Abstract
Lung volume reduction surgery (LVRS) has been shown to improve lung function and exercise tolerance in patients with severe emphysema. Some predictors of poor outcome have been described but the role of alpha1-antitrypsin (α1-AT) deficiency is still not well known. The aim of this study was to analyze the results of unilateral LVRS in our center according to the α1-AT status. The results of LVRS in 17 deficient patients and 35 nondeficient patients were analyzed at 3–6 months and 1 year after surgery. Compared with baseline, a significant improvement of FEV1, partial pressure in arterial blood (PaO2), dyspnea score and walking distance was observed in the two groups at 3–6 months after surgery and the studied parameters remained significantly improved at 1 year in the nondeficient group. By contrast, PaO2 and walking distance returned towards baseline in the deficient group at 1 year whereas improvement of FEV1 and dyspnea score was persistent. Mean values of FEV1 at baseline, 3–6 months, and 1 year were 22 ± 6%, 29 ± 11%, and 26 ± 9% and 28 ± 12%, 38 ± 17%, and 40 ± 17% predicted in the deficient group and in the non-deficient group, respectively. In conclusion, the functional benefit is short-lasting in α1-AT deficient patients after unilateral LVRS.
Keywords: alpha1-antitrypsin deficiency, lung volume reduction surgery, emphysema
Introduction
Emphysema is a progressive, debilitating disease associated with a high rate of morbidity. LVRS has been shown to be effective in providing short-term, medium-term, and long-term benefit in patients with emphysema (Cooper et al 1996; Flaherty and Martinez 2000; Yusen et al 2003; Weder 2003). The NETT study, a large, randomized, controlled trial comparing LVRS and medical therapy, has also shown that LVRS is able to provide a better 24-month functional outcome than medical treatment alone but without difference in mortality rate (NETT 2003). Moreover, this study was able to define a group of patients with high risk of mortality after LVRS (NETT 2001). While several teams have identified predictive factors of good functional results (Wang et al 1997; NETT 2003; Ingenito et al 1998, 2001; Thurnheer et al 1999), the influence of α1-AT deficiency on functional results remains debated. The studies which have focused on the results of LVRS in patients with α1-AT deficiency have yielded controversial results (Cooper et al 1996; Cassina et al 1998; Gelb et al 1999; Rischer et al 1999). Taking these results into account, the ATS–ERS statement on the standards for the diagnosis and management of individuals with α1-AT deficiency has recently concluded that LVRS offers only short-term benefits for most deficient patients and that LVRS should not be recommended in these patients pending additional studies (ATS–ERS 2003).
In 1994, a prospective program of LVRS was started at our center. The α1-AT deficient patients were not excluded a priori from the program thus giving us an opportunity to evaluate the benefit of LVRS in these patients. The aim of the present study was to analyze our results of LVRS in patients with α1-AT deficiency and to compare these results with those obtained in patients without deficiency.
Materials and methods
Patients who met the predefined selection criteria (see below), and who accepted the risks and the uncertainties of LVRS underwent either bilateral or unilateral LVRS which were performed by the same surgeon (GL). After surgery, the patients were asked to visit our center at regular intervals in order to assess their functional results. Between January 1994 and December 2001, 66 patients underwent unilateral LVRS whereas 17 patients had bilateral surgery.
In order to homogenize the data, we selected only the patients who underwent unilateral LVRS. Among the 66 patients who had unilateral LVRS, 13 patients in whom the α1-AT status was unknown were excluded from this study. One patient who underwent LVRS after lung transplantation was also excluded. Thus, 52 patients in whom the α1-AT status was known form the basis of the study. α1-AT deficiency was found in 17 patients (deficient group) whereas 35 patients had no deficiency (nondeficient group). α1-AT deficiency was defined as PiZZ phenotype or α1-AT serum level below 50 mg/ml. The files of these patients were analyzed and several items were retrieved: preoperative characteristics, perioperative and overall mortality, post-operative morbidity. The functional results of LVRS in the two groups were studied at 3–6 months and at 1 year after surgery.
Selection criteria
To be considered for LVRS, the patients had to meet the following selection criteria: physiologic evidence of severe airflow obstruction (FEV11-AT serum level below 50 ≤ 40% predicted), hyperinflation (defined as a TLC value above predicted without threshold of TLC), severe dyspnea defined as a Fletcher dyspnea score ≥ 2, and CT scan evidence of advanced emphysema with some degree of heterogeneity in the distribution of emphysema.
