPD patients. HNE-protein adduct levels were not different between the three groups. (b) Malondialdehyde (MDA) levels (mean ± SEM) in diaphragms of non-COPD patients and both mild-to-moderate and severe COPD patients. MDA levels were significantly lower in diaphragms of mild-to-moderate COPD patients (ap < 0.05) and tended to be lower in severe COPD patients (p = 0.06).
Figure 5.
Immunofluorescence analysis of 4-hydroxy-2-nonenal-protein adduct localization in diaphragm muscle of 1 representative non-COPD patient (a) and 1 representative severe COPD patient (b). The staining is localized diffusely within the muscle fibers, with some fibers staining more intensely than other fibers. Scale bar = 100 μm.
Diaphragm MDA level was significantly lower in mild-to-moderate COPD patients (n = 6) compared with non-COPD patients (n = 3). MDA level in severe COPD patients (n = 2) tended to be lower than in non-COPD patients.
Discussion
Our results demonstrate that in the diaphragm of COPD patients, catalase activity is increased and lipid peroxidation is decreased. Furthermore, with increasing severity of hyperinflation, the level of protein tyrosine nitration is reduced.
Oxidative and nitrosative stress may be expected to occur in the diaphragm of COPD patients. Firstly, antioxidative capacity may be altered. In this study, we found that the activity of catalase is increased in the diaphragm of COPD patients. This finding is in accordance with the increase in diaphragmatic antioxidant capacity after endurance training in rats (Vincent et al 1999) and mice (Salminen and Vihko 1983), and in line with progressive endurance training of the human diaphragm due to increased load over several decades (Levine et al 2001). Barreiro et al (2005) reported a tendency towards a decrease in catalase protein content with increasing severity of hyperinflation. However, catalase protein content is not directly related to catalase activity (Wilson and Johnson 2000), so our findings do not necessarily contrast with the findings by Barreiro et al.
Secondly, alterations in the level of nitrotyrosines, a marker for peroxynitrite generation, may occur. Peroxynitrite is a reaction product of NO and ·O2− . In contrast to our hypothesis, NT level was decreased with increasing severity of hyperinflation. In rat gastrocnemius muscle, endurance training increases NT formation (Vassilakopoulos et al 2003). A possible explanation is that the activity of, eg superoxide dismutase (SOD), may also be increased in the diaphragm of COPD patients, since training-induced increases in SOD activity have been reported in rat vastus lateralis (Leeuwenburgh et al 1994, 1997). This increase might decrease levels of ·O2−, leading to decreased peroxynitrite formation and thus lower NT levels.
Another consequence of an alteration in redox state that may occur in the diaphragm of COPD patients, is a change in lipid peroxidation. In this study, we found a decreased level of MDA in the diaphragm of COPD patients, but no change in HNE-protein adduct formation, which is most likely related to the small sample size. The determination of MDA and HNE are different methods, based on different principles. HNE is recognized as the most reliable marker of lipid peroxidation (Uchida and Stadtman 1992; Toyokuni et al 1995), but the number of measurements in severe COPD patients was low. For determination of MDA, we used a specific, sensitive, and reproducible HPLC method that allows a good separation between the MDA-TBA and interfering substances (Wong et al 1987). The reduced MDA levels observed in our study strongly indicate that enhanced lipid peroxidation occurs in the diaphragm of COPD patients. Since the diaphragm of COPD patients shows endurance training-like alterations (Levine et al 2002), we suggest that the lower level of lipid peroxidation also resembles the effect of endurance training. Indeed, the decreased level of lipid peroxidation in our study is in accordance with studies in skeletal muscle after endurance training in mice (Salminen and Vihko 1983) and in diaphragm muscle of endurance-trained rats (Vincent et al 1999).