The exclusion criteria for LVRS were as follows: age > 75 years, presence of giant bulla defined as a bulla > 1/3 of hemithorax on the CT scan, severe left ventricular dysfunction, or BMI < 18 kg.m−2. The levels of hypercapnia and pulmonary hypertension were not considered as exclusion criteria.
Pre- and post-operative evaluation
All patients underwent baseline pulmonary function tests, CT scan of the thorax, and lung ventilation–perfusion scan. Cardiac function evaluation was made using echocardiography and right heart catheterization. Coronary angiography was performed in case of symptoms. Pulmonary function tests included spirometry, and measurement of DLCO and thoracic gas volumes. TLC was measured by standardized body plethysmography (MedGraphics 1085 series Plethysmograph). Exercise capacity was assessed by the 6MWD test. Arterial blood gas analysis was made at rest on room air (AVL analyser, Radiometer, Copenhagen). Severity of pre- and post-operative dyspnea was assessed using the score described by Fletcher (1952). Pulmonary artery pressure and cardiac index were evaluated during right heart catheterization. Pre-operative CT and ventilation–perfusion scans were used to identify target areas for lung resection. Post-operatively, all these tests were repeated between 3 and 6 months and at 1 year except for right heart catheterization which was not performed after surgery.
Surgical procedure
All patients underwent unilateral LVRS at the same center, by the same thoracic surgeon (GL). The LVRS procedure was performed in all cases via unilateral thoracotomy. The worst areas of emphysematous lung were resected by stapling guided by the results of CT and lung perfusion scan.
Statistical analysis
Baseline characteristics are reported as mean and standard deviation. Preoperative and post-operative differences between groups were compared by means of Student’s t-test. In each group, comparisons between baseline values and values at 3–6 months and between baseline values and values at 1 year were made using paired Student’s t-test. The comparison of the relative gain of FEV1 at 3–6 months and 1 year from baseline between the two groups was analyzed using t-test (the variables are normally distributed). The proportion of patients with improved FEV1 (defined as a gain > 150 ml) and 6MWD (defined as a gain > 50 m) at 3–6 months and 1 year was assessed by a χ2 test. A p value ≤ 0.05 was considered statistically significant.
Results
Baseline characteristics of the patients are shown in Table 1. All patients were dyspneic at rest or for mild exercise (Fletcher dyspnea score = 4.2 ± 0.7 in the deficient group and 3.6 ± 0.8 in the nondeficient group; p = 0.02). We observed a severe limitation in exercise capacity in the two groups (6MWD = 237 ± 145 m and 340 ± 173 m in the deficient group and in the nondeficient groups, respectively; p = 0.04).
Table 1.
Baseline characteristics of patients with α1-AT deficiency and without deficiency
| Value | α1-AT deficient group n=17 | Nondeficient group n=35 | p |
|---|---|---|---|
| Age (years) | 56 ± 9a | 54 ± 11 | 0.62 |
| BMI (m2/kg) | 20 ± 4 | 22 ± 4 | 0.07 |
| Smoking habit (pack-years) | 18.4 ± 14 | 41.1 ± 27 | 0.007 |
| FEV1 (% pred) | 22.2 ± 5.7 | 28 ± 11.9 | 0.06 |
| FEV1 (ml) | 613 ± 163 | 907 ± 453 | 0.013 |
| FEV1 post-BD (% pred) | 24 ± 6.7 | 29.9 ± 13 | 0.09 |
| FVC (% pred) | 48 ± 11 | 50 ± 17 | 0.65 |
| RV (% pred) | 278 ± 48 | 259 ± 71 | 0.34 |
| FRC (% pred) | 201 ± 35 | 182 ± 34 | 0.06 |
| TLC (% pred) | 138 ± 18 | 129 ± 21 | 0.15 |
| IC (% pred) | 62 ± 19 | 67 ± 18 | 0.37 |
| 6MWD (m) | 237 ± 145 | 340 ± 173 | 0.039 |
| Fletcher score | 4.18 ± 0.73 | 3.57 ± 0.85 | 0.015 |
| PaO2 (mmHg) | 62 ± 9.7 | 67.6 ± 10.8 | 0.07 |
| PaCO2 (mmHg) | 40 ± 6.71 | 40.7 ± 6.1 | 0.7 |
| PAP systolic (mmHg) | 37 ± 7.3 | 36 ± 7.7 | 0.55 |
Values are expressed as mean ± standard deviation.
Abbreviations: BD, bronchodilator; pred, predicted.
The deficient patients tended to have more severe airflow obstruction than nondeficient patients (FEV1= 22.2 ± 6% predicted [pred] and 28 ± 12% pred in the deficient group and in the nondeficient group, respectively; p = 0.06). Severe hyper-inflation was observed in the two groups (TLC = 138 ± 18% pred and RV = 278 ± 48% pred in the deficient group, TLC = 129 ± 21% pred and RV = 259 ± 71% pred in the nondeficient group; p = 0.15 and p = 0.34, respectively). Mean PaO2 was 62 ± 10 mmHg in the deficient group and 68 ± 11 mmHg in the nondeficient group (p = 0.07)
One patient in the nondeficient group and 1 patient in the deficient group had severe hypercapnia defined by a PaCO2 level of more than 55 mmHg. Severe pulmonary hypertension (systolic pulmonary artery pressure > 55 mmHg) was observed in 1 patient with α1-AT deficiency and in none of the patients without deficiency. Sixteen patients in the deficient group and 12 patients in the nondeficient group had oxygen supplementation at rest. Out of the 17 patients with α1-AT deficiency, the PI phenotype was available in 15. All of them had PIZZ phenotype.
Upon CT scan examination, all patients had heterogeneously distributed emphysema, which predominated on the lower lobe in all the α1-AT deficient patients and on the lower or the upper lobes in the nondeficient group. Among the 35 patients in the latter group, 19 had predominant lower lobe lesions.
Mortality and morbidity
Hospital mortality was defined as death occurring before a patient’s discharge from the hospital after LVRS. Two patients died within this period, resulting in a mortality rate of 3.8%. These patients from the nondeficient group died at day 16 from septic shock related to peritonitis and at day 36 from bacterial pneumonia. Neither patients with PaCO2 level > 55 mmHg or pulmonary artery hypertension > 55 mmHg died during hospital stay. If we except the patients who died in the early post-operative period, the remaining 50 patients were alive at 3–6 months and at 1 year. Thus, the 1-year mortality rate among the followed patients was 3.8%.
LVRS morbidity consisted of lower respiratory tract infection and persistent air leak. No other complication was observed in our series. The average duration of chest drainage was 9.0 ± 5 days and 9.8 ± 6 days in the nondeficient group and in the deficient group, respectively. The average duration of hospital stay was 24 days in the two groups.
Concerning lower respiratory tract infection, 3 of the 17 patients in the deficient group and 4 of the 35 patients in the nondeficient group developed nosocomial purulent bronchitis or pneumonia.
Functional results
At 3–6 months, functional data were available in all patients (except in the two who died early in the nondeficient group) while the data were lacking at 1 year in 8 other patients (1 in the deficient group and 7 in the nondeficient group). While 1 of these 8 patients with nonavailable functional results at 1 year was reoperated on the controlateral side between 6 months and 1 year, the other 7 patients did not undergo their 1-year evaluation. The functional results in the two groups are shown in Table 2 and in Figures 1, 2, and 3.
Table 2.