In contrast to the reduced lipid peroxidation in diaphragm muscle of COPD patients, lipid peroxidation seems to be increased in peripheral muscle of COPD patients. For example, Allaire et al (2002) found an increase in lipofuscin content, a marker for oxidative damage to lipids, in quadriceps muscle of COPD patients. This finding contrasts with those of other studies (Rabinovich et al 2001; Couillard et al 2003), reporting equal levels of lipid peroxidation in vastus lateralis muscle of resting COPD patients as compared with controls, evaluated by the concentration of thiobarbituric acid reactive substances (TBARs). This discrepancy may be explained by different methods that were used to assess lipid peroxidation; whereas TBARs are markers of acute oxidative stress, lipofuscin, which accumulates in the cells, is a reflector of cumulative stress. Moreover, the latter study (Couillard et al 2003) reported an increase in lipid peroxidation after an exercise bout in vastus lateralis of COPD patients. Furthermore, glutathione peroxidase activity in vastus lateralis muscle of healthy subjects was increased after a single bout of exercise, whereas the antioxidant activity of COPD patients was not changed. This indicates that vastus lateralis muscle of COPD patients is more vulnerable to oxidative stress as a result of a compromised exercise-induced increase in antioxidative function. Besides determination of lipid peroxidation in the muscle, plasma lipid peroxidation can be used as a marker for free radical-induced tissue damage. We (Heunks et al 1999) reported previously that in rest there was no difference in serum MDA level between COPD patients receiving the xanthine oxidase inhibitor allopurinol and control COPD patients. However, after exhaustive exercise, MDA levels were significantly higher in the control COPD subjects, suggesting that during exercise lipid peroxidation occurs, which can be attenuated by treatment with allopurinol.
In the diaphragm of COPD patients a shift occurs from type II to type I fibers (Levine et al 1997) and it has been previously shown that type I fibers are more susceptible to lipid peroxidation (Allaire et al 2002). However, in our study we have shown decreased lipid peroxidation levels in the diaphragm of COPD patients. This might be explained by the finding that antioxidative capacity is increased, thereby protecting the muscle fibers against lipid peroxidation. In contrast, in peripheral muscles of COPD patients a shift occurs towards type II fibers (Maltais et al 1996; Whittom et al 1998; Gosker et al 2002), having a lower antioxidant capacity than type I fibers (Caillaud et al 1999; Ji et al 1992). This may explain the fact that in peripheral skeletal muscles of COPD patients more lipid peroxidation is observed, although the exact relationship between fiber type composition and antioxidant capacity remains to be elucidated in COPD. These findings together suggest that the disuse of peripheral muscles in COPD results in enhanced lipid peroxidation, whereas the chronically increased loading on the diaphragm in COPD results in a decrease in lipid peroxidation.
In summary, this study shows that in the diaphragm of COPD patients, catalase activity as a marker for antioxidant capacity is increased, associated with a decrease in oxidative and nitrosative stress. From a clinical perspective, this adaptation may be beneficial for diaphragm function, as it has been shown that oxidative stress is associated with muscle dysfunction.
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Allaire J, Maltais F, LeBlanc P, et al. Lipofuscin accumulation in the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Muscle Nerve. 2002;25:383–9. doi: 10.1002/mus.10039. [DOI] [PubMed] [Google Scholar]
- Barreiro E, de la Puente B, Minguella J, et al. Oxidative stress and respiratory muscle dysfunction in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:1116–24. doi: 10.1164/rccm.200407-887OC. [DOI] [PubMed] [Google Scholar]