Follow-up data after LVRS in the α1-AT deficient and nondeficient groups
| Group | FEV1 (% pred) | RV (% pred) | TLC (% pred) | FRC (% pred) | 6MWD (m) | dyspnea score | PaO2 (mmHg) | PaCO2 (mmHg) | |
|---|---|---|---|---|---|---|---|---|---|
| α1-AT deficiency | pre-op (n = 17) | 22.2 ± 5.7a | 278 ± 48 | 138 ± 18 | 201 ± 35 | 237 ± 145 | 4.18 ± 0.73 | 62 ± 9.7 | 40 ± 6.71 |
| 3–6 months (n = 17) | 28.9 ± 11.2* | 248 ± 57 | 132 ± 16 | 191 ± 28 | 310 ± 154* | 3.1 ± 0.9* | 67 ± 6.8* | 38 ± 4.4 | |
| 12 months (n = 16) | 25.9 ± 9.1* | 259 ± 13.5 | 133 ± 20 | 200 ± 32 | 270 ± 151 | 3.2 ± 0.6* | 65.2 ± 8.2 | 38.6 ± 4 | |
| nondeficient group | pre-op (n = 35) | 28 ± 11.9 | 259 ± 71 | 129 ± 21 | 182 ± 34 | 340 ± 173 | 3.57 ± 0.85 | 67.6 ± 10.8 | 40.7 ± 6.1 |
| 3–6 months (n = 33) | 36.6 ± 16.8* | 217 ± 71* | 119 ± 20* | 166 ± 37* | 408 ± 163* | 2.7 ± 1.2* | 72.3 ± 12.4* | 40.2 ± 6.1 | |
| 12 months (n = 26) | 39.7 ± 17.2* | 209 ± 63* | 119 ± 18* | 161 ± 32* | 421 ± 153* | 2.6 ± 1.2* | 71.8 ± 9.3* | 38.5 ± 3.5 |
Significantly different from baseline value.
Values are expressed as mean ± standard deviation.
Abbreviations: pred, predicted; pre-op, pre-operatively.
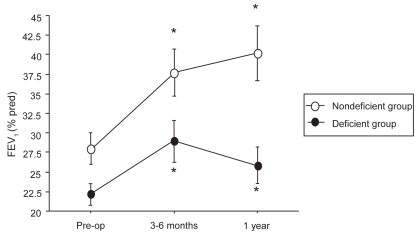
Figure 1.
Evolution of FEV1 before LVRS, at 3–6 months, and at 1 year after LVRS.
*p < 0.05 compared with pre-operative values.
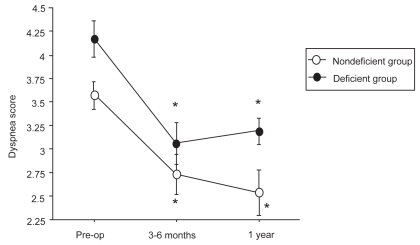
Figure 2.
Evolution of dyspnea score before LVRS, at 3–6 months, and at 1 year after LVRS.
*p < 0.05 compared with pre-operative values.
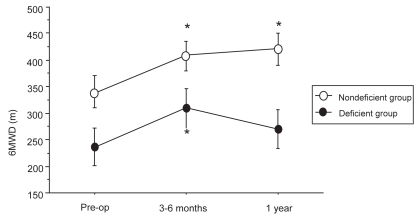
Figure 3.
Evolution of 6MWD before LVRS, at 3–6 months, and at 1 year after LVRS.
*p < 0.05 compared with pre-operative values.
Deficient group
Compared with baseline, a significant improvement of mean FEV1 at 3–6 months was observed in the α1-AT deficient group (from 22.2 ± 6% pred to 28.9 ± 11% pred; p < 0.002). A significant gain in PaO2 and 6MWD and a significant decrease in dyspnea score were also observed. At 1 year postoperative, a decline in pulmonary function values, 6MWD, and PaO2 was noted but FEV1 value and dyspnea score remained significantly improved compared with baseline.
Nondeficient group
In the nondeficient group, RV, TLC, FRC, FEV1, 6MWD, dyspnea score, and PaO2 values improved significantly at 3–6 months compared with baseline, the gain being persistent at 1 year.
Comparison between the two groups
At 3–6 months, the FEV1 values were not significantly different but a statistical difference was observed at 1 year (26 ± 9% and 40 ± 17% pred, in the deficient group and the nondeficient group respectively; p < 0.005). Compared with pre-operative mean value, the relative gain in FEV1 at 3–6 months was 29 ± 26% and 34 ± 36% pred in the deficient and in the nondeficient group, respectively (p = NS). The relative gain in FEV1 at 1 year compared with baseline tended to be higher in the nondeficient group than in the deficient group (34 ± 35% and 16 ± 24%, respectively, without reaching statistical significance; p = 0.07).
The proportion of patients with improvement in FEV1 and 6MWD in the two groups is given in Table 3. Although no statistical significance was achieved, the percentage of patients improved at 1 year tended to be higher in the non-deficient group.
Table 3.
Number of patients with persistent increase in FEV1 and 6MWD according to α1-AT deficiency status (gain expressed in comparison with baseline)
| Parameters | Group | at 6 months | at 12 months | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| gain in FEV1 | |||||
| > 150 ml | nondeficient | 13/33 | 39.4 | 9/26 | 34.6 |
| deficient | 4/17 | 23.5 | 3/16 | 18.7 | |
| gain in 6MWD | |||||
| > 50 m | nondeficient | 21/33 | 63.6 | 15/26 | 57.7 |
| deficient | 12/17 | 70.6 | 6/17 | 35.3 | |
Discussion
α1-AT deficiency is an hereditary disorder characterized by low serum levels of α1-AT and increased risk of emphysema at an early age. Augmentation therapy by regular intravenous infusion of α1-AT has been shown to increase the serum level of this protein and is recommended for the management of α1-AT deficient patients, particularly in individuals with moderate airflow obstruction (ATS–ERS 2003). A clinical efficacy in terms of survival or pulmonary function has been suggested but has never been convincingly demonstrated (Seersholm et al 1997; AADR 1998; Wencker et al 2001). Besides augmentation therapy, 2 surgical procedures may be considered in case of advanced emphysema: lung transplantation and LVRS.
In their study describing the results of 150 patients who underwent LVRS, Cooper et al (1996) stated that the benefit was less important in patients with lower lobe lesions or α1-AT deficiency. The functional results of LVRS in deficient patients have been previously analyzed in a more specific way by several authors (Cassina et al 1998; Gelb et al 1999; Ritscher et al 1999). Cassina et al (1998) noted a transient functional improvement in patients with α1-AT deficiency with return to baseline 6–12 months after surgery except for 6MWD. In contrast, Gelb et al (1999), reporting the results of LVRS in 6 α1-AT deficient patients, observed a modest improvement in some functional parameters lasting more than 22 months post-operative in 4 cases. Similarly, no important differences were observed 18 months after surgery between 8 α1-AT deficient patients and 46 patients without deficiency (Ritscher et al 1999). Recently, Tutic et al (2004) reported results of LVRS in 21 α1-AT deficient patients. A functional benefit in terms of FEV1 persisted up to 1 year after surgery but the gain of LVRS was smaller and shorter lasting than in patients with pure smoker’s emphysema. In the above-mentioned studies, baseline status of the patients was similar to that of our patients, but the surgical approach was always bilateral.
The results of the present study show that the effects of LVRS in α1-AT deficient patients are short lasting since most functional parameters returned towards baseline at 1 year after surgery. The magnitude of the gain in FEV1, walking distance, and dyspnea score in the deficient group was not lower than in nondeficient patients but the duration of improvement was shorter. Thus, our results are in accordance with those of Cassina et al (1998) but are not in opposition to those of Tutic et al (2004). In the latter study, the duration of improvement was somewhat longer but the difference might be explained by the distribution of emphysema, since better functional results have been observed after upper lobe surgery (Cooper et al 1996; McKenna et al 1997; Ingenito et al 1998; Coxson et al 2003; NETT 2003). As a matter of fact, the predominant site of destruction was in the lower lobes in all of our patients but only in 10/21 patients in the study by Tutic et al (2004). Another finding of our study was the difference found in α1-AT deficient patients between objective improvement (lung volume measurements, 6MWD, PaO2) and subjective benefit (dyspnea score) which persisted for 1 year. Such a difference had been previously reported in nondeficient patients (Gelb et al 1998, 2001; Flaherty et al 2001) and was also found by Tutic et al (2004).
By contrast with previous studies, our study analyzed the results of unilateral LVRS in deficient patients. Our surgical approach (unilateral thoracotomy) could not explain the poorer results of LVRS in α1-AT deficient patients. Indeed, the nondeficient patients had the same surgical approach, giving results in accordance with those previously reported after unilateral LVRS (relative gain in FEV1 of 20%–30% in most studies) (Flaherty and Martinez 2000). α1-AT deficient patients are known to have emphysematous lesions which predominate on lower lobes, and, as said previously, poorer 6-month results have been observed when LVRS is performed on lower lobes rather than on upper lobes (Cooper et al 1996; McKenna et al 1997; NETT 2003; Ingenito et al 1998; Coxson et al 2003). Thus, a question arises: are the functional results in deficient patients related to the location of emphysematous lesions or to the α1-AT deficiency per se? In our series, all deficient patients had predominant lower-lobe lesions whereas one third of nondeficient patients had predominant lower-lobe lesions. The results of LVRS in our nondeficient patients with predominant lower-lobe lesions showed that, as was the case in deficient patients, FEV1 returned to baseline at 1 year (data not shown). Therefore, we hypothesize that the poorer functional results in deficient patients might be related to nonapical emphysema surgery rather than to α1-AT deficiency.