- Barreiro E, Gea J, Corominas JM, et al. Nitric oxide synthases and protein oxidation in the quadriceps femoris of COPD patients. Am J Resp Cell Mol Biol. 2003;29:771–8. doi: 10.1165/rcmb.2003-0138OC. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite:the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–37. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- Caillaud C, Py G, Eydoux N, et al. Antioxidants and mitochondrial respiration in lung, diaphragm, and locomotor muscles:effect of exercise. Free Radic Biol Med. 1999;26:1292–9. doi: 10.1016/s0891-5849(98)00342-6. [DOI] [PubMed] [Google Scholar]
- Couillard A, Maltais F, Saey D, et al. Exercise-induced quadriceps oxidative stress and peripheral muscle dysfunction in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:1664–9. doi: 10.1164/rccm.200209-1028OC. [DOI] [PubMed] [Google Scholar]
- Gosker HR, van Mameren H, van Dijk PJ, et al. Skeletal muscle fibre-type shifting and metabolic profile in patients with chronic obstructive pulmonary disease. Eur Respir J. 2002;19:617–25. doi: 10.1183/09031936.02.00762001. [DOI] [PubMed] [Google Scholar]
- Heunks LM, Bast A, van Herwaarden CL, et al. Effects of emphysema and training on glutathione oxidation in the hamster diaphragm. J Appl Physiol. 2000;88:2054–61. doi: 10.1152/jappl.2000.88.6.2054. [DOI] [PubMed] [Google Scholar]
- Heunks L M, Vina J, van Herwaarden CL, et al. Xanthine oxidase is involved in exercise-induced oxidative stress in chronic obstructive pulmonary disease. Am J Physiol. 1999;277:R1697–704. doi: 10.1152/ajpregu.1999.277.6.R1697. [DOI] [PubMed] [Google Scholar]
- Hickner RC, Fisher JS, Ehsani AA, et al. Role of nitric oxide in skeletal muscle blood flow at rest and during dynamic exercise in humans. Am J Physiol. 1997;273:H405–10. doi: 10.1152/ajpheart.1997.273.1.H405. [DOI] [PubMed] [Google Scholar]
- Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9:515–40. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- Ji LL, Fu R, Mitchell EW. Glutathione and antioxidant enzymes in skeletal muscle:effects of fiber type and exercise intensity. J Appl Physiol. 1992;73:1854–9. doi: 10.1152/jappl.1992.73.5.1854. [DOI] [PubMed] [Google Scholar]
- Kobzik L, Reid MB, Bredt DS, et al. Nitric oxide in skeletal muscle. Nature. 1994;372:546–8. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- Kolbeck RC, She ZW, Callahan LA, et al. Increased superoxide production during fatigue in the perfused rat diaphragm. Am J Respir Crit Care Med. 1997;156:140–5. doi: 10.1164/ajrccm.156.1.9610041. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Fiebig R, Chandwaney R, et al. Aging and exercise training in skeletal muscle:responses of glutathione and antioxidant enzyme systems. Am J Physiol. 1994;267:R439–45. doi: 10.1152/ajpregu.1994.267.2.R439. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Hollander J, Leichtweis S, et al. Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fiber specific. Am J Physiol. 1997;272:R363–9. doi: 10.1152/ajpregu.1997.272.1.R363. [DOI] [PubMed] [Google Scholar]
- Levine S, Kaiser L, Leferovich J, et al. Cellular adaptations in the diaphragm in chronic obstructive pulmonary disease. N Engl J Med. 1997;337:1799–806. doi: 10.1056/NEJM199712183372503. [DOI] [PubMed] [Google Scholar]
- Levine S, Nguyen T, Shrager J, et al. Diaphragm adaptations elicited by severe chronic obstructive pulmonary disease:lessons for sports science. Exerc Sport Sci Rev. 2001;29:71–5. doi: 10.1097/00003677-200104000-00006. [DOI] [PubMed] [Google Scholar]
- Levine S, Gregory C, Nguyen T, et al. Bioenergetic adaptation of individual human diaphragmatic myofibers to severe COPD. J Appl Physiol. 2002;92:1205–13. doi: 10.1152/japplphysiol.00116.2001. [DOI] [PubMed] [Google Scholar]
- Maltais F, Simard AA, Simard C, et al. Oxidative capacity of the skeletal muscle and lactic acid kinetics during exercise in normal subjects and in patients with COPD. Am J Respir Crit Care Med. 1996;153:288–93. doi: 10.1164/ajrccm.153.1.8542131. [DOI] [PubMed] [Google Scholar]