Whether α1-AT deficient patients who present with severe emphysema are suitable for LVRS or for lung transplantation remains open to debate. The functional results of lung transplantation are clearly superior to what is provided by LVRS (Gelb et al 1998) but the risks of the procedure are much higher for lung transplantation. The current policy at our center is to explain the potential risks and benefits of both procedures in patients with emphysema and to favour LVRS when the selection criteria are met. In the particular case of α1-AT deficient patients, given our results, we tend to favour lung transplantation but the LVRS remains an option even though the duration of benefit is probably shorter than in nondeficient patients.
In summary, our experience suggests that the functional benefit is short lasting in α1-AT deficient patients who undergo unilateral LVRS for emphysema. Based on these results, we consider that LVRS remains an option but that lung transplantation, if possible, is more appropriate for most of these patients.
Abbreviations
- α1-AT
alpha1-antitrypsin
- BMI
body mass index
- FEV1
forced expiratory volume in one second
- FRC
functional residual capacity
- FVC
forced vital capacity
- IC
inspiratory capacity
- LVRS
lung volume reduction surgery
- PaO2
partial pressure in arterial blood
- PAP
pulmonary artery pressure
- RV
residual volume
- TLC
total lung capacity
- 6MWD
6 minute walking distance
- DLCO
diffusing capacity of the lungs for carbon monoxide
References
- [AADR] The alpha-1-Antitrypsin deficiency registry study group. Survival and FEV1 decline in individuals with severe deficiency of alpha1-antitrypsin. Am J Respir Crit Care Med. 1998;158:49–59. doi: 10.1164/ajrccm.158.1.9712017. [DOI] [PubMed] [Google Scholar]
- [ATR–ERS] American Thoracic Society/European Thoracic Society statement: standards for diagnossi and management of individuals with α1-Antitrypsin deficiency. Am J Respir Crit Care Med. 2003;168:818–900. doi: 10.1164/rccm.168.7.818. [DOI] [PubMed] [Google Scholar]
- Cassina PC, Teschler H, Konietzko N, et al. Two-year results after lung volume reduction surgery in α1-antitrypsin deficiency versus smoker’s emphysema. Eur Respir J. 1998;12:1028–1032. doi: 10.1183/09031936.98.12051028. [DOI] [PubMed] [Google Scholar]
- Cooper JD, Patterson G, Sunderesan RS, et al. Results of 150 consecutive bilateral lung volume reduction procedures in patients with severe emphysema. J Thorac Cardiovasc Surg. 1996;112:1319–30. doi: 10.1016/S0022-5223(96)70147-2. [DOI] [PubMed] [Google Scholar]
- Coxson HO, Whittall KP, Nakano Y, et al. Selection of patients for lung volume reduction surgery using a law analysis of the computed tomographic scan. Thorax. 2003;58:510–14. doi: 10.1136/thorax.58.6.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KR, Martinez FJ. Lung volume reduction surgery for emphysema. Clin Chest Med. 2000;21:819–48. doi: 10.1016/s0272-5231(05)70186-6. [DOI] [PubMed] [Google Scholar]
- Flaherty KR, Kazerooni EA, Curtis JL, et al. Short term and long term outcomes after bilateral lung volume reduction surgery. Chest. 2001;119:1337–46. doi: 10.1378/chest.119.5.1337. [DOI] [PubMed] [Google Scholar]
- Fletcher CM. The clinical diagnosis of pulmonary emphysema: an experimental study. Proc R Soc Med. 1952;45:577–84. [PubMed] [Google Scholar]
- Gaissert HA, Trulock EP, Cooper JD, et al. Comparison of early functional results after volume reduction or lung transplantation for chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg. 1996;111:296–306. doi: 10.1016/s0022-5223(96)70438-5. [DOI] [PubMed] [Google Scholar]
- Gelb AF, Brenner M, McKenna RJ, et al. Serial lung function and elastic recoil 2 years after lung volume reduction surgery for emphysema. Chest. 1998;113:1497–506. doi: 10.1378/chest.113.6.1497. [DOI] [PubMed] [Google Scholar]
- Gelb AF, McKenna RJ, Brenner M, et al. Lung function after bilateral lower lung volume reduction surgery for α1-antitrypsin emphysema. Eur Respir J. 1999;14:928–33. doi: 10.1034/j.1399-3003.1999.14d33.x. [DOI] [PubMed] [Google Scholar]
- Gelb AF, McKenna RJ, Brenner M, et al. Lung function 5 years after lung volume reduction surgery for emphysema. Am J Respir Crit Care Med. 2001;163:1562–6. doi: 10.1164/ajrccm.163.7.2009048. [DOI] [PubMed] [Google Scholar]
- Ingenito EP, Evans RB, Loring SH, et al. Relation between preoperative inspiratory lung resistance and the outcome of lung volume reduction surgery for emphysema. N Engl J Med. 1998;338:1181–5. doi: 10.1056/NEJM199804233381703. [DOI] [PubMed] [Google Scholar]
- Ingenito EP, Loring SH, Moy ML, et al. Comparison of physiological and radiological screening for lung volume reduction surgery. Am J Respir Crit Care Med. 2001;163:1068–73. doi: 10.1164/ajrccm.163.5.9911013. [DOI] [PubMed] [Google Scholar]
- McKenna RJ, Brenner M, Fischel RJ, et al. Patient selection criteria for lung volume reduction surgery. J Thorac Cardiovasc Surg. 1997;114:957–67. doi: 10.1016/S0022-5223(97)70010-2. [DOI] [PubMed] [Google Scholar]
- [NETT] National emphysema treatment trial research group. Patients at high risk of death after lung volume reduction surgery. N Engl J Med. 2001;345:1075–83. doi: 10.1056/NEJMoa11798. [DOI] [PubMed] [Google Scholar]
- [NETT] National emphysema treatment trial group. A randomised trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–73. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- Ritscher D, Hamacher J, Weder W, et al. Lung volume reduction surgery in patients with a1-antitrypsin deficiency and emphysema. Eur Respir J. 1999;14:S275. [Google Scholar]
- Seersholm N, Wencker M, Banik N, et al. Does alpha1-antitrypsin augmentation therapy slow the annual decline in FEV1 in patients with severe hereditary alpha1-antitrypsin deficiency? Eur Respir J. 1997;10:2260–3. doi: 10.1183/09031936.97.10102260. [DOI] [PubMed] [Google Scholar]
- Thurnheer R, Engel H, Weder W, et al. Role of lung perfusion scintigraphy in relation to chest computed tomography and pulmonary function in the evaluation of candidates for lung volume reduction surgery. Am J Respir Crit Care Med. 1999;159:301–10. doi: 10.1164/ajrccm.159.1.9711030. [DOI] [PubMed] [Google Scholar]
- Tutic M, Bloch KE, Lardinois D, et al. Long term results after lung volume reduction surgery in patienbts with α1-antitrypsin deficiency. J Thorac Cardiovasc Surg. 2004;128:408–13. doi: 10.1016/j.jtcvs.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Wang SC, Fischer KC, Slone RM, et al. Perfusion scintigraphy in the evaluation for lung volume reduction surgery: correlation with clinical outcome. Radiology. 1997;205:243–8. doi: 10.1148/radiology.205.1.9314992. [DOI] [PubMed] [Google Scholar]
- Wencker M, Fuhrmann B, Banik N, et al. Longitudinal follow-up of patient with α1-Protease inhibitor deficiency before and during therapy with IV α1-Protease inhibitor. Chest. 2001;119:737–44. doi: 10.1378/chest.119.3.737. [DOI] [PubMed] [Google Scholar]
- Weder W. Lung volume reduction surgery. Eur Resp Mon. 2003;26:40–6. [Google Scholar]
- Yusen R D, Lefrak SS, Gierada DS, et al. A prospective evaluation of lung volume reduction surgery in 200 consecutive patients. Chest. 2003;123:1026–37. doi: 10.1378/chest.123.4.1026. [DOI] [PubMed] [Google Scholar]