- Ottenheijm CA, Heunks LM, Sieck GC, et al. Diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:200–5. doi: 10.1164/rccm.200502-262OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich RA, Ardite E, Troosters T, et al. Reduced muscle redox capacity after endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1114–18. doi: 10.1164/ajrccm.164.7.2103065. [DOI] [PubMed] [Google Scholar]
- Reid MB, Haack KE, Franchek KM, et al. Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol. 1992;73:1797–804. doi: 10.1152/jappl.1992.73.5.1797. [DOI] [PubMed] [Google Scholar]
- Reid MB, Shoji T, Moody MR, et al. Reactive oxygen in skeletal muscle. II. Extracellular release of free radicals. J Appl Physiol. 1992;73:1805–9. doi: 10.1152/jappl.1992.73.5.1805. [DOI] [PubMed] [Google Scholar]
- Reid MB. Plasticity in skeletal, cardiac, and smooth muscle:Invited Review:Redox modulation of skeletal muscle contraction:what we know and what we don’t. J Appl Physiol. 2001;90:724–31. doi: 10.1152/jappl.2001.90.2.724. [DOI] [PubMed] [Google Scholar]
- Roy D, Perreault M, Marette A. Insulin stimulation of glucose uptake in skeletal muscles and adipose tissues in vivo is NO dependent. Am J Physiol. 1998;274:E692–9. doi: 10.1152/ajpendo.1998.274.4.E692. [DOI] [PubMed] [Google Scholar]
- Salminen A, Vihko V. Endurance training reduces the susceptibility of mouse skeletal muscle to lipid peroxidation in vitro. Acta Physiol Scand. 1983;117:109–13. doi: 10.1111/j.1748-1716.1983.tb07184.x. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Meissner G. Physiology of Nitric Oxide in Skeletal Muscle. Physiol Rev. 2001;81:209–37. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- Suko J, Drobny H, Hellmann G. Activation and inhibition of purified skeletal muscle calcium release channel by NO donors in single channel current recordings. Biochim Biophys Acta. 1999;1451:271–87. doi: 10.1016/s0167-4889(99)00098-1. [DOI] [PubMed] [Google Scholar]
- Toyokuni S, Miyake N, Hiai H, et al. The monoclonal antibody specific for the 4-hydroxy-2-nonenal histidine adduct. FEBS Lett. 1995;359:189–91. doi: 10.1016/0014-5793(95)00033-6. [DOI] [PubMed] [Google Scholar]
- Uchida K, Stadtman ER. Modification of histidine residues in proteins by reaction with 4-hydroxynonenal. Proc Natl Acad Sci U S A. 1992;89:4544–8. doi: 10.1073/pnas.89.10.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilakopoulos T, Deckman G, Kebbewar M, et al. Regulation of nitric oxide production in limb and ventilatory muscles during chronic exercise training. Am J Physiol. 2003;284:L452–7. doi: 10.1152/ajplung.00270.2002. [DOI] [PubMed] [Google Scholar]
- Vincent HK, Powers SK, Demirel HA, et al. Exercise training protects against contraction-induced lipid peroxidation in the diaphragm. Eur J Appl Physiol Occup Physiol. 1999;79:268–73. doi: 10.1007/s004210050505. [DOI] [PubMed] [Google Scholar]
- Whittom F, Jobin J, Simard PM, et al. Histochemical and morphological characteristics of the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Med Sci Sports Exerc. 1998;30:1467–74. doi: 10.1097/00005768-199810000-00001. [DOI] [PubMed] [Google Scholar]
- Wijnhoven JH, Hafmans T, Dekhuijzen PNR. A morphological study of oxidative stress in diaphragm muscle in COPD. Am J Respir Crit Care Med. 2004;169:A241. [Google Scholar]
- Wilson DO, Johnson P. Exercise modulates antioxidant enzyme gene expression in rat myocardium and liver. J Appl Physiol. 2000;88:1791–6. doi: 10.1152/jappl.2000.88.5.1791. [DOI] [PubMed] [Google Scholar]
- Wong SH, Knight JA, Hopfer SM, et al. Lipoperoxides in plasma as measured by liquid-chromatographic separation of malondialdehydethiobarbituric acid adduct. Clin Chem. 1987;33:214–20. [PubMed] [Google Scholar]
- Zhu X, Heunks LM, Versteeg EM, et al. Hypoxia-induced dysfunction of rat diaphragm:role of peroxynitrite. Am J Physiol. 2005;288:L16–26. doi: 10.1152/ajplung.00412.2003. [DOI] [PubMed] [Google Scholar]
- Zidoni E, Kremer ML. Kinetics and mechanism of catalase action. Formation of the intermediate complex. Arch Biochem Biophys. 1974;161:658–64. doi: 10.1016/0003-9861(74)90351-8. [DOI] [PubMed] [Google Scholar]